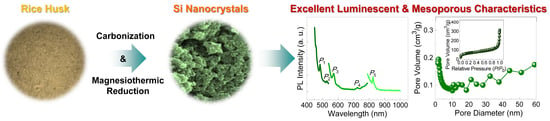
Derivation of Luminescent Mesoporous Silicon Nanocrystals from Biomass Rice Husks by Facile Magnesiothermic Reduction
Abstract
1. Introduction
2. Experimental Section
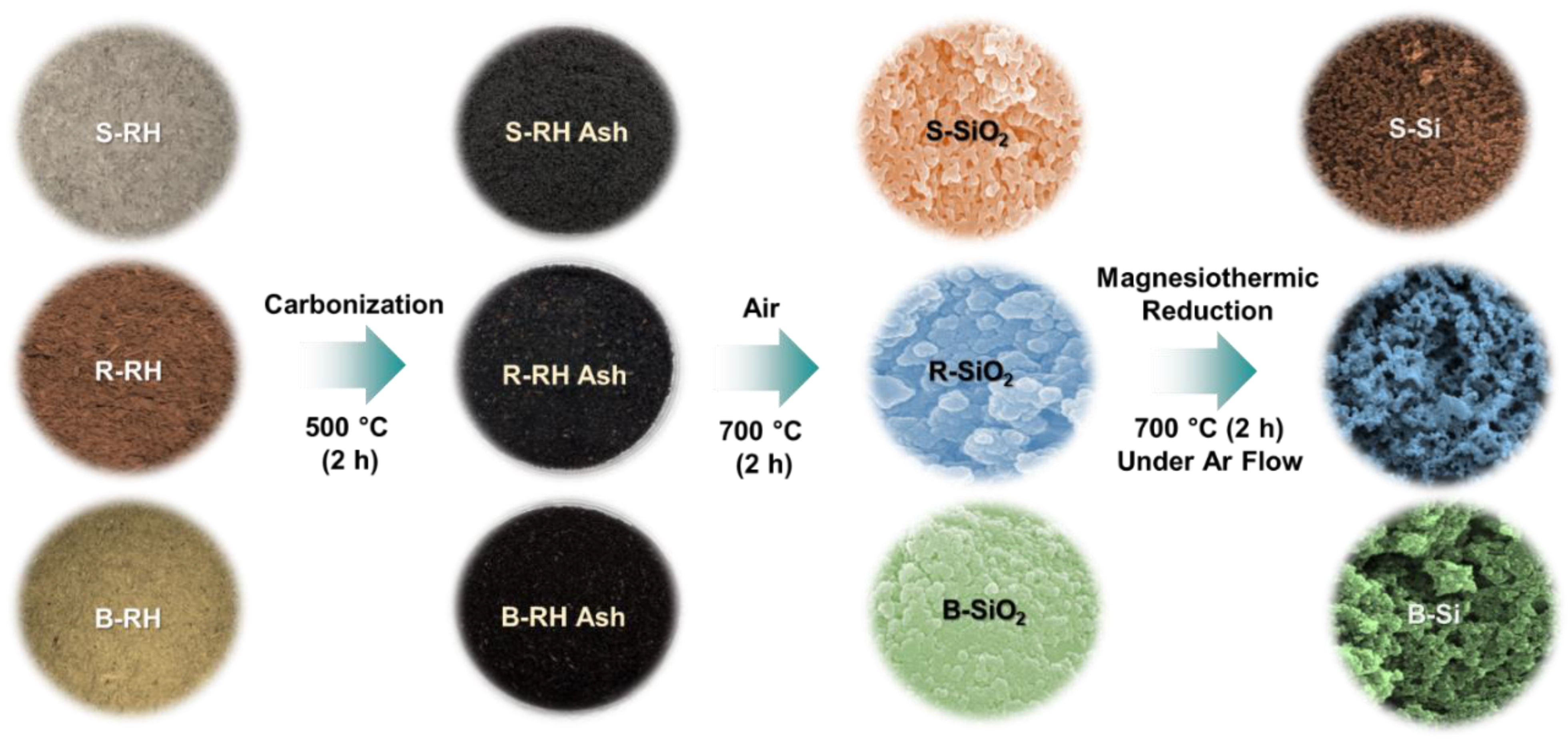
2.1. Material Preparation
2.2. Synthesis of Si Nanocrystals
2.3. Characterization of Material Properties
3. Results and Discussion
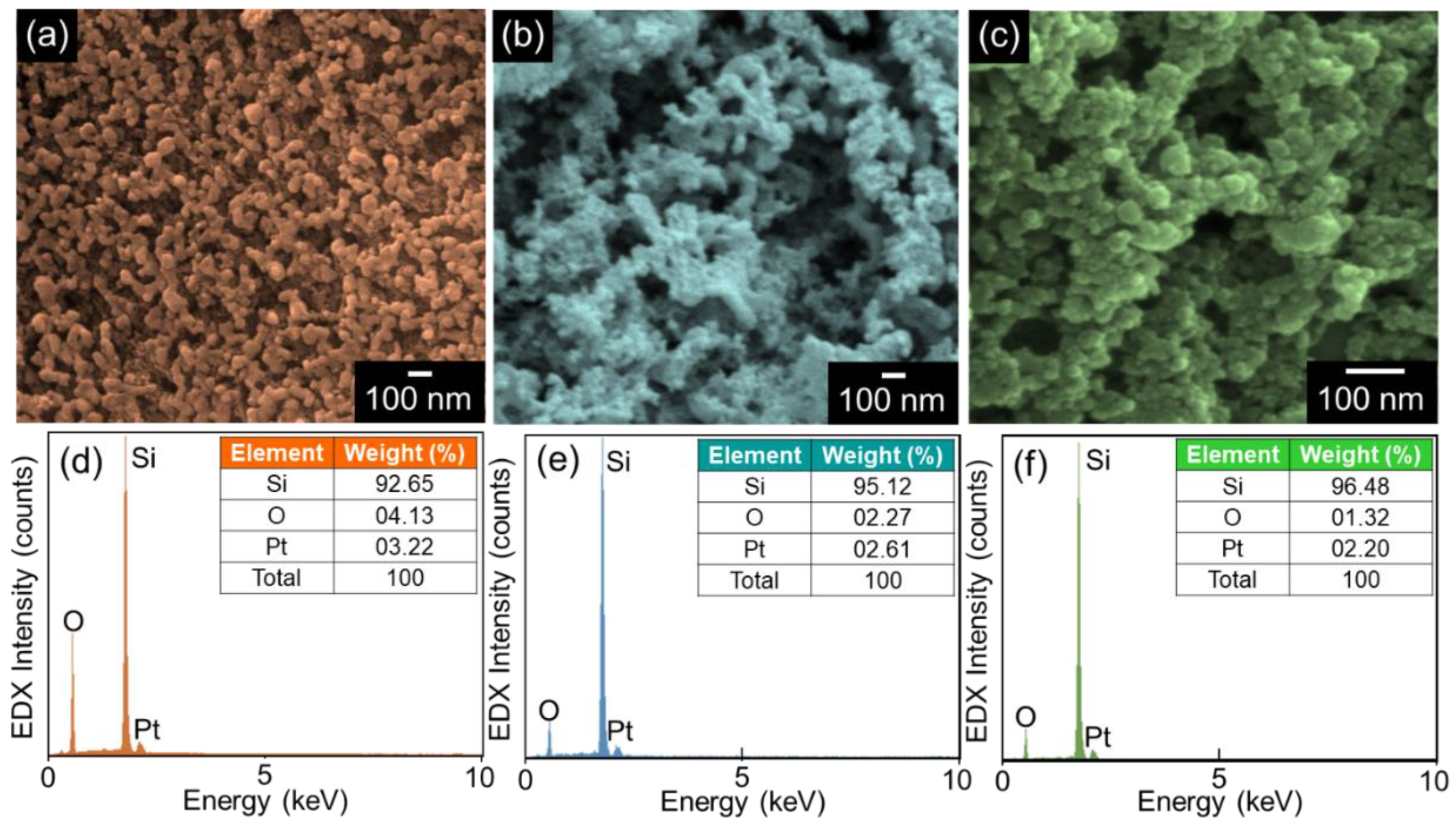
3.1. Morphological and Compositional Properties
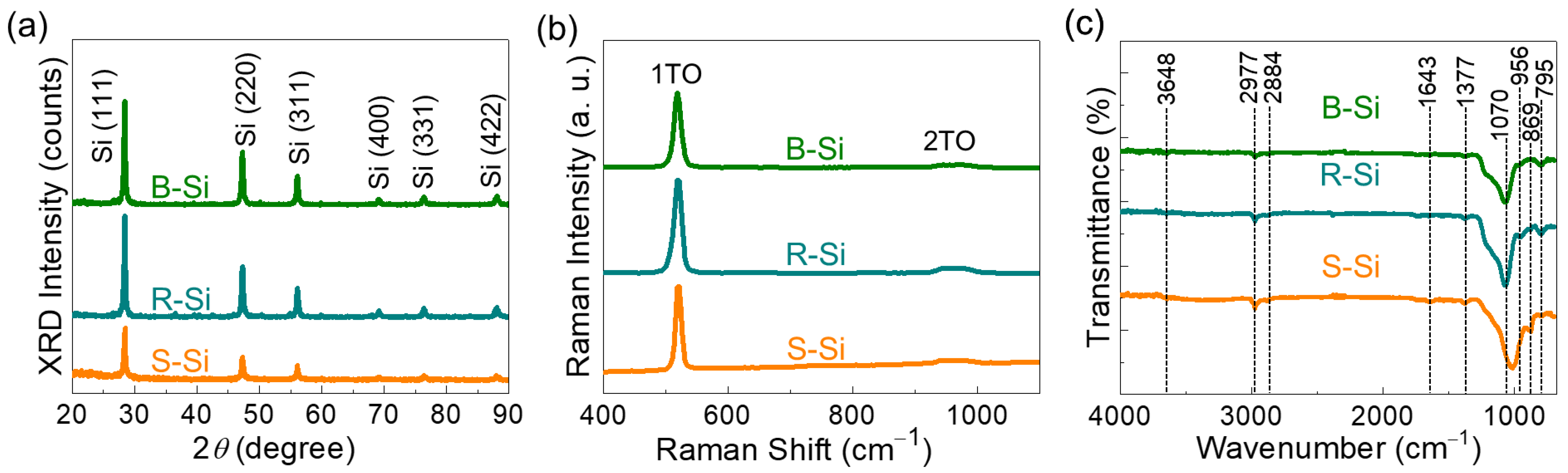
3.2. Structural and Vibrational Properties
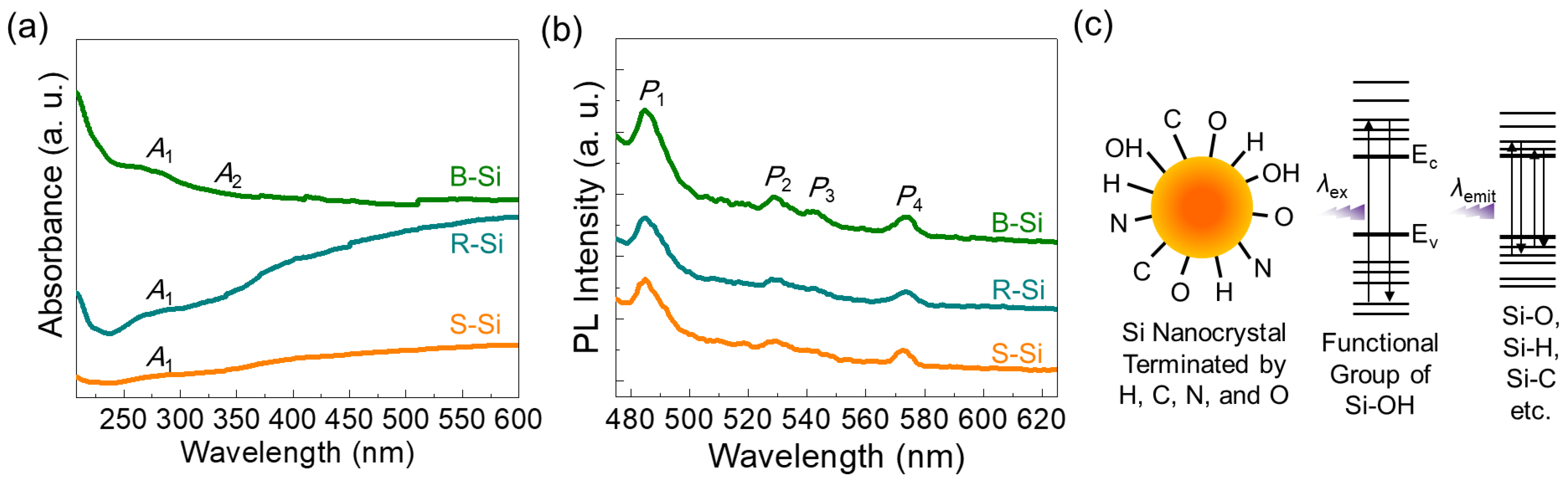
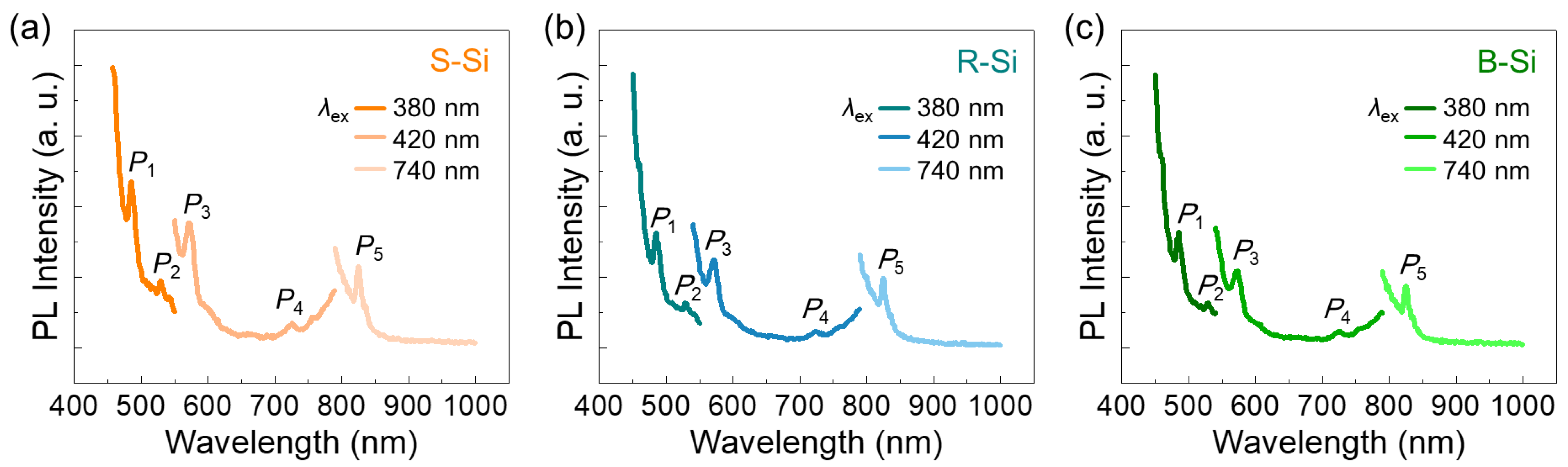
3.3. Optical Properties
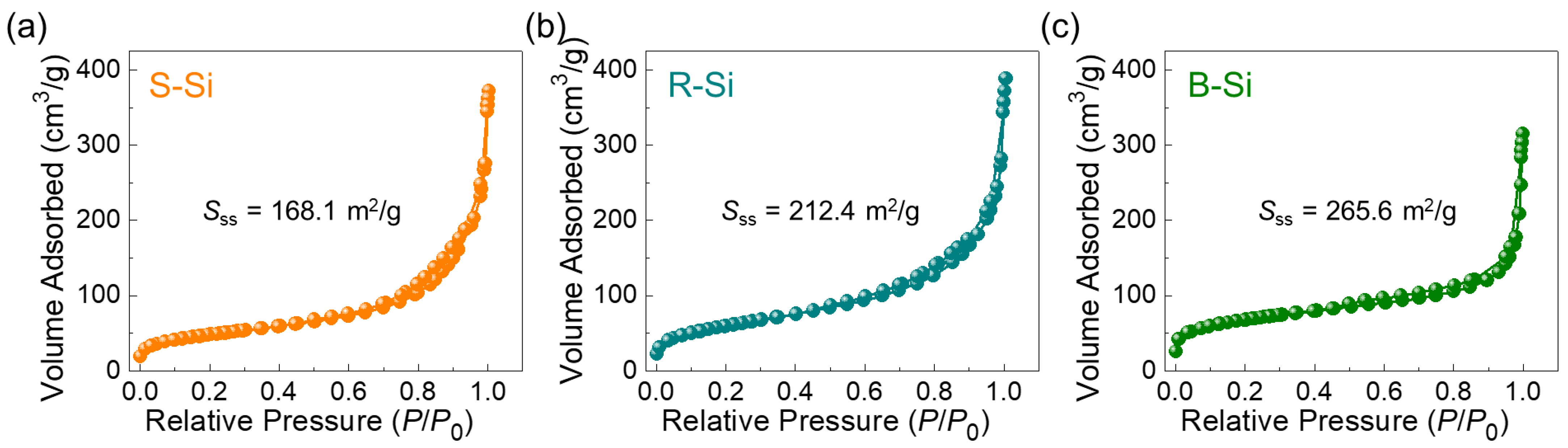
3.4. Textural Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Grim, J.Q.; Manna, L.; Moreels, I. A sustainable future for photonic colloidal nanocrystals. Chem. Soc. Rev. 2015, 44, 5897–5914. [Google Scholar] [CrossRef]
- Berencén, Y.; Prucnal, S.; Liu, F.; Skorupa, I.; Hübner, R.; Rebohle, L.; Zhou, S.; Schneider, H.; Helm, M.; Skorupa, W. Room-temperature short-wavelength infrared Si photodetector. Sci. Rep. 2017, 7, 43688. [Google Scholar] [CrossRef] [PubMed]
- Klimov, V.I.; Ivanov, S.A.; Nanda, J.; Achermann, M.; Bezel, I.; McGuire, J.A.; Piryatinski, A. Single-exciton optical gain in semiconductor nanocrystals. Nature 2007, 447, 441–446. [Google Scholar] [CrossRef]
- Barbagiovanni, E.G.; Lockwood, D.J.; Simpson, P.J.; Goncharova, L.V. Quantum confinement in Si and Ge nanostructures. J. Appl. Phys. 2012, 111, 034307. [Google Scholar] [CrossRef]
- Montalti, M.; Cantelli, A.; Battistelli, G. Nanodiamonds and silicon quantum dots: Ultrastable and biocompatible luminescent nanoprobes for long-term bioimaging. Chem. Soc. Rev. 2015, 44, 4853–4921. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Luo, T.-Y.; Zhou, M.; Abroshan, H.; Huang, J.; Kim, H.J.; Rosi, N.L.; Shao, Z.; Jin, R. Silicon Nanoparticles with Surface Nitrogen: 90% Quantum Yield with Narrow Luminescence Bandwidth and the Ligand Structure Based Energy Law. ACS Nano 2016, 10, 8385–8393. [Google Scholar] [CrossRef] [PubMed]
- Wolf, O.; Dasog, M.; Yang, Z.; Balberg, I.; Veinot, J.G.C.; Millo, O. Doping and Quantum Confinement Effects in Single Si Nanocrystals Observed by Scanning Tunneling Spectroscopy. Nano Lett. 2013, 13, 2516–2521. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, W.; Yang, H.; Jiang, X.; Yu, J.; Qin, H.; Xu, L.; Chen, K.; Huang, X.; Li, W.; et al. Performance and retention characteristics of nanocrystalline Si floating gate memory with an Al2O3 tunnel layer fabricated by plasma-enhanced atomic layer deposition. J. Appl. Phys. 2016, 119, 075702. [Google Scholar] [CrossRef]
- Kang, Y.M.; Lee, S.J.; Kim, D.Y.; Kim, T.W.; Woo, Y.D.; Wang, K.L. Formation of Si nanocrystals utilizing a Au nanoscale island etching mask. Mater. Res. Bull. 2005, 40, 193–198. [Google Scholar] [CrossRef]
- Lee, S.; Shim, Y.S.; Cho, H.Y.; Kang, T.W.; Kim, D.Y.; Lee, Y.H.; Wang, K.L. Fabrication and characterization of silicon-nanocrystal using platinum-nanomask. Thin Solid Films 2004, 451–452, 379–383. [Google Scholar] [CrossRef]
- Lee, S.; Shim, Y.S.; Cho, H.Y.; Kim, D.Y.; Kim, T.W.; Wang, K.L. Optical and Electrical Properties of Si Nanocrystals Embedded in SiO2 Layers. Jpn. J. Appl. Phys. 2003, 42, 7180–7183. [Google Scholar] [CrossRef]
- Walters, R.J.; Bourianoff, G.I.; Atwater, H.A. Field-effect electroluminescence in silicon nanocrystals. Nat. Mater. 2005, 4, 143–146. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Lin, G.-R. Si-QD Synthesis for Visible Light Emission, Color Conversion, and Optical Switching. Materials 2020, 13, 3635. [Google Scholar] [CrossRef]
- Hou, Y.; Aydin, E.; De Bastiani, M.; Xiao, C.; Isikgor, F.H.; Xue, D.-J.; Chen, B.; Chen, H.; Bahrami, B.; Chowdhury, A.H.; et al. Efficient tandem solar cells with solution-processed perovskite on textured crystalline silicon. Science 2020, 367, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Alberucci, A.; Alasgarzade, N.; Chambonneau, M.; Blothe, M.; Kämmer, H.; Matthäus, G.; Jisha, C.P.; Nolte, S. In-Depth Optical Characterization of Femtosecond-Written Waveguides in Silicon. Phys. Rev. Appl. 2020, 14, 24078. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y.; Huang, Y.; Pu, M.; Wang, Y.; Ma, X.; Li, X.; Luo, X. Crosstalk reduction of integrated optical waveguides with nonuniform subwavelength silicon strips. Sci. Rep. 2020, 10, 4491. [Google Scholar] [CrossRef]
- Ryu, J.; Jang, Y.J.; Choi, S.; Kang, H.J.; Park, H.; Lee, J.S.; Park, S. All-in-one synthesis of mesoporous silicon nanosheets from natural clay and their applicability to hydrogen evolution. NPG Asia Mater. 2016, 8, e248. [Google Scholar] [CrossRef]
- Sekar, S.; Aqueel Ahmed, A.T.; Kim, D.Y.; Lee, S. One-Pot Synthesized Biomass C-Si Nanocomposites as an Anodic Material for High-Performance Sodium-Ion Battery. Nanomaterials 2020, 10, 1728. [Google Scholar] [CrossRef] [PubMed]
- Loaiza, L.C.; Monconduit, L.; Seznec, V. Si and Ge-Based Anode Materials for Li-, Na-, and K-Ion Batteries: A Perspective from Structure to Electrochemical Mechanism. Small 2020, 16, 1905260. [Google Scholar] [CrossRef]
- Chae, S.; Choi, S.-H.; Kim, N.; Sung, J.; Cho, J. Integration of Graphite and Silicon Anodes for the Commercialization of High-Energy Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2020, 59, 110–135. [Google Scholar] [CrossRef]
- Tsuchimoto, Y.; Yano, T.-A.; Hada, M.; Nakamura, K.G.; Hayashi, T.; Hara, M. Controlling the Visible Electromagnetic Resonances of Si/SiO2 Dielectric Core–Shell Nanoparticles by Thermal Oxidation. Small 2015, 11, 4844–4849. [Google Scholar] [CrossRef]
- Kramer, N.J.; Schramke, K.S.; Kortshagen, U.R. Plasmonic Properties of Silicon Nanocrystals Doped with Boron and Phosphorus. Nano Lett. 2015, 15, 5597–5603. [Google Scholar] [CrossRef]
- Iannotti, V.; Ausanio, G.; Campana, C.; D’Orazio, F.; Hison, C.; Lucari, F.; Lanotte, L. Magnetic anisotropy in Ni–Si nanoparticle films produced by ultrashort pulsed laser deposition. J. Magn. Magn. Mater. 2008, 320, e594–e598. [Google Scholar] [CrossRef]
- Ge, M.; Rong, J.; Fang, X.; Zhang, A.; Lu, Y.; Zhou, C. Scalable preparation of porous silicon nanoparticles and their application for lithium-ion battery anodes. Nano Res. 2013, 6, 174–181. [Google Scholar] [CrossRef]
- Joo, J.; Liu, X.; Kotamraju, V.R.; Ruoslahti, E.; Nam, Y.; Sailor, M.J. Gated Luminescence Imaging of Silicon Nanoparticles. ACS Nano 2015, 9, 6233–6241. [Google Scholar] [CrossRef]
- Yang, R.; Buonassisi, T.; Gleason, K.K. Organic Vapor Passivation of Silicon at Room Temperature. Adv. Mater. 2013, 25, 2078–2083. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Kano, S.; Sugimoto, H.; Imakita, K.; Fujii, M. Size-Dependence of Acceptor and Donor Levels of Boron and Phosphorus Codoped Colloidal Silicon Nanocrystals. Nano Lett. 2016, 16, 2615–2620. [Google Scholar] [CrossRef] [PubMed]
- Erogbogbo, F.; Lin, T.; Tucciarone, P.M.; LaJoie, K.M.; Lai, L.; Patki, G.D.; Prasad, P.N.; Swihart, M.T. On-Demand Hydrogen Generation using Nanosilicon: Splitting Water without Light, Heat, or Electricity. Nano Lett. 2013, 13, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Praneetha, S.; Murugan, A.V. Development of Sustainable Rapid Microwave Assisted Process for Extracting Nanoporous Si from Earth Abundant Agricultural Residues and Their Carbon-based Nanohybrids for Lithium Energy Storage. ACS Sustain. Chem. Eng. 2015, 3, 224–236. [Google Scholar] [CrossRef]
- Wang, L.; Gao, B.; Peng, C.; Peng, X.; Fu, J.; Chu, P.K.; Huo, K. Bamboo leaf derived ultrafine Si nanoparticles and Si/C nanocomposites for high-performance Li-ion battery anodes. Nanoscale 2015, 7, 13840–13847. [Google Scholar] [CrossRef]
- Favors, Z.; Wang, W.; Bay, H.H.; Mutlu, Z.; Ahmed, K.; Liu, C.; Ozkan, M.; Ozkan, C.S. Scalable Synthesis of Nano-Silicon from Beach Sand for Long Cycle Life Li-ion Batteries. Sci. Rep. 2014, 4, 5623. [Google Scholar] [CrossRef]
- Su, A.; Li, J.; Dong, J.; Yang, D.; Chen, G.; Wei, Y. An Amorphous/Crystalline Incorporated Si/SiOx Anode Material Derived from Biomass Corn Leaves for Lithium-Ion Batteries. Small 2020, 16, 2001714. [Google Scholar] [CrossRef]
- Sekar, S.; Aqueel Ahmed, A.T.; Inamdar, A.I.; Lee, Y.; Im, H.; Kim, D.Y.; Lee, S. Activated carbon-decorated spherical silicon nanocrystal composites synchronously-derived from rice husks for anodic source of lithium-ion battery. Nanomaterials 2019, 9, 1055. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.S.; Ryou, M.-H.; Sung, Y.J.; Park, S.B.; Choi, J.W. Recycling rice husks for high-capacity lithium battery anodes. Proc. Natl. Acad. Sci. USA 2013, 110, 12229–12234. [Google Scholar] [CrossRef] [PubMed]
- Marchal, J.C.; Krug Iii, D.J.; McDonnell, P.; Sun, K.; Laine, R.M. A low cost, low energy route to solar grade silicon from rice hull ash (RHA), a sustainable source. Green Chem. 2015, 17, 3931–3940. [Google Scholar] [CrossRef]
- Bose, S.; Ganayee, M.A.; Mondal, B.; Baidya, A.; Chennu, S.; Mohanty, J.S.; Pradeep, T. Synthesis of Silicon Nanoparticles from Rice Husk and their Use as Sustainable Fluorophores for White Light Emission. ACS Sustain. Chem. Eng. 2018, 6, 6203–6210. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, Y.; Chu, B.; Sun, B.; Song, B.; Wu, S.; Su, Y.; He, Y. Plant-derived fluorescent silicon nanoparticles featuring excitation wavelength-dependent fluorescence spectra for anti-counterfeiting applications. Chem. Commun. 2016, 52, 7047–7050. [Google Scholar] [CrossRef]
- Sankar, S.; Kaur, N.; Lee, S.; Kim, D.Y. Rapid sonochemical synthesis of spherical silica nanoparticles derived from brown rice husk. Ceram. Int. 2018, 44, 8720–8724. [Google Scholar] [CrossRef]
- Sankar, S.; Lee, H.; Jung, H.; Kim, A.; Aqueel Ahmed, A.T.; Inamdar, A.I.; Kim, H.; Lee, S.; Im, H.; Kim, D.Y. Ultrathin graphene nanosheets derived from rice husks for sustainable supercapacitor electrodes. New J. Chem. 2017, 41, 13792–13797. [Google Scholar] [CrossRef]
- Jin, X.; Wang, R.; Zhang, L.; Si, R.; Shen, M.; Wang, M.; Tian, J.; Shi, J. Electron Configuration Modulation of Nickel Single Atoms for Elevated Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2020, 59, 6827–6831. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Gong, Y.; Zhang, L.; Gao, H.; Yang, G.-J.; Fang, B. Semiconductor polymeric graphitic carbon nitride photocatalysts: The “holy grail” for the photocatalytic hydrogen evolution reaction under visible light. Energy Environ. Sci. 2019, 12, 2080–2147. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Gong, S.; Mahmood, N.; Pan, L.; Zhang, X.; Zou, J.-J. Oxygen-doped nanoporous carbon nitride via water-based homogeneous supramolecular assembly for photocatalytic hydrogen evolution. Appl. Catal. B 2018, 221, 9–16. [Google Scholar] [CrossRef]
- Zhao, D.; Dong, C.-L.; Wang, B.; Chen, C.; Huang, Y.-C.; Diao, Z.; Li, S.; Guo, L.; Shen, S. Synergy of Dopants and Defects in Graphitic Carbon Nitride with Exceptionally Modulated Band Structures for Efficient Photocatalytic Oxygen Evolution. Adv. Mater. 2019, 31, 1903545. [Google Scholar] [CrossRef]
- Yang, X.; Tian, L.; Zhao, X.; Tang, H.; Liu, Q.; Li, G. Interfacial optimization of g-C3N4-based Z-scheme heterojunction toward synergistic enhancement of solar-driven photocatalytic oxygen evolution. Appl. Catal. B 2019, 244, 240–249. [Google Scholar] [CrossRef]
- Wang, D.; Sheng, T.; Chen, J.; Wang, H.-F.; Hu, P. Identifying the key obstacle in photocatalytic oxygen evolution on rutile TiO2. Nat. Catal. 2018, 1, 291–299. [Google Scholar] [CrossRef]
- Sankar, S.; Sharma, S.K.; Kaur, N.; Lee, B.; Kim, D.Y.; Lee, S.; Jung, H. Biogenerated silica nanoparticles synthesized from sticky, red, and brown rice husk ashes by a chemical method. Ceram. Int. 2016, 42, 4875–4885. [Google Scholar] [CrossRef]
- Bulyarskii, S.V.; Svetukhin, V.V.; Prikhod’ko, O.V. Spatially inhomogeneous oxygen precipitation in silicon. Semiconductors 1999, 33, 1157–1162. [Google Scholar] [CrossRef]
- Bulyarskiy, S.V.; Svetukhin, V.V. A Kinetic Model of Silicon Nanocrystal Formation. Silicon 2020. [Google Scholar] [CrossRef]
- Fokker, A.D. Die mittlere Energie rotierender elektrischer Dipole im Strahlungsfeld. Ann. Phys. 1914, 348, 810–820. [Google Scholar] [CrossRef]
- Planck, M. Über einen Satz der statistischen Dynamik und seine Erweiterung in der Quantentheorie. Sitz. Preuss. Akad. Wiss. Berl. 1917, 24, 324–341. [Google Scholar]
- Rosenbluth, M.N.; MacDonald, W.M.; Judd, D.L. Fokker-Planck Equation for an Inverse-Square Force. Phys. Rev. 1957, 107, 1–6. [Google Scholar] [CrossRef]
- Choi, J.-H.; Kim, H.-K.; Jin, E.-M.; Seo, M.W.; Cho, J.S.; Kumar, R.V.; Jeong, S.M. Facile and scalable synthesis of silicon nanowires from waste rice husk silica by the molten salt process. J. Hazard. Mater. 2020, 399, 122949. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; You, Y.; Xin, S.; Yin, Y.-X.; Zhang, J.; Wang, P.; Zheng, X.-s.; Cao, F.-F.; Guo, Y.-G. Rice husk-derived hierarchical silicon/nitrogen-doped carbon/carbon nanotube spheres as low-cost and high-capacity anodes for lithium-ion batteries. Nano Energy 2016, 25, 120–127. [Google Scholar] [CrossRef]
- Sankar, S.; Sharma, S.K.; An, N.; Lee, H.; Kim, D.Y.; Im, Y.B.; Cho, Y.D.; Ganesh, R.S.; Ponnusamy, S.; Raji, P.; et al. Photocatalytic properties of Mn-doped NiO spherical nanoparticles synthesized from sol-gel method. Optik 2016, 127, 10727–10734. [Google Scholar] [CrossRef]
- Kaur, N.; Lee, Y.; Kim, D.Y.; Lee, S. Optical bandgap tuning in nanocrystalline ZnO:Y films via forming defect-induced localized bands. Mater. Des. 2018, 148, 30–38. [Google Scholar] [CrossRef]
- Lee, S.; Kang, T.W.; Kim, D.Y. Correlation of Magnetic Properties with Microstructural Properties for Columnar-Structured (Zn1−xMnx)O/Al2O3 (0001) Thin Films. J. Cryst. Growth 2005, 284, 6–14. [Google Scholar] [CrossRef]
- Mishra, P.; Jain, K.P. First- and second-order Raman scattering in nanocrystalline silicon. Phys. Rev. B 2001, 64, 073304. [Google Scholar] [CrossRef]
- Krause, A.; Tkacheva, O.; Omar, A.; Langklotz, U.; Giebeler, L.; Dörfler, S.; Fauth, F.; Mikolajick, T.; Weber, W.M. In Situ Raman Spectroscopy on Silicon Nanowire Anodes Integrated in Lithium Ion Batteries. J. Electrochem. Soc. 2019, 166, A5378–A5385. [Google Scholar] [CrossRef]
- Tan, D.; Ma, Z.; Xu, B.; Dai, Y.; Ma, G.; He, M.; Jin, Z.; Qiu, J. Surface passivated silicon nanocrystals with stable luminescence synthesized by femtosecond laser ablation in solution. Phys. Chem. Chem. Phys. 2011, 13, 20255–20261. [Google Scholar] [CrossRef]
- Zhong, Y.; Sun, X.; Wang, S.; Peng, F.; Bao, F.; Su, Y.; Li, Y.; Lee, S.-T.; He, Y. Facile, Large-Quantity Synthesis of Stable, Tunable-Color Silicon Nanoparticles and Their Application for Long-Term Cellular Imaging. ACS Nano 2015, 9, 5958–5967. [Google Scholar] [CrossRef] [PubMed]
- Llansola Portolés, M.J.; Rodriguez Nieto, F.; Soria, D.B.; Amalvy, J.I.; Peruzzo, P.J.; Mártire, D.O.; Kotler, M.; Holub, O.; Gonzalez, M.C. Photophysical Properties of Blue-Emitting Silicon Nanoparticles. J. Phys. Chem. C 2009, 113, 13694–13702. [Google Scholar] [CrossRef]
- Shirahata, N.; Linford, M.R.; Furumi, S.; Pei, L.; Sakka, Y.; Gates, R.J.; Asplund, M.C. Laser-derived one-pot synthesis of silicon nanocrystals terminated with organic monolayers. Chem. Commun. 2009, 4684–4686. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buriak, J.M.; Stewart, M.P.; Geders, T.W.; Allen, M.J.; Choi, H.C.; Smith, J.; Raftery, D.; Canham, L.T. Lewis Acid Mediated Hydrosilylation on Porous Silicon Surfaces. J. Am. Chem. Soc. 1999, 121, 11491–11502. [Google Scholar] [CrossRef]
- Bateman, J.E.; Horrocks, B.R.; Houlton, A. Reactions of water and methanol at hydrogen-terminated silicon surfaces studied by transmission FTIR. J. Chem. Soc. Faraday Trans. 1997, 93, 2427–2431. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, G.; Wang, J.; Liang, X.; Zuo, Z.; Shi, Y. Fabrication and photocatalytic properties of silicon nanowires by metal-assisted chemical etching: Effect of H2O2 concentration. Nanoscale Res. Lett. 2012, 7, 663. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.-B.; Song, B.; Fang, B.; Jiang, A.-R.; Ji, S.-J.; He, Y. Excitation-wavelength-dependent photoluminescence of silicon nanoparticles enabled by adjustment of surface ligands. Chem. Commun. 2018, 54, 4947–4950. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Su, Y.; Yang, X.; Kang, Z.; Xu, T.; Zhang, R.; Fan, C.; Lee, S.-T. Photo and pH Stable, Highly-Luminescent Silicon Nanospheres and Their Bioconjugates for Immunofluorescent Cell Imaging. J. Am. Chem. Soc. 2009, 131, 4434–4438. [Google Scholar] [CrossRef]
- Pi, X.D.; Liptak, R.W.; Deneen Nowak, J.; Wells, N.P.; Carter, C.B.; Campbell, S.A.; Kortshagen, U. Air-stable full-visible-spectrum emission from silicon nanocrystals synthesized by an all-gas-phase plasma approach. Nanotechnology 2008, 19, 245603. [Google Scholar] [CrossRef]
- Hua, F.; Erogbogbo, F.; Swihart, M.T.; Ruckenstein, E. Organically Capped Silicon Nanoparticles with Blue Photoluminescence Prepared by Hydrosilylation Followed by Oxidation. Langmuir 2006, 22, 4363–4370. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhao, Z.; Zhao, Y.; Xie, H.; Xing, P.; Wang, D.; Yin, H. A self-driven alloying/dealloying approach to nanostructuring micro-silicon for high-performance lithium-ion battery anodes. Energy Storage Mater. 2021, 34, 768–777. [Google Scholar] [CrossRef]
- Azadeh, M.; Zamani, C.; Ataie, A.; Morante, J.R. Three-dimensional rice husk-originated mesoporous silicon and its electrical properties. Mater. Today Commun. 2018, 14, 141–150. [Google Scholar] [CrossRef]
- Kang, W.; Kim, J.-C.; Kim, D.-W. Waste glass microfiber filter-derived fabrication of fibrous yolk-shell structured silicon/carbon composite freestanding electrodes for lithium-ion battery anodes. J. Power Sources 2020, 468, 228407. [Google Scholar] [CrossRef]
- Sakamoto, M.; Terada, S.; Mizutani, T.; Saitow, K.-I. Large Field Enhancement of Nanocoral Structures on Porous Si Synthesized from Rice Husks. ACS Appl. Mater. Interfaces 2020, 13, 1105–1113. [Google Scholar] [CrossRef] [PubMed]

| Biomass | Nanostructures | Light Emission Color | References |
|---|---|---|---|
| Brown Rice Husks | Spherical Si Nanocrystals | Blue, Green, Yellow | This Work |
| Red Rice Husks | Spherical Si Nanocrystals | Blue, Green, Yellow | This Work |
| Sticky Rice Husks | Spherical Si Nanocrystals | Blue, Green, Yellow | This Work |
| Rice Husks | Si Nanoparticles | Green | [36] |
| Wheat Straws | Si Nanoparticles | Blue | [37] |
| Sugarcane Bagasse | Si Nanoparticles | Blue | [37] |
| Rice Husks | Si Nanoparticles | Blue | [37] |
| Biomass | Sss (m2/g) | Vtp (cm3/g) | References |
|---|---|---|---|
| Brown Rice Husks | 265.6 | 0.5389 | This Work |
| Red Rice Husks | 212.4 | 0.4201 | This Work |
| Sticky Rice Husks | 168.1 | 0.4103 | This Work |
| Bamboo Leaves | 302.13 | 0.526 | [30] |
| Beach Sand | 323 | - | [31] |
| Corn Leaves | 64 | - | [32] |
| Rice Husks | 288.4 | 0.35 | [53] |
| Rice Husks | 47.3 | 0.18 | [34] |
| Rice Husks | 127.05 | 0.2306 | [71] |
| Waste Glass Microfiber Filter | 66.12 | 0.64 | [72] |
| Rice Husks | 57.9 | - | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekar, S.; Lee, S. Derivation of Luminescent Mesoporous Silicon Nanocrystals from Biomass Rice Husks by Facile Magnesiothermic Reduction. Nanomaterials 2021, 11, 613. https://doi.org/10.3390/nano11030613
Sekar S, Lee S. Derivation of Luminescent Mesoporous Silicon Nanocrystals from Biomass Rice Husks by Facile Magnesiothermic Reduction. Nanomaterials. 2021; 11(3):613. https://doi.org/10.3390/nano11030613
Chicago/Turabian StyleSekar, Sankar, and Sejoon Lee. 2021. "Derivation of Luminescent Mesoporous Silicon Nanocrystals from Biomass Rice Husks by Facile Magnesiothermic Reduction" Nanomaterials 11, no. 3: 613. https://doi.org/10.3390/nano11030613
APA StyleSekar, S., & Lee, S. (2021). Derivation of Luminescent Mesoporous Silicon Nanocrystals from Biomass Rice Husks by Facile Magnesiothermic Reduction. Nanomaterials, 11(3), 613. https://doi.org/10.3390/nano11030613