Comparative Study of Carbon Nanosphere and Carbon Nanopowder on Viscosity and Thermal Conductivity of Nanofluids
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanofluid
2.3. Characterization Techniques
3. Results and Discussion
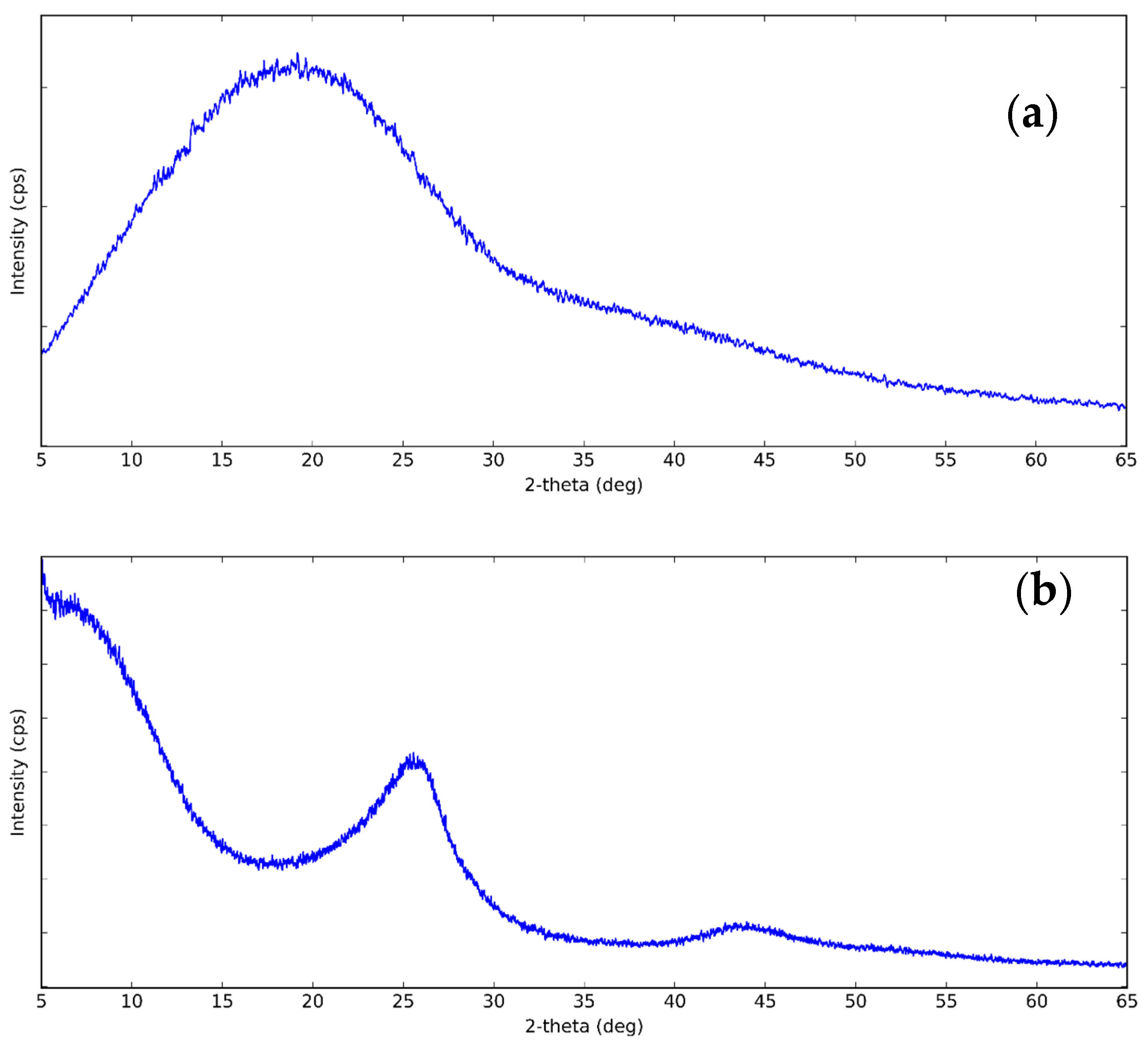
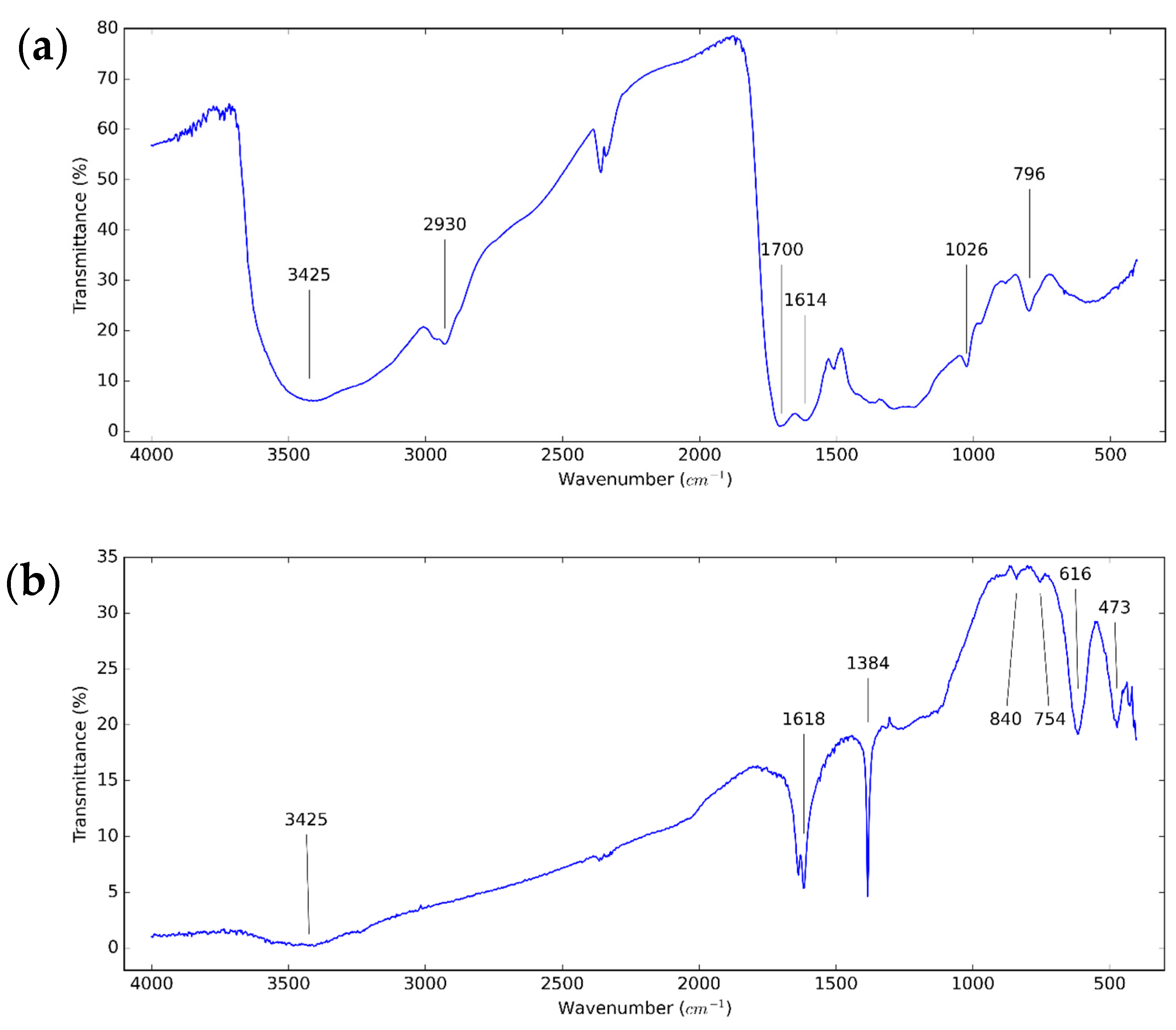
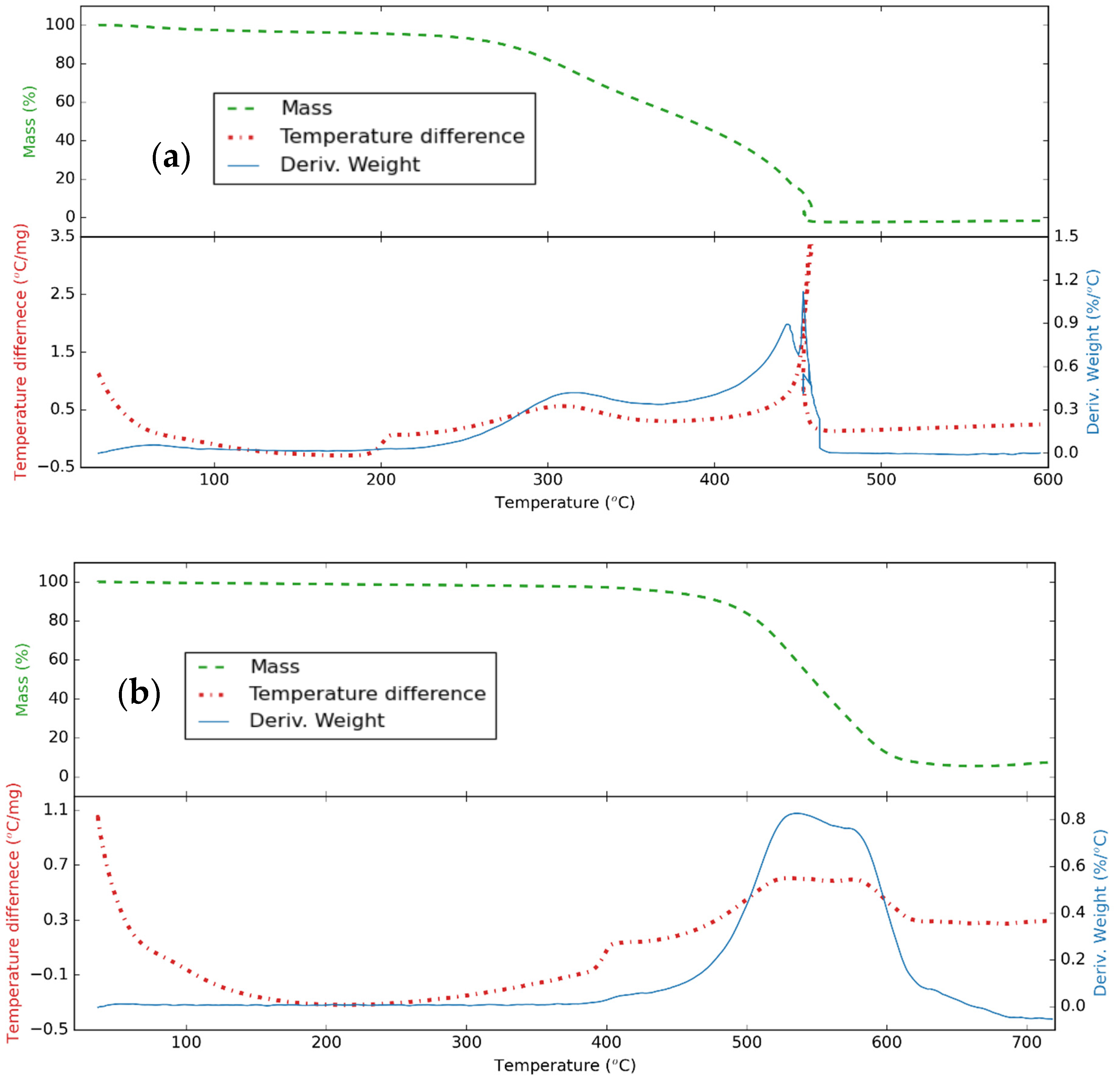
3.1. Structure of CNS and CNP
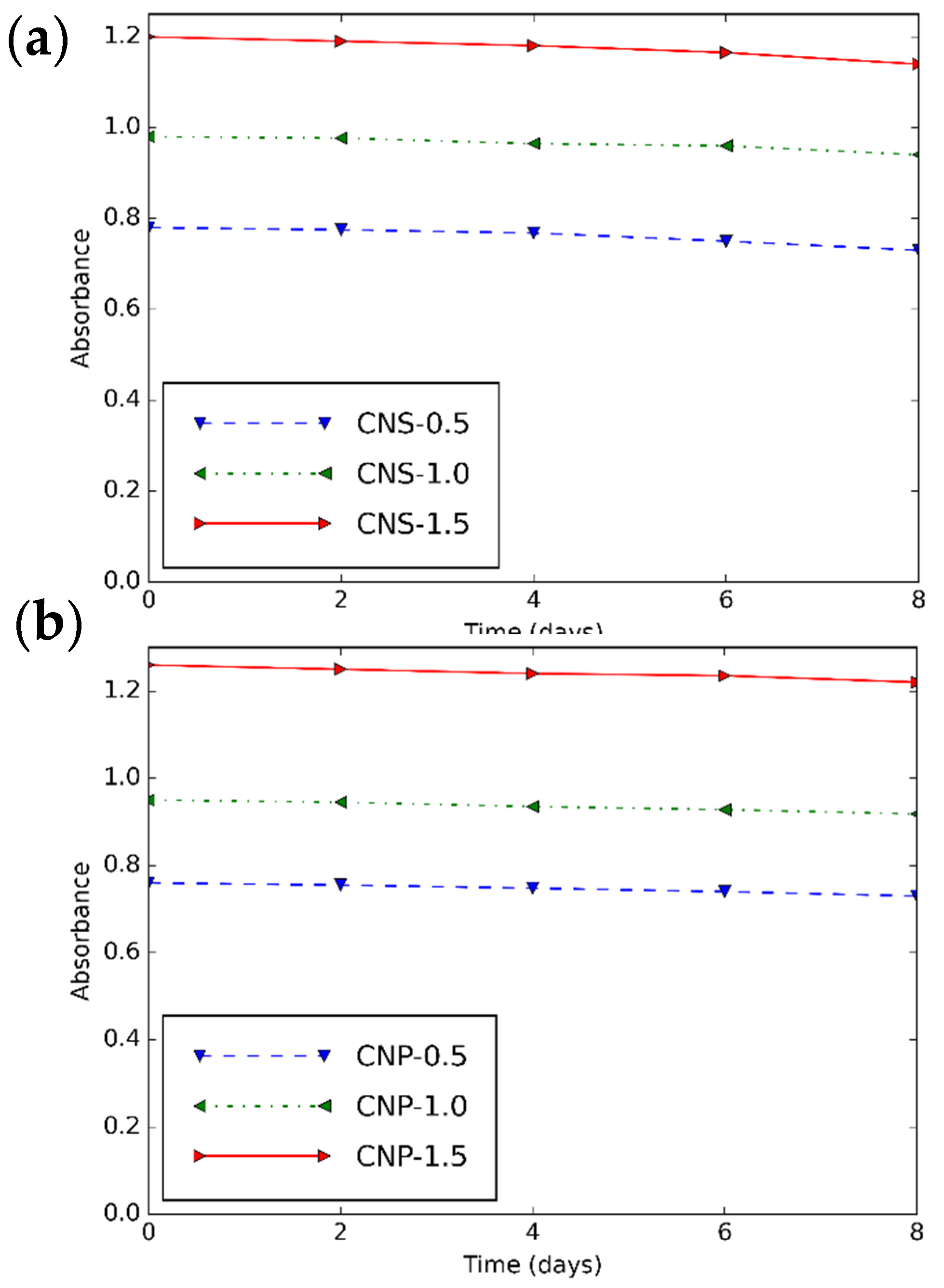
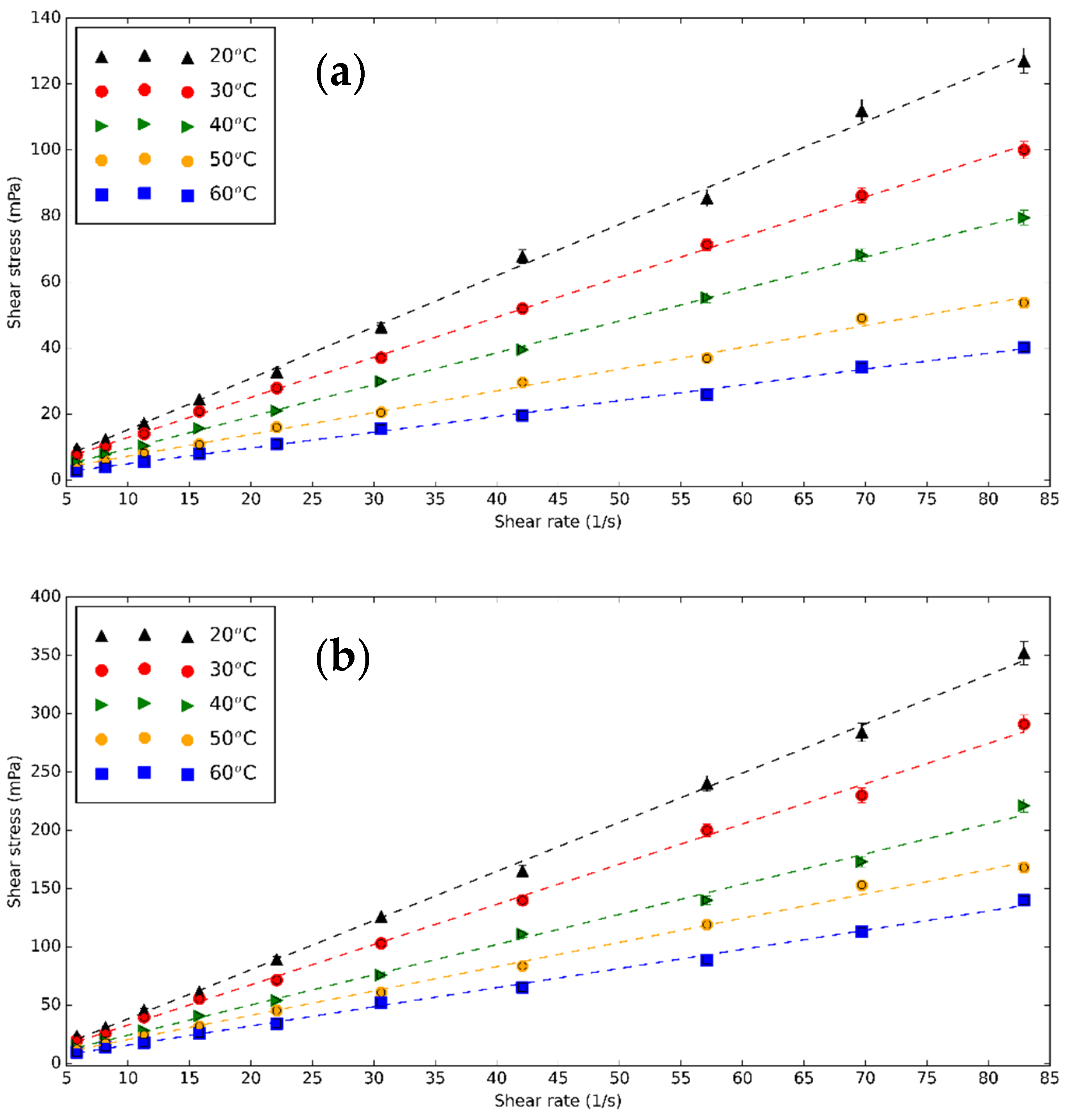
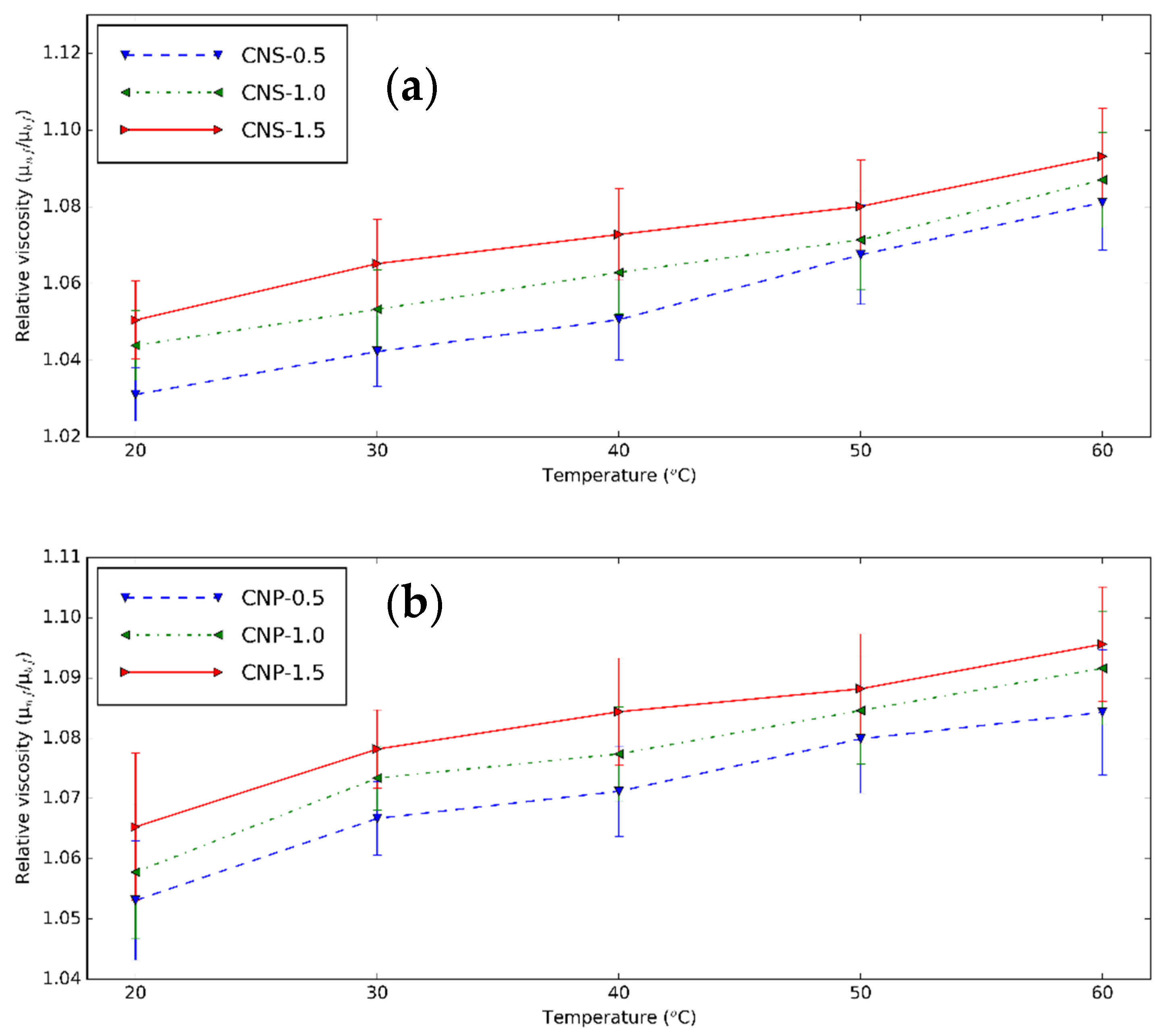
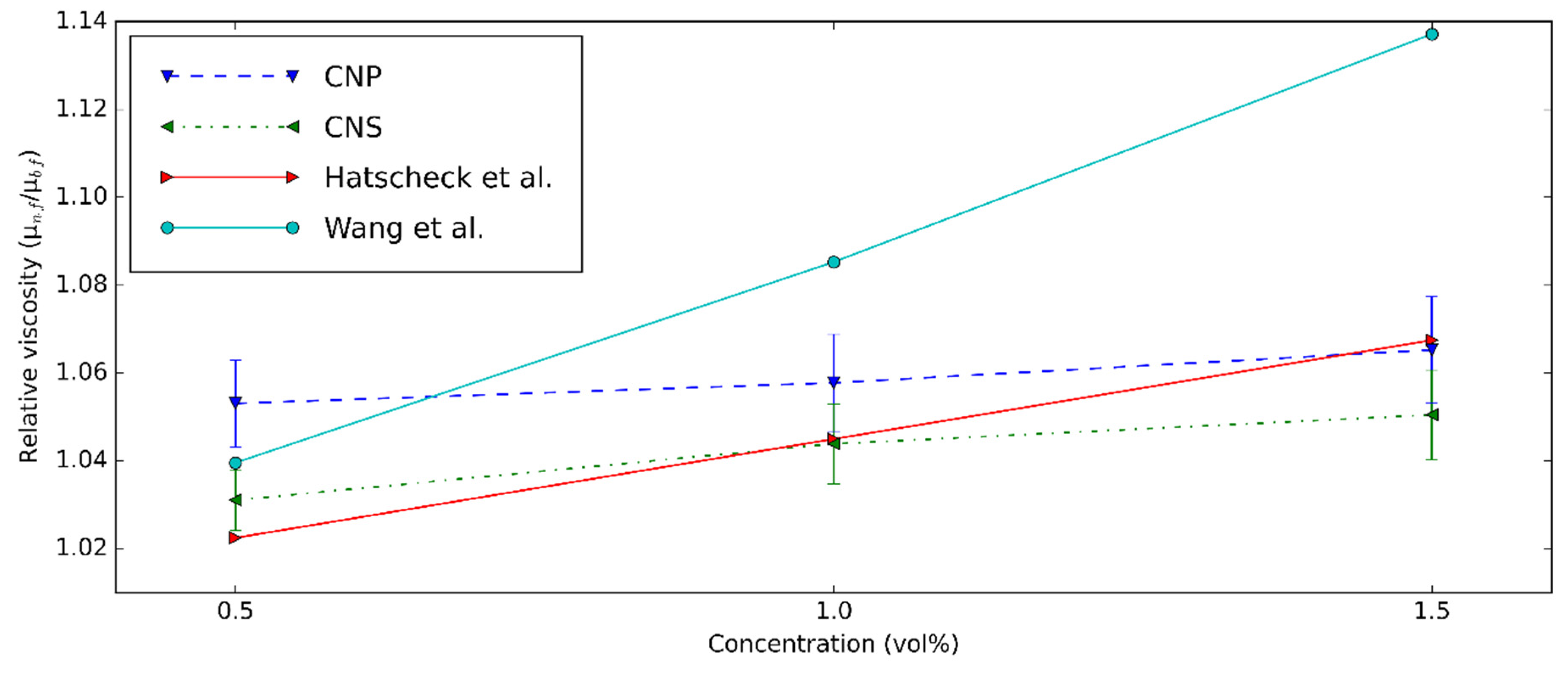
3.2. Rheological Characteristics of CNS and CNP Nanofluids
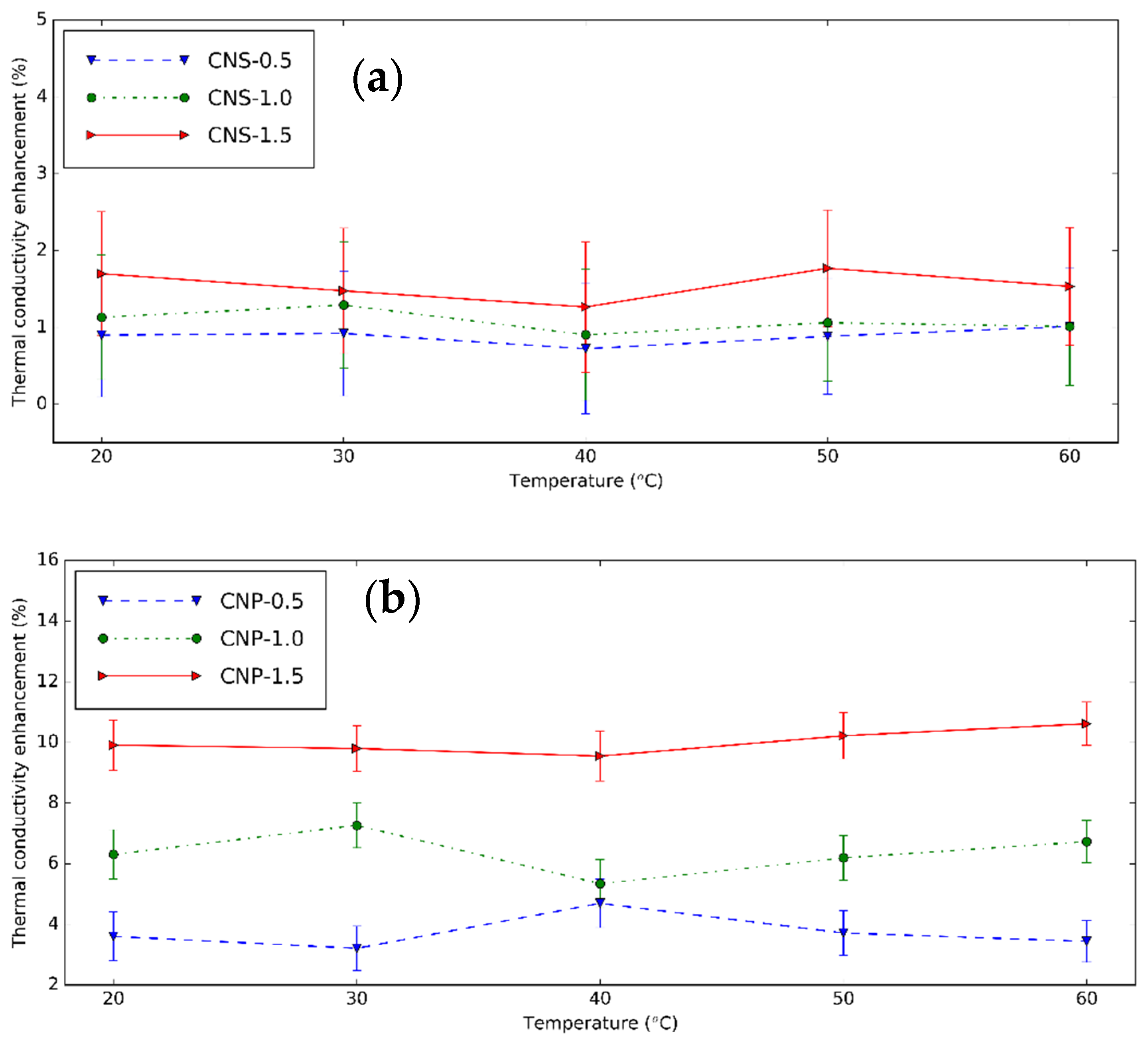
3.3. Thermal Conductivity of CNS and CNP Nanofluids
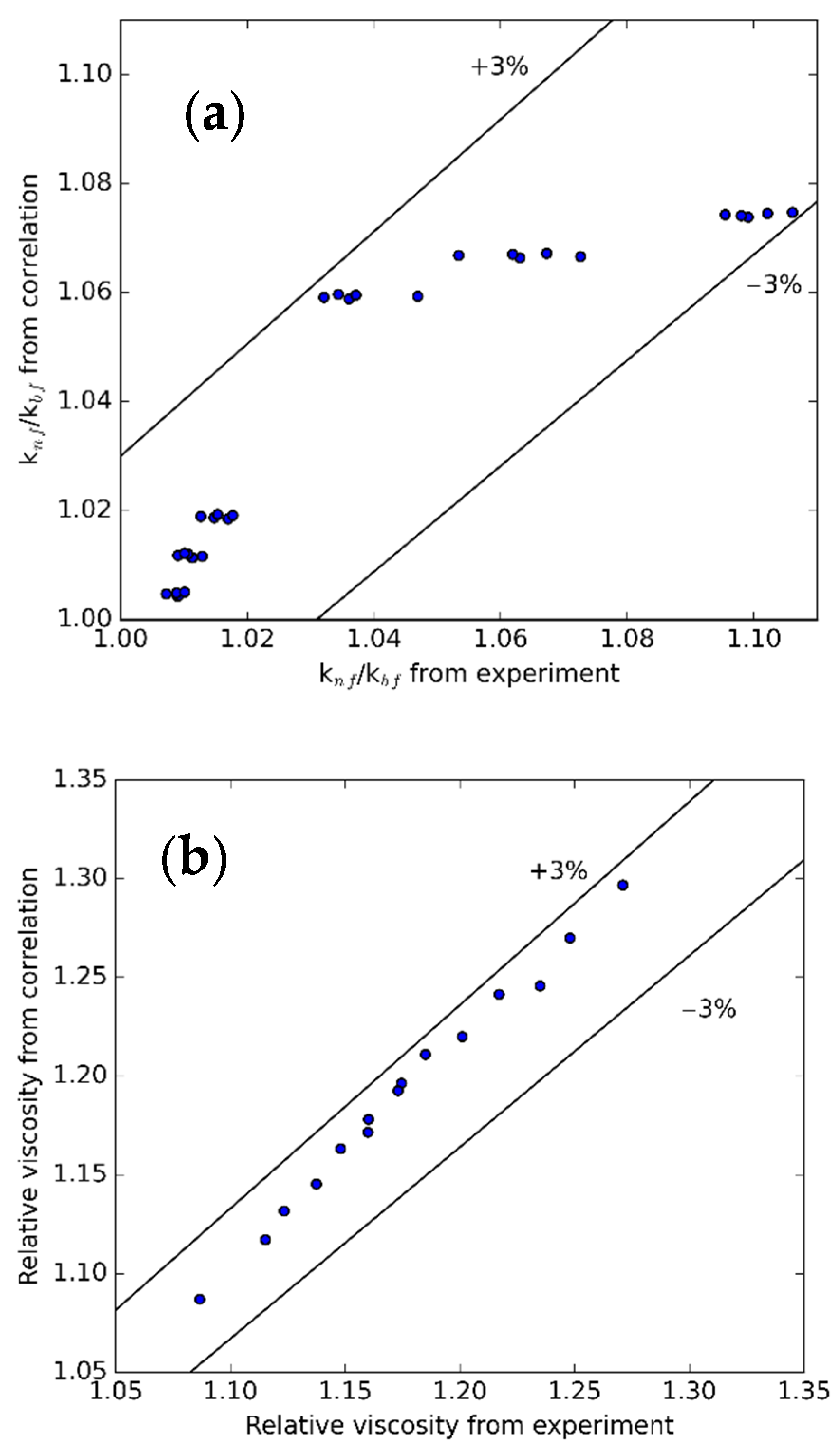
3.4. Regression Correlations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahian, O.; Kianifar, A.; Kalogirou, S.A.; Pop, I.; Wongwises, S. A review of the applications of nanofluids in solar energy. Int. J. Heat Mass Transf. 2013, 57, 582–594. [Google Scholar] [CrossRef]
- Murshed, S.; Leong, K.; Yang, C. Enhanced thermal conductivity of TiO2—water based nanofluids. Int. J. Therm. Sci. 2005, 44, 367–373. [Google Scholar] [CrossRef]
- Le Ba, T.; Mahian, O.; Wongwises, S.; Szilágyi, I.M. Review on the recent progress in the preparation and stability of graphene-based nanofluids. J. Therm. Anal. Calorim. 2020, 142, 1145–1172. [Google Scholar] [CrossRef]
- Sharma, A.K.; Tiwari, A.K.; Dixit, A.R. Rheological behaviour of nanofluids: A review. Renew. Sustain. Energy Rev. 2016, 53, 779–791. [Google Scholar] [CrossRef]
- Masuda, H.; Ebata, A.; Teramae, K.; Hishinuma, N. Alteration of Thermal Conductivity and Viscosity of Liquid by Dispersing Ultra-Fine Particles. Dispersion of Al2O3, SiO2 and TiO2 Ultra-Fine Particles. Netsu Bussei 1993, 7, 227–233. [Google Scholar] [CrossRef]
- Choi, S.U.; Eastman, J.A. Enhancing thermal conductivity of fluids with nanoparticles. In Proceedings of the 1995 International Mechanical Engineering Congress and Exhibition, San Francisco, CA, USA, 12–17 November 1995. [Google Scholar]
- Lee, S.; Choi, S.U.-S.; Li, S.; Eastman, J.A. Measuring Thermal Conductivity of Fluids Containing Oxide Nanoparticles. J. Heat Transf. 1999, 121, 280–289. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Choi, S.U.S. Thermal Conductivity of Nanoparticle—Fluid Mixture. J. Thermophys. Heat Transf. 1999, 13, 474–480. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Zhang, Z.G.; Yu, W.; Lockwood, F.E.; Grulke, E.A. Anomalous thermal conductivity enhancement in nanotube suspensions. Appl. Phys. Lett. 2001, 79, 2252–2254. [Google Scholar] [CrossRef]
- Al-Anssari, S.; Arif, M.; Wang, S.; Barifcani, A.; Iglauer, S. Stabilising nanofluids in saline environments. J. Colloid Interface Sci. 2017, 508, 222–229. [Google Scholar] [CrossRef]
- Babu, K.; Kumar, T.P. Effect of CNT concentration and agitation on surface heat flux during quenching in CNT nanofluids. Int. J. Heat Mass Transf. 2011, 54, 106–117. [Google Scholar] [CrossRef]
- Ashtiani, D.; Akhavan-Behabadi, M.; Pakdaman, M.F. An experimental investigation on heat transfer characteristics of multi-walled CNT-heat transfer oil nanofluid flow inside flattened tubes under uniform wall temperature condition. Int. Commun. Heat Mass Transf. 2012, 39, 1404–1409. [Google Scholar] [CrossRef]
- Le Ba, T.; Várady, Z.I.; Lukács, I.E.; Molnár, J.; Balczár, I.A.; Wongwises, S.; Szilágyi, I.M. Experimental investigation of rheological properties and thermal conductivity of SiO2–P25 TiO2 hybrid nanofluids. J. Therm. Anal. Calorim. 2020, 1–15. [Google Scholar] [CrossRef]
- Le Ba, T.; Alkurdi, A.Q.; Lukács, I.E.; Molnár, J.; Wongwises, S.; Gróf, G.; Szilágyi, I.M. A Novel Experimental Study on the Rheological Properties and Thermal Conductivity of Halloysite Nanofluids. Nanomaterials 2020, 10, 1834. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, H.; Wang, L.; Yang, C.-Y.; He, Y.; Yang, W.; Lee, W.P.; Zhang, L.; Huo, R. Heat Transfer Intensification Using Nanofluids. KONA Powder Part. J. 2007, 25, 23–38. [Google Scholar] [CrossRef]
- Pantzali, M.; Mouza, A.; Paras, S. Investigating the efficacy of nanofluids as coolants in plate heat exchangers (PHE). Chem. Eng. Sci. 2009, 64, 3290–3300. [Google Scholar] [CrossRef]
- Phuoc, T.X.; Massoudi, M.; Chen, R.-H. Viscosity and thermal conductivity of nanofluids containing multi-walled carbon nanotubes stabilized by chitosan. Int. J. Therm. Sci. 2011, 50, 12–18. [Google Scholar] [CrossRef]
- Razi, P.; Akhavan-Behabadi, M.; Saeedinia, M. Pressure drop and thermal characteristics of CuO–Base oil nanofluid laminar flow in flattened tubes under constant heat flux. Int. Commun. Heat Mass Transf. 2011, 38, 964–971. [Google Scholar] [CrossRef]
- Mirsaeidi, A.M.; Yousefi, F. Viscosity, thermal conductivity and density of carbon quantum dots nanofluids: An experimental investigation and development of new correlation function and ANN modeling. J. Therm. Anal. Calorim. 2021, 143, 351–361. [Google Scholar] [CrossRef]
- Devendiran, D.K.; Amirtham, V.A. A review on preparation, characterization, properties and applications of nanofluids. Renew. Sustain. Energy Rev. 2016, 60, 21–40. [Google Scholar] [CrossRef]
- Kumar, D.D.; Arasu, A.V. A comprehensive review of preparation, characterization, properties and stability of hybrid nanofluids. Renew. Sustain. Energy Rev. 2018, 81, 1669–1689. [Google Scholar] [CrossRef]
- Sajid, M.U.; Ali, H.M. Thermal conductivity of hybrid nanofluids: A critical review. Int. J. Heat Mass Transf. 2018, 126, 211–234. [Google Scholar] [CrossRef]
- Caccia, M.; Rodríguez, A.; Narciso, J. Diamond Surface Modification to Enhance Interfacial Thermal Conductivity in Al/Diamond Composites. JOM 2014, 66, 920–925. [Google Scholar] [CrossRef]
- Molina, J.; Prieto, R.; Narciso, J.; Louis, E. The effect of porosity on the thermal conductivity of Al–12wt.% Si/SiC composites. Scr. Mater. 2009, 60, 582–585. [Google Scholar] [CrossRef]
- Esfe, M.H.; Zabihi, F.; Rostamian, H.; Esfandeh, S. Experimental investigation and model development of the non-Newtonian behavior of CuO-MWCNT-10w40 hybrid nano-lubricant for lubrication purposes. J. Mol. Liq. 2018, 249, 677–687. [Google Scholar] [CrossRef]
- Eshgarf, H.; Afrand, M. An experimental study on rheological behavior of non-Newtonian hybrid nano-coolant for application in cooling and heating systems. Exp. Therm. Fluid Sci. 2016, 76, 221–227. [Google Scholar] [CrossRef]
- Esfe, M.H.; Rostamian, H.; Sarlak, M.R. A novel study on rheological behavior of ZnO-MWCNT/10w40 nanofluid for automotive engines. J. Mol. Liq. 2018, 254, 406–413. [Google Scholar] [CrossRef]
- Teng, T.-P.; Cheng, C.-M.; Pai, F.-Y. Preparation and characterization of carbon nanofluid by a plasma arc nanoparticles synthesis system. Nanoscale Res. Lett. 2011, 6, 293. [Google Scholar] [CrossRef]
- Teng, T.-P.; Lin, L.; Yu, C.-C. Preparation and Characterization of Carbon Nanofluids by Using a Revised Water-Assisted Synthesis Method. J. Nanomater. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Afrand, M.; Esfe, M.H.; Abedini, E.; Teimouri, H. Predicting the effects of magnesium oxide nanoparticles and temperature on the thermal conductivity of water using artificial neural network and experimental data. Phys. E Low-Dimensional Syst. Nanostruct. 2017, 87, 242–247. [Google Scholar] [CrossRef]
- Esfahani, N.N.; Toghraie, D.; Afrand, M. A new correlation for predicting the thermal conductivity of ZnO–Ag (50%–50%)/water hybrid nanofluid: An experimental study. Powder Technol. 2018, 323, 367–373. [Google Scholar] [CrossRef]
- Ghadimi, A.; Saidur, R.; Metselaar, H. A review of nanofluid stability properties and characterization in stationary conditions. Int. J. Heat Mass Transf. 2011, 54, 4051–4068. [Google Scholar] [CrossRef]
- Estellé, P.; Halelfadl, S.; Mare, T. Lignin as dispersant for water-based carbon nanotubes nanofluids: Impact on viscosity and thermal conductivity. Int. Commun. Heat Mass Transf. 2014, 57, 8–12. [Google Scholar] [CrossRef]
- Zawrah, M.; Khattab, R.; Girgis, L.; El Daidamony, H.; Aziz, R.E.A. Stability and electrical conductivity of water-base Al2O3 nanofluids for different applications. HBRC J. 2016, 12, 227–234. [Google Scholar] [CrossRef]
- Manasrah, A.D.; Almanassra, I.W.; Marei, N.N.; Al-Mubaiyedh, U.A.; Laoui, T.; Atieh, M.A. Surface modification of carbon nanotubes with copper oxide nanoparticles for heat transfer enhancement of nanofluids. RSC Adv. 2018, 8, 1791–1802. [Google Scholar] [CrossRef]
- Buongiorno, J.; Venerus, D.C.; Prabhat, N.; McKrell, T.J.; Townsend, J.; Christianson, R.J.; Tolmachev, Y.V.; Keblinski, P.; Hu, L.-W.; Alvarado, J.L.; et al. A benchmark study on the thermal conductivity of nanofluids. J. Appl. Phys. 2009, 106, 094312. [Google Scholar] [CrossRef]
- Özerinç, S.; Kakaç, S.; Yazıcıoğlu, A.G. Enhanced thermal conductivity of nanofluids: A state-of-the-art review. Microfluid. Nanofluidics 2009, 8, 145–170. [Google Scholar] [CrossRef]
- Shima, P.D.; Philip, J.; Raj, B. Influence of aggregation on thermal conductivity in stable and unstable nanofluids. Appl. Phys. Lett. 2010, 97, 153113. [Google Scholar] [CrossRef]
- Estellé, P.; Halelfadl, S.; Maré, T. Thermal conductivity of CNT water based nanofluids: Experimental trends and models overview. J. Therm. Eng. 2015, 1, 381. [Google Scholar] [CrossRef]
- Talaei, Z.; Mahjoub, A.R.; Rashidi, A.M.; Amrollahi, A.; Meibodi, M.E. The effect of functionalized group concentration on the stability and thermal conductivity of carbon nanotube fluid as heat transfer media. Int. Commun. Heat Mass Transf. 2011, 38, 513–517. [Google Scholar] [CrossRef]
- Nanda, J.; Maranville, C.; Bollin, S.C.; Sawall, D.; Ohtani, H.; Remillard, J.T.; Ginder, J.M. Thermal Conductivity of Single-Wall Carbon Nanotube Dispersions: Role of Interfacial Effects. J. Phys. Chem. C 2008, 112, 654–658. [Google Scholar] [CrossRef]
- Aravind, S.S.J.; Baskar, P.; Baby, T.T.; Sabareesh, R.K.; Das, S.; Ramaprabhu, S. Investigation of Structural Stability, Dispersion, Viscosity, and Conductive Heat Transfer Properties of Functionalized Carbon Nanotube Based Nanofluids. J. Phys. Chem. C 2011, 115, 16737–16744. [Google Scholar] [CrossRef]
- Chen, L.; Xie, H. Surfactant-free nanofluids containing double- and single-walled carbon nanotubes functionalized by a wet-mechanochemical reaction. Thermochim. Acta 2010, 497, 67–71. [Google Scholar] [CrossRef]
- Nasiri, A.; Shariaty-Niasar, M.; Rashidi, A.; Khodafarin, R. Effect of CNT structures on thermal conductivity and stability of nanofluid. Int. J. Heat Mass Transf. 2012, 55, 1529–1535. [Google Scholar] [CrossRef]
- Brzóska, K.; Jóźwiak, B.; Golba, A.; Dzida, M.; Boncel, S. Thermophysical Properties of Nanofluids Composed of Ethylene Glycol and Long Multi-Walled Carbon Nanotubes. Fluids 2020, 5, 241. [Google Scholar] [CrossRef]
- Wang, Y.; Su, F.; Wood, C.D.; Lee, A.J.Y.; Zhao, X.S. Preparation and Characterization of Carbon Nanospheres as Anode Materials in Lithium-Ion Secondary Batteries. Ind. Eng. Chem. Res. 2008, 47, 2294–2300. [Google Scholar] [CrossRef]
- Jin, Y.Z.; Gao, C.; Hsu, W.K.; Zhu, Y.; Huczko, A.; Bystrzejewski, M.; Roe, M.; Lee, C.Y.; Acquah, S.; Kroto, H.; et al. Large-scale synthesis and characterization of carbon spheres prepared by direct pyrolysis of hydrocarbons. Carbon 2005, 43, 1944–1953. [Google Scholar] [CrossRef]
- Pol, V.G.; Motiei, M.; Gedanken, A.; Calderon-Moreno, J.; Yoshimura, M. Carbon spherules: Synthesis, properties and mechanistic elucidation. Carbon 2004, 42, 111–116. [Google Scholar] [CrossRef]
- Serp, P.; Feurer, R.; Kalck, P.; Kihn, Y.; Faria, J.; Figueiredo, J.L. Chemical vapour deposition process for the production of carbon nanospheres. Carbon 2001, 39, 621–626. [Google Scholar] [CrossRef]
- Ma, X.; Xu, F.; Chen, L.; Zhang, Y.; Zhang, Z.; Qian, J.; Qian, Y. Easy nickel substrate-assisted growth of uniform carbon microspheres and their spectroscopic properties. Carbon 2006, 44, 2861–2864. [Google Scholar] [CrossRef]
- Zhou, Z.; Yan, Q.; Su, F.; Zhao, X.S. Replicating novel carbon nanostructures with 3D macroporous silica template. J. Mater. Chem. 2005, 15, 2569–2574. [Google Scholar] [CrossRef]
- Tosheva, L.; Parmentier, J.; Valtchev, V.; Vix-Guterl, C.; Patarin, J. Carbon spheres prepared from zeolite Beta beads. Carbon 2005, 43, 2474–2480. [Google Scholar] [CrossRef]
- Kim, B.J.; Chang, J.Y. Preparation of Carbon Nanospheres from Diblock Copolymer Micelles with Cores Containing Curable Acetylenic Groups. Macromolecules 2006, 39, 90–94. [Google Scholar] [CrossRef]
- Zhang, X.; Manohar, S.K. Microwave synthesis of nanocarbons from conducting polymers. Chem. Commun. 2006, 2477–2479. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.; Wang, H.; Luo, P.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D.; et al. Carbon Dots for Multiphoton Bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Brundavanam, S.; Shah, M.; Laava, I.; Fawcett, D. Photothermal response of CVD synthesized carbon (nano)spheres/aqueous nanofluids for potential application in direct solar absorption collectors: A preliminary investigation. Nanotechnology, Sci. Appl. 2012, 5, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.A.; Mhlanga, S.D.; Coville, N.J. Carbon spheres. Mater. Sci. Eng. R Rep. 2010, 70, 1–28. [Google Scholar] [CrossRef]
- Bakos, L.P.; Justh, N.; Hernádi, K.; Kiss, G.; Réti, B.; Erdélyi, Z.; Parditka, B.; Szilágyi, I.M. Core-shell carbon nanosphere-TiO2 composite and hollow TiO2nanospheres prepared by atomic layer deposition. J. Phys.Conf. Ser. 2016, 764, 012005. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Li, M.; Zhao, Z. Hydrothermal preparation of highly porous carbon spheres from hemp (Cannabis sativa L.) stem hemicellulose for use in energy-related applications. Ind. Crop. Prod. 2015, 65, 216–226. [Google Scholar] [CrossRef]
- Juangsa, F.B.; Muroya, Y.; Ryu, M.; Morikawa, J.; Nozaki, T. Comparative study of thermal conductivity in crystalline and amorphous nanocomposite. Appl. Phys. Lett. 2017, 110, 253105. [Google Scholar] [CrossRef]
- Mohammad, R.; Kandasamy, R. Nanoparticle shapes on electric and magnetic force in water, ethylene glycol and engine oil based Cu, Al2O3 and SWCNTs. J. Mol. Liq. 2017, 237, 54–64. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, H.; Liu, Y.; Sun, X.; Zhang, D.; Xue, D. First identification of primary nanoparticles in the aggregation of HMF. Nanoscale Res. Lett. 2012, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Zhan, Z.; Zou, M.; Zhang, L.; Zhao, S. Green synthesis of stable and biocompatible fluorescent carbon dots from peanut shells for multicolor living cell imaging. New J. Chem. 2015, 40, 1698–1703. [Google Scholar] [CrossRef]
- Kaçan, E.; Kutahyali, C. Adsorption of strontium from aqueous solution using activated carbon produced from textile sewage sludges. J. Anal. Appl. Pyrolys. 2012, 97, 149–157. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chem. A Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, R.C.; De Almeida, C.F.; De Siqueira, A.B.; Treu-Filho, O.; Caires, F.J.; De Carvalho, C.T. Thermal and spectroscopic study of the 3,4-(methylenedioxy)cinnamate compounds of transition metals in the solid state. Thermochim. Acta 2014, 596, 56–62. [Google Scholar] [CrossRef]
- Kuila, T.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Recent advances in the efficient reduction of graphene oxide and its application as energy storage electrode materials. Nanoscale 2013, 5, 52–71. [Google Scholar] [CrossRef]
- Varga, M.; Izak, T.; Vretenar, V.; Kozak, H.; Holovsky, J.; Artemenko, A.; Hulman, M.; Skakalova, V.; Lee, D.S.; Kromka, A. Diamond/carbon nanotube composites: Raman, FTIR and XPS spectroscopic studies. Carbon 2017, 111, 54–61. [Google Scholar] [CrossRef]
- Mohan, V.B.; Jakisch, L.; Jayaraman, K.; Bhattacharyya, D. Role of chemical functional groups on thermal and electrical properties of various graphene oxide derivatives: A comparative x-ray photoelectron spectroscopy analysis. Mater. Res. Express 2018, 5, 035604. [Google Scholar] [CrossRef]
- Afrand, M.; Toghraie, D.; Ruhani, B. Effects of temperature and nanoparticles concentration on rheological behavior of Fe3O4 –Ag/EG hybrid nanofluid: An experimental study. Exp. Therm. Fluid Sci. 2016, 77, 38–44. [Google Scholar] [CrossRef]
- Nguyen, C.; Desgranges, F.; Roy, G.; Galanis, N.; Maré, T.; Boucher, S.; Mintsa, H.A. Temperature and particle-size dependent viscosity data for water-based nanofluids—Hysteresis phenomenon. Int. J. Heat Fluid Flow 2007, 28, 1492–1506. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Li, T.; Zeng, M.; Sundén, B. Effect of various surfactants on stability and thermophysical properties of nanofluids. J. Therm. Anal. Calorim. 2020, 1–14. [Google Scholar] [CrossRef]
- Hatschek, E. The general theory of viscosity of two-phase systems. Trans. Faraday Soc. 1913, 9, 80–92. [Google Scholar] [CrossRef]
- Pak, B.C.; Cho, Y.I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transf. 1998, 11, 151–170. [Google Scholar] [CrossRef]
- Maxwell, J.C.A. A Treatise on Electricity and Magnetism. Nature 1873, 7, 478–480. [Google Scholar] [CrossRef]
- Azmi, W.H.; Sharma, K.V.; Mamat, R.; Alias, A.B.S.; Misnon, I.I. Correlations for thermal conductivity and viscosity of water based nanofluids. IOP Conf. Ser. Mater. Sci. Eng. 2012, 36. [Google Scholar] [CrossRef]

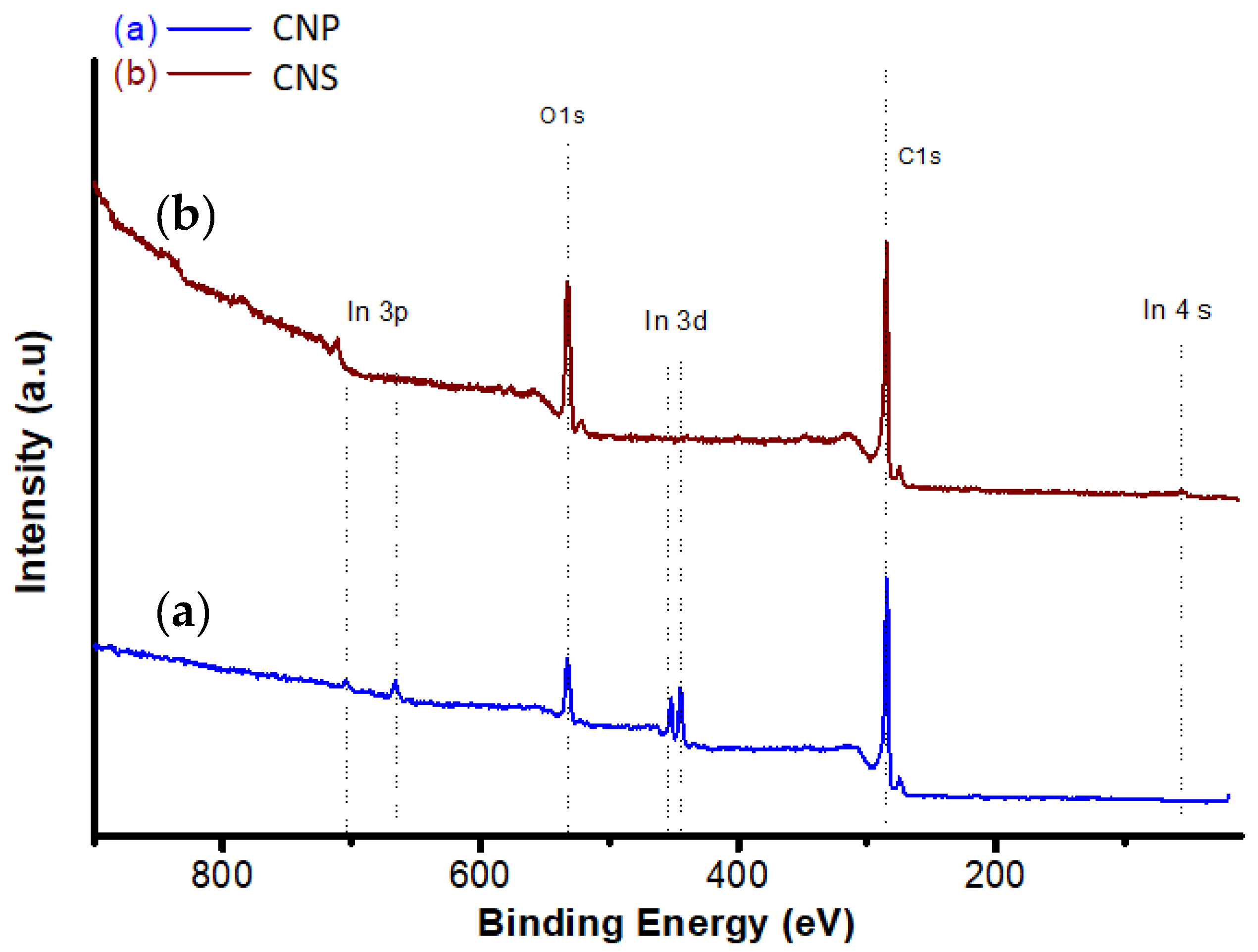
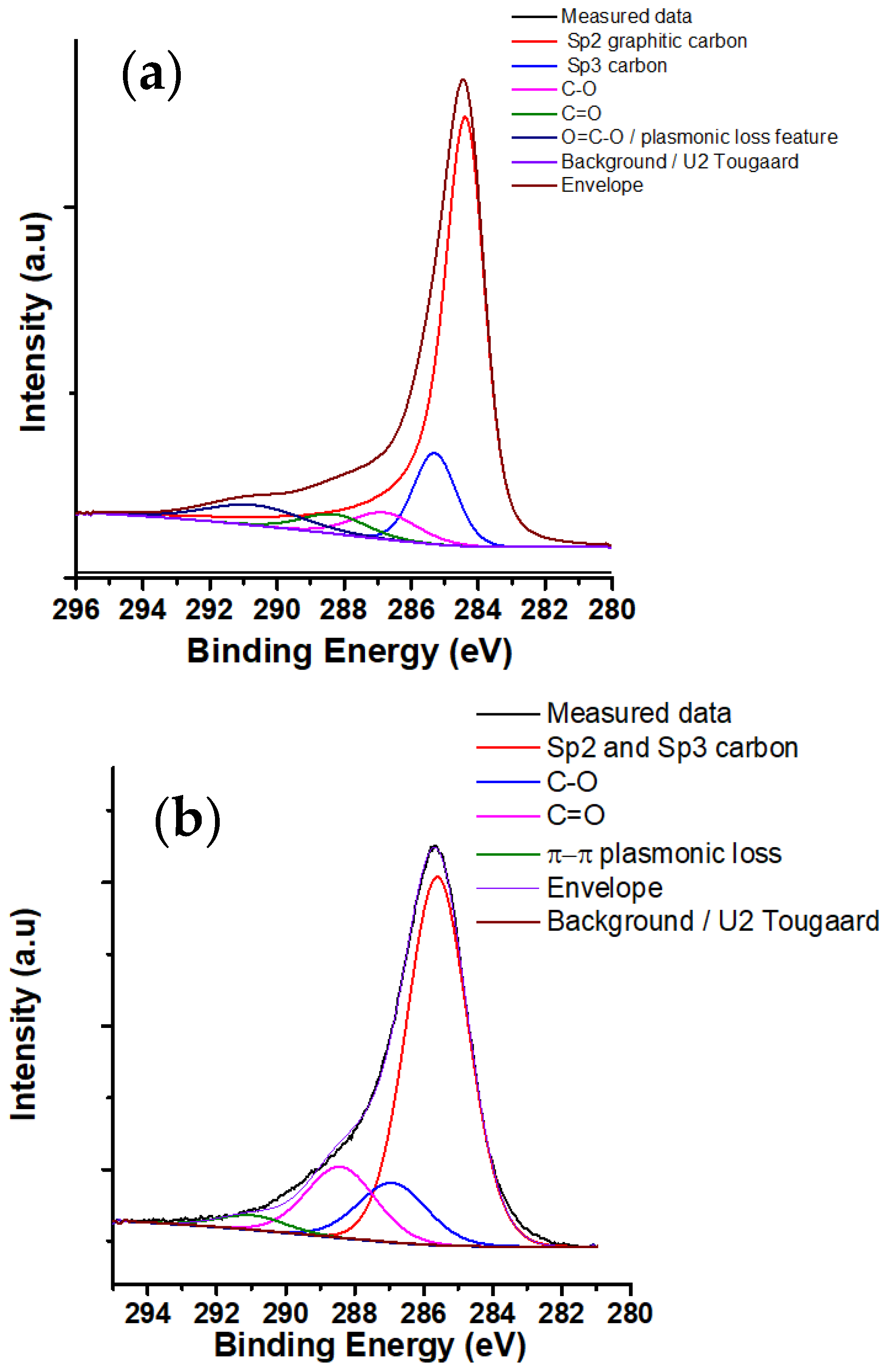


| Nanoparticles | SBET | Atomic Percent (XPS) | Atomic Percent (EDX) | ||
|---|---|---|---|---|---|
| (m2/g) | C | O | C | O | |
| CNS | 9 | 76.9 | 23.1 | 81.4 | 18.6 |
| CNP | 106 | 90.7 | 9.3 | 91.6 | 8.4 |
| Nanoparticles | C 1s (%) | O 1s (%) | |||||
|---|---|---|---|---|---|---|---|
| sp3 | sp2 | C–O | C=O | O–C=O | C=O | O–H | |
| CNP | 72.1 | 12.3 | 5.5 | 4.0 | 6.1 | 35.4 | 64.6 |
| CNS | 69.3 | 12.6 | 15.0 | 3.1 | 8.2 | 91.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ba, T.L.; Bohus, M.; Lukács, I.E.; Wongwises, S.; Gróf, G.; Hernadi, K.; Szilágyi, I.M. Comparative Study of Carbon Nanosphere and Carbon Nanopowder on Viscosity and Thermal Conductivity of Nanofluids. Nanomaterials 2021, 11, 608. https://doi.org/10.3390/nano11030608
Ba TL, Bohus M, Lukács IE, Wongwises S, Gróf G, Hernadi K, Szilágyi IM. Comparative Study of Carbon Nanosphere and Carbon Nanopowder on Viscosity and Thermal Conductivity of Nanofluids. Nanomaterials. 2021; 11(3):608. https://doi.org/10.3390/nano11030608
Chicago/Turabian StyleBa, Thong Le, Marcell Bohus, István Endre Lukács, Somchai Wongwises, Gyula Gróf, Klara Hernadi, and Imre Miklós Szilágyi. 2021. "Comparative Study of Carbon Nanosphere and Carbon Nanopowder on Viscosity and Thermal Conductivity of Nanofluids" Nanomaterials 11, no. 3: 608. https://doi.org/10.3390/nano11030608
APA StyleBa, T. L., Bohus, M., Lukács, I. E., Wongwises, S., Gróf, G., Hernadi, K., & Szilágyi, I. M. (2021). Comparative Study of Carbon Nanosphere and Carbon Nanopowder on Viscosity and Thermal Conductivity of Nanofluids. Nanomaterials, 11(3), 608. https://doi.org/10.3390/nano11030608









