Evaluation of Physicochemical Properties of Amphiphilic 1,4-Dihydropyridines and Preparation of Magnetoliposomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Magnetic Nanoparticles Synthesis and Characterization
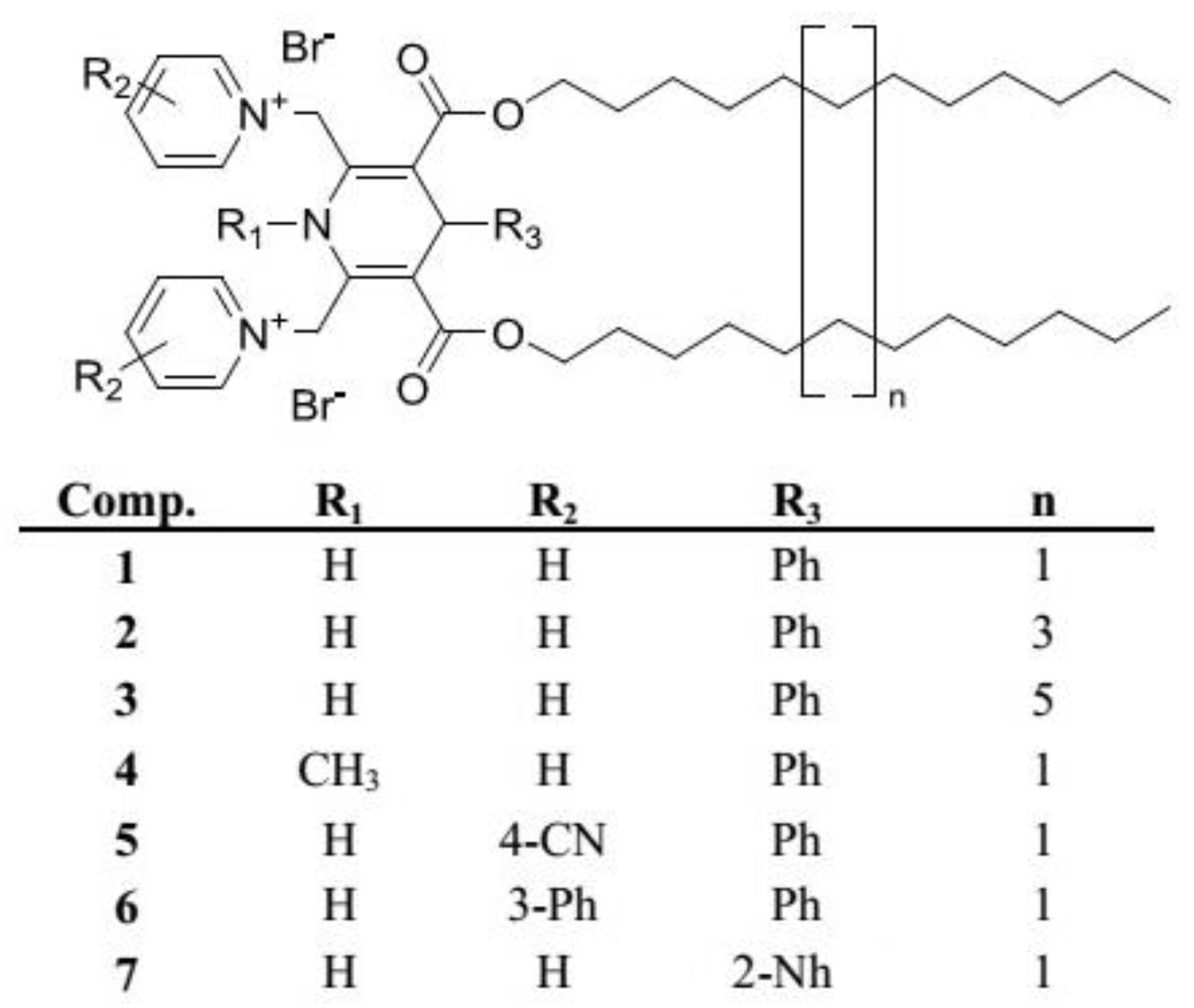
2.3. Synthesis of 1,4-DHP Amphiphiles
2.4. Thermal Analysis of 1,4-DHP Amphiphiles
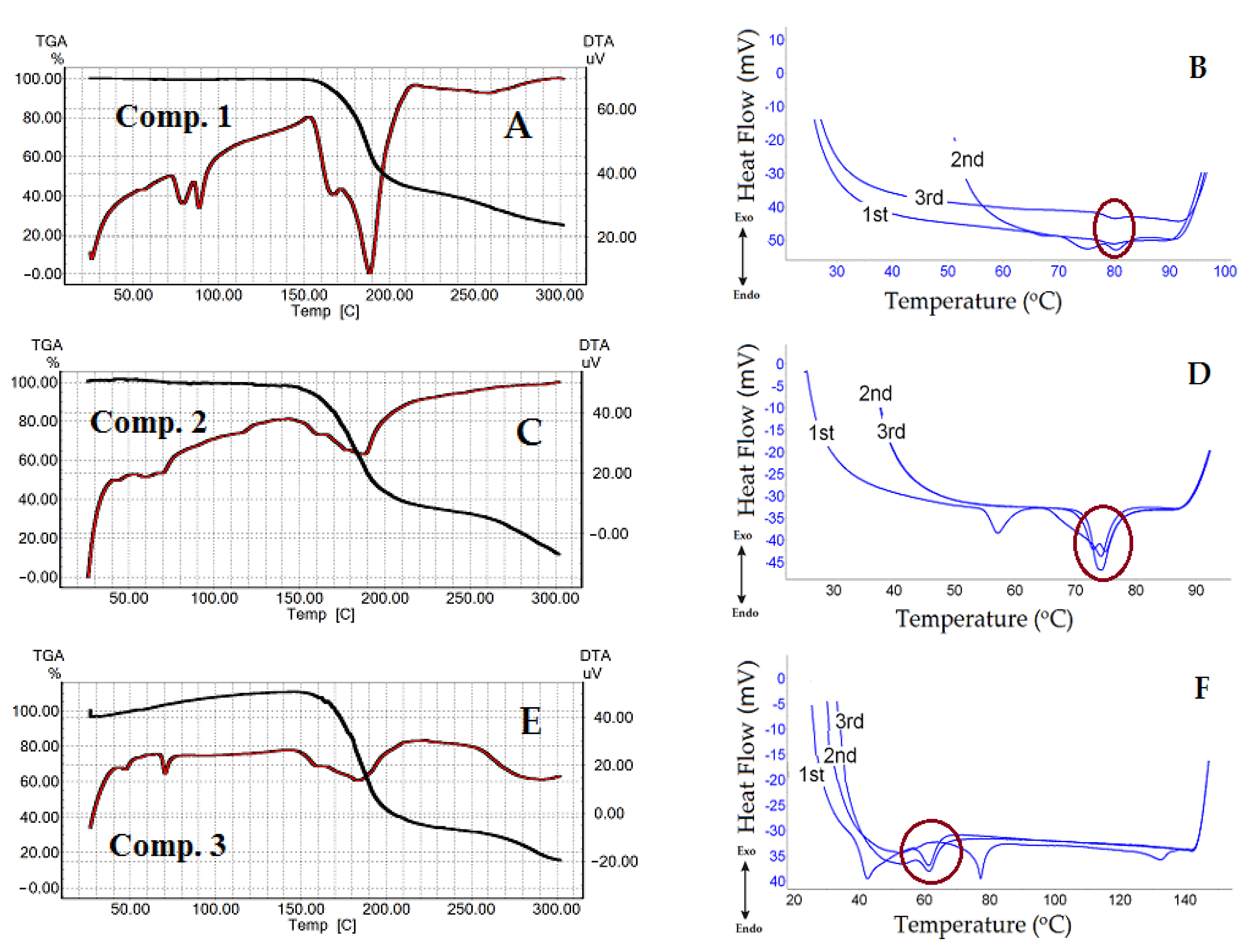
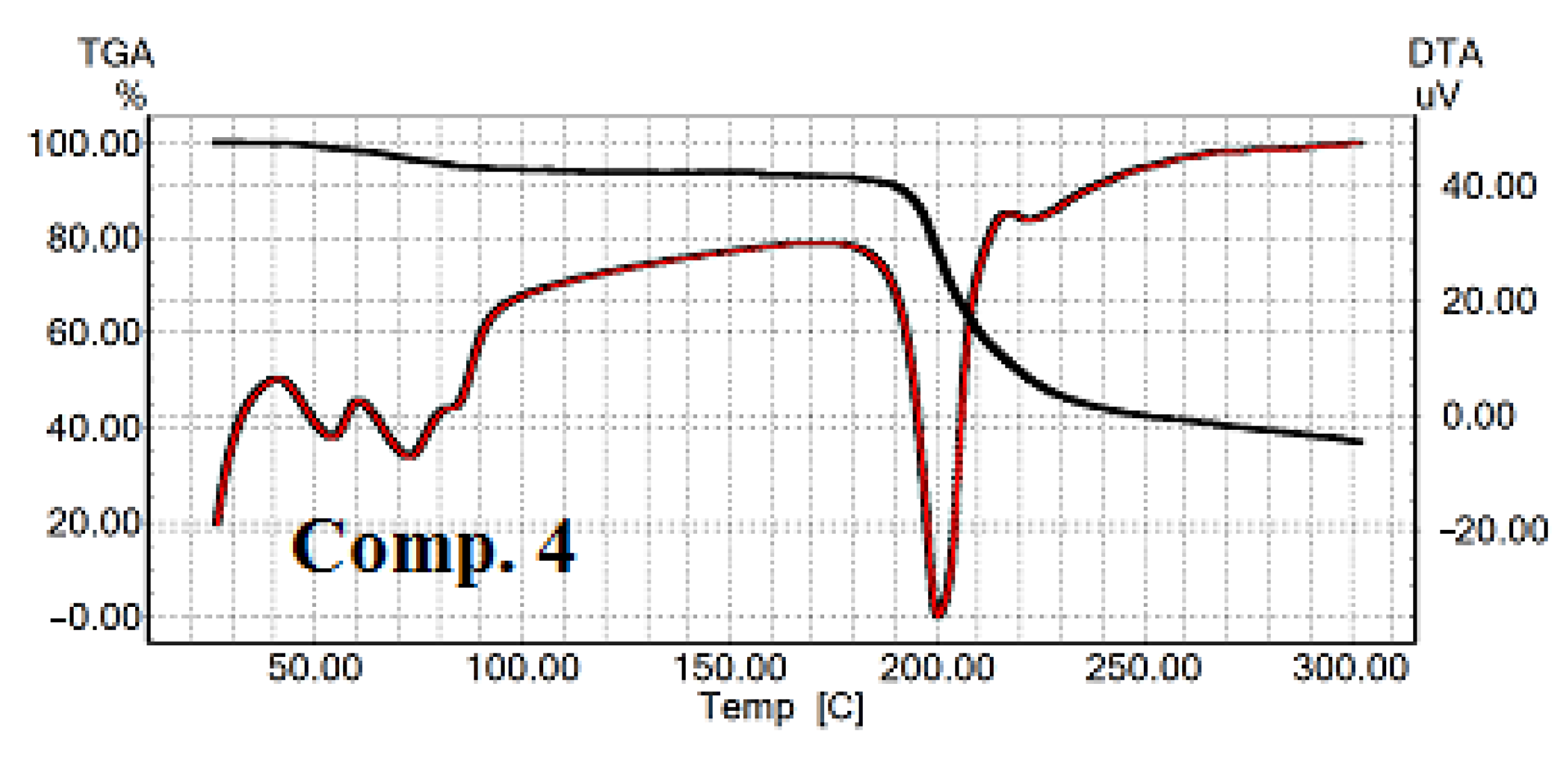
2.4.1. Thermogravimetric and Differential Thermal Analysis for the Tested 1,4-DHP Amphiphiles
2.4.2. Differential Scanning Calorimetry for the Tested 1,4-DHP Amphiphiles
2.5. Characterization of Monolayers Formed by 1,4-DHP Amphiphiles or Surface Pressure–Area (π–A) Isotherms
2.6. Magnetoliposome Preparation
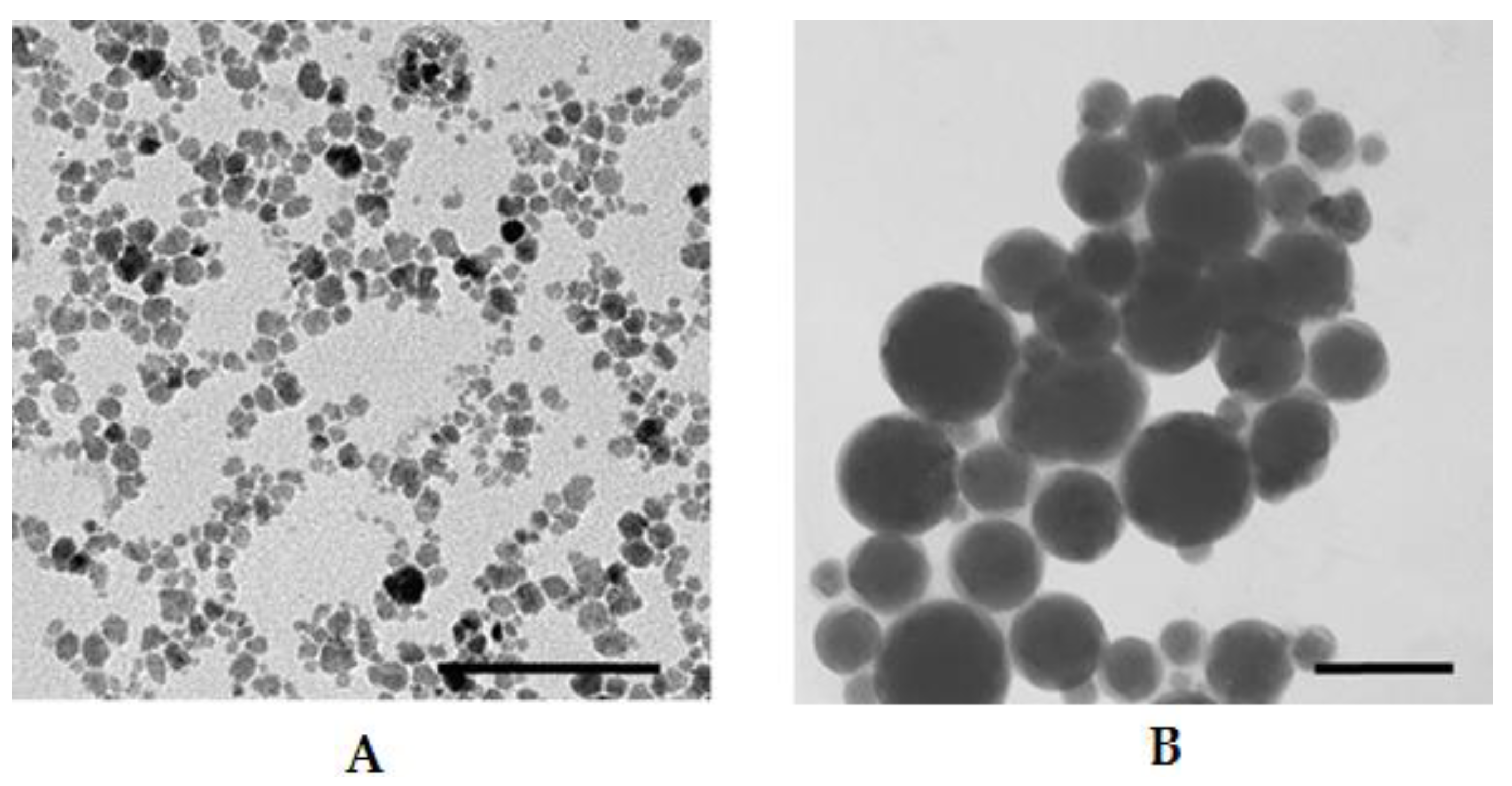
2.7. Characterization of Liposomes by Dynamic Light Scattering (DLS) and Transmission Electron Microscopy
3. Results and Discussion
3.1. Magnetic Nanoparticle Synthesis and Characterization
3.2. Synthesis of 1,4-DHP Derivatives
- 1,4-DHPs with different alkyl chain lengths at 3,5-ester moieties of 1,4-DHP (C12, C14 and C16) (comps. 1–3);
- variation of the N-substituent at position 1 of 1,4-DHP (N-H or N-CH3) (comps. 1 and 4);
- variation of the substituents at pyridinium moieties as cationic head groups at positions 2 and 6 of 1,4-DHP (H, 4-CN and 3-Ph) (comps. 1, 5 and 6);
- variation of the substituent at position 4 of 1,4-DHP (Ph and Nh) (comps. 1 and 7).
3.3. Thermal Analysis of 1,4-DHP Amphiphiles
3.4. Surface Pressure–Area Isotherms, Mechanical Properties of Monolayers
3.5. Magnetoliposome Preparation, Evaluation and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laouini, A.; Jaafar-Maalej, C.; Limayem-Blouza, I.; Star, S.; Charcosset, C.; Fessi, H. Preparation, chararacterization and aplication of liposomes. J. Colloid Sci. Biotehnol. 2012, 1, 147–168. [Google Scholar] [CrossRef]
- Monteiro, N.; Martins, A.; Reis, R.L.; Neves, N.M. Liposomes in tissue engineering and regenerative medicine. J. R. Soc. Interface 2014, 11, 20140459. [Google Scholar] [CrossRef] [PubMed]
- Rucins, M.; Dimitrijevs, P.; Pajuste, K.L.; Petrichenko, O.; Jackevica, L.; Gulbe, A.; Kibilda, S.; Smits, K.; Plotniece, M.; Tirzite, D.; et al. Contribution of molecular structure to self-assembling and biological properties of bifunctional lipid-like 4-(N-alkylpyridinium)-1,4-dihydropyridines. Pharmaceutics 2019, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef]
- Skouras, A.; Mourtas, S.; Markoutsa, E.; De Golstein, M.C.; Wallon, C.; Sarah, C.; Antimisiaris, S.G. Magnetoliposomes with high USPIO entrapping efficiency, stability and magnetic properties. Nanomedicine: NBM 2011, 7, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Hofmann-Amtenbrink, M.; Hofmann, H.; Montet, X. Superparamagnetic nanoparticles—A tool for early diagnostics. Swiss Med. Wkly. 2010, 140, w13081. [Google Scholar] [CrossRef] [PubMed]
- Babincová, N.; Sourivong, P.; Babinec, P.; Bergemann, C.; Babincová, M.; Durdík, Š. Application of magnetoliposomes with encapsulated doxorubicin for integrated chemotherapy and hyperthermia of rat C6 glioma. Z. Naturforsch. C J. Biosci. 2018, 73, 265–271. [Google Scholar] [CrossRef]
- Gordon, R.; Hogan, C.E.; Neal, M.L.; Anantharam, V.; Kanthasamy, A.G.; Kanthasamy, A. A simple magnetic separation method for high-yield isolation of pure primary microglia. J. Neurosci. Methods 2011, 194, 287–296. [Google Scholar] [CrossRef]
- Nardoni, M.; della Valle, E.; Liberti, M.; Relucenti, M.; Casadei, M.A.; Paolicelli, P.; Apollonio, F.; Petralito, S. Can pulsed electromagnetic fields trigger on-demand drug release from high-Tm magnetoliposomes? Nanomaterials 2018, 8, 196. [Google Scholar] [CrossRef]
- Ding, H.; Sagar, V.; Agudelo, M.; Polakka-Kanthikeel, S.; Subba Rao Atluri, V.; Raymond, A.; Samikkannu, T.; Nair, M.P. Enhanced blood–brain barrier transmigration using a novel transferrin embedded fluorescent magneto-liposome nanoformulation. Nanotechnology 2014, 25, 055101. [Google Scholar] [CrossRef]
- Fan, Z.; Fu, P.P.; Yu, H.; Raya, P.C. Theranostic nanomedicine for cancer detection and treatment. J. Food Drug Anal. 2014, 22, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, L.B.; Thomsen, M.S.; Moos, T. Targeted drug delivery to the brain using magnetic nanoparticles. Ther. Deliv. 2015, 6, 1145–1155. [Google Scholar] [CrossRef]
- Garcia-Pinel, B.; Jabalera, Y.; Ortiz, R.; Cabeza, L.; Jimenez-Lopez, C.; Melguizo, C.; Prados, J. Biomimetic magnetoliposomes as oxaliplatin nanocarriers: In vitro study for potential application in colon cancer. Pharmaceutics 2020, 12, 589. [Google Scholar] [CrossRef]
- Cardoso, B.D.; Rodrigues, A.R.O.; Almeida, B.G.; Amorim, C.O.; Amaral, V.S.; Castanheira, E.M.S.; Coutinho, P.J.G. Stealth magnetoliposomes based on calcium-substituted magnesium ferrite nanoparticles for curcumin transport and release. Int. J. Mol. Sci. 2020, 21, 3641. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.; Bajaj, A.; Indi, S.S.; Bhattacharya, S. Synthesis of novel dimeric cationic lipids based on an aromatic backbone between the hydrocarbon chains and headgroup. Tetrahedron Lett. 2006, 47, 8401–8405. [Google Scholar] [CrossRef]
- De Smedt, S.C.; Demeester, J.; Hennik, W.E. Cationic polymer based gene delivery systems. Pharm. Res. 2000, 17, 113–126. [Google Scholar] [CrossRef]
- Ibraheem, D.; Elaissari, H.; Fessi, H. Gene therapy and DNA delivery systems. Int. J. Pharm. 2014, 459, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New developments in liposomal drug delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef]
- Ilies, M.A.; Johnson, B.H.; Makori, F.; Miller, A.; Seitz, W.A.; Thompson, E.B.; Balaban, A.T. Pyridinium cationic lipids in gene delivery: An in vitro and in vivo comparison of transfection efficiency versus a tetraalkylammonium congener. Arch. Biochem. Biophys. 2005, 435, 217–226. [Google Scholar] [CrossRef]
- Damen, M.; Groenen, A.J.J.; van Dongen, S.F.M.; Nolte, R.J.M.; Scholte, B.J.; Feiters, M.C. Transfection by cationic gemini lipids and surfactants. MedChemComm 2018, 9, 1404–1425. [Google Scholar] [CrossRef]
- Inglut, C.T.; Sorrin, A.J.; Kuruppu, T.; Vig, S.; Cicalo, J.; Ahmad, H.; Huang, H.-C. Immunological and toxicological considerations for the design of liposomes. Nanomaterials 2020, 10, 190. [Google Scholar] [CrossRef]
- Hyvönen, Z.; Plotniece, A.; Reine, I.; Chekavichus, B.; Duburs, G.; Urtti, A. Novel cationic amphiphilic 1,4-dihydropyridine derivatives for DNA delivery. Biochim. Biophys. Acta 2000, 1509, 451–466. [Google Scholar] [CrossRef]
- Hyvönen, Z.; Ruponen, M.; Rönkkö, S.; Suhonen, P.; Urtti, A. Extracellular and intracellular factors influencing gene transfection mediated by 1,4-dihydropyridine amphiphiles. Eur. J. Pharm. Sci. 2002, 15, 449–460. [Google Scholar] [CrossRef]
- Mishra, A.P.; Bajpai, A.; Rai, A.K. 1,4-Dihydropyridine: A dependable heterocyclic ring with the promising and most anticipable therapeutic effects. Mini-Rev. Med. Chem. 2019, 19, 1219–1254. [Google Scholar] [CrossRef] [PubMed]
- Godfraind, T. Discovery and development of calcium channel blockers. Front. Pharmacol. 2017, 29, 286. [Google Scholar] [CrossRef] [PubMed]
- Klusa, V. Atypical 1,4-dihydropyridine derivatives, an approach to neuroprotection and memory enhancement. Pharmacol. Res. 2016, 113, 754–759. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Li, Y.; Wang, F.; Yang, F.; Xu, W. Synthesis and radioprotective activity of mitochondria targeted dihydropyridines in vitro. Int. J. Mol. Sci. 2017, 25, 2233. [Google Scholar] [CrossRef]
- Leonova, E.; Ošiņa, K.; Duburs, G.; Bisenieks, E.; Germini, D.; Vassetzky, Y.; Sjakste, N. Metal ions modify DNA-protecting and mutagen-scavenging capacities of the AV-153 1,4-dihydropyridine. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 845, 403077. [Google Scholar] [CrossRef]
- Milkovic, L.; Vukovic, T.; Zarkovic, N.; Tatzber, F.; Bisenieks, E.; Kalme, Z.; Bruvere, I.; Ogle, Z.; Poikans, J.; Velena, A.; et al. Antioxidative 1,4-dihydropyridine derivatives modulate oxidative stress and growth of human osteoblast-like cells in vitro. Antioxidants 2018, 7, 123. [Google Scholar] [CrossRef]
- Manna, D.; Akhtar, S.; Maiti, P.; Mondal, S.; Kumar Mandal, T.; Ghosh, R. Anticancer activity of a 1,4-dihydropyridine in DMBA-induced mouse skin tumor model. Anticancer Drugs 2020, 31, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Lentz, F.; Reiling, N.; Spengler, G.; Kincses, A.; Csonka, A.; Molnár, J.; Hilgeroth, A. Dually acting nonclassical 1,4-dihydropyridines promote the anti-tuberculosis (Tb) activities of clofazimine. Molecules 2019, 24, 2873. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Casado, J.; Chueca, E.; Salillas, S.; Velázquez-Campoy, A.; Angarica, V.E.; Bénejat, L.; Guignard, J.; Giese, A.; Sancho, J.; et al. Repurposing dihydropyridines for treatment of helicobacter pylori infection. Pharmaceutics 2019, 11, 681. [Google Scholar] [CrossRef] [PubMed]
- Triggle, D.J. 1,4-Dihydropyridine as calcium channel ligands and privileged structures. Cell. Mol. Neurobiol. 2003, 23, 293–303. [Google Scholar] [CrossRef]
- Triggle, D.J. The 1,4-dihydropyridine nucleus: A pharmacophoric template part. 1. Actions at ion channels. Mini-Rev. Med. Chem. 2003, 3, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Duburs, G.; Vīgante, B.; Plotniece, A.; Krauze, A.; Sobolevs, A.; Briede, J.; Kluša, V.; Velēna, A. Dihydropyridine derivatives as bioprotectors. Chem. Today 2008, 26, 68–70. [Google Scholar]
- Cindric, M.; Cipak, A.; Serly, J.; Plotniece, A.; Jaganjac, M.; Mrakovcic, L.; Lovakovic, T.; Dedic, A.; Soldo, I.; Duburs, G.; et al. Reversal of multidrug resistance in murine lymphoma cell by amphiphilic dihydropyridine antioxidant derivative. Anticancer Res. 2010, 30, 4063–4070. [Google Scholar]
- Pajuste, K.; Hyvönen, Z.; Petrichenko, O.; Kaldre, D.; Rucins, M.; Cekavicus, B.; Ose, V.; Skrivele, B.; Gosteva, M.; Morin-Picardat, E.; et al. Gene delivery agents possessing antiradical activity: Self-assembling cationic amphiphilic 1,4-dihydropyridine derivatives. New J. Chem. 2013, 37, 3062–3075. [Google Scholar] [CrossRef]
- Bruvere, I.; Bisenieks, E.; Poikans, J.; Uldrikis, J.; Plotniece, A.; Pajuste, K.; Rucins, M.; Vigante, B.; Kalme, Z.; Gosteva, M.; et al. Dihydropyridine derivatives as cell growth modulators in vitro. Oxid. Med. Cell. Longev. 2017, 2017, 4069839. [Google Scholar] [CrossRef]
- Jansone, B.; Kadish, I.; van Groen, T.; Beitnere, U.; Moore, D.R.; Plotniece, A.; Pajuste, K.; Klusa, V. A Novel 1,4-Dihydropyridine derivative improves spatial learning and memory and modifies brain protein expression in wild type and transgenic APPSweDI mice. PLoS ONE 2015, 10, e0127686. [Google Scholar] [CrossRef]
- Beaune, G.; Dubertret, B.; Clément, O.; Vayssettes, C.; Cabuil, V.; Ménager, C. Giant vesicles containing magnetic nanoparticles and quantum dots: Feasibility and tracking by fiber confocal fluorescence microscopy. Angew. Chem. Int. Ed. 2007, 46, 5421–5424. [Google Scholar] [CrossRef] [PubMed]
- Petrichenko, O.; Plotniece, A.; Pajuste, K.; Ose, V.; Cebers, A. Formation of magnetoliposomes using self-assembling 1,4-dihydropyridine derivative and maghemite γ-Fe2O3 nanoparticles. Chem. Heterocycl. Compd. 2015, 51, 672–677. [Google Scholar] [CrossRef]
- Petrichenko, O.; Erglis, K.; Cebers, A.; Plotniece, A.; Pajuste, K.; Bealle, G.; Menager, C.; Dubois, E.; Perzynski, R. Bilayer properties of giant magnetic liposomes formed by cationic pyridine amphiphile and probed by active deformation under magnetic forces. Eur. Phys. J. E 2013, 36, 9. [Google Scholar] [CrossRef]
- Bee, A.; Massart, R.; Neveu, S. Synthesis of very small fine maghemite particles. JMMM 1995, 149, 6–9. [Google Scholar] [CrossRef]
- Răcuciu, M.; Creangă, D.E.; Airinei, A. Citric-acid-coated magnetite nanoparticles for biological applications. Eur. Phys. J. E 2006, 21, 117–121. [Google Scholar] [CrossRef]
- Pajuste, K.; Plotniece, A.; Kore, K.; Intenberga, L.; Cekavicus, B.; Kaldre, D.; Duburs, G.; Sobolev, A. Use of pyridinium ionic liquids as catalysts for the synthesis of 3,5-bis(dodecyloxycarbonyl)-1,4-dihydropyridine derivative. CEJC 2011, 9, 143–148. [Google Scholar] [CrossRef]
- De Luca, M.; Ioele, G.; Ragno, G. 1,4-Dihydropyridine antihypertensive drugs: Recent advances in photostabilization strategies. Pharmaceutics 2019, 11, 85. [Google Scholar] [CrossRef]
- De Luca, M.; Ioele, G.; Spatari, C.; Ragno, G. Photodegradation of 1,4-dihydropyridine antihypertensive drugs: An updated review. Int. J. Pharm. Pharm. Sci. 2018, 10, 8–18. [Google Scholar] [CrossRef]
- Rucins, M.; Smits, R.; Sipola, A.; Vigante, B.; Domracheva, I.; Turovska, B.; Muhamadejev, R.; Pajuste, K.; Plotniece, M.; Sobolev, A.; et al. Pleiotropic properties of amphiphilic dihydropyridines, dihydropyridones and aminovinylcarbonyl compounds. Oxid. Med. Cell. Longev. 2020, 2020, 8413713. [Google Scholar] [CrossRef]
- Plotniece, A.; Pajuste, K.; Kaldre, D.; Cekavicus, B.; Vigante, B.; Turovska, B.; Belyakov, S.; Sobolev, A.; Dubur, G. Oxidation of cationic 1,4-dihydropyridine derivatives as model compounds for putative gene delivery agents. Tetrahedron 2009, 65, 8344–8349. [Google Scholar] [CrossRef]
- Pardo-Jiménez, V.; Barrientos, C.; Pérez-Cruz, K.; Navarrete-Encina, P.A.; Olea-Azar, C.; Nuñez-Vergara, L.J.; Squella, J.A. Synthesis and electrochemical oxidation of hybrid compounds: Dihydropyridinefused coumarins. Electrochim. Acta 2014, 125, 457–464. [Google Scholar] [CrossRef]
- Stradyn’, Y.P.; Beilis, Y.I.; Uldrikis, Y.R.; Dubur, G.Y.; Sausin’, A.E.; Chekavichus, B.S. Voltamperometry of 1,4-dihydropyridine derivatives—II. Electronic and steric effects in the electrooxidation of 4-substituted 1,4-dihydropyridines. Chem. Heterocycl. Compd. 1975, 11, 1299–1303. [Google Scholar] [CrossRef]
- Turovska, B.; Goba, I.; Turovskis, I.; Grinberga, S.; Belyakov, S.; Stupnikova, S.; Liepinsh, T.; Stradins, J. Electrochemical oxidation of 4-monoalkyl-substituted 1,4-dihydropyridines. Chem. Heterocycl. Compd. 2008, 44, 1483–1490. [Google Scholar] [CrossRef]
- Petrichenko, O.; Rucins, M.; Vezane, A.; Timofejeva, I.; Sobolev, A.; Cekavicus, B.; Pajuste, K.; Plotniece, M.; Gosteva, M.; Kozlovska, T.; et al. Studies of the physicochemical and structural properties of self-assembling cationic pyridine derivatives as gene delivery agents. Chem. Phys. Lipids 2015, 191, 25–37. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nano 2019, 10, 11. [Google Scholar] [CrossRef]
- Lin, X.; Gu, N. Surface properties of encapsulating hydrophobic nanoparticles regulate the main phase transition temperature of lipid bilayers: A simulation study. Nano Res. 2014, 7, 1195–1204. [Google Scholar] [CrossRef]
- Ballweg, S.; Sezgin, E.; Doktorova, M.; Covino, R.; Reinhard, J.; Wunnicke, D.; Hänelt, I.; Levental, I.; Hummer, G.; Ernst, R. Regulation of lipid saturation without sensing membrane fluidity. Nat. Commun. 2020, 11, 756. [Google Scholar] [CrossRef] [PubMed]
- Tirzite, D.; Koronova, J.; Plotniece, A. Influence of some quaternised 1,4-dihydropyridines derivatives on liposomes and erythrocyte membranes. Biochem. Mol. Biol. Int. 1998, 45, 849–856. [Google Scholar] [PubMed]
- Capuzzi, G.; Fratini, E.; Pini, F.; Baglioni, P.; Casnati, A.; Teixeira, J. Counterion complexation by calixarene ligands in cesium and potassium dodecyl micelles. A small angle neutron scattering study. Langmuir 2000, 16, 188–194. [Google Scholar] [CrossRef]
- Vitovic, P.; Subjakova, V.; Hianik, T. The physical properties of lipid monolayers and bilayers containing calixarenes sensitive to cytochrome c. Gen. Physiol. Biophys. 2013, 32, 189–200. [Google Scholar] [CrossRef][Green Version]
- Nagarajan, R. Molecular packing parameter and surfactant self-assembly: The neglected role of the surfactant tail. Langmuir 2002, 18, 31–38. [Google Scholar] [CrossRef]
- Šegota, S.; Težak, D. Spontaneous formation of vesicles. Adv. Colloid Interface Sci. 2006, 121, 51–75. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.S.M.; Cardoso, B.D.; Rodrigues, A.R.O.; Amorim, C.O.; Amaral, V.S.; Almeida, B.G.; Queiroz, M.-J.R.P.; Martinho, O.; Baltazar, F.; Calhelha, R.C.; et al. Magnetoliposomes containing calcium ferrite nanoparticles for applications in breast cancer therapy. Pharmaceutics 2019, 11, 477. [Google Scholar] [CrossRef] [PubMed]


| Comp. | Transitions Temperatures Range, °C | ||||
|---|---|---|---|---|---|
| Transition | 1st Heating (Process) | 2nd Heating (Process) | 3rd Heating (Process) | All Coolings (Process) | |
| 1 | 1st 2nd 3rd | 65.22–70.05 ↓ 70.05–78.08 ↓ 78.10–82.70 ↓ | 77.21–82.96 ↓ | 77.00–82.70 ↓ | 65.00–60.00 (weak signal) |
| 2 | 1st 2nd | 55.20–60.15 ↓ 69.90–76.42 ↓ | 71.97–76.83 ↓ | 70.00–78.00 ↓ | 75.27–72.02 ↑ |
| 3 | 1st 2nd | 37.31–57.42 ↓ 70.86–81.57 ↓ | 57.42–66.44 ↓ | 56.82–66.71 ↓ | 63.53–55.89 (weak signal) |
| 6 | 1st | 64.20–76.32 ↓ | 59.14–74.98 ↓ | 59.58–74.54 ↓ | 56.53–44.63 ↑ |
| Comps. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| P ± SD, mN/m | 46.62 ± 0.02 | 46.53 ± 0.75 | 46.33 ± 0.58 | 48.53 ± 0.68 | 53.06 ± 0.68 | 44.81 ± 0.44 | 47.13 ± 0.74 |
| ± SD, mN/m | 156.65 ± 1.24 | 170.46 ± 2.37 | 160.18 ± 1.58 | 170.44 ± 3.95 | 209.98 ± 1.69 | 120.71 ± 3.11 | 189.12 ± 2.92 |
| Comps. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| MMA ± SD, Å2 | 82.77 ± 0.60 | 82.48 ± 1.19 | 86.91 ± 1.50 | 95.01 ± 0.94 | 82.91 ± 0.61 | 96.64 ± 1.08 | 76.65 ± 1.59 |
| p * | 0.51 | 0.51 | 0.48 | 0.44 | 0.51 | 0.43 | 0.55 |
| “Empty” Liposomes | PdI | Z-ave DH, nm | Distr. Peaks (max) Mean DH, nm (%) | ||
|---|---|---|---|---|---|
| Peak 1 (%) | Peak 2 (%) | Peak 3 (%) | |||
| Comp. 1 * | 0.263 ± 0.050 | 146.4 ± 1.4 | 188 (97) | 4757 (3) | – |
| Comp. 1 | 0.263 ± 0.013 | 150.0 ± 8.1 | 190 (97) | 3246 (3) | – |
| Comp. 2 | 0.486 ± 0.007 | 106.9 ± 8.3 | 216 (79) | 34 (18) | 4491 (3) |
| Comp. 3 | 0.431 ± 0.013 | 219.2 ± 5.4 | 347 (95) | 46 (5) | – |
| Comp. 7 | 0.428 ± 0.017 | 290.6 ± 4.6 | 557 (93) | 34 (2) | 2829 (5) |
| Liposomes | PdI | Z-ave DH, nm | Distr. Peaks (max) Mean DH, nm (%) | ||
|---|---|---|---|---|---|
| Peak 1 (%) | Peak 2 (%) | Peak 3 (%) | |||
| Comp. 1 | 0.291 ± 0.016 | 299.3 ± 1.8 | 432 (93) | 58 (7) | – |
| Comp. 2 | 0.246 ± 0.017 | 149.6 ± 1.0 | 204 (94) | 38 (6) | – |
| Comp. 3 | 0.286 ± 0.001 | 137.6 ± 0.1 | 201 (99) | 4896 (1) | – |
| Comp. 7 | 0.521 ± 0.003 | 143.8 ± 0.7 | 316 (70) | 54 (25) | 4567 (5) |
| FF–citr | 0.181 ± 0.006 | 38.4. ± 0.4 | 47 (100) | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrichenko, O.; Plotniece, A.; Pajuste, K.; Rucins, M.; Dimitrijevs, P.; Sobolev, A.; Sprugis, E.; Cēbers, A. Evaluation of Physicochemical Properties of Amphiphilic 1,4-Dihydropyridines and Preparation of Magnetoliposomes. Nanomaterials 2021, 11, 593. https://doi.org/10.3390/nano11030593
Petrichenko O, Plotniece A, Pajuste K, Rucins M, Dimitrijevs P, Sobolev A, Sprugis E, Cēbers A. Evaluation of Physicochemical Properties of Amphiphilic 1,4-Dihydropyridines and Preparation of Magnetoliposomes. Nanomaterials. 2021; 11(3):593. https://doi.org/10.3390/nano11030593
Chicago/Turabian StylePetrichenko, Oksana, Aiva Plotniece, Karlis Pajuste, Martins Rucins, Pavels Dimitrijevs, Arkadij Sobolev, Einars Sprugis, and Andrejs Cēbers. 2021. "Evaluation of Physicochemical Properties of Amphiphilic 1,4-Dihydropyridines and Preparation of Magnetoliposomes" Nanomaterials 11, no. 3: 593. https://doi.org/10.3390/nano11030593
APA StylePetrichenko, O., Plotniece, A., Pajuste, K., Rucins, M., Dimitrijevs, P., Sobolev, A., Sprugis, E., & Cēbers, A. (2021). Evaluation of Physicochemical Properties of Amphiphilic 1,4-Dihydropyridines and Preparation of Magnetoliposomes. Nanomaterials, 11(3), 593. https://doi.org/10.3390/nano11030593






