Laser Ablation-Assisted Synthesis of Plasmonic Si@Au Core-Satellite Nanocomposites for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Si@AuNPs:
2.1.1. Laser Synthesis of Bare Si and Au NPs:
2.1.2. Modification of Si NPs:
2.1.3. Formation of Si@Au NPs:
2.1.4. Physicochemical Characterizations:
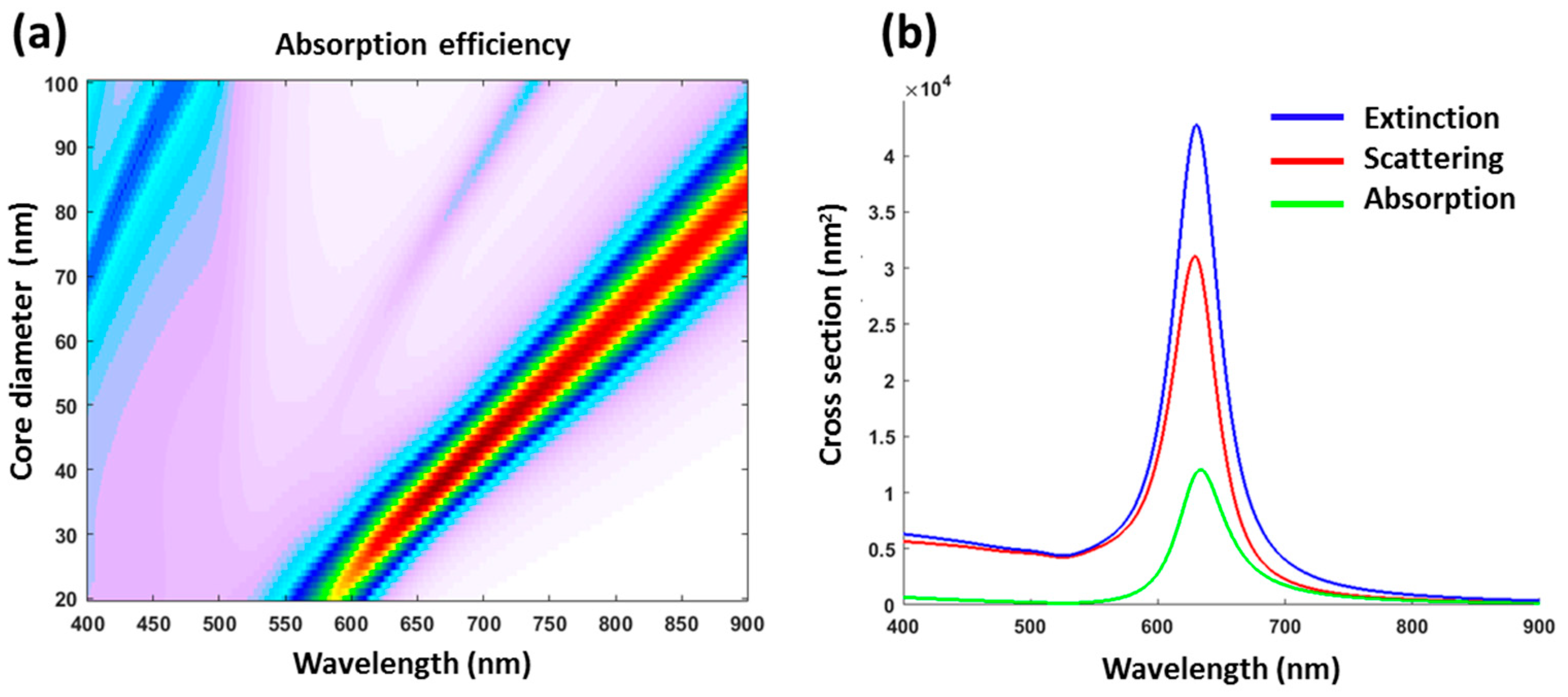
2.2. Optical Simulation: Mie Theory Modeling
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.; Lowery, A.; Halas, N.; West, J.; Drezek, R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 2005, 5, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, K.; Follen, M.; Aaron, J.; Pavlova, I.; Malpica, A.; Lotan, R.; Richards-Kortum, R. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res. 2003, 63, 1999–2004. [Google Scholar] [PubMed]
- Gobin, A.M.; Lee, M.H.; Halas, N.J.; James, W.D.; Drezek, R.A.; West, J.L. Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy. Nano Lett. 2007, 7, 1929–1934. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, X.; Wang, X.; Ku, G.; Gill, K.L.; O’Neal, D.P.; Stoica, G.; Wang, L.V. Photoacoustic tomography of a nanoshell contrast agent in the in vivo rat brain. Nano Lett. 2004, 4, 1689–1692. [Google Scholar] [CrossRef]
- Gao, N.; Chen, Y.; Li, L.; Guan, Z.; Zhao, T.; Zhou, N.; Yuan, P.; Yao, S.Q.; Xu, Q.-H. Shape-dependent two-photon photoluminescence of single gold nanoparticles. J. Phys. Chem. C 2014, 118, 13904–13911. [Google Scholar] [CrossRef]
- Dasgupta, S.; Auth, T.; Gompper, G. Shape and orientation matter for the cellular uptake of nonspherical particles. Nano Lett. 2014, 14, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, S.J.; Averitt, D.; Westcott, S.L.; Halas, N.J. Nanoengineering of optical resonances. Chem. Phys. Lett. 1998, 288, 243–247. [Google Scholar] [CrossRef]
- Yu, M.; Zheng, J. Clearance pathways and tumor targeting of imaging nanoparticles. ACS Nano 2015, 9, 6655–6674. [Google Scholar] [CrossRef] [PubMed]
- James, W.D.; Hirsch, L.R.; West, J.L.; O’Neal, P.D.; Payne, J.D. Application of INAA to the build-up and clearance of gold nanoshells in clinical studies in mice. J. Radioanal. Nucl. Chem. 2007, 271, 455–459. [Google Scholar] [CrossRef]
- Lv, W.; Phelan, P.E.; Swaminathan, R.; Otanicar, T.P.; Taylor, R.A. Multifunctional core-shell nanoparticle suspensions for efficient absorption. J. Sol. Energy Eng. 2013, 135, 021004. [Google Scholar] [CrossRef]
- Chaabani, W.; Chehaidar, A.; Plain, J. Comparative theoretical study of the optical properties of silicon/gold, silica/gold core/shell and gold spherical nanoparticles. Plasmonics 2016, 11, 1525–1535. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Delaporte, P.; Grojo, D.; Torres, R.; Sarnet, T.; Sentis, M. Nanofabrication with pulsed lasers. Nanoscale Res. Lett. 2010, 5, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser synthesis and processing of colloids: Fundamentals and applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef] [PubMed]
- Geohegan, D.B.; Puretzky, A.A.; Duscher, G.; Pennycook, S.J. Time-resolved imaging of gas phase nanoparticle synthesis by laser ablation. Appl. Phys. Lett. 1998, 72, 2987–2989. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Meunier, M. Visible Photoluminescence from Nanostructured Si-Based Layers Produced by Air Optical Breakdown on Silicon. Appl. Phys. Lett. 2003, 82, 1619–1621. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Meunier, M. Laser-induced treatment of silicon in air and formation of Si/SiOx photoluminescent nanostructured layers. Mater. Sci. Eng. B 2003, 101, 60–64. [Google Scholar] [CrossRef]
- Fojtik, A.; Henglein, A. Laser Ablation of Films and Suspended Particles in Solvent-Formation of Cluster and Colloid Solutions. Ber. Bunsenges. Phys. Chem. 1993, 97, 252. [Google Scholar]
- Dolgaev, S.I.; Simakin, A.V.; Vornov, V.V.; Shafeev, G.A.; Bozon-Verduraz, F. Nanoparticles Produced by Laser Ablation of Solids in Liquid Environment. Appl. Surf. Sci. 2002, 186, 546–551. [Google Scholar] [CrossRef]
- Kabashin, V.K.; Meunier, M. Synthesis of Colloidal Nanoparticles during Femtosecond Laser Ablation of Gold in Water. J. Appl. Phys. 2003, 94, 7941. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Timoshenko, V.Y. What Theranostic applications could ultrapure laser-synthesized Si nanoparticles have in cancer? Nanomedicine 2016, 11, 2247–2250. [Google Scholar] [CrossRef] [PubMed]
- Kabashin, A.V.; Singh, A.; Swihart, M.T.; Zavestovskaya, I.N.; Prasad, P.N. Laser-processed nanosilicon: A multifunctional nanomaterial for energy and healthcare. ACS Nano 2019, 13, 9841–9867. [Google Scholar] [CrossRef]
- Kabashin, V.K.; Meunier, M. Laser ablation-based synthesis of functionalized colloidal nanomaterials in biocompatible solutions. J. Photochem. Photobiol. A 2006, 182, 330–334. [Google Scholar] [CrossRef]
- Baati, T.; Al-Kattan, A.; Esteve, M.A.; Njim, L.; Ryabchikov, Y.; Chaspoul, F.; Hammami, M.; Sentis, M.; Kabashin, A.V.; Braguer, D. Ultrapure laser-synthesized Si-based nanomaterials for biomedical applications: In vivo assessment of safety and biodistribution. Sci. Rep. 2016, 6, 1–13. [Google Scholar]
- Al-Kattan, A.; Ryabchikov, Y.V.; Baati, T.; Chirvony, V.; Sánchez-Royo, J.F.; Sentis, M.; Braguer, D.; Timoshenko, V.Y.; Estève, M.-A.; Kabashin, A.V. Ultrapure Laser-Synthesized Si Nanoparticles with Variable Oxidation States for Biomedical Applications. J. Mater. Chem. B 2016, 4, 7852–7858. [Google Scholar] [CrossRef]
- Popov, A.A.; Tselikov, G.; Dumas, N.; Berard, C.; Metwally, K.; Jones, N.; Al-Kattan, A.; Larrat, B.; Braguer, D.; Mensah, S.; et al. Laser- synthesized TiN nanoparticles as promising plasmonic alternative for biomedical applications. Sci. Rep. 2019, 9, 1194. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Popov, A.A.; Shipunova, V.O.; Tikhonowski, G.V.; Mirkasymov, A.B.; Popova-Kuznetsova, E.A.; Klimentov, S.M.; Kabashin, A.V.; Deyev, S.M. Laser-synthesized TiN nanoparticles for biomedical applications: Evaluation of safety, biodistribution and pharmacokinetics. Mater. Sci. Eng. C 2020, 120, 111717. [Google Scholar] [CrossRef]
- Amendola, V.; Scaramuzza, S.; Litti, L.; Meneghetti, M.; Zuccolotto, G.; Rosato, A.; Nicolato, E.; Marzola, P.; Fracasso, G.; Anselmi, C.; et al. Magneto-plasmonic Au-Fe alloy nanoparticles designed for multimodal SERS-MRI-CT imaging. Small 2014, 10, 2476–2486. [Google Scholar] [CrossRef]
- Scaramuzza, S.; Agnoli, S.; Amendola, V. Metastable alloy nanoparticles, metal-oxide nanocrescents and nanoshells generated by laser ablation in liquid solution: Influence of the chemical environment on structure and composition. Phys. Chem. Chem. Phys. 2015, 17, 28076–28087. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Yang, J.; Lu, S.-H.; Niu, K.-Y.; Liu, Y.; Sun, J.; Du, X.-W. Laser Synthesis of Gold/Oxide Nanocomposites. J. Mater. Chem 2010, 20, 1103–1106. [Google Scholar] [CrossRef]
- Jo, Y.K.; Wen, S.-B. Formation of Core−Shell Micro/Nano Particles through Pulsed-Laser Deposition in Liquid. J. Phys. D Appl. Phys. 2013, 46, 035302. [Google Scholar] [CrossRef]
- Kutrovskaya, S.; Arakelian, S.; Kucherik, A.; Osipov, A.; Evlyukhin, A.; Kavokin, A.V. The synthsis of hybrid gold-silicon nano particles in liquid. Sci. Rep. 2017, 7, 10284. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, E.; Abderrafi, K.; Abargues, R.; Valdés, J.L.; Martínez-Pastor, J.P. Laser-Ablation-Induced Synthesis of SiO2-Capped Noble Metal Nanoparticles in a Single Step. Langmuir 2010, 26, 7458–7463. [Google Scholar]
- Liu, P.; Chen, H.; Wang, H.; Yan, J.; Lin, Z.; Yang, G. Fabrication of Si/Au Core/Shell Nanoplasmonic Structures with Ultrasensitive Surface-Enhanced Raman Scattering for Monolayer Molecule Detection. J. Phys. Chem. C 2015, 119, 1234–1246. [Google Scholar] [CrossRef]
- Sylvestre, J.; Poulin, S.; Kabashin, A.V.; Sacher, E.; Meunier, M.; Luong, J.H.T. Surface Chemistry of Gold Nanoparticles Produced by Laser Ablation in Aqueous Media. J. Phys. Chem. B 2004, 108, 16864–16869. [Google Scholar] [CrossRef]
- Metwally, K.; Mensah, S.; Baffou, G. Fluence threshold for photothermal bubble generation using plasmonic nanoparticles. J. Phys. Chem. C. 2015, 119, 28586–28596. [Google Scholar] [CrossRef]
- Le, R.E.; Etchegoin, P. Principles of Surface-Enhanced Raman Spectroscopy: And Related Plasmonic Effects; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Lachaine, R.; Boulais, E.; Rioux, D.; Boutopoulos, C.; Meunier, M. Computational design of durable spherical nanoparticles with optimal material, shape, and size for ultrafast plasmon-enhanced nanocavitation. ACS Photonics 2016, 3, 2158–2169. [Google Scholar] [CrossRef]
- Moroz, A. Electron mean free path in a spherical shell geometry. J. Phys. Chem. C 2008, 112, 10641–10652. [Google Scholar] [CrossRef]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Ung, B.; Sheng, Y. Interference of surface waves in a metallic nanoslit. Opt. Express 2007, 15, 1182–1190. [Google Scholar] [CrossRef]
- Raschke, G.; Brogl, S.; Susha, A.S.; Rogach, A.; Klar, T.; Feldmann, J.; Fieres, B.; Petkov, N.; Bein, T.; Nichtl, A.; et al. Gold nanoshells improve single nanoparticle molecular sensors. Nano Lett. 2004, 4, 1853–1857. [Google Scholar] [CrossRef]
- Westcott, S.L.; Oldenburg, S.J.; Lee, R.; Halas, N.J. Formation and adsorption of clusters of gold nanoparticles onto functionalized silica nanoparticles. Langmuir 1998, 14, 5396–5401. [Google Scholar] [CrossRef]
- Bailly, A.-L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.-A. In vivo evaluation of safety, biodistribution and pharmacokinetics of laser-synthesized gold nanoparticles. Sci. Rep. 2019, 9, 12890. [Google Scholar] [CrossRef] [PubMed]
- Kharin, A.Y.; Lysenko, V.V.; Rogov, A.; Ryabchikov, Y.V.; Geloen, A.; Tishchenko, I.; Marty, O.; Sennikov, P.G.; Kornev, R.A.; Zavestovskaya, I.N.; et al. Bi-Modal Nonlinear Optical Contrast from Si Nanoparticles for Cancer Theranostics. Adv. Opt. Mater. 2019, 7, 1801728. [Google Scholar] [CrossRef]
- Tamarov, K.P.; Osminkina, L.A.; Zinovyev, S.V.; Maximova, K.A.; Kargina, J.V.; Gongalsky, M.B.; Ryabchikov, Y.; Al-Kattan, A.; Sviridov, A.P.; Sentis, M.; et al. Radio Frequency Radiation-Induced Hyperthermia Using Si Nanoparticle-Based Sensitizers for Mild Cancer Therapy. Sci. Rep. 2015, 4, 7034. [Google Scholar] [CrossRef]
- Kögler, M.; Ryabchikov, Y.V.; Uusitalo, S.; Popov, A.; Popov, A.; Tselikov, G.; Välimaa, A.-L.; Al-Kattan, A.; Hiltunen, J.; Laitinen, R.; et al. Bare laser-synthesized Au-based nanoparticles as non-disturbing SERS probes for Bacteria Detection. J. Biophotonics 2018, 11, e201700225. [Google Scholar] [CrossRef] [PubMed]
- Patskovsky, S.; Kabashin, A.V.; Meunier, M.; Luong, J.H.T. Near-infrared surface plasmon resonance sensing on a silicon platform. Sens. Actuators B Chem. 2004, 97, 409–414. [Google Scholar] [CrossRef]
- Patskovsky, S.; Kabashin, A.V.; Meunier, M.; Luong, J.H.T. Si-based surface plasmon resonance sensing with two surface plasmon polariton modes. Appl. Opt. 2003, 42, 6905. [Google Scholar] [CrossRef] [PubMed]
- Liedberg, B.; Nylander, C.; Lunström, I. Surface plasmon resonance for gas detection and biosensing. Sens. Actuators 1983, 4, 299–304. [Google Scholar] [CrossRef]
- Kabashin, A.V.; Kochergin, V.E.; Nikitin, P.I. Surface plasmon resonance bio- and chemical sensors with phase-polarisation contrast. Sens. Actuators B Chem. 1999, 54, 51–56. [Google Scholar] [CrossRef]
- Law, W.C.; Markowicz, P.; Yong, K.T.; Roy, I.; Baev, A.; Patskovsky, S.; Kabashin, A.V.; Ho, H.P.; Prasad, P.N. Wide dynamic range phase-sensitive surface plasmon resonance biosensor based on measuring the modulation harmonics. Biosens. Bioelectron. 2007, 23, 627–632. [Google Scholar] [CrossRef] [PubMed]

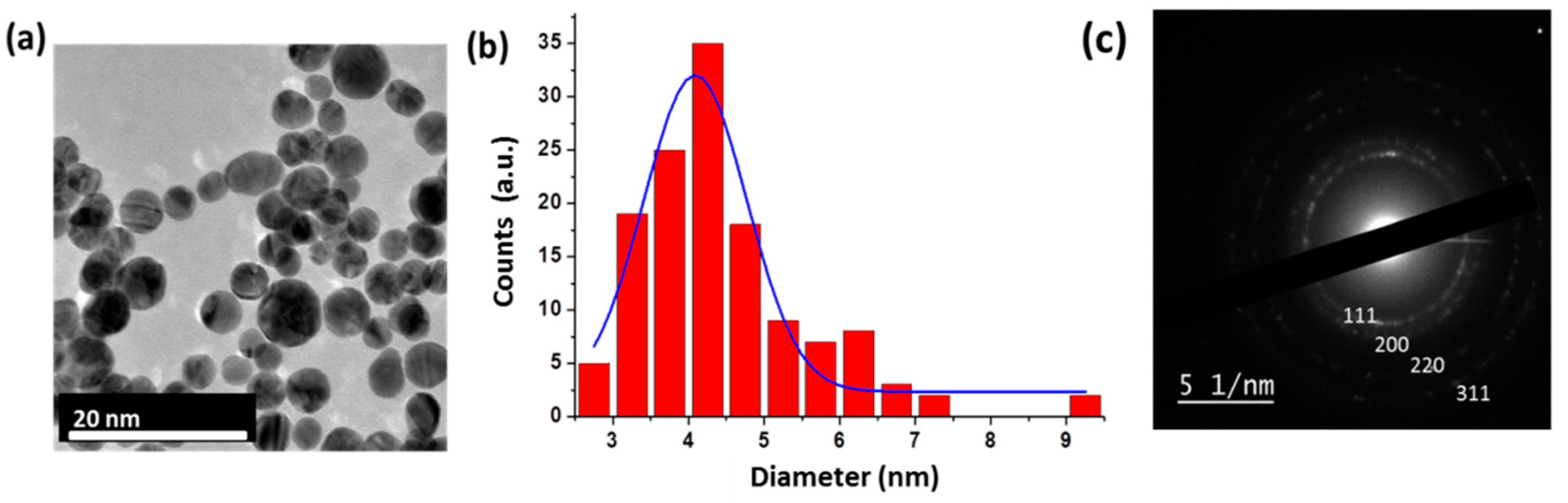
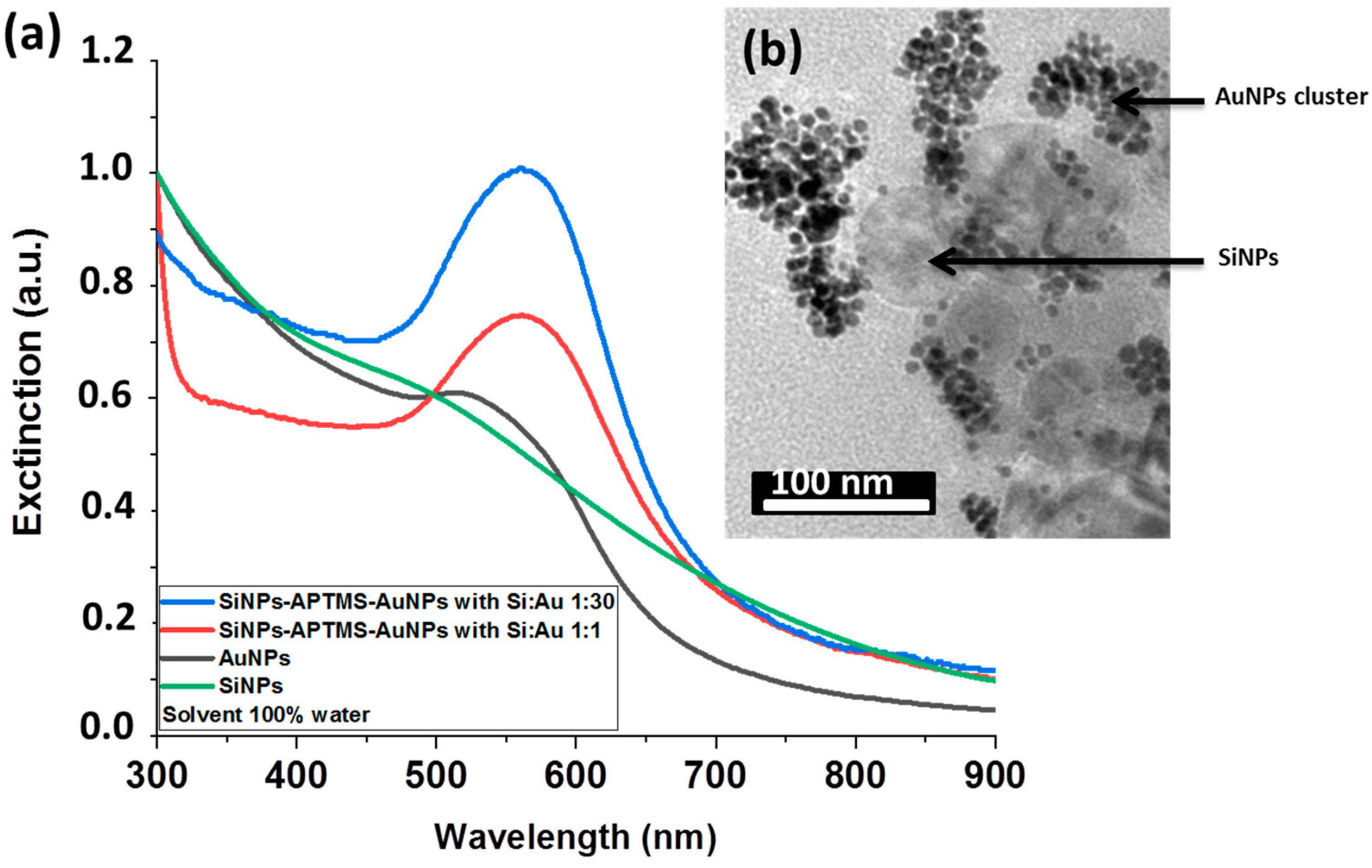
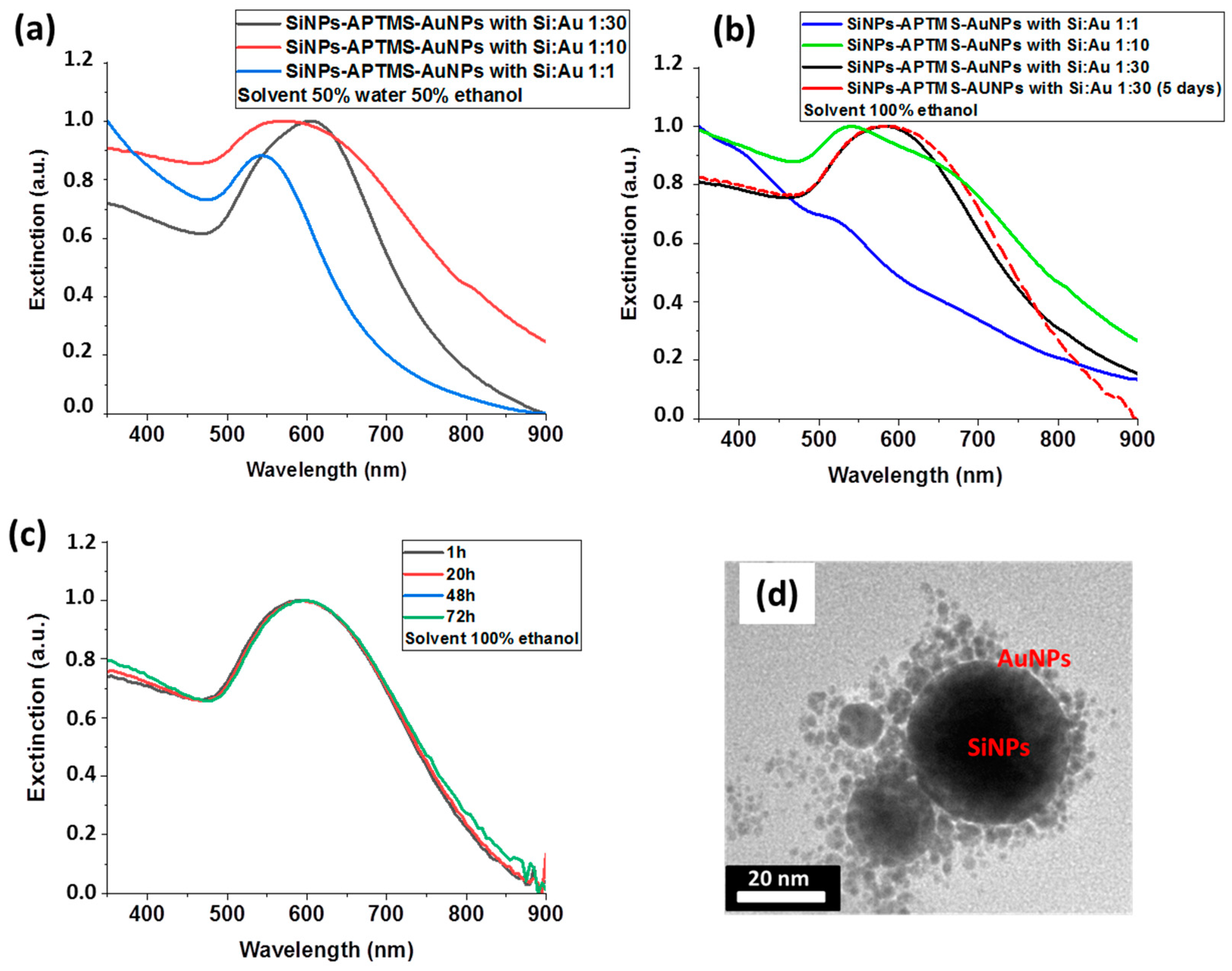
| Ring | dhkl (Å) | hkl | a (Å) | Space Group |
|---|---|---|---|---|
| 1 | 3.13 | 111 | 5.42 | Cubic diamond Fd3m |
| 2 | 1.91 | 220 | 5.40 | Cubic diamond Fd3m |
| 3 | 1.37 | 400 | 5.47 | Cubic diamond Fd3m |
| Ring | dhkl (Å) | hkl | a (Å) | Space Group |
|---|---|---|---|---|
| 1 | 2.38 | 111 | 4.08 | Cubic fcc Fm3m |
| 2 | 2.04 | 200 | 4.08 | Cubic fcc Fm3m |
| 3 | 1.46 | 420 | 4.08 | Cubic fcc Fm3m |
| 4 | 1.22 | 311 | 4.08 | Cubic fcc Fm3m |
| APTMS Volume | Ratio of Water to Ethanol | ||
|---|---|---|---|
| 100:0 | 50:50 | 0:100 | |
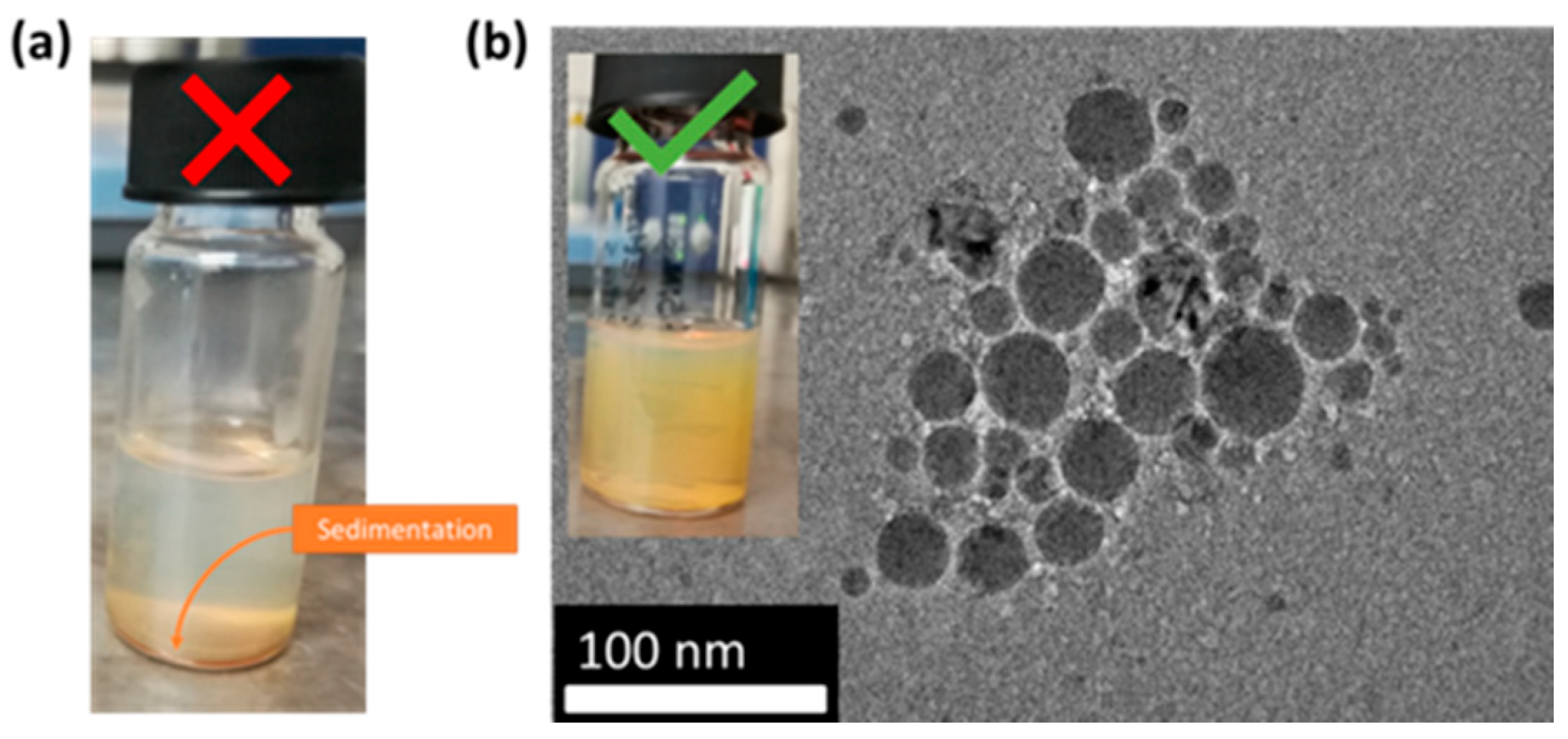
| 1 mL | Jellification/Complete sedimentation | _ | _ |
| 0.5 mL | Jellification/Partial sedimentation | _ | _ |
| 80 µL | Stable | Stable | Stable |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Kattan, A.; Tselikov, G.; Metwally, K.; Popov, A.A.; Mensah, S.; Kabashin, A.V. Laser Ablation-Assisted Synthesis of Plasmonic Si@Au Core-Satellite Nanocomposites for Biomedical Applications. Nanomaterials 2021, 11, 592. https://doi.org/10.3390/nano11030592
Al-Kattan A, Tselikov G, Metwally K, Popov AA, Mensah S, Kabashin AV. Laser Ablation-Assisted Synthesis of Plasmonic Si@Au Core-Satellite Nanocomposites for Biomedical Applications. Nanomaterials. 2021; 11(3):592. https://doi.org/10.3390/nano11030592
Chicago/Turabian StyleAl-Kattan, Ahmed, Gleb Tselikov, Khaled Metwally, Anton A. Popov, Serge Mensah, and Andrei V. Kabashin. 2021. "Laser Ablation-Assisted Synthesis of Plasmonic Si@Au Core-Satellite Nanocomposites for Biomedical Applications" Nanomaterials 11, no. 3: 592. https://doi.org/10.3390/nano11030592
APA StyleAl-Kattan, A., Tselikov, G., Metwally, K., Popov, A. A., Mensah, S., & Kabashin, A. V. (2021). Laser Ablation-Assisted Synthesis of Plasmonic Si@Au Core-Satellite Nanocomposites for Biomedical Applications. Nanomaterials, 11(3), 592. https://doi.org/10.3390/nano11030592







