The Importance of Thermal Treatment on Wet-Kneaded Silica–Magnesia Catalyst and Lebedev Ethanol-to-Butadiene Process
Abstract
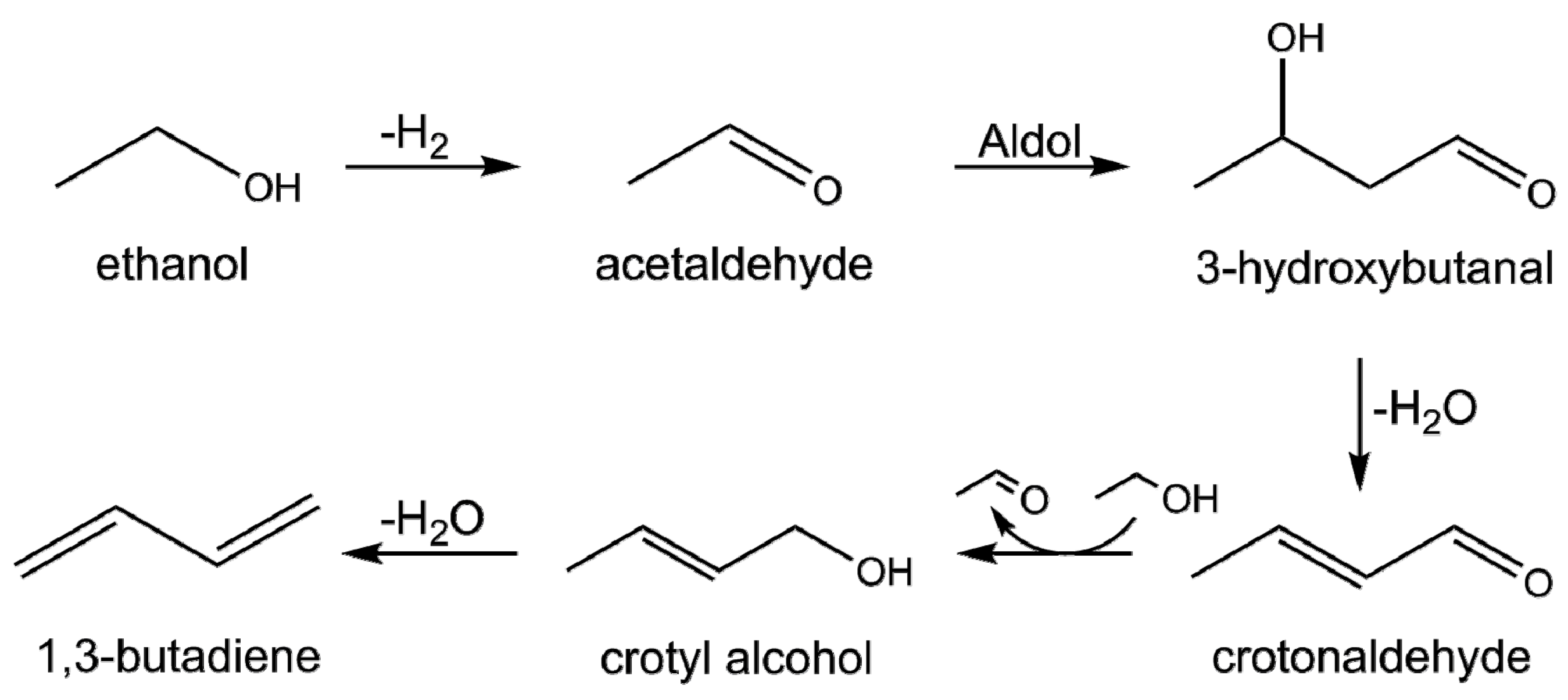
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Preparation
2.3. Characterization
2.4. Activity Test
3. Results and Discussion
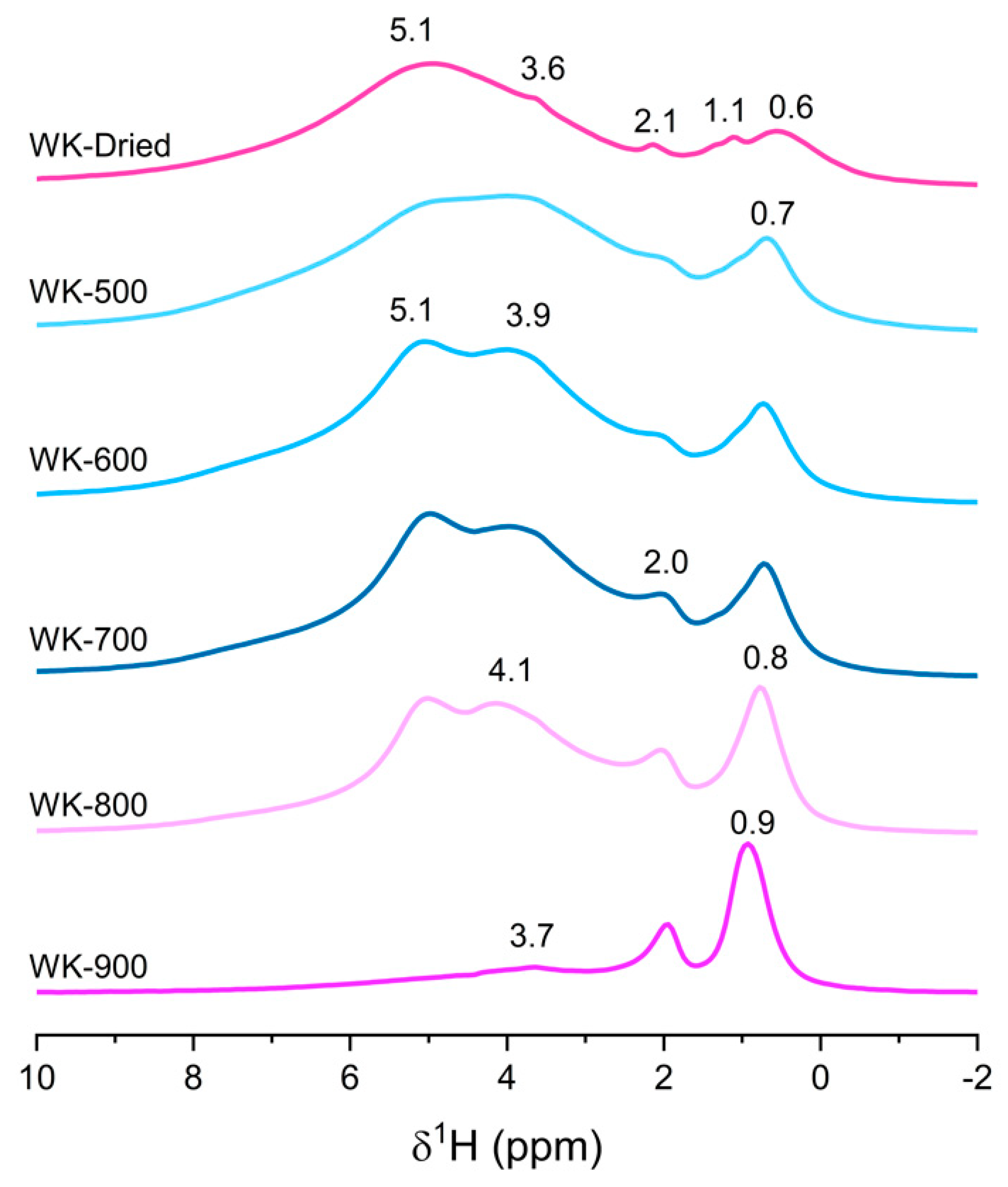
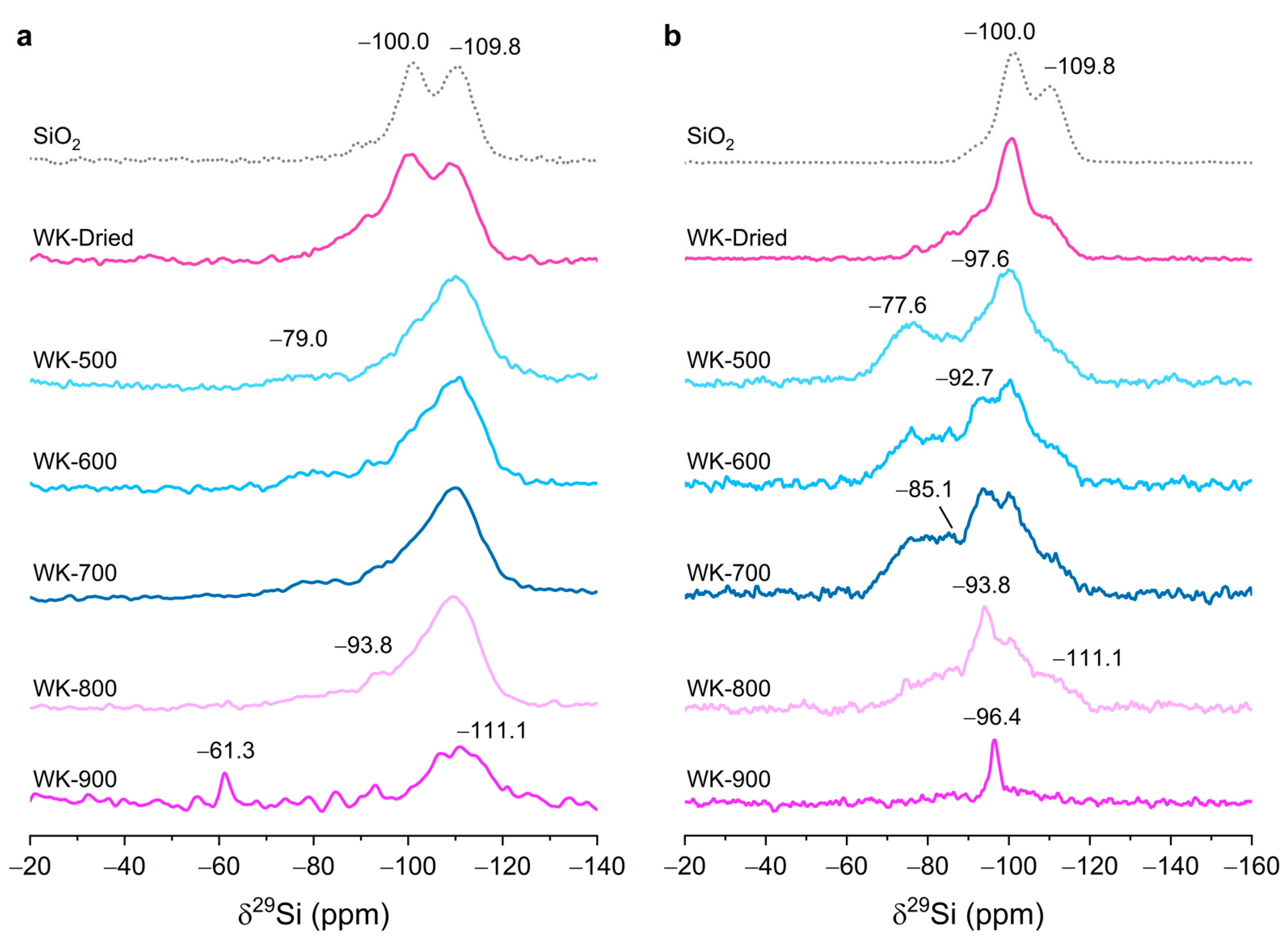
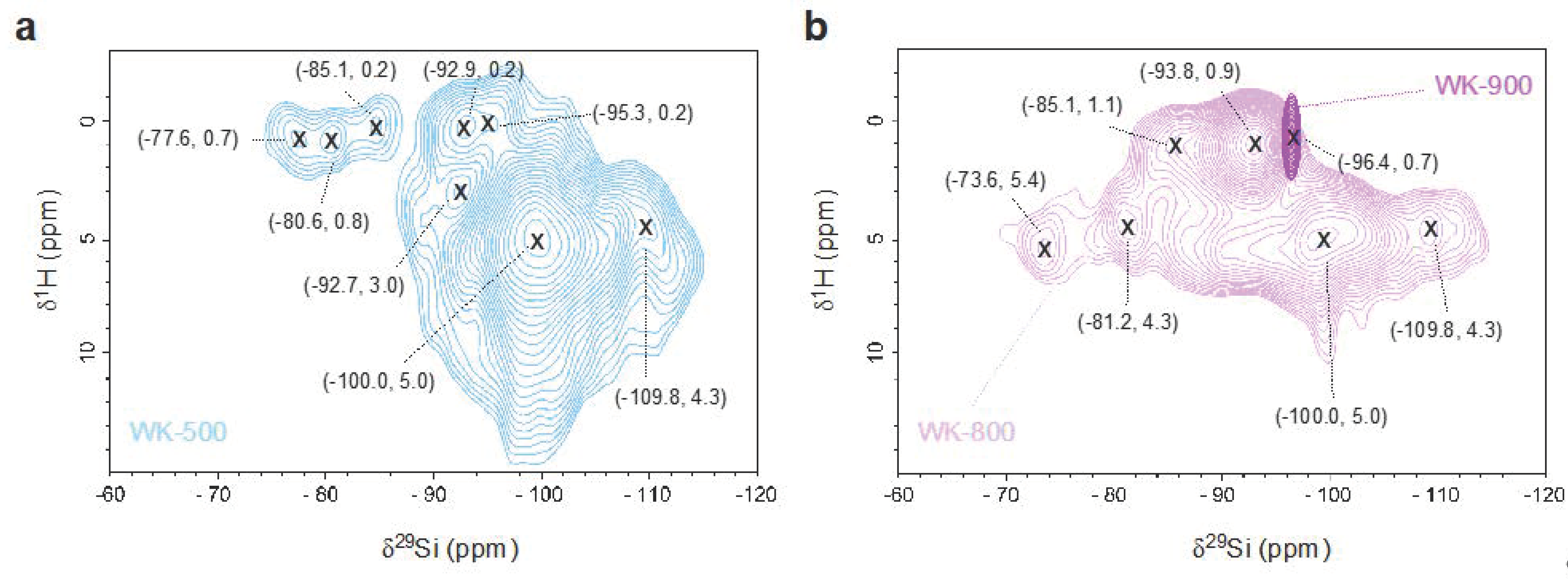
3.1. Effect of Thermal Treatment on Wet-Kneaded SiO2–MgO Catalyst
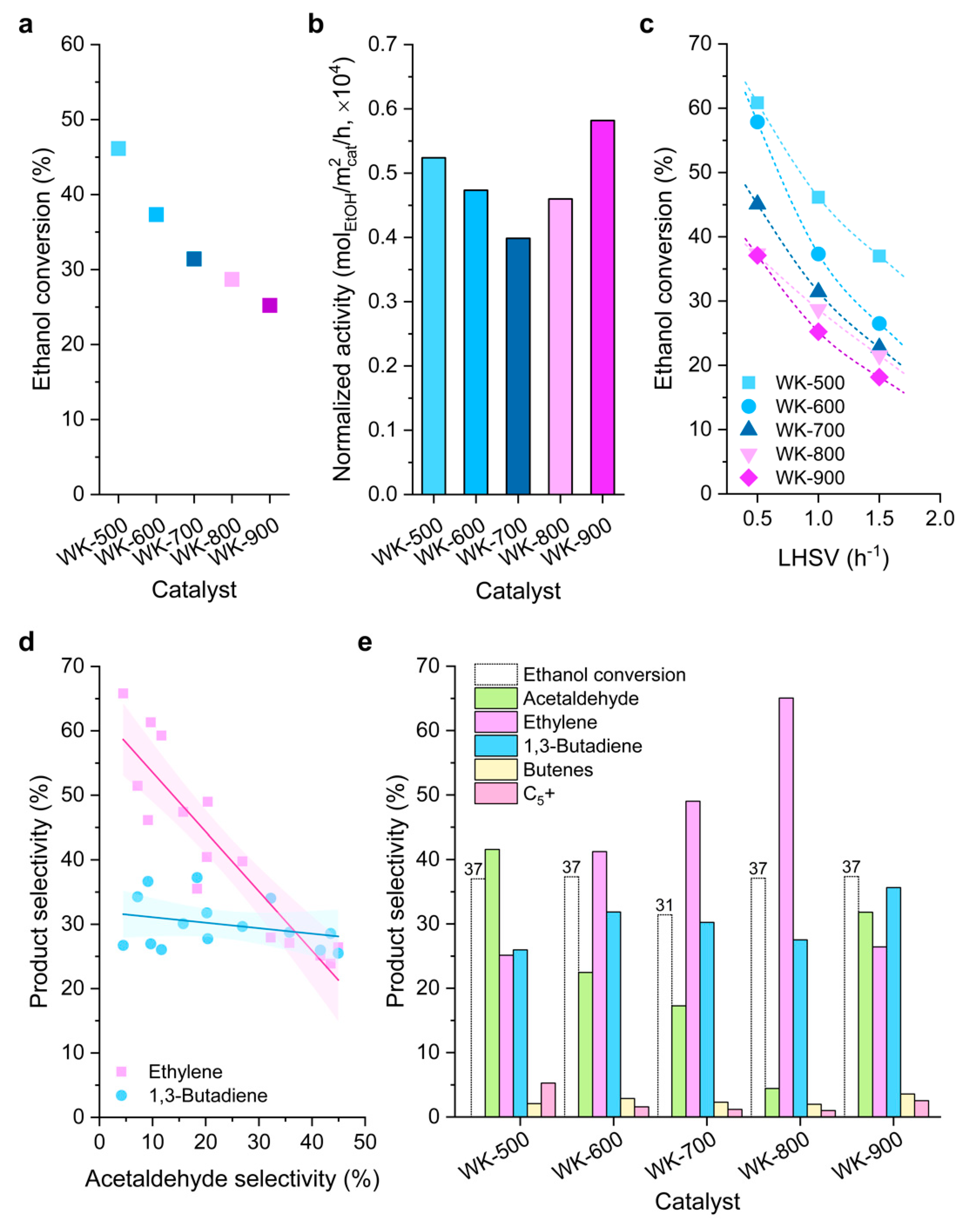
3.2. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruijnincx, P.C.A.; Weckhuysen, B.M. Shale Gas Revolution: An Opportunity for the Production of Biobased Chemicals? Angew. Chemie Int. Ed. 2013, 52, 11980–11987. [Google Scholar] [CrossRef]
- Angelici, C.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Chemocatalytic Conversion of Ethanol into Butadiene and Other Bulk Chemicals. ChemSusChem 2013, 6, 1595–1614. [Google Scholar] [CrossRef]
- Cespi, D.; Passarini, F.; Vassura, I.; Cavani, F. Butadiene from biomass, a life cycle perspective to address sustainability in the chemical industry. Green Chem. 2016, 18, 1625–1638. [Google Scholar] [CrossRef]
- Makshina, E.V.; Dusselier, M.; Janssens, W.; Degrève, J.; Jacobs, P.A.; Sels, B.F. Review of old chemistry and new catalytic advances in the on-purpose synthesis of butadiene. Chem. Soc. Rev. 2014, 43, 7917–7953. [Google Scholar] [CrossRef] [PubMed]
- Shylesh, S.; Gokhale, A.A.; Scown, C.D.; Kim, D.; Ho, C.R.; Bell, A.T. From Sugars to Wheels: The Conversion of Ethanol to 1,3-Butadiene over Metal-Promoted Magnesia-Silicate Catalysts. ChemSusChem 2016, 9, 1462–1472. [Google Scholar] [CrossRef] [PubMed]
- Pomalaza, G.; Arango Ponton, P.; Capron, M.; Dumeignil, F. Ethanol-to-butadiene: The reaction and its catalysts. Catal. Sci. Technol. 2020, 10, 4860–4911. [Google Scholar] [CrossRef]
- Sharma, B.; Larroche, C.; Dussap, C.-G. Comprehensive assessment of 2G bioethanol production. Bioresour. Technol. 2020, 313, 123630. [Google Scholar] [CrossRef] [PubMed]
- Quattlebaum, W.M.; Toussaint, W.J.; Dunn, J.T. Deoxygenation of Certain Aldehydes and Ketones: Preparation of Butadiene and Styrene. J. Am. Chem. Soc. 1947, 69, 593–599. [Google Scholar] [CrossRef]
- Kyriienko, P.I.; Larina, O.V.; Soloviev, S.O.; Orlyk, S.M.; Calers, C.; Dzwigaj, S. Ethanol Conversion into 1,3-Butadiene by the Lebedev Method over MTaSiBEA Zeolites (M = Ag, Cu, Zn). ACS Sustain. Chem. Eng. 2017, 5, 2075–2083. [Google Scholar] [CrossRef]
- Yan, T.; Dai, W.; Wu, G.; Lang, S.; Hunger, M.; Guan, N.; Li, L. Mechanistic Insights into One-Step Catalytic Conversion of Ethanol to Butadiene over Bifunctional Zn-Y/Beta Zeolite. ACS Catal. 2018, 8, 2760–2773. [Google Scholar] [CrossRef]
- Taifan, W.E.; Li, Y.; Baltrus, J.P.; Zhang, L.; Frenkel, A.I.; Baltrusaitis, J. Operando Structure Determination of Cu and Zn on Supported MgO/SiO2 Catalysts during Ethanol Conversion to 1,3-Butadiene. ACS Catal. 2019, 9, 269–285. [Google Scholar] [CrossRef]
- Cabello González, G.M.; Concepción, P.; Villanueva Perales, A.L.; Martínez, A.; Campoy, M.; Vidal-Barrero, F. Ethanol conversion into 1,3-butadiene over a mixed Hf-Zn catalyst: Effect of reaction conditions and water content in ethanol. Fuel Process. Technol. 2019, 193, 263–272. [Google Scholar] [CrossRef]
- Ochoa, J.V.; Malmusi, A.; Recchi, C.; Cavani, F. Understanding the Role of Gallium as a Promoter of Magnesium Silicate Catalysts for the Conversion of Ethanol into Butadiene. ChemCatChem 2017, 9, 2128–2135. [Google Scholar] [CrossRef]
- Angelici, C.; Velthoen, M.E.Z.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Effect of preparation method and CuO promotion in the conversion of ethanol into 1,3-butadiene over SiO2-MgO catalysts. ChemSusChem 2014, 1–12. [Google Scholar] [CrossRef]
- Angelici, C.; Velthoen, M.E.Z.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Influence of acid–base properties on the Lebedev ethanol-to-butadiene process catalyzed by SiO2–MgO materials. Catal. Sci. Technol. 2015, 5, 2869–2879. [Google Scholar] [CrossRef]
- Taifan, W.E.; Bučko, T.; Baltrusaitis, J. Catalytic conversion of ethanol to 1,3-butadiene on MgO: A comprehensive mechanism elucidation using DFT calculations. J. Catal. 2017, 346, 78–91. [Google Scholar] [CrossRef]
- Abdulrazzaq, H.T.; Rahmani Chokanlu, A.; Frederick, B.G.; Schwartz, T.J. Reaction Kinetics Analysis of Ethanol Dehydrogenation Catalyzed by MgO–SiO2. ACS Catal. 2020, 10, 6318–6331. [Google Scholar] [CrossRef]
- Natta, G.; Rigamonti, R. Studio roentgenografico e chimico dei catalizzatori usati per la produzione del butadiene dall’alcool. Chim. Ind. 1947, 29, 239–243. [Google Scholar]
- Chung, S.-H.; Angelici, C.; Hinterding, S.O.M.; Weingarth, M.; Baldus, M.; Houben, K.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Role of Magnesium Silicates in Wet-Kneaded Silica–Magnesia Catalysts for the Lebedev Ethanol-to-Butadiene Process. ACS Catal. 2016, 6, 4034–4045. [Google Scholar] [CrossRef]
- Janssens, W.; Makshina, E.V.; Vanelderen, P.; De Clippel, F.; Houthoofd, K.; Kerkhofs, S.; Martens, J.A.; Jacobs, P.A.; Sels, B.F. Ternary Ag/MgO-SiO2 Catalysts for the Conversion of Ethanol into Butadiene. ChemSusChem 2015, 8, 994–1008. [Google Scholar] [CrossRef] [PubMed]
- Velasquez Ochoa, J.; Bandinelli, C.; Vozniuk, O.; Chieregato, A.; Malmusi, A.; Recchi, C.; Cavani, F. An analysis of the chemical, physical and reactivity features of MgO-SiO2 catalysts for butadiene synthesis with the Lebedev process. Green Chem. 2016, 18, 1653–1663. [Google Scholar] [CrossRef]
- Van Rossum, B.-J.; Förster, H.; de Groot, H.J.M. High-Field and High-Speed CP-MAS13C NMR Heteronuclear Dipolar-Correlation Spectroscopy of Solids with Frequency-Switched Lee–Goldburg Homonuclear Decoupling. J. Magn. Reson. 1997, 124, 516–519. [Google Scholar] [CrossRef]
- Li, S.; Men, Y.; Wang, J.; Liu, S.; Wang, X.; Ji, F.; Chai, S.; Song, Q. Morphological control of inverted MgO-SiO2 composite catalysts for efficient conversion of ethanol to 1,3-butadiene. Appl. Catal. A Gen. 2019, 577, 1–9. [Google Scholar] [CrossRef]
- Van Aken, P.A.; Langenhorst, F. Nanocrystalline, porous periclase aggregates as product of brucite dehydration. Eur. J. Mineral. 2001, 13, 329–341. [Google Scholar] [CrossRef]
- Ball, M.C.; Taylor, H.F.W. The dehydration of brucite. Mineral. Mag. J. Mineral. Soc. 1961, 32, 754–766. [Google Scholar] [CrossRef]
- Yamaguchi, G.; Tokuda, T. Electron Microscopic Study of the Dehydration of Brucite and the Recrystallization of Periclase upon Further Heating. Bull. Chem. Soc. Jpn. 1964, 37, 399–403. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Kim, H.N.; Lee, S.K. Atomic structure and dehydration mechanism of amorphous silica: Insights from 29Si and 1H solid-state MAS NMR study of SiO2 nanoparticles. Geochim. Cosmochim. Acta 2013, 120, 39–64. [Google Scholar] [CrossRef]
- Sorte, E.G.; Rimsza, J.M.; Alam, T.M. Computational and Experimental 1H-NMR Study of Hydrated Mg-Based Minerals. Molecules 2020, 25, 933. [Google Scholar] [CrossRef]
- Dumas, A.; Martin, F.; Le Roux, C.; Micoud, P.; Petit, S.; Ferrage, E.; Brendlé, J.; Grauby, O.; Greenhill-Hooper, M. Phyllosilicates synthesis: A way of accessing edges contributions in NMR and FTIR spectroscopies. Example of synthetic talc. Phys. Chem. Miner. 2013, 40, 361–373. [Google Scholar] [CrossRef]
- Phillips, B.L.; Burnley, P.C.; Worminghaus, K.; Navrotsky, A. 29 Si and 1 H NMR spectroscopy of high-pressure hydrous magnesium silicates. Phys. Chem. Miner. 1997, 24, 179–190. [Google Scholar] [CrossRef]
- Grüninger, H.; Liu, Z.; Brauckmann, J.O.; Fei, H.; Ballaran, T.B.; Martin, T.; Siegel, R.; Kentgens, A.P.M.; Frost, D.J.; Senker, J. Hydroxyl Defects and Oxide Vacancies within Ringwoodite—toward Understanding the Defect Chemistry of Spinel-Type Oxides. J. Phys. Chem. C 2020, 124, 12001–12009. [Google Scholar] [CrossRef]
- Wu, S.; Peng, Y.-K.; Chen, T.-Y.; Mo, J.; Large, A.; McPherson, I.; Chou, H.-L.; Wilkinson, I.; Venturini, F.; Grinter, D.; et al. Removal of Hydrogen Poisoning by Electrostatically Polar MgO Support for Low-Pressure NH3 Synthesis at a High Rate over the Ru Catalyst. ACS Catal. 2020, 10, 5614–5622. [Google Scholar] [CrossRef]
- Nishizawa, J.; Ikeda-Fukazawa, T. Surface structures and properties of forsterite in crystalline and glassy states. Chem. Phys. Lett. 2019, 714, 197–201. [Google Scholar] [CrossRef]
- Liu, T.; Gautam, S.; Wang, H.-W.; Anovitz, L.M.; Mamontov, E.; Allard, L.F.; Cole, D.R. Structure and dynamics of water on the forsterite surface. Phys. Chem. Chem. Phys. 2018, 20, 27822–27829. [Google Scholar] [CrossRef] [PubMed]
- Poirier, M.; Millot, Y.; Silva Gomes, E.; Jaber, M.; Herledan, V.; Laugel, G.; Micoud, P.; Martin, F.; Lauron-Pernot, H.; Toulhoat, H. Complementarity of Density Functional Theory and Nuclear Magnetic Resonance Tools To Probe the Nano-Layered Silicates Surface Chemistry and Morphology. J. Phys. Chem. C 2020, 124, 267–286. [Google Scholar] [CrossRef]
- Temuujin, J.; Okada, K.; MacKenzie, K.J.D. Role of Water in the Mechanochemical Reactions of MgO–SiO2 Systems. J. Solid State Chem. 1998, 138, 169–177. [Google Scholar] [CrossRef]
- D’Espinose de la Caillerie, J.-B.; Kermarec, M.; Clause, O.; de la Caillerie, E.; Kermarec, M.; Clause, O. 29Si NMR Observation of an Amorphous Magnesium Silicate Formed during Impregnation of Silica with Mg(I1) in Aqueous Solution. J. Phys. Chem 1995, 99, 17273–17281. [Google Scholar] [CrossRef]
- Chen, L.; Ye, G.; Zhou, W.; Dijkmans, J.; Sels, B.; Malfliet, A.; Guo, M. Influence of MgO precursors on mechanically activated forsterite synthesis. Ceram. Int. 2015, 41, 12651–12657. [Google Scholar] [CrossRef]
- Walling, S.A.; Kinoshita, H.; Bernal, S.A.; Collier, N.C.; Provis, J.L. Structure and properties of binder gels formed in the system Mg(OH)2–SiO2–H2O for immobilisation of Magnox sludge. Dalt. Trans. 2015, 44, 8126–8137. [Google Scholar] [CrossRef]
- Davis, M.C.; Brouwer, W.J.; Wesolowski, D.J.; Anovitz, L.M.; Lipton, A.S.; Mueller, K.T. Magnesium silicate dissolution investigated by 29Si MAS, 1H–29Si CPMAS, 25Mg QCPMG, and 1H–25Mg CP QCPMG NMR. Phys. Chem. Chem. Phys. 2009, 11, 7013. [Google Scholar] [CrossRef]
- Chabrol, K.; Gressier, M.; Pebere, N.; Menu, M.-J.; Martin, F.; Bonino, J.-P.; Marichal, C.; Brendle, J. Functionalization of synthetic talc-like phyllosilicates by alkoxyorganosilane grafting. J. Mater. Chem. 2010, 20, 9695. [Google Scholar] [CrossRef]
- MacKenzie, K.J.D.; Bradley, S.; Hanna, J.V.; Smith, M.E. Magnesium analogues of aluminosilicate inorganic polymers (geopolymers) from magnesium minerals. J. Mater. Sci. 2013, 48, 1787–1793. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Chlique, C.; Wyrzykowski, M.; Dauzères, A.; Pochard, I.; Cau-Dit-Coumes, C. Characterization of magnesium silicate hydrate (M-S-H). Cem. Concr. Res. 2019, 116, 309–330. [Google Scholar] [CrossRef]
- Magi, M.; Lippmaa, E.; Samoson, A.; Engelhardt, G.; Grimmer, a.R. Solid-state high-resolution silicon-29 chemical shifts in silicates. J. Phys. Chem. 1984, 88, 1518–1522. [Google Scholar] [CrossRef]
- Stebbins, J.F.; Panero, W.R.; Smyth, J.R.; Frost, D.J. Forsterite, wadsleyite, and ringwoodite (Mg2SiO4): 29Si NMR constraints on structural disorder and effects of paramagnetic impurity ions. Am. Mineral. 2009, 94, 626–629. [Google Scholar] [CrossRef]
- Szabó, B.; Novodárszki, G.; Pászti, Z.; Domján, A.; Valyon, J.; Hancsók, J.; Barthos, R. MgO−SiO2 Catalysts for the Ethanol to Butadiene Reaction: The Effect of Lewis Acid Promoters. ChemCatChem 2020, 12, 5686–5696. [Google Scholar] [CrossRef]
- Chabanas, M.; Quadrelli, E.A.; Fenet, B.; Copéret, C.; Thivolle-Cazat, J.; Basset, J.-M.; Lesage, A.; Emsley, L. Molecular Insight Into Surface Organometallic Chemistry Through the Combined Use of 2D HETCOR Solid-State NMR Spectroscopy and Silsesquioxane Analogues. Angew. Chemie Int. Ed. 2001, 40, 4493. [Google Scholar] [CrossRef]
- Kvisle, S.; Aguero, A.; Sneeden, R.P.A. Transformation of ethanol into 1,3-butadiene over magnesium oxide/silica catalysts. Appl. Catal. 1988, 43, 117–131. [Google Scholar] [CrossRef]
- Larina, O.V.; Kyriienko, P.I.; Trachevskii, V.V.; Vlasenko, N.V.; Soloviev, S.O. Effect of Mechanochemical Treatment on Acidic and Catalytic Properties of MgO-SiO2 Composition in the Conversion of Ethanol To 1,3-Butadiene. Theor. Exp. Chem. 2016, 51, 387–393. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, B.; Tan, T. Conversion of Ethanol and Acetaldehyde to Butadiene over MgO-SiO2 Catalysts: Effect of Reaction Parameters and Interaction between MgO and SiO2 on Catalytic Performance. ACS Sustain. Chem. Eng. 2017, 5, 722–733. [Google Scholar] [CrossRef]
- Taifan, W.E.; Baltrusaitis, J. In Situ Spectroscopic Insights on the Molecular Structure of the MgO/SiO2 Catalytic Active Sites during Ethanol Conversion to 1,3-Butadiene. J. Phys. Chem. C 2018, 122, 20894–20906. [Google Scholar] [CrossRef]
- Kanezashi, M.; Yamamoto, A.; Yoshioka, T.; Tsuru, T. Characteristics of ammonia permeation through porous silica membranes. AIChE J. 2009, 59, 1204–1212. [Google Scholar] [CrossRef]
- Bräuer, P.; Ng, P.L.; Situmorang, O.; Hitchcock, I.; D’Agostino, C. Effect of Al content on number and location of hydroxyl acid species in zeolites: A DRIFTS quantitative protocol without the need for molar extinction coefficients. RSC Adv. 2017, 7, 52604–52613. [Google Scholar] [CrossRef]
- Carmignano, O.; Vieira, S.; Brandão, P.R.; Bertoli, A.; Lago, R. Serpentinites: Mineral Structure, Properties and Technological Applications. J. Braz. Chem. Soc. 2020, 31, 2–14. [Google Scholar] [CrossRef]
- Lainé, M.; Balan, E.; Allard, T.; Paineau, E.; Jeunesse, P.; Mostafavi, M.; Robert, J.-L.; Le Caër, S. Reaction mechanisms in swelling clays under ionizing radiation: Influence of the water amount and of the nature of the clay mineral. RSC Adv. 2017, 7, 526–534. [Google Scholar] [CrossRef]
- Huang, X.; Men, Y.; Wang, J.; An, W.; Wang, Y. Highly active and selective binary MgO–SiO2 catalysts for the production of 1,3-butadiene from ethanol. Catal. Sci. Technol. 2017, 7, 168–180. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Q.; Xu, H.; Li, L.; Dong, J.; Li, J. Adsorption of CO2, CH4, and N2 on Gas Diameter Grade Ion-Exchange Small Pore Zeolites. J. Chem. Eng. Data 2012, 57, 3701–3709. [Google Scholar] [CrossRef]
- Sato, K.; Hunger, M. Carbon dioxide adsorption in open nanospaces formed by overlap of saponite clay nanosheets. Commun. Chem. 2020, 3, 91. [Google Scholar] [CrossRef]
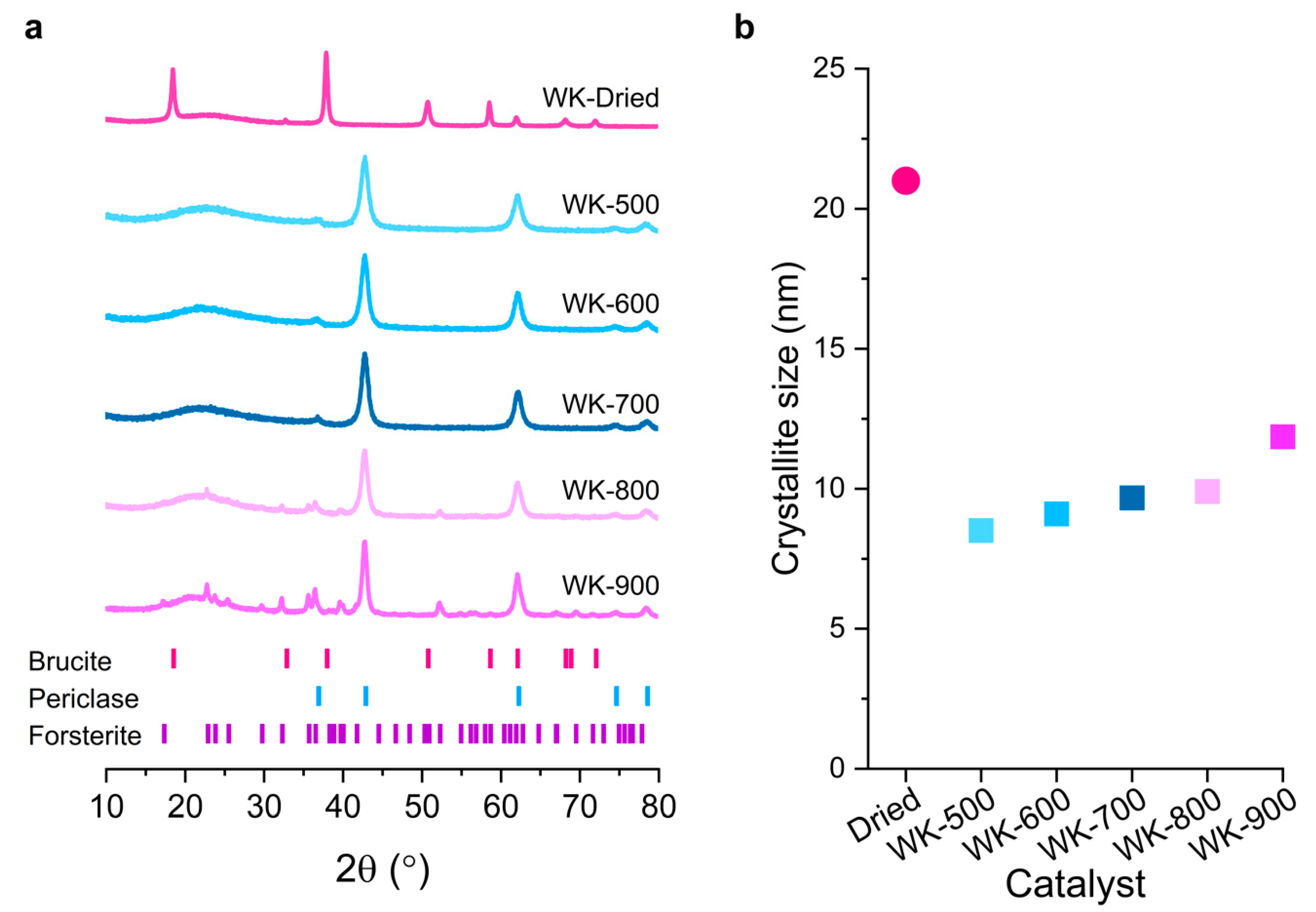
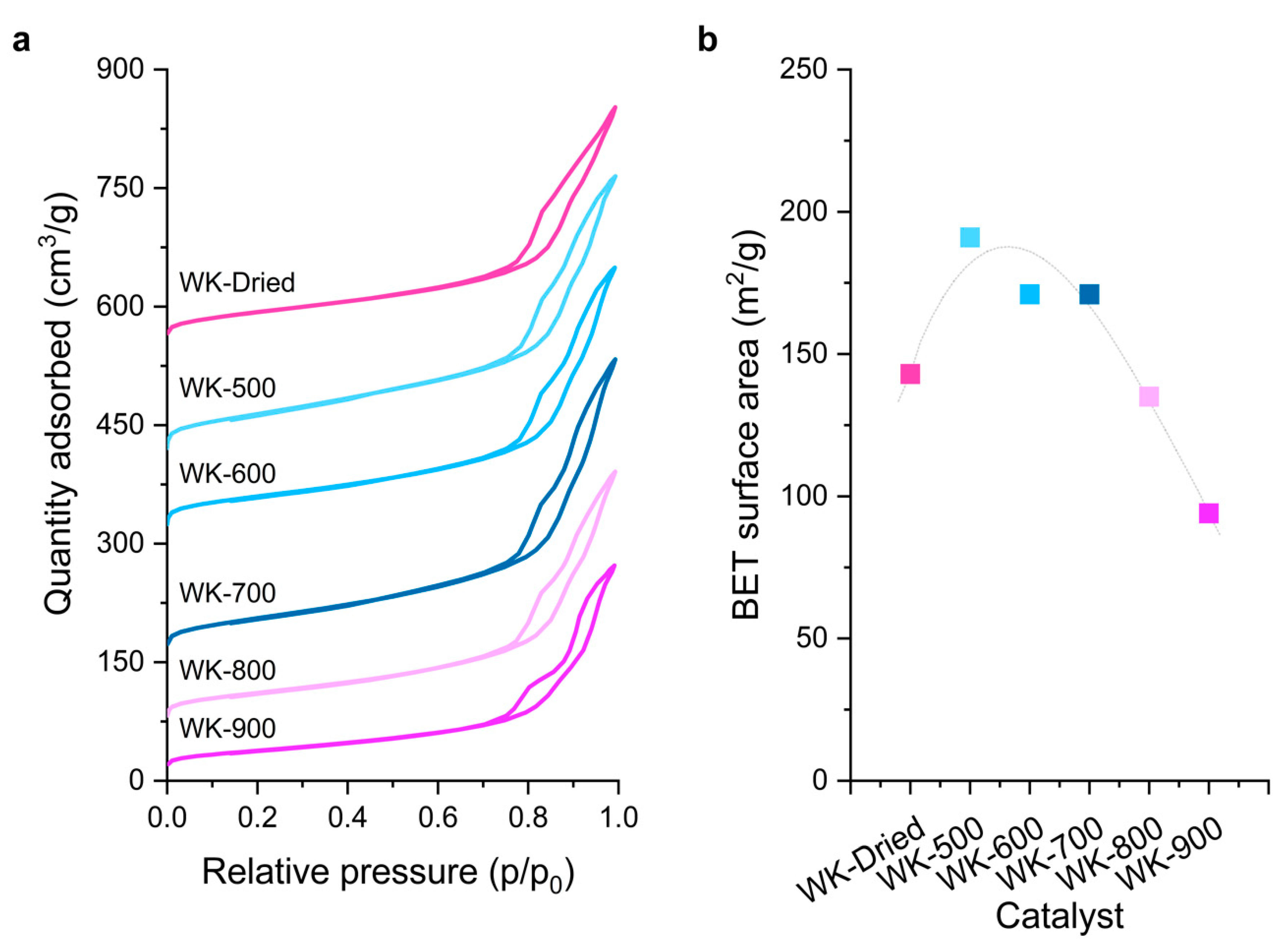
| Catalyst | Crystallite Size 1 (nm) | BET Surface Area 2 (m2/g) | Microporous Area (m2/g) | Total Surface Area (m2/g) | Micropore Volume (cm3/g) | Total Pore Volume 2 (cm3/g) |
|---|---|---|---|---|---|---|
| WK-Dried 3 | 21 | 143 | 22 | 165 | 0.04 | 0.37 |
| WK-500 | 8.5 | 191 | 27 | 218 | 0.06 | 0.44 |
| WK-600 | 9.1 | 171 | 23 | 194 | 0.06 | 0.43 |
| WK-700 | 9.6 | 171 | 28 | 199 | 0.05 | 0.44 |
| WK-800 | 9.9 | 135 | 19 | 154 | 0.04 | 0.38 |
| WK-900 | 11.9 | 94 | 17 | 111 | 0.03 | 0.30 |
| Observed 29Si Chemical Shift (ppm) | Assignment | Structure | Literature |
|---|---|---|---|
| −109.8 | Silica (Q4) | Si–(OSi)4 | [28] |
| −100.0 | Silica (Q3) | Si–(OSi)3(OH) | [28] |
| −97.6 | Talc | Mg3Si4O10(OH) | [43] |
| −96.4 | Stevensite | Mgx(Mg3−x◻x)Si4O10(OH)2 (◻ = defect site) | [42] |
| −93.8 | Lizardite | (Mg3Si2O5(OH)4) | [40] |
| −85–−92 | Amorphous hydrous magnesium silicates | - | [38,44] |
| −82.0 | Enstatite | MgSiO3 | [45] |
| −77.6 | Intermediate betweenforsterite and enstatite | (Mg2SiO4)x(MgSiO3)1−x | [39] |
| −61.3 | Forsterite | Mg2SiO4 | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, S.-H.; Ramirez, A.; Shoinkhorova, T.; Mukhambetov, I.; Abou-Hamad, E.; Telalovic, S.; Gascon, J.; Ruiz-Martínez, J. The Importance of Thermal Treatment on Wet-Kneaded Silica–Magnesia Catalyst and Lebedev Ethanol-to-Butadiene Process. Nanomaterials 2021, 11, 579. https://doi.org/10.3390/nano11030579
Chung S-H, Ramirez A, Shoinkhorova T, Mukhambetov I, Abou-Hamad E, Telalovic S, Gascon J, Ruiz-Martínez J. The Importance of Thermal Treatment on Wet-Kneaded Silica–Magnesia Catalyst and Lebedev Ethanol-to-Butadiene Process. Nanomaterials. 2021; 11(3):579. https://doi.org/10.3390/nano11030579
Chicago/Turabian StyleChung, Sang-Ho, Adrian Ramirez, Tuiana Shoinkhorova, Ildar Mukhambetov, Edy Abou-Hamad, Selevedin Telalovic, Jorge Gascon, and Javier Ruiz-Martínez. 2021. "The Importance of Thermal Treatment on Wet-Kneaded Silica–Magnesia Catalyst and Lebedev Ethanol-to-Butadiene Process" Nanomaterials 11, no. 3: 579. https://doi.org/10.3390/nano11030579
APA StyleChung, S.-H., Ramirez, A., Shoinkhorova, T., Mukhambetov, I., Abou-Hamad, E., Telalovic, S., Gascon, J., & Ruiz-Martínez, J. (2021). The Importance of Thermal Treatment on Wet-Kneaded Silica–Magnesia Catalyst and Lebedev Ethanol-to-Butadiene Process. Nanomaterials, 11(3), 579. https://doi.org/10.3390/nano11030579






