Magnetic Mesoporous Carbon/β-Cyclodextrin–Chitosan Nanocomposite for Extraction and Preconcentration of Multi-Class Emerging Contaminant Residues in Environmental Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples and Sample Collection
2.3. UA-MSPDE Preconcentration Procedure
2.4. Quality Assurance/Quality Control (QA/QC)
3. Results and Discussion
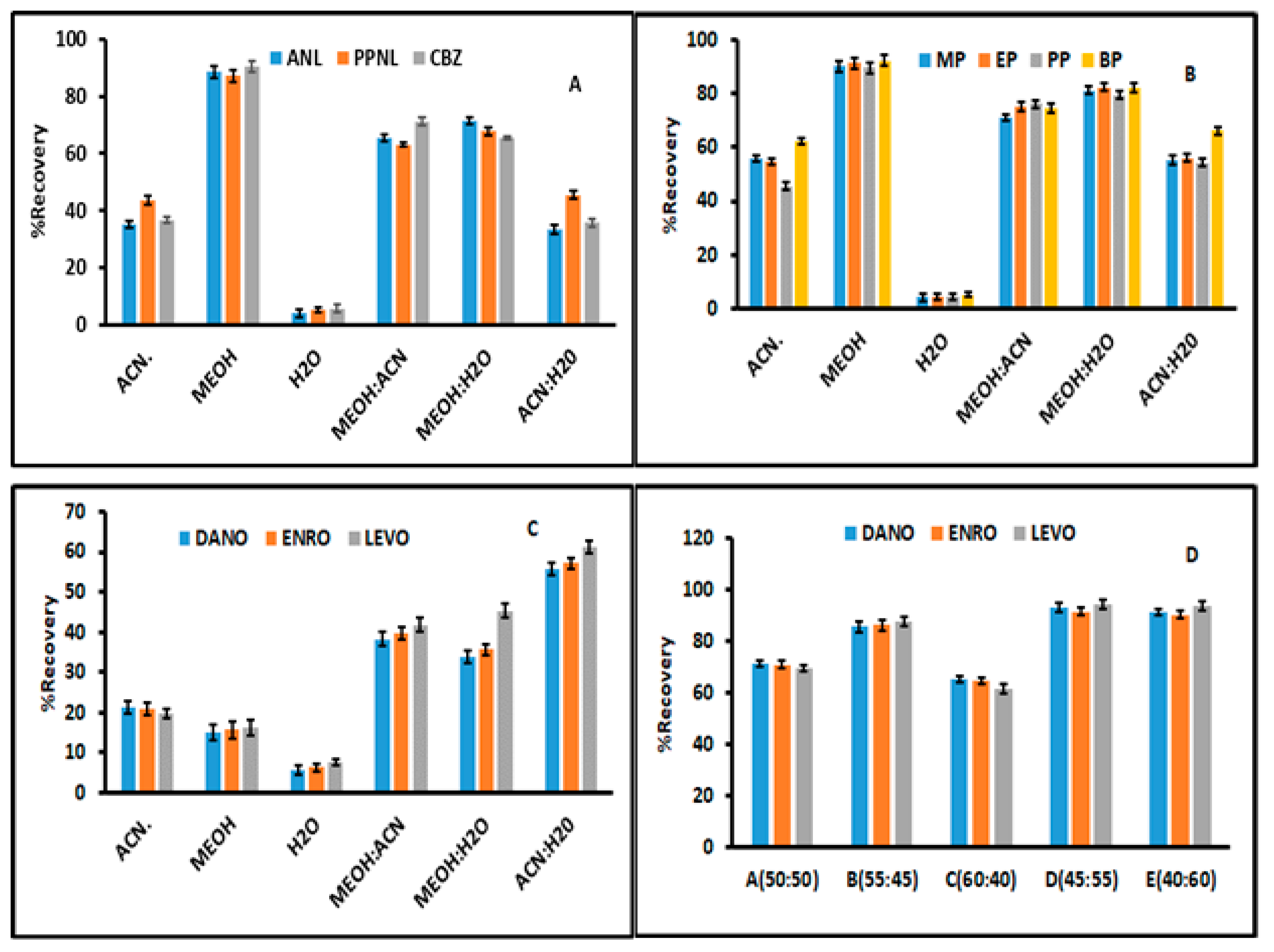
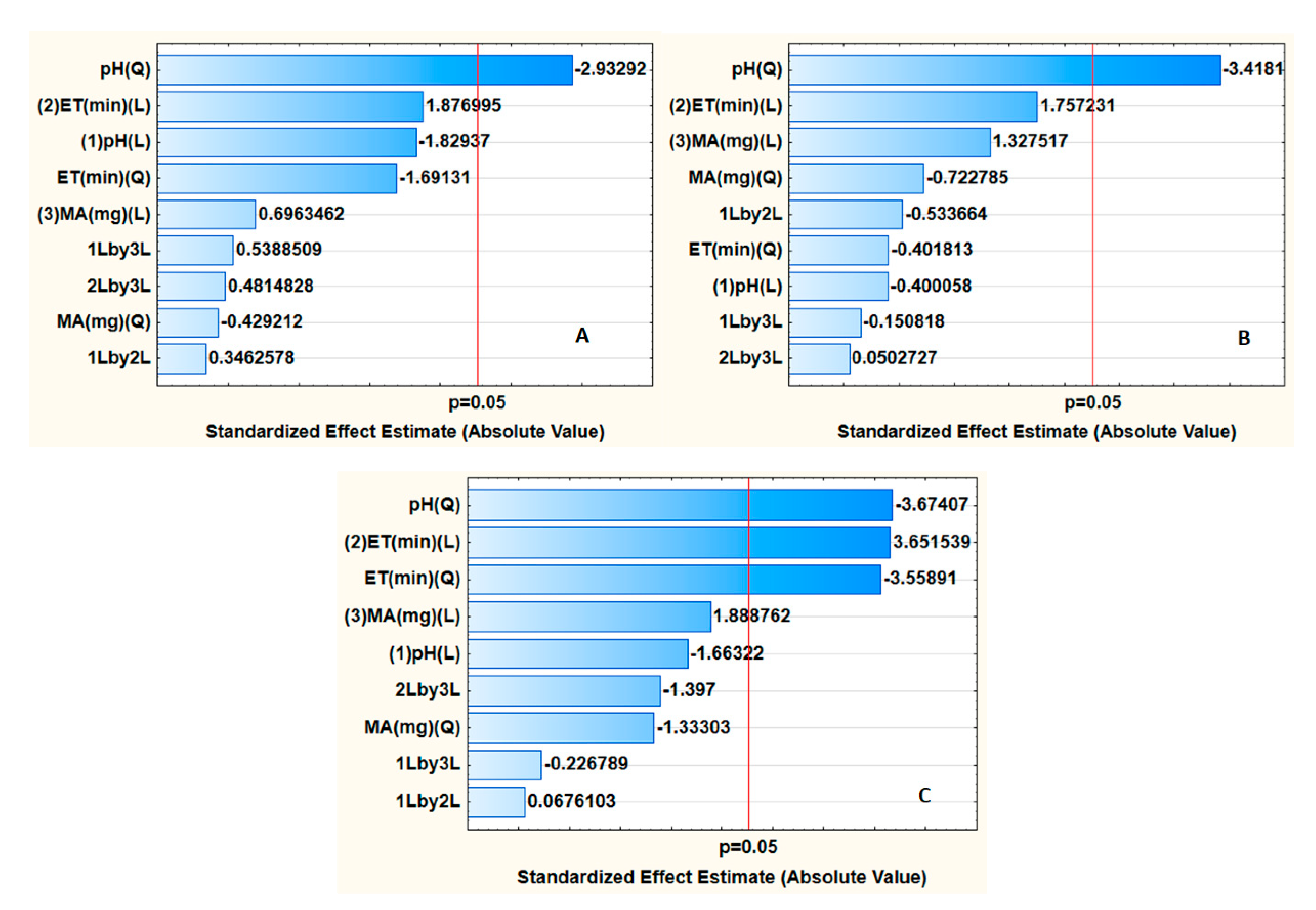
3.1. Optimization of Desorption Conditions
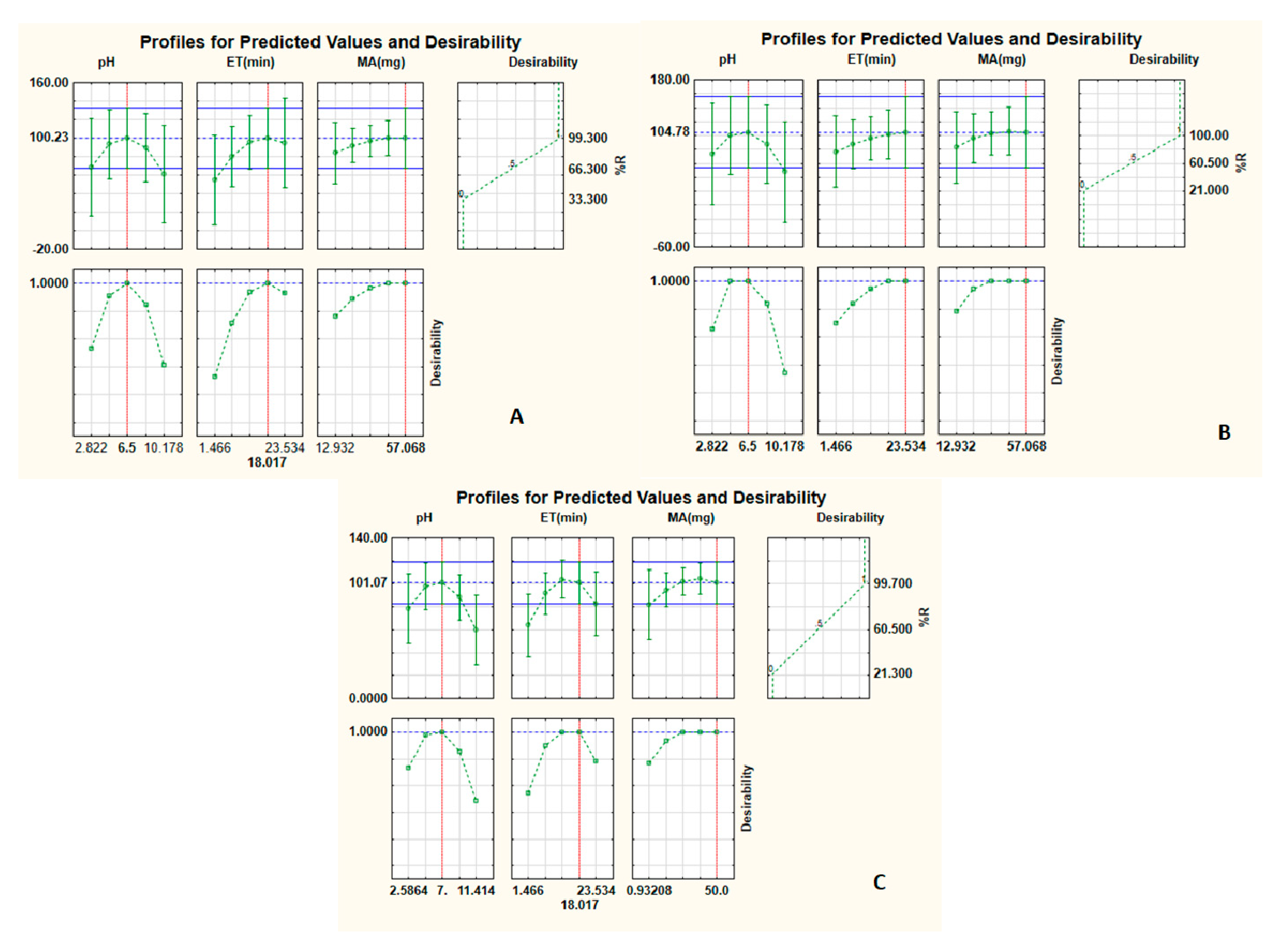
3.2. Optimization of the Preconcentration Procedure
3.3. Validation of the Preconcentration Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Proctor, K.; Petrie, B.; Barden, R.; Arnot, T.; Kasprzyk-Hordern, B. Multi-residue ultra-performance liquid chromatography coupled with tandem mass spectrometry method for comprehensive multi-class anthropogenic compounds of emerging concern analysis in a catchment-based exposure-driven study. Anal. Bioanal. Chem. 2019, 411, 7061–7086. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Guo, S.; Li, D.; Wo, R.; Zhao, R.; Jiang, W. Composite Material that Comprised Metal–Organic Nanotubes and a Sponge as a High-Performance Adsorbent for the Extraction of Pharmaceuticals and Personal Care Products from Environmental Water Samples. Chem. Asian J. 2019, 14, 1487–1495. [Google Scholar] [CrossRef]
- Petrie, B.; Youdan, J.; Barden, R.; Kasprzyk-Hordern, B. Multi-residue analysis of 90 emerging contaminants in liquid and solid environmental matrices by ultra-high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1431, 64–78. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Kosma, C.; Lambropoulou, D. Seasonal occurrence, removal, mass loading and environmental risk assessment of 55 pharmaceuticals and personal care products in a municipal wastewater treatment plant in Central Greece. Sci. Total Environ. 2016, 543, 547–569. [Google Scholar] [CrossRef]
- Parpounas, A.; Litskas, V.; Hapeshi, E.; Michael, C.; Fatta-Kassinos, D. Assessing the presence of enrofloxacin and ciprofloxacin in piggery wastewater and their adsorption behaviour onto solid materials, with a newly developed chromatographic method. Environ. Sci. Pollut. Res. 2017, 24, 23371–23381. [Google Scholar] [CrossRef]
- Wood, T.P.; Duvenage, C.S.J.; Rohwer, E. The occurrence of anti-retroviral compounds used for HIV treatment in South African surface water. Environ. Pollut. 2015, 199, 235–243. [Google Scholar] [CrossRef]
- Rimayi, C.; Chimuka, L.; Gravell, A.; Fones, G.R.; Mills, G.A. Use of the Chemcatcher® passive sampler and time-of-flight mass spectrometry to screen for emerging pollutants in rivers in Gauteng Province of South Africa. Environ. Monit. Assess. 2019, 191, 388. [Google Scholar] [CrossRef]
- Ojemaye, C.Y.; Petrik, L. Occurrences, levels and risk assessment studies of emerging pollutants (pharmaceuticals, perfluoroalkyl and endocrine disrupting compounds) in fish samples from Kalk Bay harbour, South Africa. Environ. Pollut. 2019, 252, 562–572. [Google Scholar] [CrossRef]
- Mpupa, A.; Mashile, G.P.; Nomngongo, P.N. Ultrasound-assisted dispersive solid phase nanoextraction of selected personal care products in wastewater followed by their determination using high performance liquid chromatography-diode array detector. J. Hazard. Mater. 2019, 370, 33–41. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Mdluli, P.S.; Chimuka, L. Experimental and theoretical study of molecular interactions between 2-vinyl pyridine and acidic pharmaceuticals used as multi-template molecules in molecularly imprinted polymer. React. Funct. Polym. 2016, 103, 33–43. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Chimuka, L. Simultaneous determination of naproxen, ibuprofen and diclofenac in wastewater using solid-phase extraction with high performance liquid chromatography. Water Sa 2017, 43, 264–274. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Muthwa, S.F.; Chimuka, L. Determination of triclosan and ketoprofen in river water and wastewater by solid phase extraction and high performance liquid chromatography. S. Afr. J. Chem. 2014, 67. [Google Scholar]
- Amdany, R.; Moya, A.; Cukrowska, E.; Chimuka, L. Optimization of the temperature for the extraction of pharmaceuticals from wastewater by a hollow fiber silicone membrane. Anal. Lett. 2015, 48, 2343–2356. [Google Scholar] [CrossRef]
- Amdany, R.; Chimuka, L.; Cukrowska, E.; Kohoutek, J.; Vrana, B. Investigating the temporal trends in PAH, PCB and OCP concentrations in Hartbeespoort Dam, South Africa, using semipermeable membrane devices (SPMDs). Water 2014, 40, 425–436. [Google Scholar] [CrossRef]
- Amdany, R.; Chimuka, L.; Cukrowska, E. Determination of naproxen, ibuprofen and triclosan in wastewater using the polar organic chemical integrative sampler (POCIS): A laboratory calibration and field application. Water 2014, 40, 407–414. [Google Scholar] [CrossRef]
- Medrano, L.C.; Flores-Aguilar, J.F.; Islas, G.; Rodríguez, J.A.; Ibarra, I.S. Solid-Phase Extraction and Large-Volume Sample Stacking-Capillary Electrophoresis for Determination of Artificial Sweeteners in Water Samples. Food Anal. Methods 2019, 12, 526–533. [Google Scholar] [CrossRef]
- Valimaña-Traverso, J.; Amariei, G.; Boltes, K.; García, M.Á.; Marina, M.L. Enantiomer stability and combined toxicity of duloxetine and econazole on Daphnia magna using real concentrations determined by capillary electrophoresis. Sci. Total Environ. 2019, 670, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Morés, L.; da Silva, A.C.; Merib, J.; Dias, A.N.; Carasek, E. A natural and renewable biosorbent phase as a low-cost approach in disposable pipette extraction technique for the determination of emerging contaminants in lake water samples. J. Sep. Sci. 2019, 42, 1404–1411. [Google Scholar] [CrossRef]
- Rashvand, M.; Vosough, M. Graphene oxide–polyaniline nanocomposite as a potential sorbent for dispersive solid-phase extraction and determination of selected pharmaceutical and personal care products in wastewater samples using HPLC with a diode-array detector. Anal. Methods 2016, 8, 1898–1907. [Google Scholar] [CrossRef]
- Chen, J.; Deng, W.; Li, X.; Wang, X.; Xiao, Y. Hexafluoroisopropanol/Brij-35 based supramolecular solvent for liquid-phase microextraction of parabens in different matrix samples. J. Chromatogr. A 2019, 1591, 33–43. [Google Scholar] [CrossRef]
- Mijangos, L.; Ziarrusta, H.; Olivares, M.; Zuloaga, O.; Möder, M.; Etxebarria, N.; Prieto, A. Simultaneous determination of 41 multiclass organic pollutants in environmental waters by means of polyethersulfone microextraction followed by liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2018, 410, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kaur, R.; Grover, A.; Rani, S.; Malik, A.K.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction/GC-MS method for rapid determination of broad polarity spectrum multi-class emerging pollutants in various aqueous samples. J. Sep. Sci. 2019, 42, 2407–2417. [Google Scholar] [CrossRef]
- Arismendi, D.; Becerra-Herrera, M.; Cerrato, I.; Richter, P. Simultaneous determination of multiresidue and multiclass emerging contaminants in waters by rotating-disk sorptive extraction–derivatization-gas chromatography/mass spectrometry. Talanta 2019, 201, 480–489. [Google Scholar] [CrossRef]
- Kotowska, U.; Kapelewska, J.; Kotowski, A.; Pietuszewska, E. Rapid and sensitive analysis of hormones and other emerging contaminants in groundwater using ultrasound-assisted emulsification microextraction with solidification of floating organic droplet followed by GC-MS Detection. Water 2019, 11, 1638. [Google Scholar] [CrossRef]
- Lee, S.; Kim, K.; Jeon, J.; Moon, H.-B. Optimization of suspect and non-target analytical methods using GC/TOF for prioritization of emerging contaminants in the Arctic environment. Ecotoxicol. Environ. Saf. 2019, 181, 11–17. [Google Scholar] [CrossRef]
- Khan, W.A.; Arain, M.B.; Yamini, Y.; Shah, N.; Kazi, T.G.; Pedersen-Bjergaard, S.; Tajik, M. Hollow fiber-based liquid phase microextraction followed by analytical instrumental techniques for quantitative analysis of heavy metal ions and pharmaceuticals. J. Pharm. Anal. 2019, 10, 109–122. [Google Scholar] [CrossRef]
- Dimpe, K.M.; Nomngongo, P.N. Application of activated carbon-decorated polyacrylonitrile nanofibers as an adsorbent in dispersive solid-phase extraction of fluoroquinolones from wastewater. J. Pharm. Anal. 2019, 9, 117–126. [Google Scholar] [CrossRef]
- Lekota, M.W.; Dimpe, K.M.; Nomngongo, P.N. MgO-ZnO/carbon nanofiber nanocomposite as an adsorbent for ultrasound-assisted dispersive solid-phase microextraction of carbamazepine from wastewater prior to high-performance liquid chromatographic detection. J. Anal. Sci. Technol. 2019, 10, 25. [Google Scholar] [CrossRef]
- Portillo-Castillo, O.J.; Castro-Ríos, R.; Chávez-Montes, A.; González-Horta, A.; Cavazos-Rocha, N.; de Torres, N.H.W.; Garza-Tapia, M. Developments of solid-phase microextraction fiber coatings for environmental pharmaceutical and personal care products analysis. Rev. Anal. Chem. 2018, 37. [Google Scholar] [CrossRef]
- Wang, Z.; He, M.; Chen, B.; Hu, B. Azo-linked porous organic polymers/polydimethylsiloxane coated stir bar for extraction of benzotriazole UltraViolet absorbers from environmental water and soil samples followed by high performance liquid chromatography-diode array detection. J. Chromatogr. A 2019, 1616, 460793. [Google Scholar] [CrossRef]
- Selahle, S.K.; Nomngongo, P.N. Supramolecular Solvent Based Liquid-Liquid Microextraction for Preconcentration of Selected Fluoroquinolone Antibiotics in Environmental Water Sample Prior to High Performance Liquid Chromatographic Determination. Curr. Anal. Chem. 2019, 15, 607–615. [Google Scholar] [CrossRef]
- Gissawong, N.; Boonchiangma, S.; Mukdasai, S.; Srijaranai, S. Vesicular supramolecular solvent-based microextraction followed by high performance liquid chromatographic analysis of tetracyclines. Talanta 2019, 200, 203–211. [Google Scholar] [CrossRef]
- Jakubus, A.; Gromelski, M.; Jagiello, K.; Puzyn, T.; Stepnowski, P.; Paszkiewicz, M. Dispersive solid-phase extraction using multi-walled carbon nanotubes combined with liquid chromatography–mass spectrometry for the analysis of β-blockers: Experimental and theoretical studies. Microchem. J. 2019, 146, 258–269. [Google Scholar] [CrossRef]
- Zhao, R.; Ma, T.; Li, S.; Tian, Y.; Zhu, G. Porous Aromatic Framework Modified Electrospun Fiber Membrane as a Highly Efficient and Reusable Adsorbent for Pharmaceuticals and Personal Care Products Removal. Acs Appl. Mater. Interfaces 2019, 11, 16662–16673. [Google Scholar] [CrossRef]
- Delhiraja, K.; Vellingiri, K.; Boukhvalov, D.W.; Philip, L. Development of Highly Water Stable Graphene Oxide-Based Composites for the Removal of Pharmaceuticals and Personal Care Products. Ind. Eng. Chem. Res. 2019, 58, 2899–2913. [Google Scholar] [CrossRef]
- Mashile, G.; Mpupa, A.; Nomngongo, P. In-syringe micro solid-phase extraction method for the separation and preconcentration of parabens in environmental water samples. Molecules 2018, 23, 1450. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, N.; Lan, Y.; Yang, W.; Yang, Z.; Li, X.; Zhou, X.; Liu, Y.; Shen, J.; Zhang, X. Adsorption of three selected pharmaceuticals and personal care products (PPCPs) onto MIL-101 (Cr)/natural polymer composite beads. Sep. Purif. Technol. 2017, 177, 272–280. [Google Scholar] [CrossRef]
- Seo, P.W.; Bhadra, B.N.; Ahmed, I.; Khan, N.A.; Jhung, S.H. Adsorptive removal of pharmaceuticals and personal care products from water with functionalized metal-organic frameworks: Remarkable adsorbents with hydrogen-bonding abilities. Sci. Rep. 2016, 6, 34462. [Google Scholar] [CrossRef] [PubMed]
- Mashile, G.P.; Dimpe, K.M.; Nomngongo, P.N. A Biodegradable Magnetic Nanocomposite as a Superabsorbent for the Simultaneous Removal of Selected Fluoroquinolones from Environmental Water Matrices: Isotherm, Kinetics, Thermodynamic Studies and Cost Analysis. Polymers. 2020, 12, 1102. [Google Scholar] [CrossRef]
- Pascale, R.; Bianco, G.; Coviello, D.; Cristina Lafiosca, M.; Masi, S.; Mancini, I.M.; Bufo, S.A.; Scrano, L.; Caniani, D. Validation of a liquid chromatography coupled with tandem mass spectrometry method for the determination of drugs in wastewater using a three-phase solvent system. J. Sep. Sci. 2020, 43, 886–895. [Google Scholar] [CrossRef]
- Petrović, M.; Hernando, M.D.; Díaz-Cruz, M.S.; Barceló, D. Liquid chromatography–tandem mass spectrometry for the analysis of pharmaceutical residues in environmental samples: A review. J. Chromatogr. A 2005, 1067, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.-S.; Koenig, B.G.; Metcalfe, C.D. Analysis of acidic drugs in the effluents of sewage treatment plants using liquid chromatography–electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2002, 952, 139–147. [Google Scholar] [CrossRef]
- Sacher, F.; Lange, F.T.; Brauch, H.-J.; Blankenhorn, I. Pharmaceuticals in groundwaters: Analytical methods and results of a monitoring program in Baden-Württemberg, Germany. J. Chromatogr. A 2001, 938, 199–210. [Google Scholar] [CrossRef]
- Selahle, S.K.; Nomngongo, P.N. Determination of fluoroquinolones in the environmental samples using vortex assisted dispersive liquid-liquid microextraction coupled with high performance liquid chromatography. Int. J.Environ. Anal. Chem. 2020, 100, 282–294. [Google Scholar] [CrossRef]
- Rimayi, C.; Odusanya, D.; Weiss, J.M.; de Boer, J.; Chimuka, L. Contaminants of emerging concern in the Hartbeespoort Dam catchment and the uMngeni River estuary 2016 pollution incident, South Africa. Sci. Total Environ. 2018, 627, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Lekota, M.W.; Mpupa, A.; Dimpe, K.M.; Nomngongo, P.N. Preparation of ferric oxide-aluminium oxide carbon nanofiber nanocomposites for ultrasound-assisted dispersive magnetic solid phase extraction of 17-beta estradiol in wastewater. Emerg. Contam. 2020, 6, 162–171. [Google Scholar] [CrossRef]
- Jiang, W.; Cui, W.-R.; Liang, R.-P.; Qiu, J.-D. Zwitterionic surface charge regulation in ionic covalent organic nanosheets: Synergistic adsorption of fluoroquinolone antibiotics. Chem. Eng. J. 2020, 128034. [Google Scholar] [CrossRef]
- Li, Z.; Xu, F.; Liu, Z.; Qin, C.; Ren, H.; Li, Y. Facile synthesis of novel porous self-assembling hydrogen-bonding covalent organic polymers and their applications towards fluoroquinolone antibiotics adsorption. RSC Adv. 2018, 8, 33516–33522. [Google Scholar] [CrossRef]
- Wen, A.; Li, G.; Wu, D.; Yu, Y.; Yang, Y.; Hu, N.; Wang, H.; Chen, J.; Wu, Y. Sulphonate functionalized covalent organic framework-based magnetic sorbent for effective solid phase extraction and determination of fluoroquinolones. J. Chromatogr. A 2020, 1612, 460651. [Google Scholar] [CrossRef]
- Manouchehri, M.; Seidi, S.; Rouhollahi, A.; Noormohammadi, H.; Shanehsaz, M. Micro solid phase extraction of parabens from breast milk samples using Mg-Al layered double hydroxide functionalized partially reduced graphene oxide nanocomposite. Food Chem. 2020, 314, 126223. [Google Scholar] [CrossRef]
- Pang, J.; Liao, Y.; Huang, X.; Ye, Z.; Yuan, D. Metal-organic framework-monolith composite-based in-tube solid phase microextraction on-line coupled to high-performance liquid chromatography-fluorescence detection for the highly sensitive monitoring of fluoroquinolones in water and food samples. Talanta 2019, 199, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Miossec, C.; Lanceleur, L.; Monperrus, M. Multi-residue analysis of 44 pharmaceutical compounds in environmental water samples by solid-phase extraction coupled to liquid chromatography-tandem mass spectrometry. J. Sep. Sci. 2019, 42, 1853–1866. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Wang, Y.; Li, Y.; Yu, C.; Sun, Q. Simultaneous analysis of multiclass contaminants of emerging concern in sediments by liquid chromatography with tandem quadrupole mass spectrometry. Environ. Toxicol. Chem. 2019, 38, 1409–1422. [Google Scholar] [CrossRef]
- Offiong, N.-A.O.; Inam, E.J.; Edet, J.B. Preliminary Review of Sources, Fate, Analytical Challenges and Regulatory Status of Emerging Organic Contaminants in Aquatic Environments in Selected African Countries. Chem. Afr. 2019, 2, 573–585. [Google Scholar] [CrossRef]
- Pugajeva, I.; Rusko, J.; Perkons, I.; Lundanes, E.; Bartkevics, V. Determination of pharmaceutical residues in wastewater using high performance liquid chromatography coupled to quadrupole-Orbitrap mass spectrometry. J. Pharm. Biomed. Anal. 2017, 133, 64–74. [Google Scholar] [CrossRef]
- Radwan, E.K.; Ibrahim, M.B.M.; Adel, A.; Farouk, M. The occurrence and risk assessment of phenolic endocrine-disrupting chemicals in Egypt’s drinking and source water. Environ. Sci. Pollut. Res. 2020, 27, 1776–1788. [Google Scholar] [CrossRef] [PubMed]
- Younes, H.A.; Mahmoud, H.M.; Abdelrahman, M.M.; Nassar, H.F. Seasonal occurrence, removal efficiency and associated ecological risk assessment of three antibiotics in a municipal wastewater treatment plant in Egypt. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100239. [Google Scholar] [CrossRef]
- Abdallah, M.A.-E.; Nguyen, K.-H.; Ebele, A.J.; Atia, N.N.; Ali, H.R.H.; Harrad, S. A single run, rapid polarity switching method for determination of 30 pharmaceuticals and personal care products in waste water using Q-Exactive Orbitrap high resolution accurate mass spectrometry. J. Chromatogr. A 2019, 1588, 68–76. [Google Scholar] [CrossRef] [PubMed]
- K’Oreje, K.O.; Kandie, F.J.; Vergeynst, L.; Abira, M.A.; Van Langenhove, H.; Okoth, M.; Demeestere, K. Occurrence, fate and removal of pharmaceuticals, personal care products and pesticides in wastewater stabilization ponds and receiving rivers in the Nzoia Basin, Kenya. Sci. Total Environ. 2018, 637–638, 336–348. [Google Scholar]
- K’oreje, K.O.; Vergeynst, L.; Ombaka, D.; De Wispelaere, P.; Okoth, M.; Van Langenhove, H.; Demeestere, K. Occurrence patterns of pharmaceutical residues in wastewater, surface water and groundwater of Nairobi and Kisumu city, Kenya. Chemosphere 2016, 149, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Valcárcel, Y.; Valdehíta, A.; Becerra, E.; López de Alda, M.; Gil, A.; Gorga, M.; Petrovic, M.; Barceló, D.; Navas, J.M. Determining the presence of chemicals with suspected endocrine activity in drinking water from the Madrid region (Spain) and assessment of their estrogenic, androgenic and thyroidal activities. Chemosphere 2018, 201, 388–398. [Google Scholar] [CrossRef]
- Čelić, M.; Gros, M.; Farré, M.; Barceló, D.; Petrović, M. Pharmaceuticals as chemical markers of wastewater contamination in the vulnerable area of the Ebro Delta (Spain). Sci. Total Environ. 2019, 652, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Ziarrusta, H.; Val, N.; Dominguez, H.; Mijangos, L.; Prieto, A.; Usobiaga, A.; Etxebarria, N.; Zuloaga, O.; Olivares, M. Determination of fluoroquinolones in fish tissues, biological fluids, and environmental waters by liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 6359–6370. [Google Scholar] [CrossRef]
- Mandaric, L.; Diamantini, E.; Stella, E.; Cano-Paoli, K.; Valle-Sistac, J.; Molins-Delgado, D.; Bellin, A.; Chiogna, G.; Majone, B.; Diaz-Cruz, M.S.; et al. Contamination sources and distribution patterns of pharmaceuticals and personal care products in Alpine rivers strongly affected by tourism. Sci. Total Environ. 2017, 590–591, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, M.; Li, Y.; Rehman, M.S.U.; Zubair, M.; Mustafa, G.; Nazar, M.F.; Yu, C.-P.; Sun, Q. Occurrence, spatial variation and risk assessment of pharmaceuticals and personal care products in urban wastewater, canal surface water, and their sediments: A case study of Lahore, Pakistan. Sci. Total Environ. 2019, 688, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Riaz, L.; Mahmood, T.; Kamal, A.; Shafqat, M.; Rashid, A. Industrial release of fluoroquinolones (FQs) in the waste water bodies with their associated ecological risk in Pakistan. Environ. Toxicol. Pharm. 2017, 52, 14–20. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, L.; Wang, B.; Liu, C.S.; Jia, Y.; Zhai, N.; Blaney, L.; Yu, G. Efficient multiresidue determination method for 168 pharmaceuticals and metabolites: Optimization and application to raw wastewater, wastewater effluent, and surface water in Beijing, China. Environ. Pollut. 2020, 261, 114113. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, H.; Du, J.; Qu, Y.; Shen, C.; Tan, F.; Chen, J.; Quan, X. Occurrence, removal, and risk assessment of antibiotics in 12 wastewater treatment plants from Dalian, China. Environ. Sci. Pollut. Res. 2017, 24, 16478–16487. [Google Scholar] [CrossRef]
- Lu, J.; Mao, H.; Li, H.; Wang, Q.; Yang, Z. Occurrence of and human exposure to parabens, benzophenones, benzotriazoles, triclosan and triclocarban in outdoor swimming pool water in Changsha, China. Sci. Total Environ. 2017, 605–606, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Ben, W.; Zhu, B.; Yuan, X.; Zhang, Y.; Yang, M.; Qiang, Z. Occurrence, removal and risk of organic micropollutants in wastewater treatment plants across China: Comparison of wastewater treatment processes. Water Res. 2018, 130, 38–46. [Google Scholar] [CrossRef]
- Liao, C.; Shi, J.; Wang, X.; Zhu, Q.; Kannan, K. Occurrence and distribution of parabens and bisphenols in sediment from northern Chinese coastal areas. Environ. Pollut. 2019, 253, 759–767. [Google Scholar] [CrossRef]
- Marta-Sanchez, A.V.; Caldas, S.S.; Schneider, A.; Cardoso, S.M.V.S.; Primel, E.G. Trace analysis of parabens preservatives in drinking water treatment sludge, treated, and mineral water samples. Environ. Sci. Pollut. Res. 2018, 25, 14460–14470. [Google Scholar] [CrossRef]
- Reichert, G.; Mizukawa, A.; Antonelli, J.; de Goulart, F.A.B.; Filippe, T.C.; de Azevedo, J.C.R. Determination of Parabens, Triclosan, and Lipid Regulators in a Subtropical Urban River: Effects of Urban Occupation. Water Air Soil Pollut. 2020, 231, 1–11. [Google Scholar] [CrossRef]
- Chaves, M.d.J.S.; Barbosa, S.C.; de Melo Mallinowski, M.; Volpato, D.; Castro, Í.B.; dos Santos Franco, T.C.R.; Primel, E.G. Pharmaceuticals and personal care products in a Brazilian wetland of international importance: Occurrence and environmental risk assessment. Sci. Total Environ. 2020, 734, 139374. [Google Scholar] [CrossRef]
- Reis-Santos, P.; Pais, M.; Duarte, B.; Caçador, I.; Freitas, A.; Pouca, A.S.V.; Barbosa, J.; Leston, S.; Rosa, J.; Ramos, F. Screening of human and veterinary pharmaceuticals in estuarine waters: A baseline assessment for the Tejo estuary. Mar. Pollut. Bull. 2018, 135, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Comtois-Marotte, S.; Chappuis, T.; Duy, S.V.; Gilbert, N.; Lajeunesse, A.; Taktek, S.; Desrosiers, M.; Veilleux, É.; Sauvé, S. Analysis of emerging contaminants in water and solid samples using high resolution mass spectrometry with a Q Exactive orbital ion trap and estrogenic activity with YES-assay. Chemosphere 2017, 166, 400–411. [Google Scholar] [CrossRef]
- Krogh, J.; Lyons, S.; Lowe, C.J. Pharmaceuticals and personal care products in municipal wastewater and the marine receiving environment near Victoria Canada. Front. Mar. Sci. 2017, 4, 415. [Google Scholar] [CrossRef]
- Nakata, H.; Kannan, K.; Jones, P.D.; Giesy, J.P. Determination of fluoroquinolone antibiotics in wastewater effluents by liquid chromatography–mass spectrometry and fluorescence detection. Chemosphere 2005, 58, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Giebułtowicz, J.; Stankiewicz, A.; Wroczyński, P.; Nałęcz-Jawecki, G. Occurrence of cardiovascular drugs in the sewage-impacted Vistula River and in tap water in the Warsaw region (Poland). Environ. Sci. Pollut. Res. 2016, 23, 24337–24349. [Google Scholar] [CrossRef]
- Kot-Wasik, A.; Jakimska, A.; Śliwka-Kaszyńska, M. Occurrence and seasonal variations of 25 pharmaceutical residues in wastewater and drinking water treatment plants. Environ. Monit. Assess. 2016, 188, 661. [Google Scholar] [CrossRef] [PubMed]
- Kapelewska, J.; Kotowska, U.; Karpińska, J.; Kowalczuk, D.; Arciszewska, A.; Świrydo, A. Occurrence, removal, mass loading and environmental risk assessment of emerging organic contaminants in leachates, groundwaters and wastewaters. Microchem. J. 2018, 137, 292–301. [Google Scholar] [CrossRef]
- Wagil, M.; Kumirska, J.; Stolte, S.; Puckowski, A.; Maszkowska, J.; Stepnowski, P.; Białk-Bielińska, A. Development of sensitive and reliable LC-MS/MS methods for the determination of three fluoroquinolones in water and fish tissue samples and preliminary environmental risk assessment of their presence in two rivers in northern Poland. Sci. Total Environ. 2014, 493, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Gaffney, V.; Cardoso, V.V.; Cardoso, E.; Teixeira, A.P.; Martins, J.; Benoliel, M.J.; Almeida, C.M.M. Occurrence and behaviour of pharmaceutical compounds in a Portuguese wastewater treatment plant: Removal efficiency through conventional treatment processes. Environ. Sci. Pollut. Res. 2017, 24, 14717–14734. [Google Scholar] [CrossRef]
- Jonkers, N.; Sousa, A.; Galante-Oliveira, S.; Barroso, C.M.; Kohler, H.-P.E.; Giger, W. Occurrence and sources of selected phenolic endocrine disruptors in Ria de Aveiro, Portugal. Environ. Sci. Pollut. Res. 2010, 17, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Guzel, E.Y.; Cevik, F.; Daglioglu, N. Determination of pharmaceutical active compounds in Ceyhan River, Turkey: Seasonal, spatial variations and environmental risk assessment. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 1980–1995. [Google Scholar] [CrossRef]
- Gardner, M.; Jones, V.; Comber, S.; Scrimshaw, M.D.; Coello-Garcia, T.; Cartmell, E.; Lester, J.; Ellor, B. Performance of UK wastewater treatment works with respect to trace contaminants. Sci. Total Environ. 2013, 456, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef]
| Analytes | Class | Chemical Structures | Molecular Mass (g mol−1) | pKa Values |
|---|---|---|---|---|
| Danofloxacin | Fluoroquinolones |  | 357.37 | 6.22 and 9.43 |
| Enrofloxacin | Fluoroquinolones |  | 359.4 | 6.19 and 7.59 |
| Levofloxacin | Fluoroquinolones | 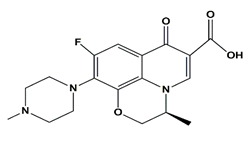 | 361.368 | 6.02 and 8.15 |
| Atenolol | β-blockers |  | 266.336 | 9.6 |
| Propranolol hydrochloride | β-blockers | 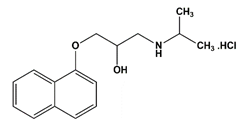 | 295.80 | 9.4 |
| Carbamazepine | Anticonvulsant |  | 236.27 | 13.9 |
| Methyl paraben | Preservatives | 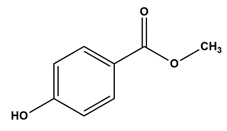 | 152.15 | 8.3 |
| Ethyl paraben | Preservatives | 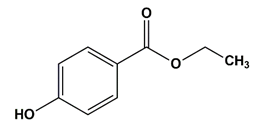 | 166.17 | 8.3 |
| Propyl paraben | Preservatives | 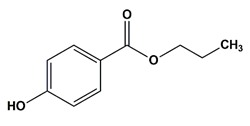 | 180.20 | 8.2 |
| Butyl paraben | Preservatives | 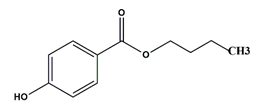 | 194.23 | 8.2 |
| Analytical Performances | β-Blockers and Anticonvulsants | Fluoroquinolones | Parabens |
|---|---|---|---|
| LODs (ng L−1) | 0.1–0.7 | 0.45–1.1 | 0.2–0.8 |
| LOQs (ng L−1) | 0.33–2.3 | 1.5–3.7 | 0.67–2.7 |
| Linearity (µg L−1) | LOQ-400 | LOQ-1000 | LOQ-300 |
| R2 | 0.9987–0.9991 | 0.9979–0.9990 | 0.9987–0.9993 |
| Repeatability (%RSD) | 1.9–2.5 | 1.8–3.4 | 1.5–2.7 |
| Reproducibility (%RSD) | 3.1–4.3 | 2.8–4.4 | 2.9–4.4 |
| Adsorbent/Method | Mass of Adsorbent (mg) | Analytes | LOD (µg L−1) | Linearity (µg L−1) | Correlation Coefficient (R2) | Refs. |
|---|---|---|---|---|---|---|
| MM-CMC/IT-SPME-HPLC-FLD | N/A | DANO, ENRO | 0.14–0.61 | 0.001–5.0 | 0.9980 | [51] |
| Oasis HLB-SPE-LC/MS/MS | 60 | Atenolol, carbamazepine | 1.01–69.30 | 1.87–138.6 | 0.9669–0.9999 | [52] |
| Carbowax 20M/FPSE/GC-MS | Not indicated | EP, BP | 0.009–0.021 | 0,05–500 | 0.9992–0.9997 | [22] |
| OasisHLB/ RDSE/GC-MS | 40 | MP, EP, PP, BP | 0.02–0.15 | 0.06–0.44 | 0.9904–0.9989 | [23] |
| Mixed mode cationic exchange cartidges (MXC) | 60 | MP | 0.01 | 0.06–1122 | 0.9999 | [1] |
| C18,Floracil, QuEChERS/UPLC-QqQ-MS | 50 mg | Beta-blockers: Atenolol, propranolol; Preservatives: BP, MP, PP; Anticonvulsant: carbamazepine | 0.093–0.12 | 1.0–200.0 | >0.95 | [53] |
| MMPC/Cyc-Chit/HPLC-DAD | 50 mg | Beta-blockers: atenolol, propranolol | 0.0001–0.0007 | LOQ-400 | 0.9987–0.9991 | This work |
| Parabens: MP, EP, PP, BP | 0.0001–0.0007 | LOQ-300 | 0.9987–0.9993 | |||
| Anticonvulsant: carbamazepine | 0.0003 | LOQ-350 | 0.9989 | |||
| Quinolones: DANO, LEVO, ENRO | 0.00045–0.0011 | LOQ-1000 | 0.9987–0.9990 |
| Samples | Analytes | Initial (ng L−1) | Found (ng L−1) a | %R | %RSD | Found (ng L−1) b | %R | %RSD |
|---|---|---|---|---|---|---|---|---|
| Influent | β-blockers, anticonvulsants | ND-28.9 | 42.7–72.1 | 86.3–94.4 | 2.8–4.5 | 93.3–118 | 87.8–93.3 | 1.6–3.4 |
| Fluoroquinolones | ND-7.33 | 4.87–12.1 | 93.8–97.3 | 3.9–4.2 | 19.1–33.9 | 92.8–100 | 3.7–4.3 | |
| Parabens | 1.37–937 | 46.2–984 | 89.7–94.3 | 2.3–3.1 | 92.7–1078 | 91.3–96.1 | 2.6–3.5 | |
| Effluent | Β-blockers, anticonvulsants | ND-7.65 | 48.4–55.4 | 95.4–98.7 | 3.1–4.3 | 95.5–105 | 96.8–97.1 | 1.7–4.9 |
| Fluoroquinolones | ND-2.07 | 4.92–6.88 | 96.2–98.3 | 2.4–3.2 | 19.3–21.3 | 94.4–98.1 | 2.1–3.1 | |
| Parabens | ND-43.1 | 48.7–92.7 | 97.3–99.1 | 1.9–2.4 | 96.5–141 | 96.5–97.9 | 1.8–2.8 | |
| Tap water | β-blockers, anticonvulsants | ND | 48.9–49.6 | 97.7–99.1 | 2.6–3.5 | 98.9–99.5 | 98.0–99.5 | 2.0–4.0 |
| Fluoroquinolones | ND | 4.91–4.98 | 98.1–99.6 | 1.4–1.8 | 19.8–19.9 | 98.9–99.5 | 1.7–1.8 | |
| Parabens | ND-4.81 | 49.0–53.8 | 97.9–99.3 | 1.9–2.4 | 97.8–104 | 97.8–99.3 | 1.3–1.6 | |
| River water | β-blockers, anticonvulsants | ND-4.92 | 49.5–53.3 | 97.0–98.9 | 2.2–4.2 | 99.1–102 | 97.0–99.1 | 1.7–2.9 |
| Fluoroquinolones | ND-3.12 | 4.94–8.02 | 94.8–98.7 | 1.9–2.5 | 19.4–22.4 | 95.6–96.3 | 1.0–1.4 | |
| Parabens | ND-40.1 | 48.5–88.8 | 96.6–98.8 | 1.7–2.4 | 97.1–137 | 96.6–98.2 | 1.3–1.9 |
| Country | β-Blockers | Anticonvulsants | Fluoroquinolones | Parabens | References |
|---|---|---|---|---|---|
| South Africa | 0.96–39,000 | 4.0–94 | 110–2257 | 0–1988 | [31,36,44,45,54] |
| Latvia | 0–150 | 18–50 | 250–400 | - | [55] |
| Egypt | 0–187 | 0–342 | - | 0–6380 | [56,57,58] |
| Kenya | - | 0–430 | - | 30–1160 | [59,60] |
| Spain | 10–6066 | 28–283 | 0–2153 | 14–720 | [21,61,62,63] |
| Italy | 0–57 | 0–137 | - | - | [64] |
| Pakistan | 0.99–452 | 11–15 | 2–37,000 | 110–228 | [65,66] |
| China | 0–995 | 23–115 | 0–2032 | 0–5960 | [65,66,67,68,69,70,71] |
| Brazil | 0.02–1.89 | 69 | 90–788 | [72,73,74,75] | |
| Canada | 114 | 20 | 34 | - | [76,77,78] |
| Poland | 69–205 | 2.0 | 248.7 | 0.01–5.03 | [79,80,81,82] |
| Portugal | 220–690 | 0.32–1.60 | 2.1–51 | [83,84] | |
| Turkey | 0.92–24.25 | 17,000–33,000 | [85] | ||
| United Kingdom | 93–388 | 13–56 | 180 | 2642–11,601 | [86,87] |
| South Africa | 0–28 | - | 0–43 | 0–937 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashile, G.P.; Mpupa, A.; Nomngongo, P.N. Magnetic Mesoporous Carbon/β-Cyclodextrin–Chitosan Nanocomposite for Extraction and Preconcentration of Multi-Class Emerging Contaminant Residues in Environmental Samples. Nanomaterials 2021, 11, 540. https://doi.org/10.3390/nano11020540
Mashile GP, Mpupa A, Nomngongo PN. Magnetic Mesoporous Carbon/β-Cyclodextrin–Chitosan Nanocomposite for Extraction and Preconcentration of Multi-Class Emerging Contaminant Residues in Environmental Samples. Nanomaterials. 2021; 11(2):540. https://doi.org/10.3390/nano11020540
Chicago/Turabian StyleMashile, Geaneth Pertunia, Anele Mpupa, and Philiswa Nosizo Nomngongo. 2021. "Magnetic Mesoporous Carbon/β-Cyclodextrin–Chitosan Nanocomposite for Extraction and Preconcentration of Multi-Class Emerging Contaminant Residues in Environmental Samples" Nanomaterials 11, no. 2: 540. https://doi.org/10.3390/nano11020540
APA StyleMashile, G. P., Mpupa, A., & Nomngongo, P. N. (2021). Magnetic Mesoporous Carbon/β-Cyclodextrin–Chitosan Nanocomposite for Extraction and Preconcentration of Multi-Class Emerging Contaminant Residues in Environmental Samples. Nanomaterials, 11(2), 540. https://doi.org/10.3390/nano11020540







