Synthesis and Characterization of Nanomaterial Based on Halloysite and Hectorite Clay Minerals Covalently Bridged
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Ht-NH2
2.2. Synthesis of Ht-Allyl
2.3. Synthesis of HNTs-Ht
2.4. Loading of Ciprofloxacin on HNTs, Ht and HNTs-Ht
2.5. Loading of Silver Ions on HNTs, Ht and HNTs-Ht
3. Results and Discussion
3.1. Synthesis and Characterization of Ht-Allyl Nanomaterial
3.2. Synthesis and Characterization of HNTs-Ht Nanomaterial
3.3. Adsorption Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Zhou, C.; Liu, M. Self-assembled structures of halloysite nanotubes: Towards the development of high-performance biomedical materials. J. Mater. Chem. B 2020, 8, 838–851. [Google Scholar]
- Santos, A.C.; Pereira, I.; Reis, S.; Veiga, F.; Saleh, M.; Lvov, Y. Biomedical potential of clay nanotube formulations and their toxicity assessment. Expert Opin. Drug Deliv. 2019, 16, 1169–1182. [Google Scholar]
- Massaro, M.; Colletti, C.G.; Guernelli, S.; Lazzara, G.; Liu, M.; Nicotra, G.; Noto, R.; Parisi, F.; Pibiri, I.; Spinella, C.; et al. Photoluminescent hybrid nanomaterials from modified halloysite nanotubes. J. Mater. Chem. C 2018, 6, 7377–7384. [Google Scholar]
- Stavitskaya, A.; Batasheva, S.; Vinokurov, V.; Fakhrullina, G.; Sangarov, V.; Lvov, Y.; Fakhrullin, R. Antimicrobial applications of clay nanotube-based composites. Nanomaterials 2019, 9, 708. [Google Scholar]
- Stavitskaya, A.; Shakhbazova, C.; Cherednichenko, Y.; Nigamatzyanova, L.; Fakhrullina, G.; Khaertdinov, N.; Kuralbayeva, G.; Filimonova, A.; Vinokurov, V.; Fakhrullin, R. Antibacterial properties and in vivo studies of tannic acid-stabilized silver–halloysite nanomaterials. Clay Miner. 2020, 55, 112–119. [Google Scholar]
- Massaro, M.; Noto, R.; Riela, S. Past, present and future perspectives on halloysite clay minerals. Molecules 2020, 25, 4863. [Google Scholar]
- Riela, S.; Barattucci, A.; Barreca, D.; Campagna, S.; Cavallaro, G.; Lazzara, G.; Massaro, M.; Pizzolanti, G.; Salerno, T.M.G.; Bonaccorsi, P.; et al. Boosting the properties of a fluorescent dye by encapsulation into halloysite nanotubes. Dye. Pigment. 2021, 187, 109094. [Google Scholar]
- Massaro, M.; Barone, G.; Barra, V.; Cancemi, P.; Di Leonardo, A.; Grossi, G.; Lo Celso, F.; Schenone, S.; Viseras Iborra, C.; Riela, S. Pyrazole[3,4-d]pyrimidine derivatives loaded into halloysite as potential cdk inhibitors. Int. J. Pharm. 2021, 120281. [Google Scholar] [CrossRef]
- Jinhua, W.; Xiang, Z.; Bing, Z.; Yafei, Z.; Rui, Z.; Jindun, L.; Rongfeng, C. Rapid adsorption of cr (vi) on modified halloysite nanotubes. Desalination 2010, 259, 22–28. [Google Scholar]
- Cataldo, S.; Lazzara, G.; Massaro, M.; Muratore, N.; Pettignano, A.; Riela, S. Functionalized halloysite nanotubes for enhanced removal of lead(ii) ions from aqueous solutions. Appl. Clay Sci. 2018, 156, 87–95. [Google Scholar]
- Zhang, J.; Zhou, C.H.; Petit, S.; Zhang, H. Hectorite: Synthesis, modification, assembly and applications. Appl. Clay Sci. 2019, 177, 114–138. [Google Scholar]
- Glotov, A.; Levshakov, N.; Stavitskaya, A.; Artemova, M.; Gushchin, P.; Ivanov, E.; Vinokurov, V.; Lvov, Y. Templated self-assembly of ordered mesoporous silica on clay nanotubes. Chem. Commun. 2019, 55, 5507–5510. [Google Scholar]
- Li, S.; Mu, B.; Wang, X.; Kang, Y.; Wang, A. Fabrication of eco-friendly betanin hybrid materials based on palygorskite and halloysite. Materials 2020, 13, 4649. [Google Scholar]
- Lisuzzo, L.; Wicklein, B.; Lo Dico, G.; Lazzara, G.; del Real, G.; Aranda, P.; Ruiz-Hitzky, E. Functional biohybrid materials based on halloysite, sepiolite and cellulose nanofibers for health applications. Dalton Trans. 2020, 49, 3830–3840. [Google Scholar]
- Massaro, M.; Colletti, C.G.; Fiore, B.; La Parola, V.; Lazzara, G.; Guernelli, S.; Zaccheroni, N.; Riela, S. Gold nanoparticles stabilized by modified halloysite nanotubes for catalytic applications. Appl. Organomet. Chem. 2019, 33, e4665. [Google Scholar]
- Colletti, C.G.; Massaro, M.; Lazzara, G.; Cavallaro, G.; Milioto, S.; Pibiri, I.; Noto, R.; Riela, S. Synthesis, characterization and study of covalently modified triazole laponite® edges. Appl. Clay Sci. 2020, 187, 105489. [Google Scholar]
- de Castro Silva, F.; Brandão Lima, L.C.; Silva-Filho, E.C.; Fonseca, M.G.; Jaber, M. Through alizarin-hectorite pigments: Influence of organofunctionalization on fading. Colloids Surf. Physicochem. Eng. Asp. 2020, 587, 124323. [Google Scholar]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Effects of halloysite content on the thermo-mechanical performances of composite bioplastics. Appl. Clay Sci. 2020, 185, 105416. [Google Scholar]
- Massaro, M.; Cavallaro, G.; Colletti, C.G.; D'Azzo, G.; Guernelli, S.; Lazzara, G.; Pieraccini, S.; Riela, S. Halloysite nanotubes for efficient loading, stabilization and controlled release of insulin. J. Colloid Interface Sci. 2018, 524, 156–164. [Google Scholar]
- Massaro, M.; Colletti, C.G.; Buscemi, G.; Cataldo, S.; Guernelli, S.; Lazzara, G.; Liotta, L.F.; Parisi, F.; Pettignano, A.; Riela, S. Palladium nanoparticles immobilized on halloysite nanotubes covered by a multilayer network for catalytic applications. New J. Chem. 2018, 42, 13938–13947. [Google Scholar]
- Cavallaro, G.; Lazzara, G.; Milioto, S. Exploiting the colloidal stability and solubilization ability of clay nanotubes/ionic surfactant hybrid nanomaterials. J. Phys. Chem. C 2012, 116, 21932–21938. [Google Scholar]
- Pasbakhsh, P.; Churchman, G.J.; Keeling, J.L. Characterisation of properties of various halloysites relevant to their use as nanotubes and microfibre fillers. Appl. Clay Sci. 2013, 74, 47–57. [Google Scholar]
- Vo, V.S.; Mahouche-Chergui, S.; Nguyen, V.H.; Naili, S.; Carbonnier, B. Crucial role of covalent surface functionalization of clay nanofillers on improvement of the mechanical properties of bioepoxy resin. ACS Sustain. Chem. Eng. 2019, 7, 15211–15220. [Google Scholar]
- Horue, M.; Cacicedo, M.L.; Fernandez, M.A.; Rodenak-Kladniew, B.; Torres Sánchez, R.M.; Castro, G.R. Antimicrobial activities of bacterial cellulose–silver montmorillonite nanocomposites for wound healing. Mater. Sci. Eng. C 2020, 116, 111152. [Google Scholar]
- Joo, Y.; Sim, J.H.; Jeon, Y.; Lee, S.U.; Sohn, D. Opening and blocking the inner-pores of halloysite. Chem. Commun. 2013, 49, 4519–4521. [Google Scholar]
- Massaro, M.; Buscemi, G.; Arista, L.; Biddeci, G.; Cavallaro, G.; D'Anna, F.; Di Blasi, F.; Ferrante, A.; Lazzara, G.; Rizzo, C.; et al. Multifunctional carrier based on halloysite/laponite hybrid hydrogel for kartogenin delivery. ACS Med. Chem. Lett. 2019, 10, 419–424. [Google Scholar]
- Peighambardoust, S.J.; Zahed-Karkaj, S.; Peighambardoust, S.H.; Ebrahimi, Y.; Peressini, D. Characterization of carboxymethyl cellulose-based active films incorporating non-modified and ag or cu-modified cloisite 30b and montmorillonite nanoclays. Iran. Polym. J. 2020, 29, 1087–1097. [Google Scholar]
- Gulen, B.; Demircivi, P. Synthesis and characterization of montmorillonite/ciprofloxacin/tio2 porous structure for controlled drug release of ciprofloxacin tablet with oral administration. Appl. Clay Sci. 2020, 197, 105768. [Google Scholar]
- Maged, A.; Kharbish, S.; Ismael, I.S.; Bhatnagar, A. Characterization of activated bentonite clay mineral and the mechanisms underlying its sorption for ciprofloxacin from aqueous solution. Environ. Sci. Pollut. Res. 2020, 27, 32980–32997. [Google Scholar]
- Valdés, L.; Pérez, I.; de Ménorval, L.C.; Altshuler, E.; Fossum, J.O.; Rivera, A. A simple way for targeted delivery of an antibiotic: In vitro evaluation of a nanoclay-based composite. PLoS ONE 2017, 12, e0187879. [Google Scholar]
- Xiong, Z.-C.; Yang, Z.-Y.; Zhu, Y.-J.; Chen, F.-F.; Zhang, Y.-G.; Yang, R.-L. Ultralong hydroxyapatite nanowires-based paper co-loaded with silver nanoparticles and antibiotic for long-term antibacterial benefit. ACS Appl. Mater. Interf. 2017, 9, 22212–22222. [Google Scholar]

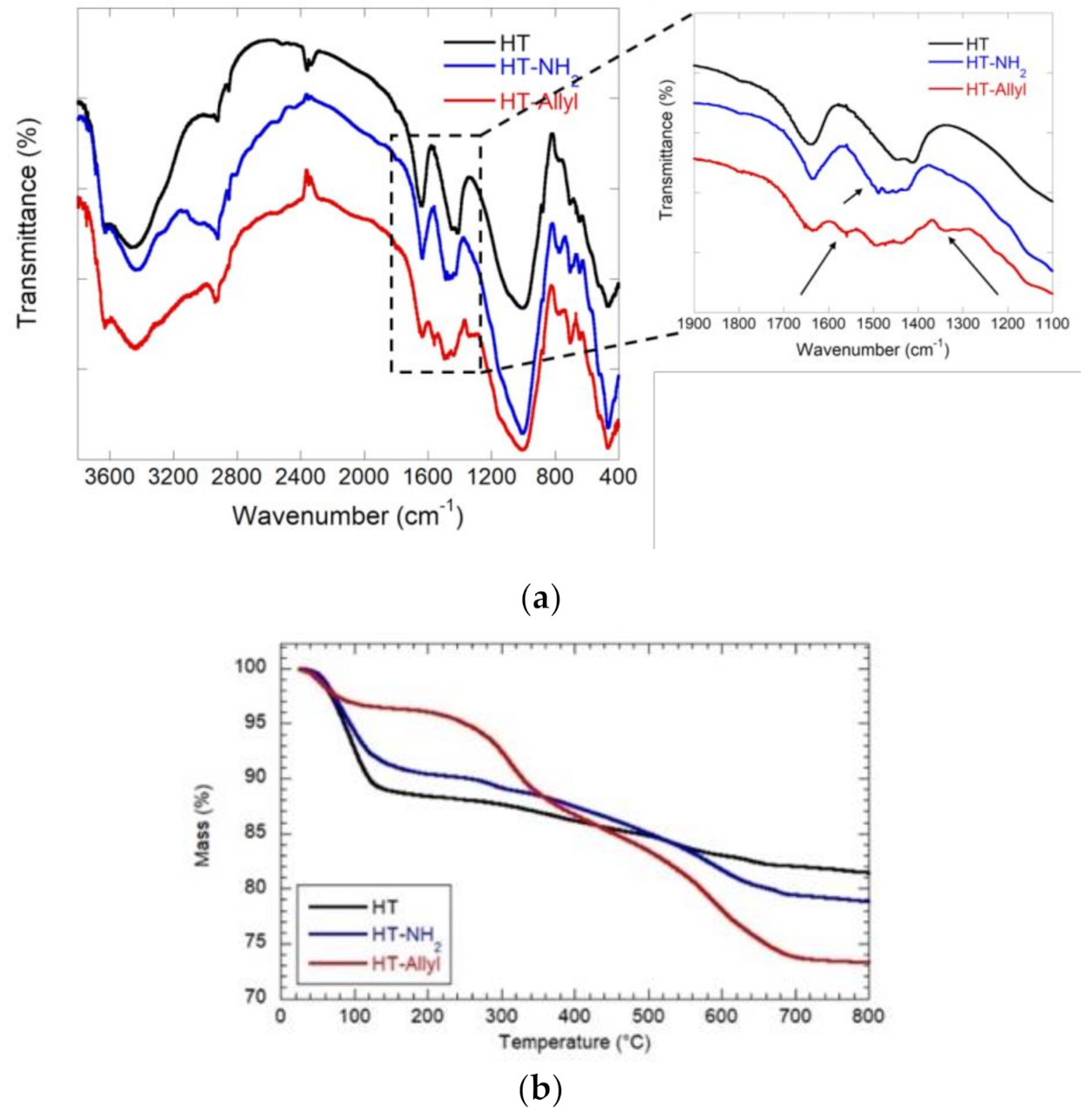

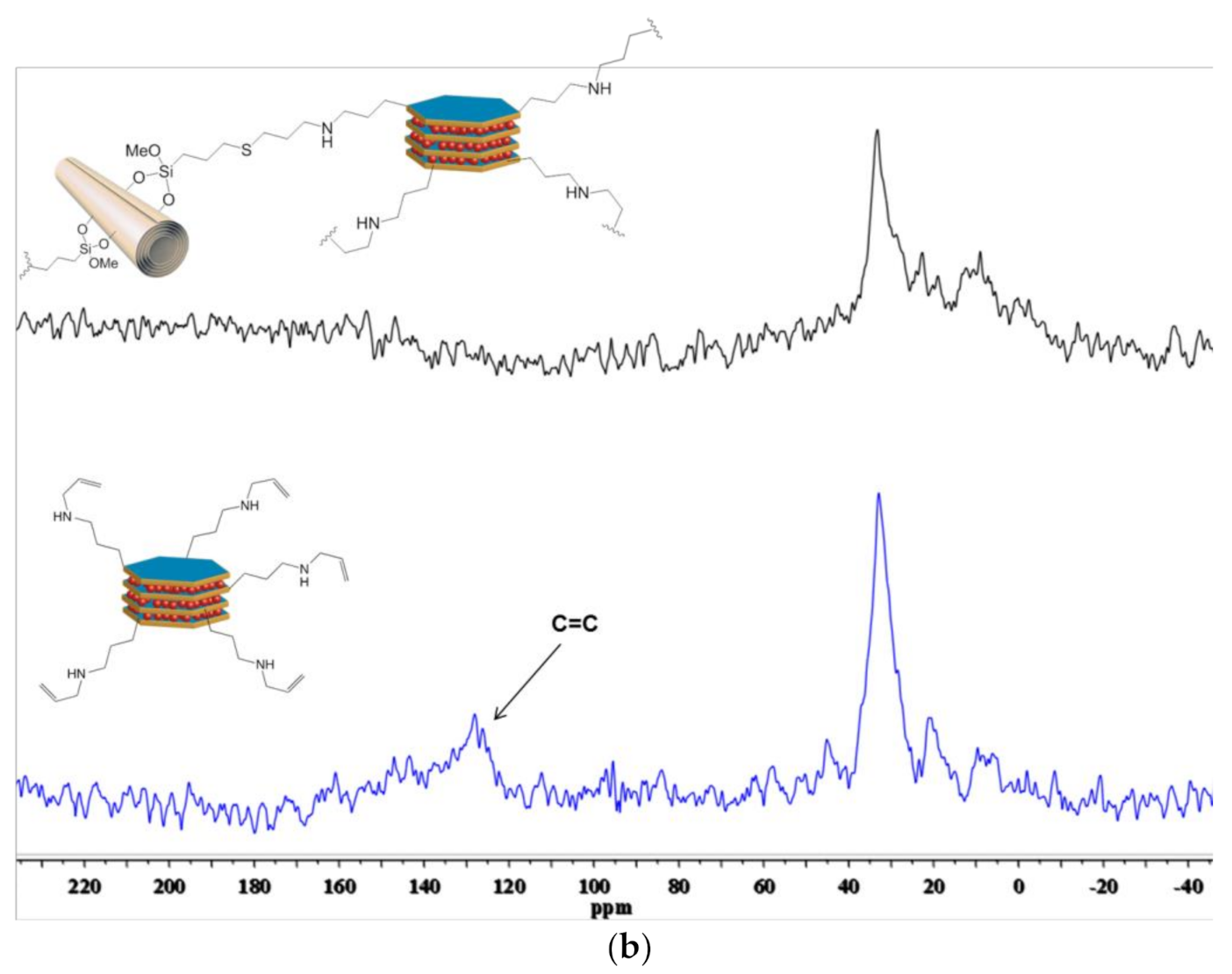
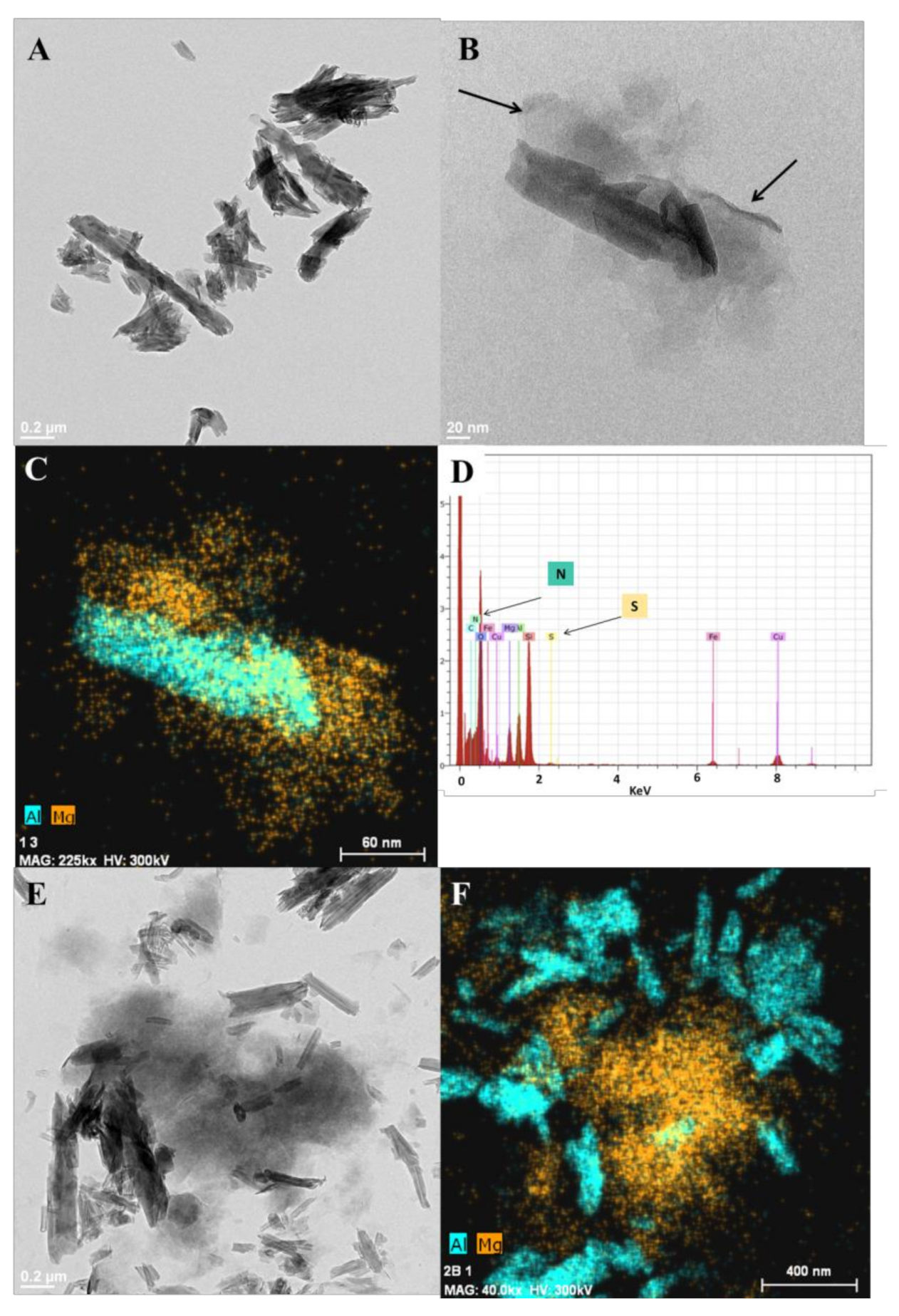
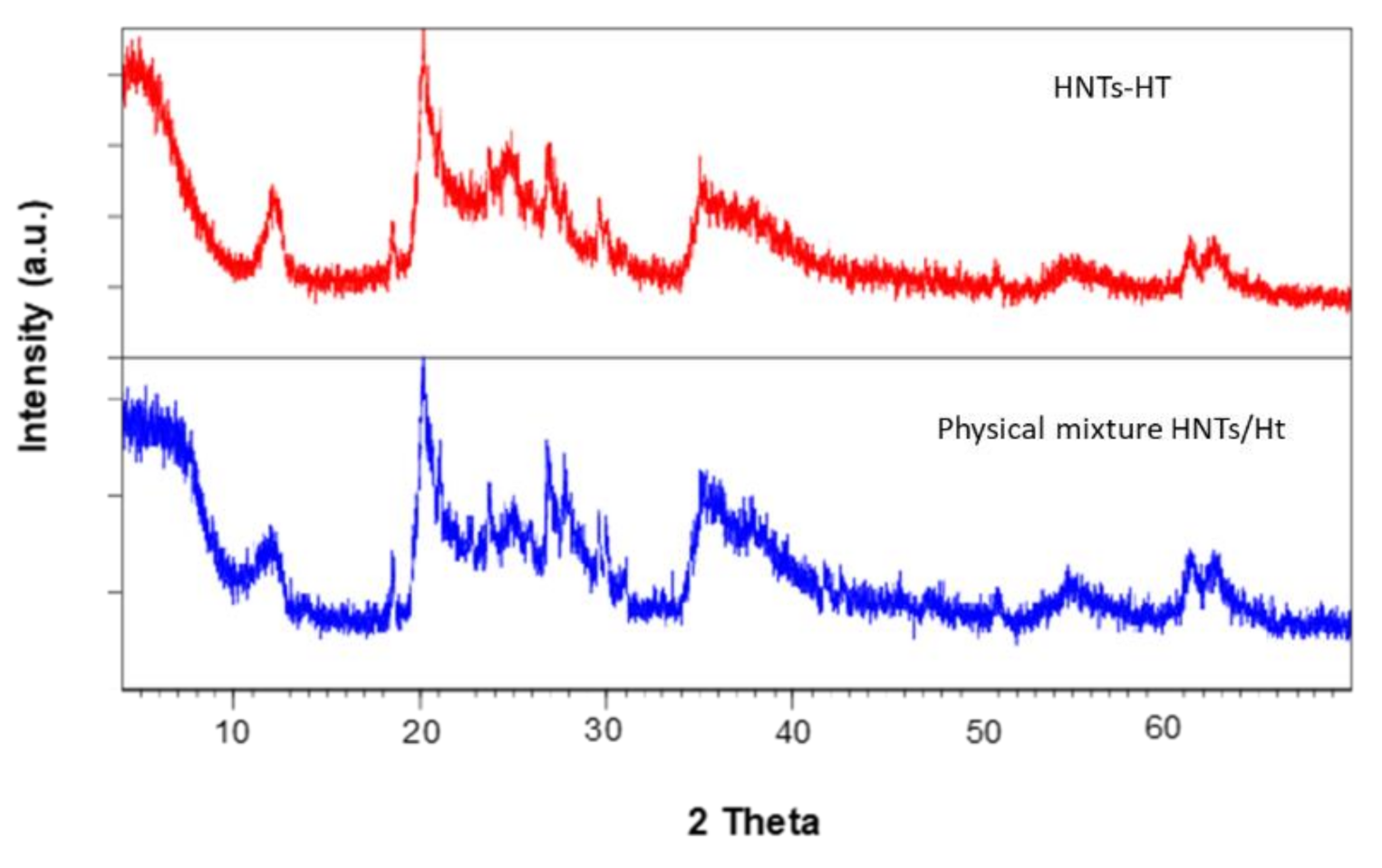
| Material | ML150 (wt%) | MR800 (wt%) | MD800 (wt%) |
|---|---|---|---|
| HT | 11.09 | 82.0 | 6.91 |
| HT-NH2 | 8.76 | 79.4 | 11.84 |
| HT-Allyl | 3.58 | 73.8 | 22.62 |
| Material | Hydrodynamic Diameter (nm) | ζ−Potential (mV) |
|---|---|---|
| Ht | 432 ± 80 | −36.9 ± 1.9 |
| Ht-Allyl | 484 ± 89 | −15.0 ± 1.4 |
| HNTs | 421 ± 91 | −34.4 ± 1.8 |
| HNTs-SH | 427 ± 95 | −27.1 ± 1.1 |
| HNTs-Ht | 453 ± 96 | −17.3 ± 1.4; −35.5 ± 1.7 |
| Entry | Sample | mg | Ciprofloxacin (wt%) a | Ag+ ions (wt%) b |
|---|---|---|---|---|
| 1 | HNTs | 16 | 2.6 | <1 |
| 2 | Ht | 16 | <1 | 7 |
| 3 | HNTs-Ht | 32 | 2.6 | 8 |
| 4 | HNTs/Ht | 32 | <1 | 7.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massaro, M.; Viseras Iborra, C.; Cavallaro, G.; Colletti, C.G.; García-Villén, F.; Lazzara, G.; Riela, S. Synthesis and Characterization of Nanomaterial Based on Halloysite and Hectorite Clay Minerals Covalently Bridged. Nanomaterials 2021, 11, 506. https://doi.org/10.3390/nano11020506
Massaro M, Viseras Iborra C, Cavallaro G, Colletti CG, García-Villén F, Lazzara G, Riela S. Synthesis and Characterization of Nanomaterial Based on Halloysite and Hectorite Clay Minerals Covalently Bridged. Nanomaterials. 2021; 11(2):506. https://doi.org/10.3390/nano11020506
Chicago/Turabian StyleMassaro, Marina, Cesar Viseras Iborra, Giuseppe Cavallaro, Carmelo Giuseppe Colletti, Fátima García-Villén, Giuseppe Lazzara, and Serena Riela. 2021. "Synthesis and Characterization of Nanomaterial Based on Halloysite and Hectorite Clay Minerals Covalently Bridged" Nanomaterials 11, no. 2: 506. https://doi.org/10.3390/nano11020506
APA StyleMassaro, M., Viseras Iborra, C., Cavallaro, G., Colletti, C. G., García-Villén, F., Lazzara, G., & Riela, S. (2021). Synthesis and Characterization of Nanomaterial Based on Halloysite and Hectorite Clay Minerals Covalently Bridged. Nanomaterials, 11(2), 506. https://doi.org/10.3390/nano11020506










