Structural, Optical, and Antibacterial Efficacy of Pure and Zinc-Doped Copper Oxide Against Pathogenic Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Zn-Doped CuO-NSs
2.3. Screening of Antibacterial Activity
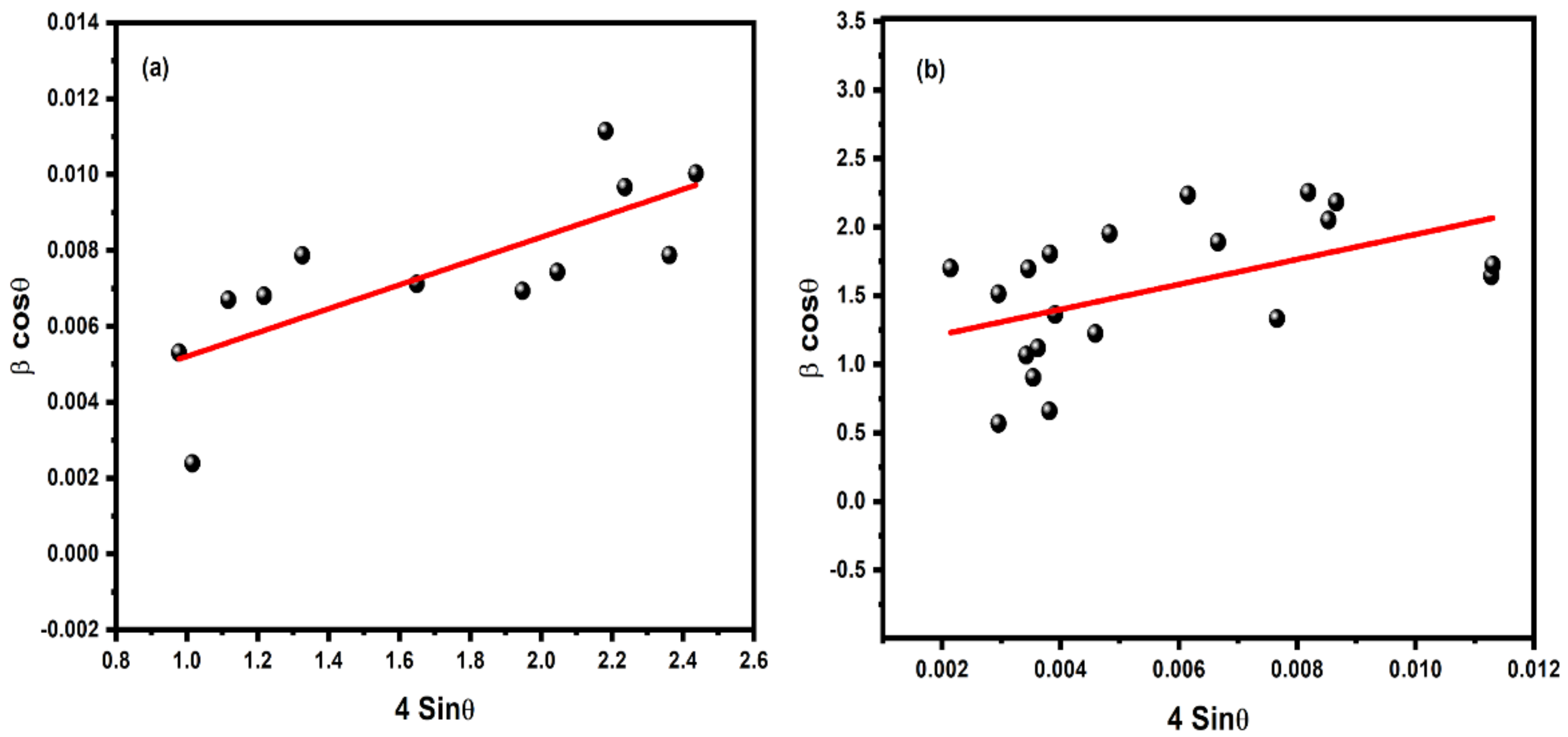
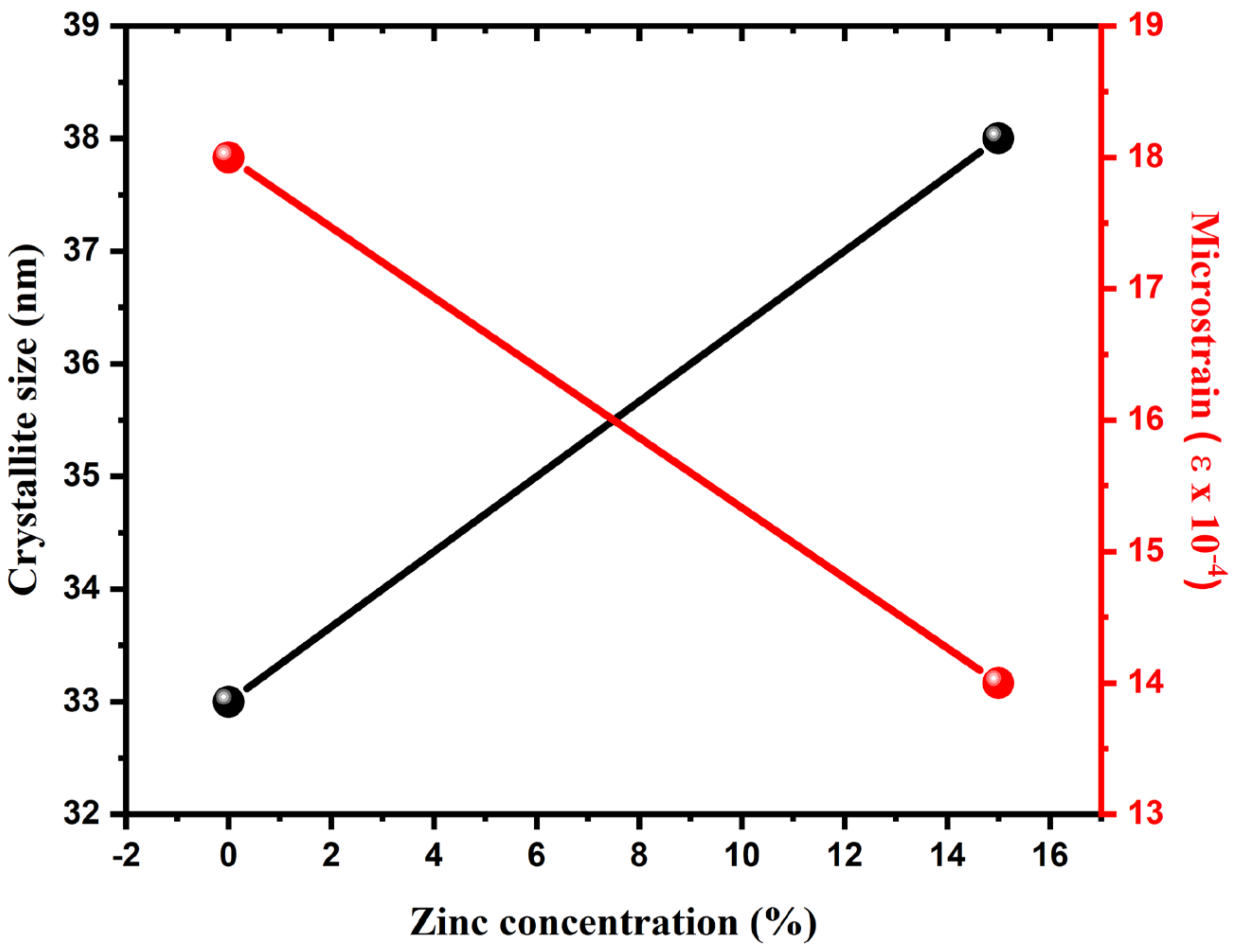
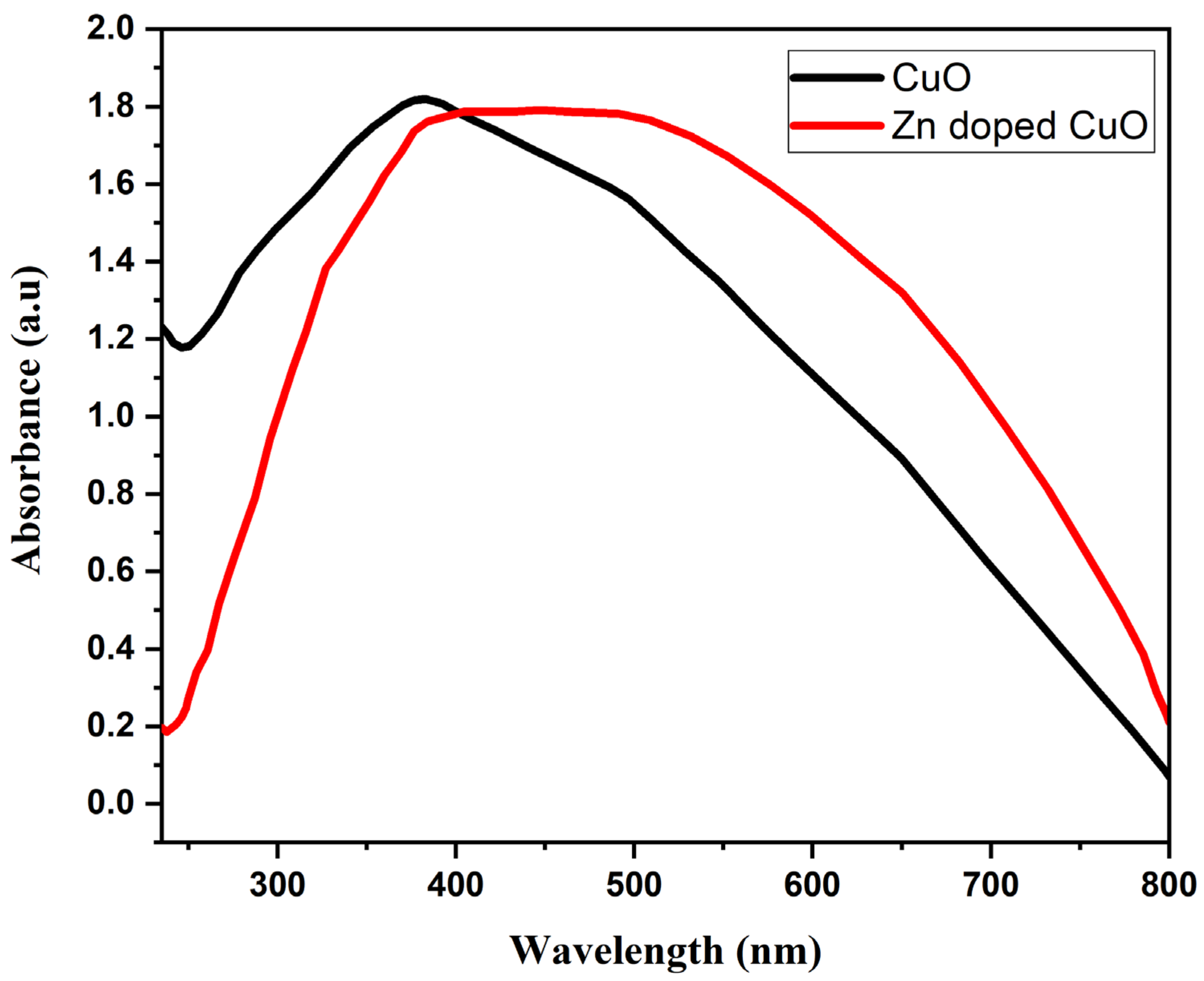
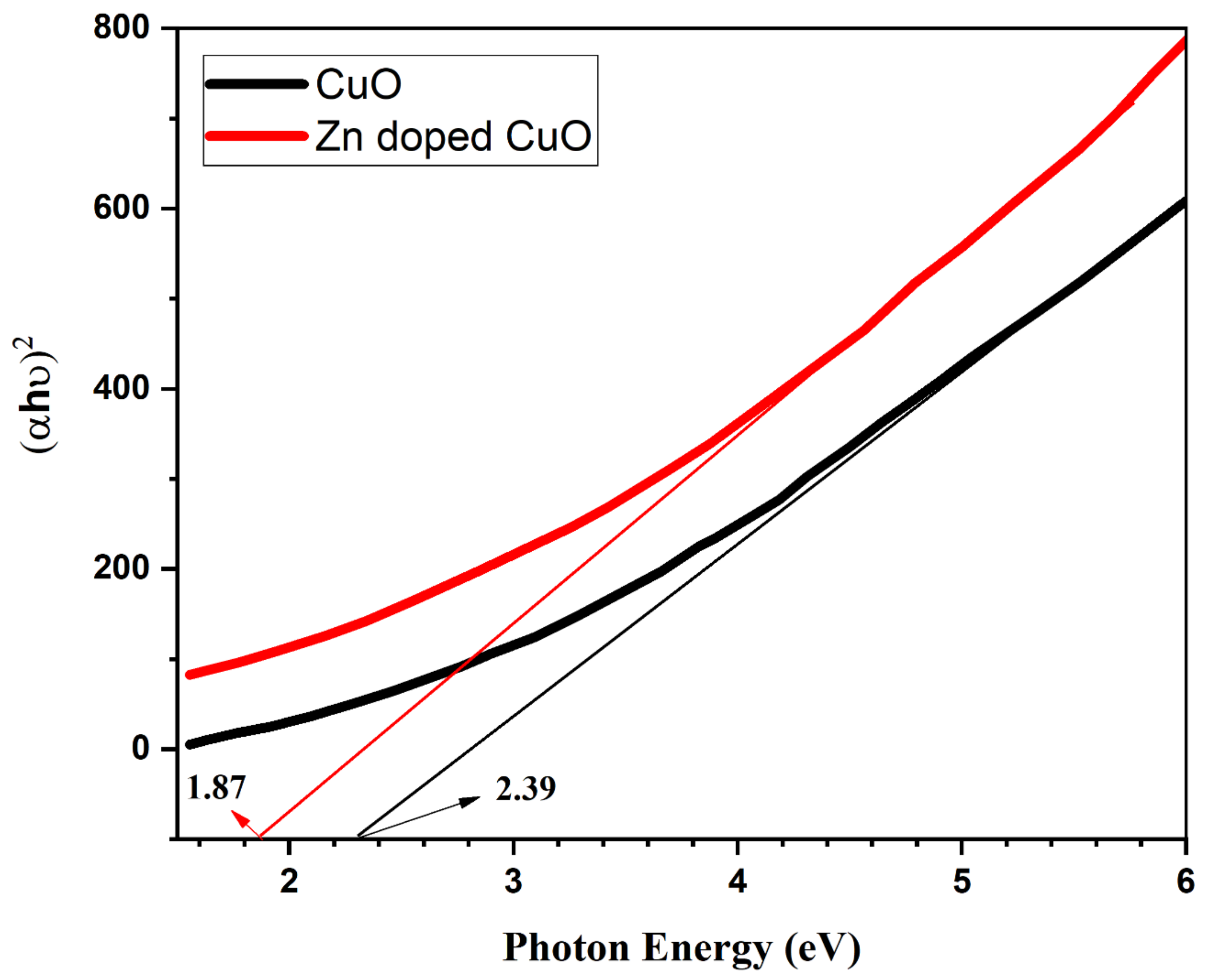
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rejith, S.; Krishnan, C. Optical characterizations of Zn-doped CuO nanoparticles. Sci. Acta Xaver 2013, 4, 91. [Google Scholar]
- Magdalane, C.M.; Kaviyarasu, K.; Vijaya, J.J.; Siddhardha, B.; Jeyaraj, B. Photocatalytic activity of binary metal oxide nanocomposites of CeO2/CdO nanospheres: Investigation of optical and antimicrobial activity. J. Photochem. Photobiol. B Biol. 2016, 163, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Ahmed, B.; Khan, M.S.; Al-Shaeri, M.; Musarrat, J. Inhibition of growth and biofilm formation of clinical bacterial isolates by NiO nanoparticles synthesized from Eucalyptus globulus plants. Microb. Pathog. 2017, 111, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wu, L.; Ding, F.; Li, Q.; Wang, P.; Li, J.; Lu, Z.; Han, H. Surface-imprinted SiO2@ Ag nanoparticles for the selective detection of BPA using surface enhanced Raman scattering. Sens. Actuators B Chem. 2018, 258, 566–573. [Google Scholar] [CrossRef]
- Bayansal, F.; Gülen, Y.; Şahin, B.; Kahraman, S.; Çetinkara, H. CuO nanostructures grown by the SILAR method: Influence of Pb-doping on the morphological, structural and optical properties. J. Alloys Compd. 2015, 619, 378–382. [Google Scholar] [CrossRef]
- Armelao, L.; Barreca, D.; Bertapelle, M.; Bottaro, G.; Sada, C.; Tondello, E. A sol–gel approach to nanophasic copper oxide thin films. Thin Solid Films 2003, 442, 48–52. [Google Scholar] [CrossRef]
- Balamurugan, B.; Mehta, B.; Shivaprasad, S. Surface-modified CuO layer in size-stabilized single-phase Cu2O nanoparticles. Appl. Phys. Lett. 2001, 79, 3176–3178. [Google Scholar] [CrossRef]
- Bahoosh, S.; Apostolov, A.; Apostolova, I.; Wesselinowa, J. Theory of phonon properties in doped and undoped CuO nanoparticles. Phys. Lett. A 2012, 376, 2252–2255. [Google Scholar] [CrossRef]
- Joseph, D.P.; Venkateswaran, C.; Sambasivam, S.; Choi, B.C. Effect of Fe alloying on the structural, optical, electrical and magnetic properties of spray-deposited CuO thin films. J. Korean Phys. Soc. 2012, 61, 449–454. [Google Scholar] [CrossRef]
- Jiang, X.; Herricks, T.; Xia, Y. CuO nanowires can be synthesized by heating copper substrates in air. Nano Lett. 2002, 2, 1333–1338. [Google Scholar] [CrossRef]
- Jan, T.; Iqbal, J.; Ismail, M.; Badshah, N.; Mansoor, Q.; Arshad, A.; Ahkam, Q.M. Synthesis, physical properties and antibacterial activity of metal oxides nanostructures. Mater. Sci. Semicond. Process. 2014, 21, 154–160. [Google Scholar] [CrossRef]
- Li, B.-X.; Wang, Y.-Y.; Wang, Y.-F. Facile synthesis and photocatalytic property of CuO nanostructure arrays. Acta Phys. Chim. Sin. 2009, 25, 2366–2372. [Google Scholar]
- Liu, J.; Jin, J.; Deng, Z.; Huang, S.-Z.; Hu, Z.-Y.; Wang, L.; Wang, C.; Chen, L.-H.; Li, Y.; Van Tendeloo, G. Tailoring CuO nanostructures for enhanced photocatalytic property. J. Colloid Interface Sci. 2012, 384, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003. [Google Scholar] [CrossRef] [PubMed]
- Hassan, I.A.; Parkin, I.P.; Nair, S.P.; Carmalt, C.J. Antimicrobial activity of copper and copper (I) oxide thin films deposited via aerosol-assisted CVD. J. Mater. Chem. B 2014, 2, 2855–2860. [Google Scholar] [CrossRef]
- Santo, C.E.; Morais, P.V.; Grass, G. Isolation and characterization of bacteria resistant to metallic copper surfaces. Appl. Environ. Microbiol. 2010, 76, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Hans, M.; Erbe, A.; Mathews, S.; Chen, Y.; Solioz, M.; Mücklich, F. Role of copper oxides in contact killing of bacteria. Langmuir 2013, 29, 16160–16166. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanopart. Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Applerot, G.; Lipovsky, A.; Dror, R.; Perkas, N.; Nitzan, Y.; Lubart, R.; Gedanken, A. Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Adv. Funct. Mater. 2009, 19, 842–852. [Google Scholar] [CrossRef]
- Zhao, X.; Ren, X.; Zhu, R.; Luo, Z.; Ren, B. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria-mediated apoptosis in zebrafish embryos. Aquat. Toxicol. 2016, 180, 56–70. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Schiek, M.; Al-Shamery, K.; Kunat, M.; Traeger, F.; Wöll, C. Water adsorption on the hydroxylated H-(1 × 1) O-ZnO (0001 [combining macron]) surface. PCCP 2006, 8, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Jan, T.; Iqbal, J.; Mansoor, Q.; Ismail, M.; Naqvi, M.S.H.; Gul, A.; Naqvi, S.F.-u.-H.; Abbas, F. Synthesis, physical properties and antibacterial activity of Ce doped CuO: A novel nanomaterial. J. Phys. D Appl. Phys. 2014, 47, 355301. [Google Scholar] [CrossRef]
- Malka, E.; Perelshtein, I.; Lipovsky, A.; Shalom, Y.; Naparstek, L.; Perkas, N.; Patick, T.; Lubart, R.; Nitzan, Y.; Banin, E. Eradication of multi-drug resistant bacteria by a novel Zn-doped CuO nanocomposite. Small 2013, 9, 4069–4076. [Google Scholar] [CrossRef] [PubMed]
- Eshed, M.; Lellouche, J.; Gedanken, A.; Banin, E. A Zn-doped CuO nanocomposite shows enhanced antibiofilm and antibacterial activities against streptococcus mutans compared to nanosized CuO. Adv. Funct. Mater. 2014, 24, 1382–1390. [Google Scholar] [CrossRef]
- Fterich, M.; Nasr, F.B.; Lefi, R.; Toumi, M.; Guermazi, S. Effect of concentration of hexamethylenetetramine in structure, microstructure and optical properties of CuO nanoparticles synthesized by hydrothermal route. Mater. Sci. Semicond. Process. 2016, 43, 114–122. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, S.; Soni, S.; Sharma, S.; Singh, R.C. Influence of different milling media on structural, morphological and optical properties of the ZnO nanoparticles synthesized by ball milling process. Mater. Sci. Semicond. Process. 2019, 98, 29–38. [Google Scholar] [CrossRef]
- Vieillard, J.; Bouazizi, N.; Morshed, M.N.; Clamens, T.; Desriac, F.; Bargougui, R.; Thebault, P.; Lesouhaitier, O.; Le Derf, F.; Azzouz, A. CuO nanosheets modified with amine and thiol grafting for high catalytic and antibacterial activities. Ind. Eng. Chem. Res. 2019, 58, 10179–10189. [Google Scholar] [CrossRef]
- Bouazizi, N.; Vieillard, J.; Thebault, P.; Desriac, F.; Clamens, T.; Bargougui, R.; Couvrat, N.; Thoumire, O.; Brun, N.; Ladam, G. Silver nanoparticle embedded copper oxide as an efficient core–shell for the catalytic reduction of 4-nitrophenol and antibacterial activity improvement. Dalton Trans. 2018, 47, 9143–9155. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Harunsani, M.H.; Tan, A.L.; Hojamberdiev, M.; Azamay, S.; Ahmad, N. Antibacterial activities of zinc oxide and Mn-doped zinc oxide synthesized using Melastoma malabathricum (L.) leaf extract. Bioprocess Biosyst. Eng. 2020, 43, 1499–1508. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Asim, T.; Chen, Y.; Khan, M.; Adil, S.F. Green synthesis of ZnO hierarchical microstructures by Cordia myxa and their antibacterial activity. Saudi J. Biol. Sci. 2019, 26, 1364–1371. [Google Scholar] [CrossRef]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H.A. A review on enhancing the antibacterial activity of ZnO: Mechanisms and microscopic investigation. Nanoscale Res. Lett. 2020, 15, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, D.; Boothroyd, A. Single crystal growth of Zn-doped CuO by the floating-zone method. J. Cryst. Growth 2003, 250, 77–82. [Google Scholar] [CrossRef]
- Din, S.U.; Sajid, M.; Imran, M.; Iqbal, J.; Shah, B.A.; Shah, S. One step facile synthesis, characterization and antimicrobial properties of Mg-doped CuO nanostructures. Mater. Res. Express 2019, 6, 085022. [Google Scholar] [CrossRef]
- Barry, A.L.; Craig, W.A.; Nadler, H.; Reller, L.B.; Sanders, C.C.; Swenson, J.M. Methods for Determining Bactericidal Activity of Antimicrobial Agents: Approved Guideline; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 1999; Volume 19. [Google Scholar]
- Ma, C.-W.; Chang, C.-M.; Huang, P.-C.; Yang, Y.-J. Sea-urchin-like ZnO nanoparticle film for dye-sensitized solar cells. J. Nanomater. 2015, 2015. [Google Scholar] [CrossRef]
- Pung, S.; Ong, C.; Isha, K.M.; Othman, M. Synthesis and characterization of Cu-doped ZnO nanorods. Sains Malays. 2014, 43, 273–281. [Google Scholar]
- Shirsath, S.R.; Pinjari, D.V.; Gogate, P.R.; Sonawane, S.H.; Pandit, A.B. Ultrasound assisted synthesis of doped TiO2 nano-particles: Characterization and comparison of effectiveness for photocatalytic oxidation of dyestuff effluent. Ultrason. Sonochem. 2013, 20, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Umar, A.; Harraz, F.A.; Ibrahim, A.A.; Almas, T.; Kumar, R.; Al-Assiri, M.; Baskoutas, S. Iron-doped titanium dioxide nanoparticles as potential scaffold for hydrazine chemical sensor applications. Coatings 2020, 10, 182. [Google Scholar] [CrossRef]
- Prajapati, B.; Kumar, S.; Kumar, M.; Chatterjee, S.; Ghosh, A.K. Investigation of the physical properties of Fe: TiO 2-diluted magnetic semiconductor nanoparticles. J. Mater. Chem. C 2017, 5, 4257–4267. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Jiang, T.; Jia, H.; Yu, M. The preparation of M (M: Mn 2+, Cd 2+, Zn 2+)-doped CuO nanostructures via the hydrothermal method and their properties. J. Mater. Sci. Mater. Electron. 2016, 27, 2138–2145. [Google Scholar] [CrossRef]
- Deng, M.-J.; Wang, C.-C.; Ho, P.-J.; Lin, C.-M.; Chen, J.-M.; Lu, K.-T. Facile electrochemical synthesis of 3D nano-architectured CuO electrodes for high-performance supercapacitors. J. Mater. Chem. A 2014, 2, 12857–12865. [Google Scholar] [CrossRef]
- Dubal, D.P.; Gund, G.S.; Holze, R.; Lokhande, C.D. Mild chemical strategy to grow micro-roses and micro-woolen like arranged CuO nanosheets for high performance supercapacitors. J. Power Sources 2013, 242, 687–698. [Google Scholar] [CrossRef]
- Liu, W.; Tang, X.; Tang, Z.; Chu, F.; Zeng, T.; Tang, N. Role of oxygen defects in magnetic property of Cu doped ZnO. J. Alloy. Compd. 2014, 615, 740–744. [Google Scholar] [CrossRef]
- Zheng, L.; Zheng, Y.; Chen, C.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K.; Zhu, J. Network structured SnO2/ZnO heterojunction nanocatalyst with high photocatalytic activity. Inorg. Chem. 2009, 48, 1819–1825. [Google Scholar] [CrossRef]
- Navarro, R.; Del Valle, F.; Fierro, J. Photocatalytic hydrogen evolution from CdS–ZnO–CdO systems under visible light irradiation: Effect of thermal treatment and presence of Pt and Ru cocatalysts. Int. J. Hydrog. Energy 2008, 33, 4265–4273. [Google Scholar] [CrossRef]
- Iqbal, J.; Jan, T.; Ul-Hassan, S.; Ahmed, I.; Mansoor, Q.; Umair Ali, M.; Abbas, F.; Ismail, M. Facile synthesis of Zn doped CuO hierarchical nanostructures: Structural, optical and antibacterial properties. Aip. Adv. 2015, 5, 127112. [Google Scholar] [CrossRef]
- Perelshtein, I.; Applerot, G.; Perkas, N.; Wehrschuetz-Sigl, E.; Hasmann, A.; Gübitz, G.; Gedanken, A. CuO–cotton nanocomposite: Formation, morphology, and antibacterial activity. Surf. Coat. Technol. 2009, 204, 54–57. [Google Scholar] [CrossRef]
- Singh, D.P.; Srivastava, O.N. Synthesis and optical properties of different CuO (ellipsoid, ribbon and sheet like) nanostructures. J. Nanosci. Nanotechnol. 2009, 9, 5345–5350. [Google Scholar] [CrossRef] [PubMed]
- Al-Amri, S.; Shahnawaze Ansari, M.; Rafique, S.; Aldhahri, M.; Rahimuddin, S.; Azam, A.; Memic, A. Ni doped CuO nanoparticles: Structural and optical characterizations. Curr. Nanosci. 2015, 11, 191–197. [Google Scholar] [CrossRef]
- Yayapao, O.; Thongtem, T.; Phuruangrat, A.; Thongtem, S. Sonochemical synthesis of Dy-doped ZnO nanostructures and their photocatalytic properties. J. Alloy. Compd. 2013, 576, 72–79. [Google Scholar] [CrossRef]
- Valgas, C.; de Souza, S.M.; Smânia, E.F.; Smânia, A., Jr. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef]
- Cowan, S.T. Cowan and Steel’s Manual for the Identification of Medical Bacteria; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]



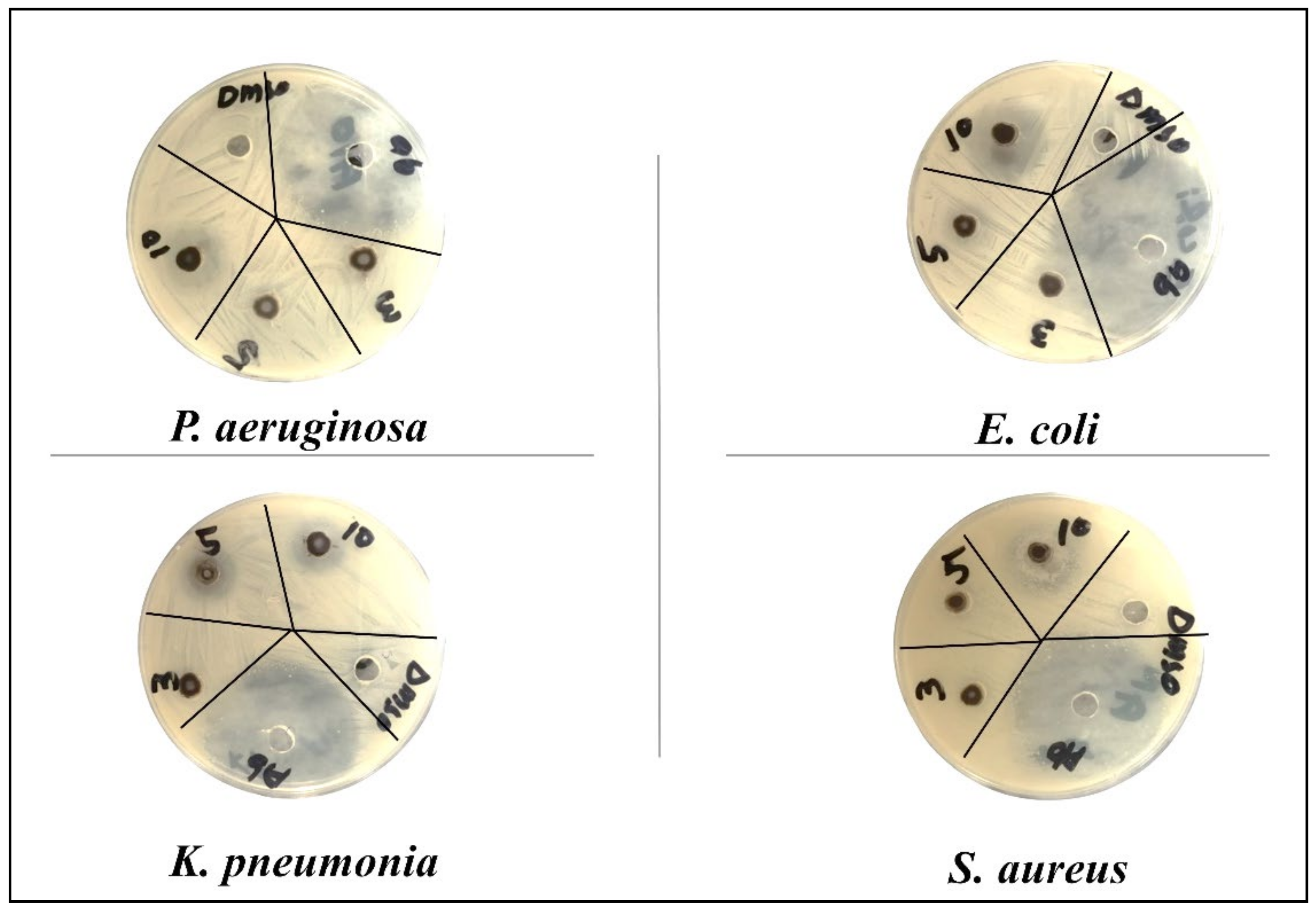
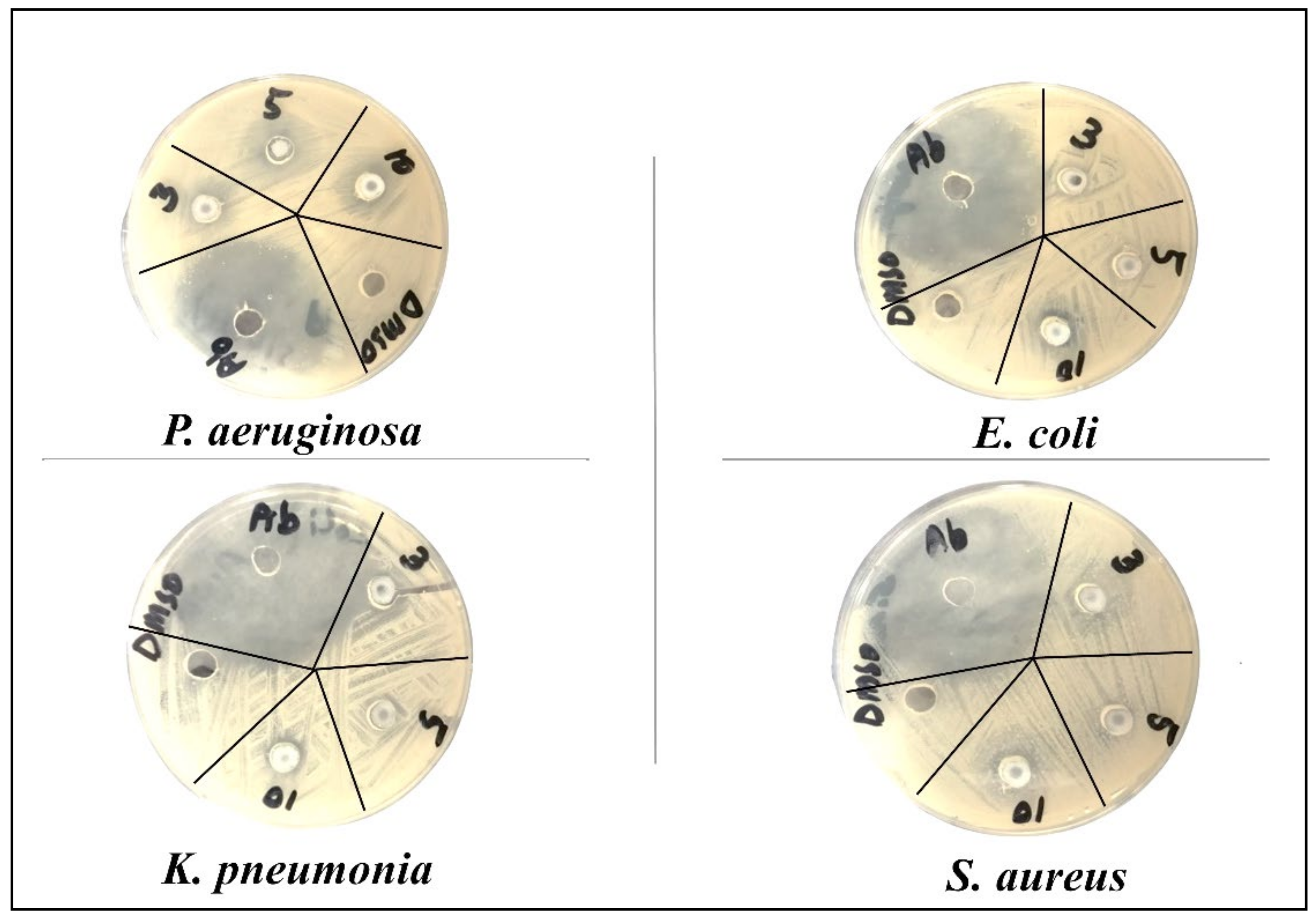
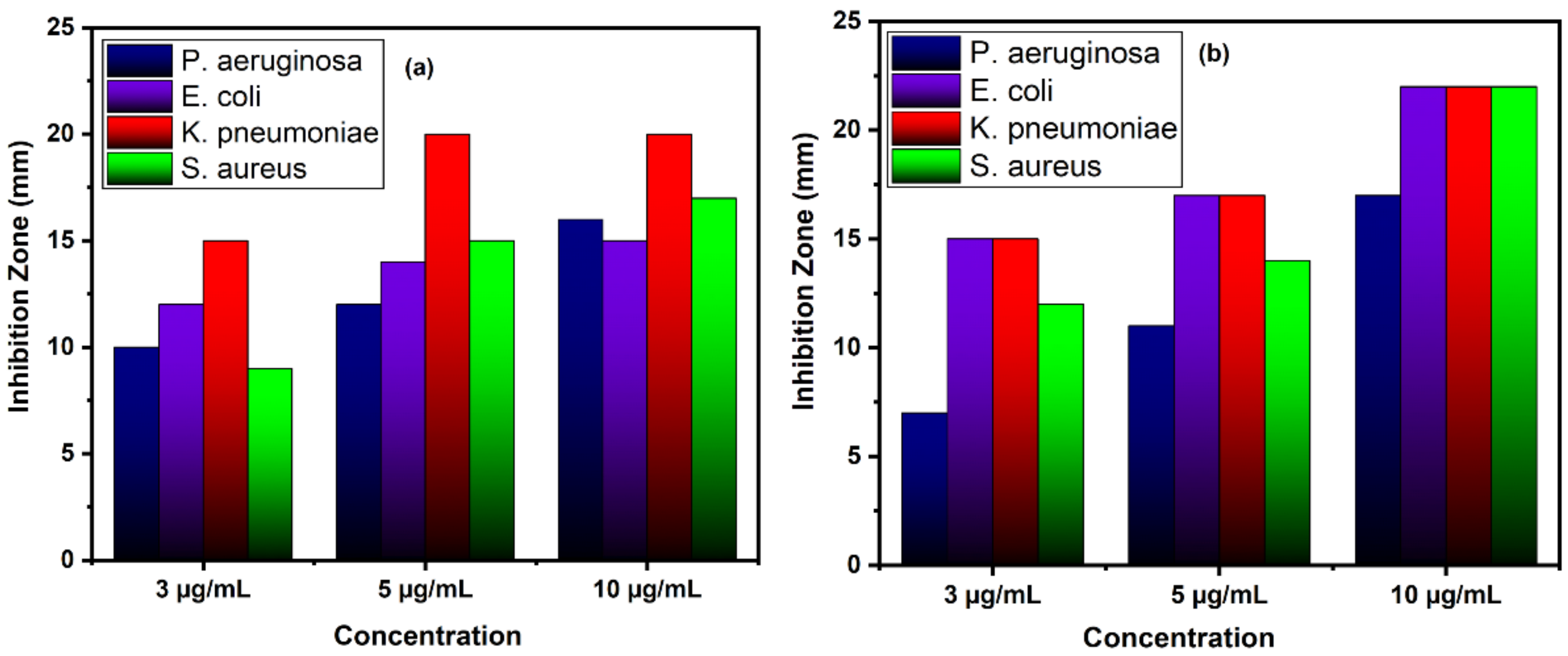
| Bacteria | CuO | Zn Doped CuO | ||||||
|---|---|---|---|---|---|---|---|---|
| 3 mg/mL | 5 mg/mL | 10 mg/mL | 3 mg/mL | 5 mg/mL | 10 mg/mL | |||
| Gram negative | P. aeruginosa | Inhibition zone (mm) | 10 ± 0.12 | 12 ± 0.20 | 16 ± 0.21 | 7 ± 0.15 | 11 ± 0.13 | 17 ± 0.14 |
| E. coli | 12 ± 0.11 | 14 ± 0.11 | 15 ± 0.20 | 15 ± 0.21 | 17 ± 0.11 | 22 ± 0.21 | ||
| K. pneumoniae | 15 ± 0.20 | 20 ± 0.15 | 20 ± 0.20 | 15 ± 0.12 | 17 ± 0.20 | 22 ± 0.20 | ||
| Gram positive | S. aureus | 9 ± 0.13 | 15 ± 0.11 | 17 ± 0.13 | 12 ± 0.14 | 14 ± 0.18 | 22 ± 0.13 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, A.; Ahmad, P.; Alharthi, A.I.; Muhammad, S.; Khandaker, M.U.; Rehman, M.; Faruque, M.R.I.; Din, I.U.; Alotaibi, M.A.; Alzimami, K.; et al. Structural, Optical, and Antibacterial Efficacy of Pure and Zinc-Doped Copper Oxide Against Pathogenic Bacteria. Nanomaterials 2021, 11, 451. https://doi.org/10.3390/nano11020451
Khalid A, Ahmad P, Alharthi AI, Muhammad S, Khandaker MU, Rehman M, Faruque MRI, Din IU, Alotaibi MA, Alzimami K, et al. Structural, Optical, and Antibacterial Efficacy of Pure and Zinc-Doped Copper Oxide Against Pathogenic Bacteria. Nanomaterials. 2021; 11(2):451. https://doi.org/10.3390/nano11020451
Chicago/Turabian StyleKhalid, Awais, Pervaiz Ahmad, Abdulrahman I. Alharthi, Saleh Muhammad, Mayeen Uddin Khandaker, Mubasher Rehman, Mohammad Rashed Iqbal Faruque, Israf Ud Din, Mshari A. Alotaibi, Khalid Alzimami, and et al. 2021. "Structural, Optical, and Antibacterial Efficacy of Pure and Zinc-Doped Copper Oxide Against Pathogenic Bacteria" Nanomaterials 11, no. 2: 451. https://doi.org/10.3390/nano11020451
APA StyleKhalid, A., Ahmad, P., Alharthi, A. I., Muhammad, S., Khandaker, M. U., Rehman, M., Faruque, M. R. I., Din, I. U., Alotaibi, M. A., Alzimami, K., & Bradley, D. A. (2021). Structural, Optical, and Antibacterial Efficacy of Pure and Zinc-Doped Copper Oxide Against Pathogenic Bacteria. Nanomaterials, 11(2), 451. https://doi.org/10.3390/nano11020451










