First-Principle Insight into Ga-Doped MoS2 for Sensing SO2, SOF2 and SO2F2
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Geometric Structure of Gas Molecules, MoS2, and Ga-MoS2 Monolayer
3.2. Adsorption Analysis of Ga-MoS2 Monolayer to Gas Molecules
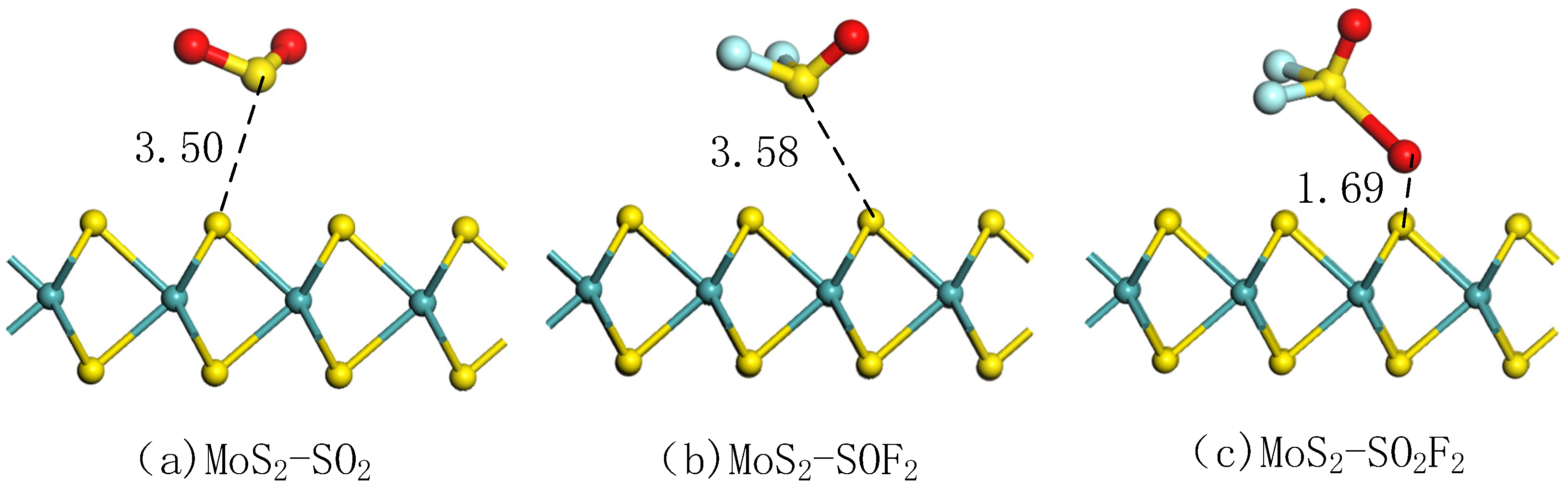
3.2.1. Adsorption Analysis of MoS2 Monolayer to Gas Molecules
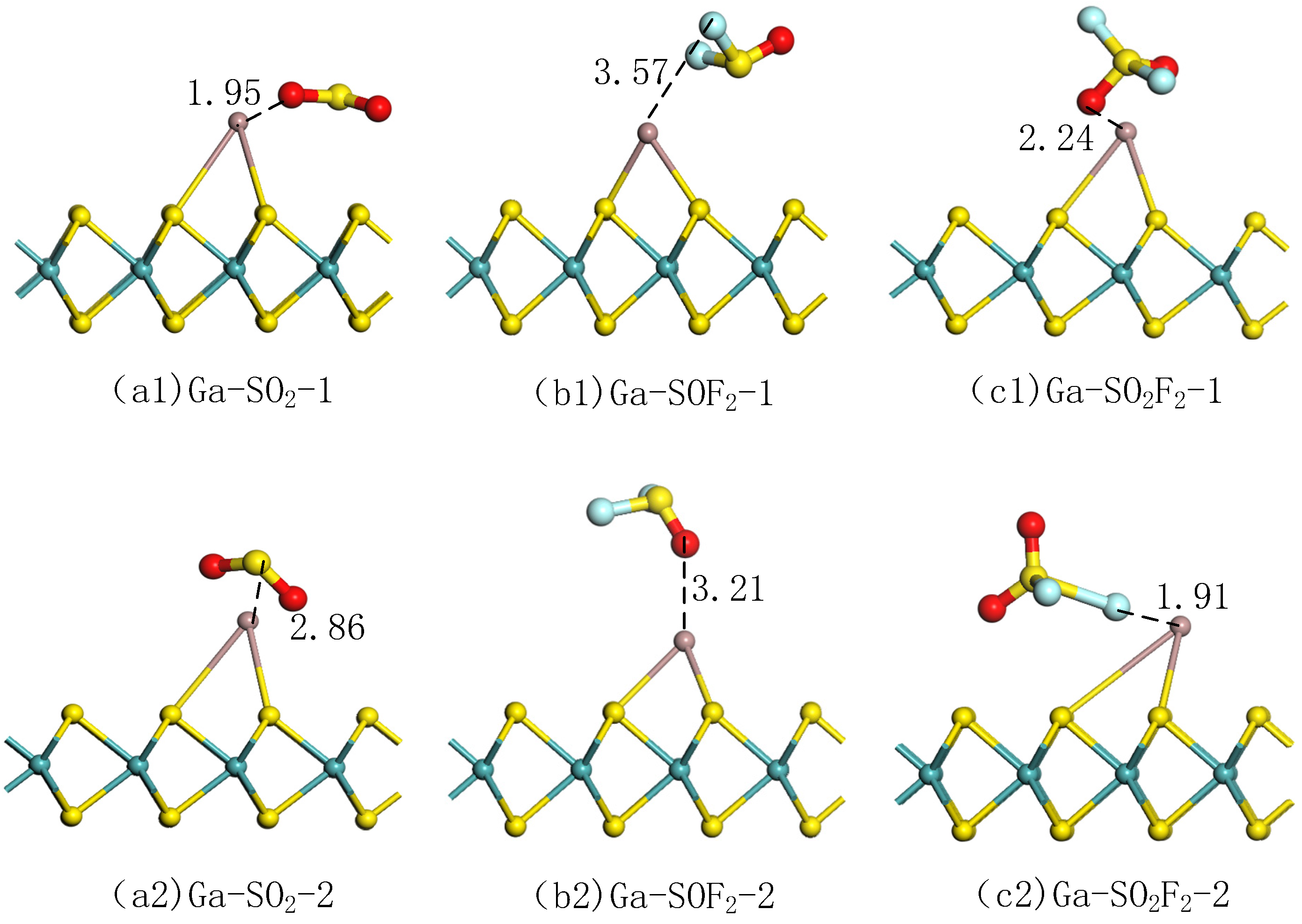
3.2.2. Selection of Adsorption Modes for Gas Molecules in Ga-MoS2 Monolayer
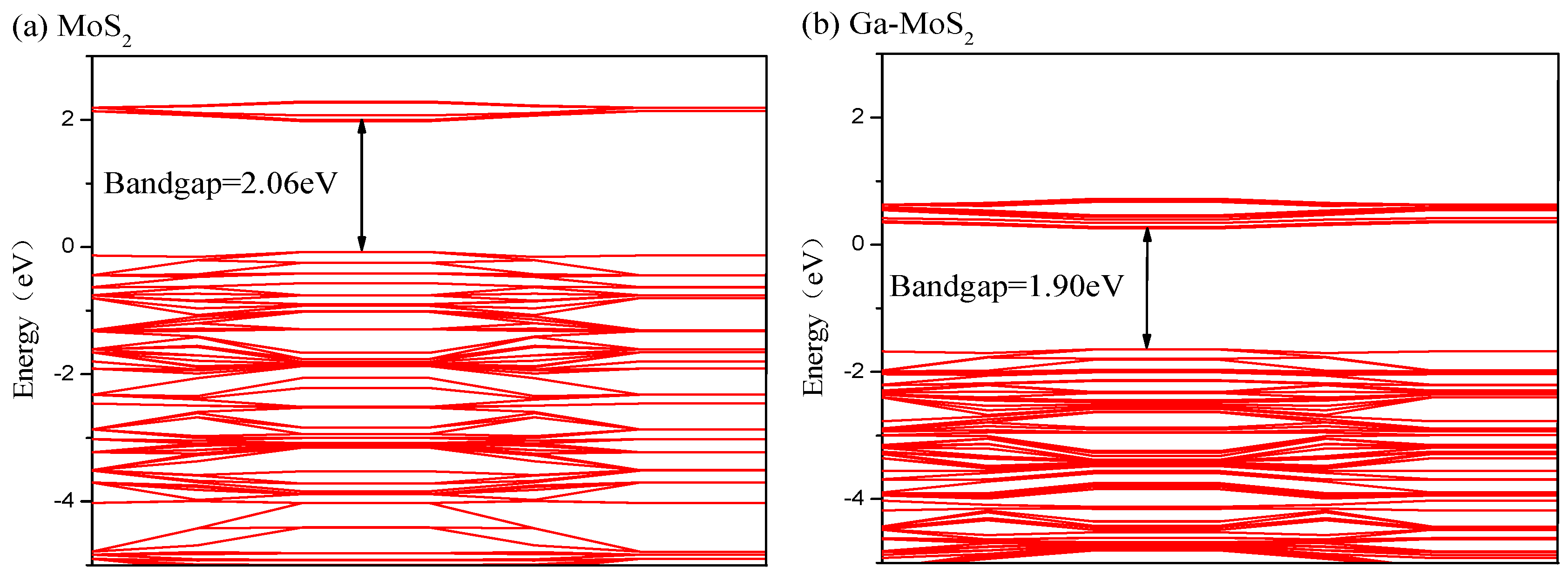
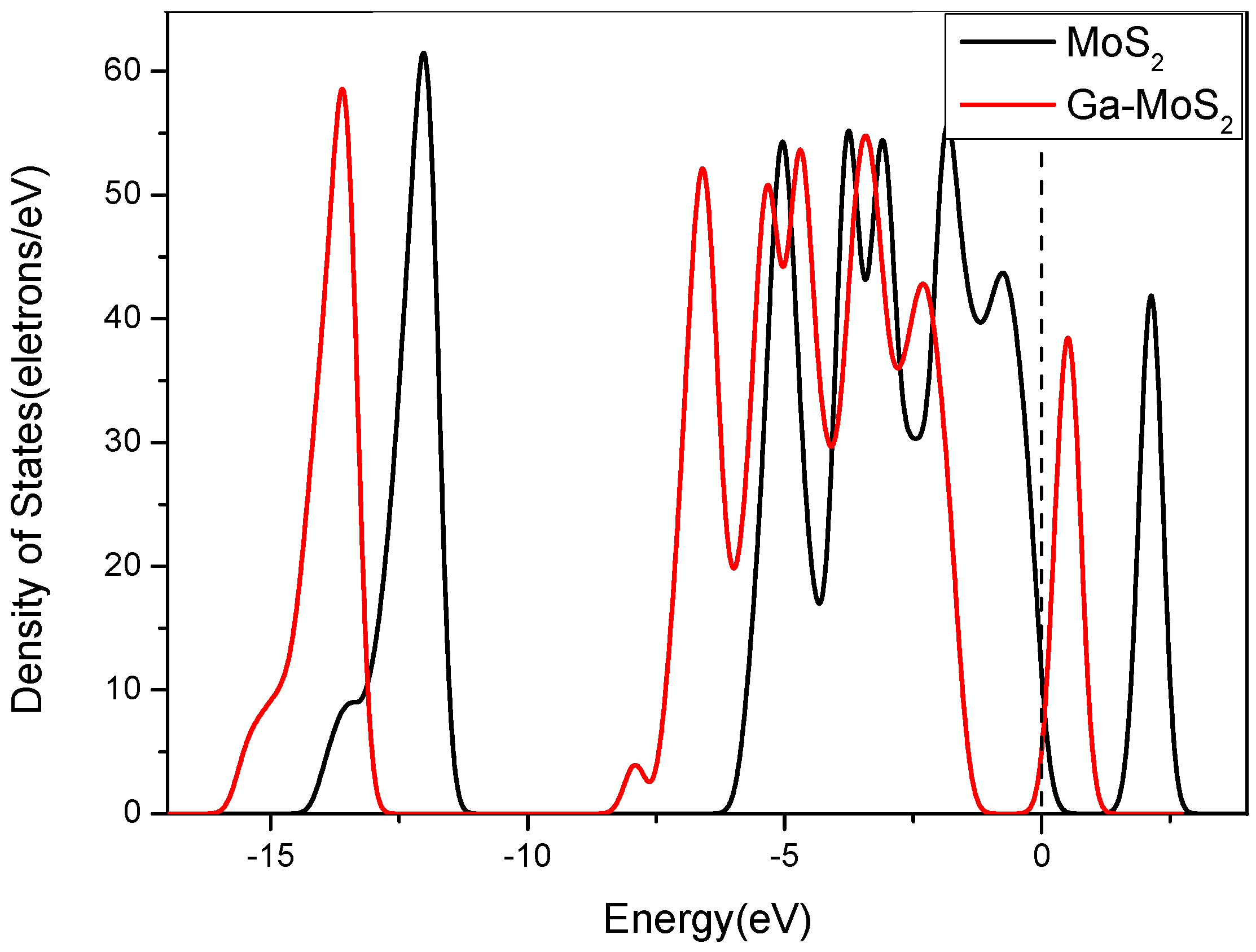
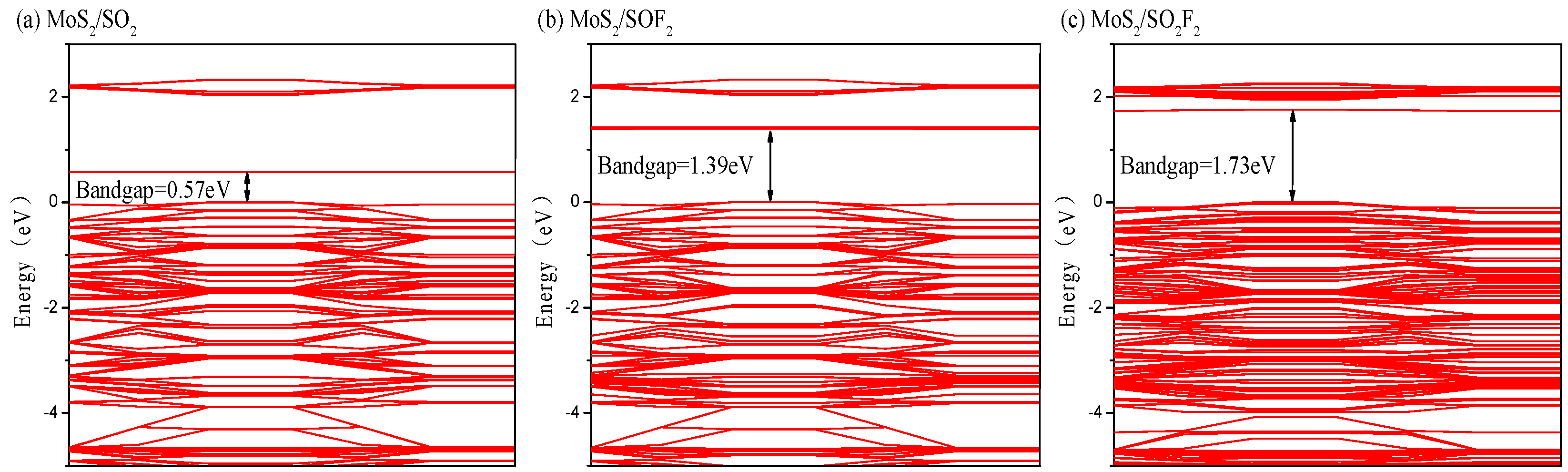
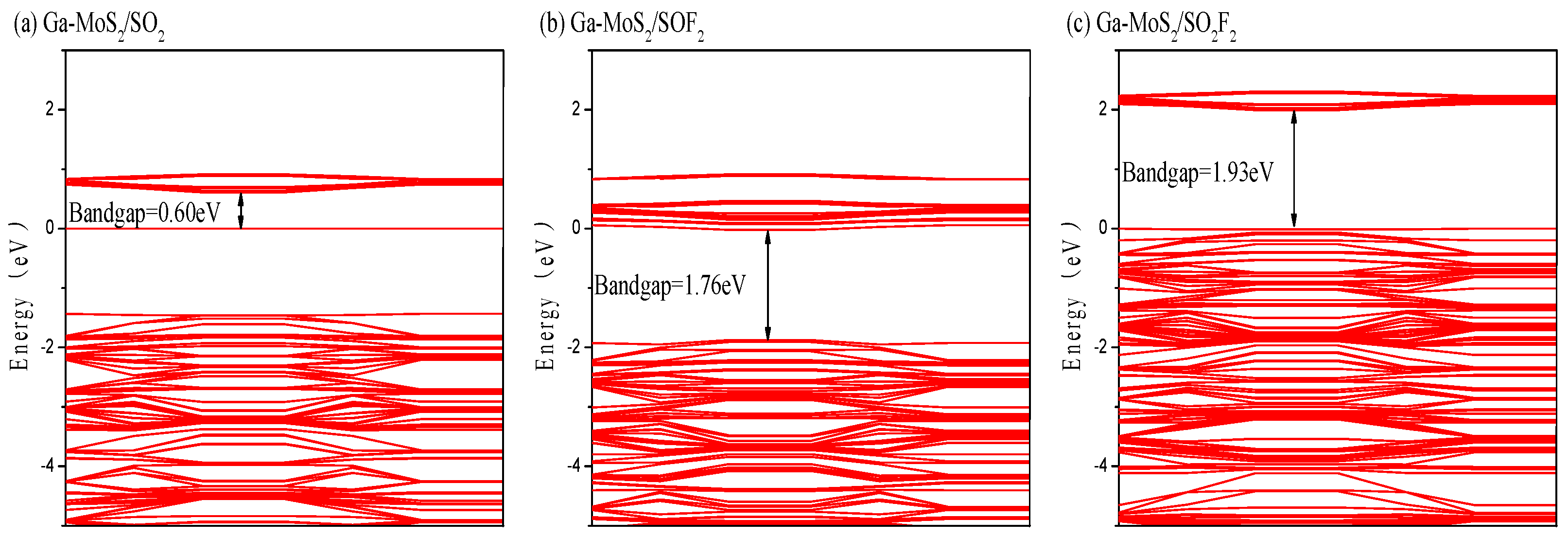
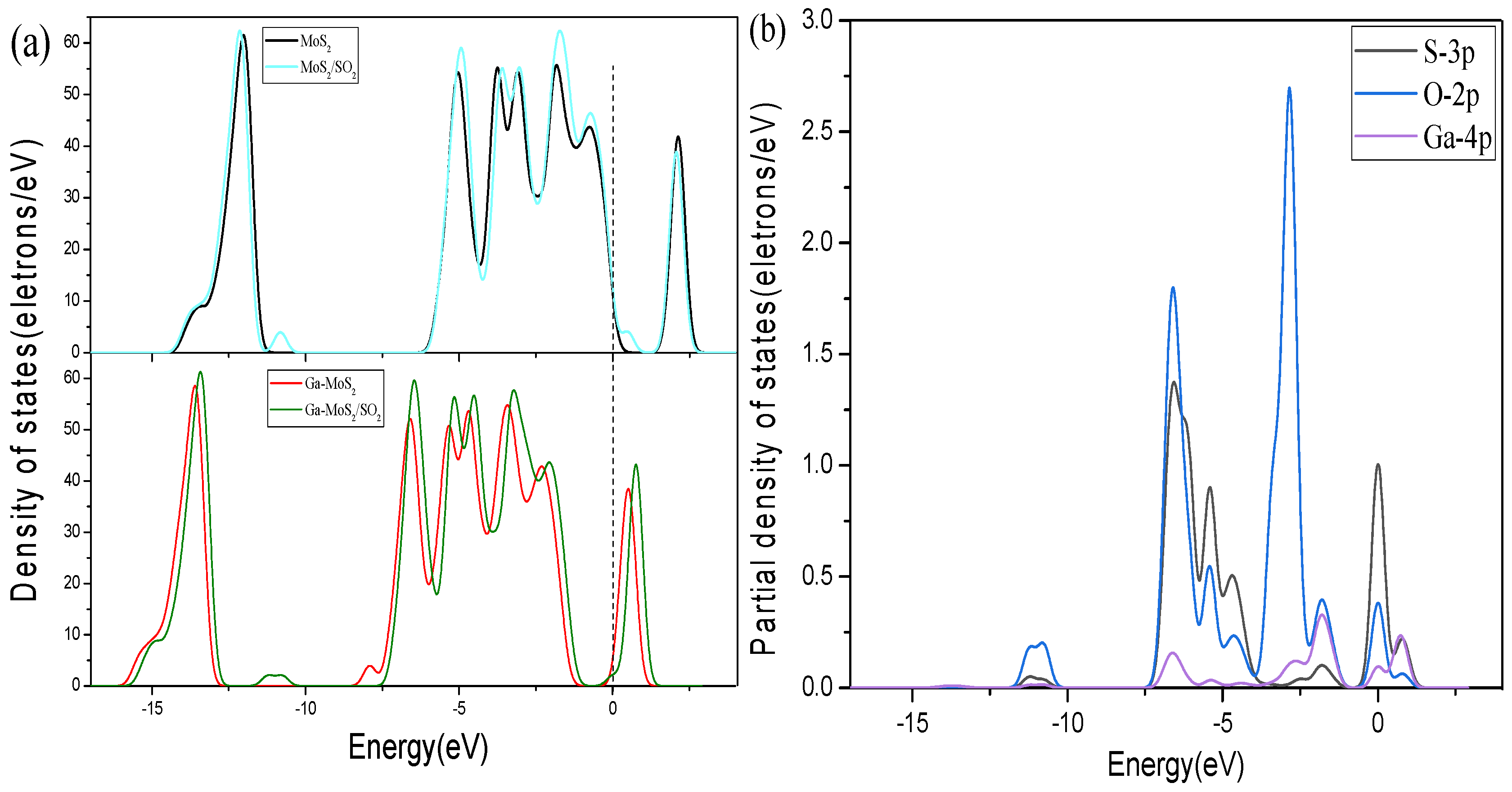
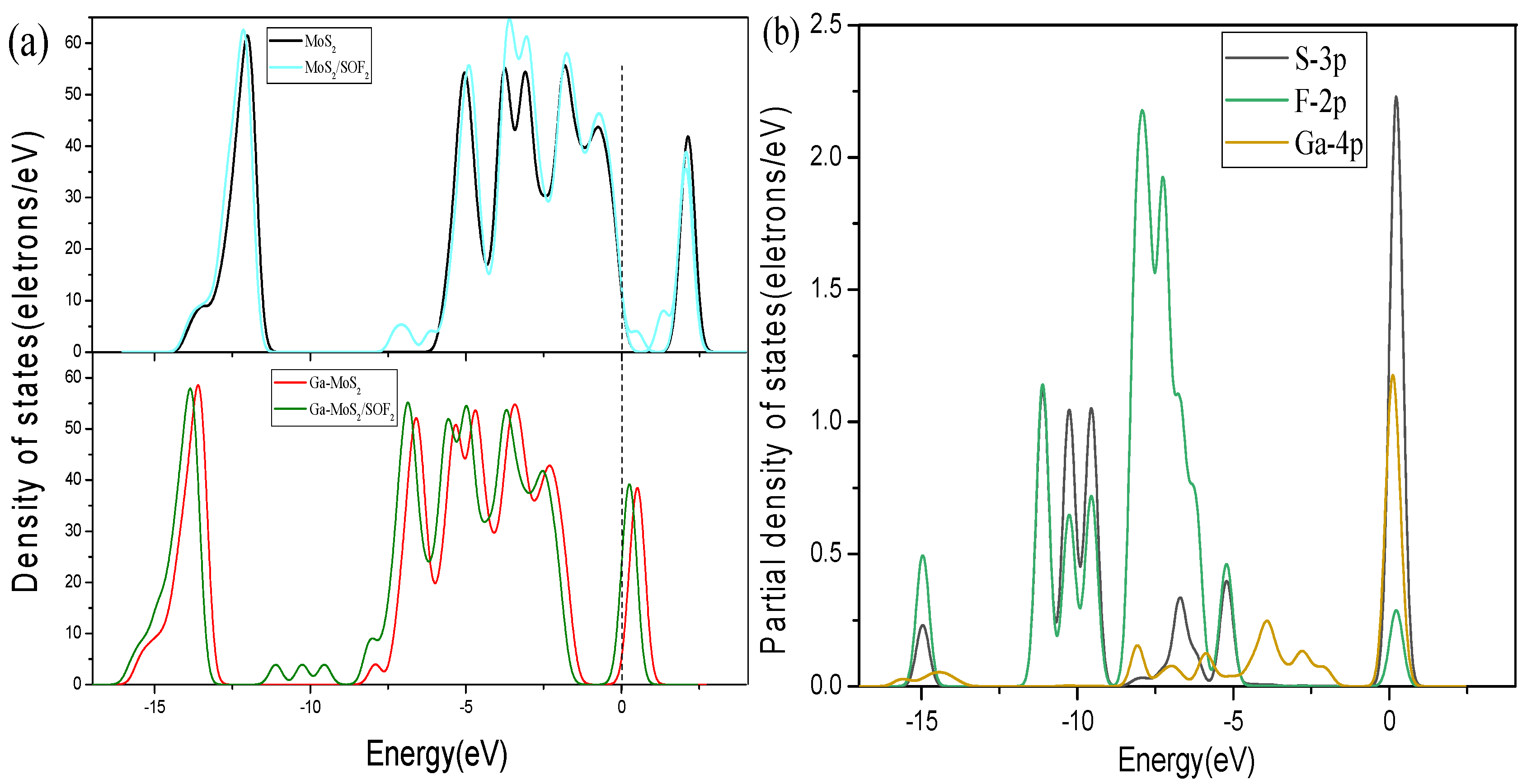
3.2.3. Energy Band and DOS Analysis
4. Conclusions
- Ga dopant was most likely to be adsorbed onto the MoS2 monolayer through TMo site.
- The intrinsic MoS2 had a weak adsorption capacity for the target gases.
- SO2 molecule tends to approach the doped model with S atoms. SOF2 molecule prefers to approach the doped model with O atoms. SO2F2 molecule is likely to approach the doped model with F atoms.
- The conductivity of the material could be improved to varying degrees by adsorbing SO2, SOF2, while adsorption of SO2F2 had little effect on the conductivity of the material. The of SO2 and SO2F2 were both negative, indicating that Ga-MoS2 could adsorb the above two gases more stably. The of SOF2 is positive, which proves that Ga-MoS2 had some difficulty in adsorption of SOF2. The Ga-MoS2 can be used as an excellent gas-sensing material for SO2 and SO2F2 molecules.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, F.P.; Li, H.T.; Zhang, M.X.; Li, C.; Yao, Q.; Tang, J. Establishment of Reax force field of SF6 gas over-thermal decomposition. J. Phys. D Appl. Phys. 2020, 26, 125540. [Google Scholar]
- Zhang, X.X.; Cui, Z.L.; Yi, L.; Li, Y.L.; Xiao, H.Y.; Chan, D.C. Theoretical study of the interaction of SF6 molecule on Ag (1 1 1) surfaces. Appl. Surf. Sci. 2018, 457, 745–751. [Google Scholar] [CrossRef]
- Liu, H.C.; Zhou, Q.; Zhang, Q.Y.; Hong, C.X.; Xu, L.N.; Jin, L.F.; Chen, W.G. Synthesis, Characterization and Enhanced Sensing Properties of a NiO/ZnO p-n Junctions Sensor for the SF6 Decomposition Byproducts SO2, SO2F2, and SOF2. Sensors 2017, 17, 913. [Google Scholar] [CrossRef]
- Wang, J.X.; Zhou, Q.; Zeng, W. Competitive adsorption of SF6 decompositions on Ni-doped ZnO (100) surface: Computational and experimental study. Appl. Surf. Sci. 2019, 479, 185–197. [Google Scholar] [CrossRef]
- Tang, J.; Zeng, F.; Pan, J.; Zhang, X.X.; Yao, Q.; He, J.; Hou, X. Correlation analysis between formation process of SF6 decomposed components and partial discharge qualities. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 864–875. [Google Scholar] [CrossRef]
- Dasgupta, U.; Chatterjee, S.; Pal, A.J. Thin-film formation of 2D MoS2 and its application as a hole-transport layer in planar perovskite solar cells. Sol. Energ. Mat. Sol. C 2017, 172, 353–360. [Google Scholar] [CrossRef]
- Zeng, F.P.; Li, H.T.; Zhang, M.X.; Lei, Z.C.; Li, C.; Tang, J. Isotope Tracing Experimental Study on the Effects of Trace H2O on the Over-Thermal Decomposition of SF6. J. Phys. D Appl. Phys. 2020, 53, 355501. [Google Scholar] [CrossRef]
- Abbasi, A.; Sardroodi, J.J. Adsorption of O3, SO2 and SO3 gas molecules on MoS2 monolayers: A computational investigation. Appl. Surf. Sci. 2019, 469, 781–791. [Google Scholar] [CrossRef]
- Zhang, X.X.; Ren, J.B.; Tang, J.; Sun, C.X. Kernel statistical uncorrelated optimum discriminant vectors algorithm for GIS PD recognition. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 206–213. [Google Scholar] [CrossRef]
- Chen, D.C.; Tang, J.; Zhang, X.X.; Li, Y.; Liu, H.J. Detecting decompositions of sulfur hexafluoride using MoS2 monolayer as gas sensor. IEEE Sens. J. 2019, 19, 39–46. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Jiang, C.X.; Wu, J.F. Layer-by-layer assembled In2O3 nanocubes/flower-like MoS2 nanofilm for room temperature formaldehyde sensing. Sens. Actuators B Chem. 2018, 273, 176–184. [Google Scholar] [CrossRef]
- Fan, C.; Liu, G.Z.; Zhang, Y.H.; Wang, M.J. Synthesis and gas-responsive characteristics to methanol and isopropanol of bean-sprout-like MoS2. Mater. Lett. 2017, 209, 8–10. [Google Scholar] [CrossRef]
- Wu, S.Y.; Zeng, F.P.; Tang, J.; Yao, Q.; Miao, Y. Triangle Fault Diagnosis Method for SF6 Gas Insulated Equipment. IEEE Trans. Power Del. 2019, 34, 1470–1477. [Google Scholar] [CrossRef]
- Zhang, X.X.; Gui, Y.G.; Dong, X.C. Preparation and application of TiO2 nanotube array gas sensor for SF6-insulated equipment detection: A review. Nanoscale Res. Lett. 2016, 11, 302. [Google Scholar] [CrossRef]
- Cho, B.; Yoon, J.; Lim, S.K.; Kim, A.R.; Kim, D.H.; Park, S.G.; Kwon, J.D.; Lee, Y.J.; Lee, K.H.; Lee, B.H. Chemical sensing of 2D graphene/MoS2 heterostructure device. ACS Appl. Mater. Inter. 2015, 7, 16775–16780. [Google Scholar] [CrossRef]
- Zeng, F.P.; Lei, Z.C.; Yang, X.; Tang, J.; Yao, Q.; Miao, Y.L. Evaluating the DC Partial Discharge Based on SF6 Decomposition Characteristics. IEEE Trans. Power Del. 2019, 34, 1383–1392. [Google Scholar] [CrossRef]
- Wu, P.; Yin, N.Q.; Li, P.; Cheng, W.J.; Huang, M. The adsorption and diffusion behavior of noble metal adatoms (Pd, Pt, Cu, Ag and Au) on a MoS2 monolayer: A first-principles study. Phys. Chem. Chem. Phys. 2017, 19, 20713–20722. [Google Scholar] [CrossRef]
- Wang, J.X.; Zhou, Q.; Lu, Z.R.; Wei, Z.J.; Zeng, W. Gas sensing performances and mechanism at atomic level of Au-MoS2 microspheres. Appl. Surf. Sci. 2019, 490, 124–136. [Google Scholar] [CrossRef]
- Abbas, H.G.; Debela, T.T.; Hussain, S.; Hussain, I. Inorganic molecule (O2, NO) adsorption on nitrogen- and phosphorus-doped MoS2 monolayer using first principle calculations. RSC Adv. 2018, 8, 38656–38666. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Q.; Wang, J.X.; Xu, L.N.; Zeng, W. Adsorption of SO2 molecule on Ni-doped and Pd-doped graphene based on first-principle study. Appl. Surf. Sci. 2020, 517, 146180. [Google Scholar] [CrossRef]
- Li, T.; Gui, Y.; Zhao, W.; Tang, C.; Dong, X. Palladium modified MoS2 monolayer for adsorption and scavenging of SF6 decomposition products: A DFT study. Phys. E 2020, 123, 114178. [Google Scholar] [CrossRef]
- Azofra, L.M.; Sun, C.H.; Cavallo, L.; MacFarlane, D.R. Feasibility of N2 binding and reduction to ammonia on Fe-deposited MoS2 2D sheets: A DFT study. Chem. Eur. J. 2017, 23, 8275–8279. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zeng, W.; Chen, W.G.; Xu, L.N.; Kumar, R.; Umar, A. High sensitive and low-concentration sulfur dioxide (SO2) gas sensor application of heterostructure NiO-ZnO nanodisks. Sens. Act. B Chem. 2019, 298, 126870. [Google Scholar] [CrossRef]
- Wang, J.X.; Zhou, Q.; Wei, Z.J.; Xu, L.N.; Zeng, W. Experimental and theoretical studies of Zn-doped MoO3 hierarchical microflower with excellent sensing performances to carbon monoxide. Ceram. Inter. 2020, 46, 29222–29232. [Google Scholar] [CrossRef]
- Wang, M.; Wang, W.; Ji, M.; Cheng, X. Adsorption of phenol and hydrazine upon pristine and X-decorated (X=Sc, Ti, Cr and Mn) MoS2 monolayer. Appl. Surf. Sci. 2018, 439, 350–363. [Google Scholar] [CrossRef]
- Chen, D.C.; Zhang, X.X.; Tang, J.; Cui, H.; Li, Y. Noble metal (Pt or Au)-doped monolayer MoS2 as a promising adsorbent and gas-sensing material to SO2, SOF2 and SO2F2: A DFT study. Appl. Phys. A Mater. Sci. Process. 2018, 124, 194. [Google Scholar] [CrossRef]
- Gui, X.X.; Zhou, Q.; Peng, S.D.; Xu, L.N.; Zeng, W. Dissolved gas analysis in transformer oil using Sb-doped graphene: A DFT study. Appl. Surf. Sci. 2020, 533, 147509. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Lai, X.; Liu, B.; Chen, Y.; Wang, F.; Wang, R.; Zhang, L. Direct observation of the hysteretic Fermi level modulation in monolayer MoS2 field effect transistors. Curr. Appl. Physics. 2020, 20, 293–303. [Google Scholar] [CrossRef]
- Gui, Y.G.; Wang, Y.; Duan, S.K.; Tang, C.; Zhou, Q.; Xu, L.N.; Zhang, X.X. Ab Initio Study of SOF2 and SO2F2 Adsorption on Co-MoS2. ACS Omega 2019, 4, 2517–2522. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, L.N.; Umar, A.; Chen, W.G.; Kumar, R. Pt nanoparticles decorated SnO2 nanoneedles for efficient CO gas sensing applications. Sens. Act. B Chem. 2018, 256, 656–664. [Google Scholar] [CrossRef]
- Jiang, C.X.; Zhang, D.Z.; Yin, N.L.; Yao, Y.; Shaymurat, T.; Zhou, X.Y. Acetylene Gas-Sensing Properties of Layer-by-Layer Self-Assembled Ag-Decorated Tin Dioxide/Graphene Nanocomposite Film. Nanomaterials 2017, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhou, Q.; Wang, J.X.; Xu, L.N.; Zeng, W. Enhanced ethanol sensing and mechanism of Cr-doped ZnO nanorods: Experimental and computational study. Ceram. Int. 2017, 43, 14873–14879. [Google Scholar]
- Zhou, Q.; Chen, W.G.; Xu, L.N.; Kumar, R.; Gui, Y.G.; Zhao, Z.Y.; Tang, C.; Zhu, S.P. Highly sensitive carbon monoxide (CO) gas sensors based on Ni and Zn doped SnO2 nanomaterials. Ceram. Int. 2018, 44, 4392–4399. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.X.; Zhang, G.Z.; Tang, J. Pd-doped MoS2 monolayer: A promising candidate for DGA in transformer oil based on DFT method. Appl. Surf. Sci. 2019, 470, 1035–1042. [Google Scholar] [CrossRef]
- Gui, X.X.; Zhou, Q.; Peng, S.D.; Xu, L.N.; Zeng, W. Adsorption behavior of Rh-doped MoS2 monolayer towards SO2, SOF2, SO2F2 based on DFT study. Phys. E 2020, 122, 114224. [Google Scholar] [CrossRef]
- Wang, J.X.; Zhou, Q.; Lu, Z.R.; Wei, Z.J.; Zeng, W. Adsorption of H2O molecule on TM (Au, Ag) doped-MoS2 monolayer: A firstprinciples study. Phys. E 2019, 113, 72–78. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zeng, W.; Li, Y.Q. Hydrothermal synthesis and controlled growth of hierarchical 3D flowerlike MoS2 nanospheres assisted with CTAB and their NO2 gas sensing. Appl. Surf. Sci. 2018, 455, 276–282. [Google Scholar] [CrossRef]
- Cui, H.; Jia, P.F. Doping effect of small Rhn (n = 1–4) clusters on the geometric and electronic behaviors of MoS2 monolayer: A firstprinciples study. Appl. Surf. Sci. 2020, 526, 146659. [Google Scholar] [CrossRef]
- Zhou, Q.; Hong, C.X.; Yao, Y.; Hussain, S.; Xu, L.N.; Zhang, Q.Y.; Gui, Y.G.; Wang, M.S. Hierarchically MoS2 nanospheres assembled from nanosheets for superior CO gas-sensing properties. Mater. Res. Bull. 2018, 30, 132–139. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zeng, W.; Li, Y.Q. The hydrothermal synthesis of 3D hierarchical porous MoS2 microspheres assembled by nanosheets with excellent gas sensing properties. J. Alloys Compd. 2018, 307, 355–362. [Google Scholar] [CrossRef]
- Wei, Z.J.; Zhou, Q.; Zeng, W. Hierarchical WO3-NiO microflower for high sensitivity detection of SF6 decomposition byproduct H2S. Nanotechnology. 2020, 31, 215701. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.G.; Liu, D.K.; Li, X.D.; Tang, C.; Zhou, Q. DFT-based study on H2S and SOF2 adsorption on Si-MoS2 monolayer. Results Phys. 2019, 13, 102225. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, W.G.; Li, J.; Peng, S.D.; Lu, Z.R.; Yang, Z.; Xu, L.N. Highly sensitive hydrogen sulfide sensor based on titanium dioxide nanomaterials. J. Nanoelectron. Optoelectron. 2018, 2417, 1784–1788. [Google Scholar] [CrossRef]
- Zhao, B.; Li, C.Y.; Liu, L.L.; Zhou, B.; Zhang, Q.K.; Chen, Z.Q.; Tang, Z. Adsorption of gas molecules on Cu impurities embedded monolayer MoS2: A first-principles study. Appl. Surf. Sci. 2016, 158, 280–287. [Google Scholar] [CrossRef]
- Wang, J.X.; Zhou, Q.; Xu, L.N.; Gao, X.; Zeng, W. Gas sensing mechanism of dissolved gases in transformer oil on Ag-MoS2 monolayer. Phys. E 2019, 118, 113947. [Google Scholar] [CrossRef]
- Sahoo, M.P.K.; Wang, J.; Zhang, Y.J.; Shimada, T.; Kitamura, T. Modulation of gas adsorption and magnetic properties of monolayer-MoS2 by antisite defect and strain. J. Phys. Chem. C. 2016, 120, 14113–14121. [Google Scholar] [CrossRef]
- Qian, H.; Lu, W.H.; Wei, X.X.; Chen, W.; Deng, J. H2S and SO2 adsorption on Pt-MoS2 adsorbent for partial discharge elimination: A DFT study. Results Phys. 2019, 12, 107–112. [Google Scholar] [CrossRef]
- Park, J.; Mun, J.H.; Shin, J.S.; Kang, S.W. Highly sensitive two-dimensional MoS2 gas sensor decorated with Pt nanoparticles. R. Soc. Open Sci. 2018, 12, 181462. [Google Scholar] [CrossRef]
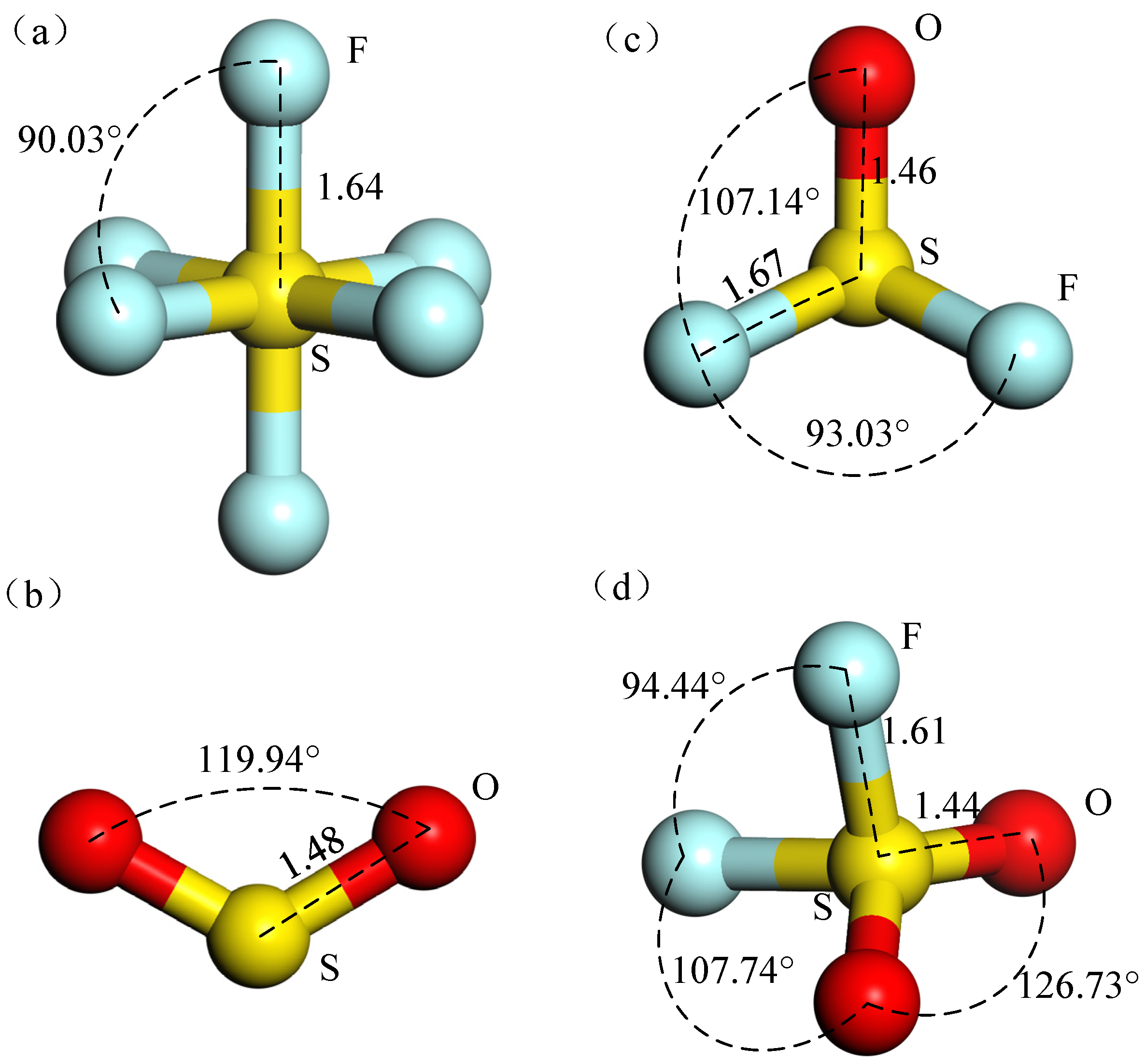

| Gas Molecule | Bond Length (Å) | Bond Angle (°) | ||
|---|---|---|---|---|
| SF6 | S-F | 1.64 | F-S-F | 90.3 |
| SO2 | S-O | 1.48 | O-S-O | 119.94 |
| SOF2 | S-F | 1.67 | O-S-F | 107.14 |
| S-O | 1.46 | F-S-F | 93.039 | |
| SO2F2 | S-F | 1.61 | O-S-O | 126.73 |
| S-O | 1.44 | O-S-F | 107.74 | |
| - | F-S-F | 94.44 | ||
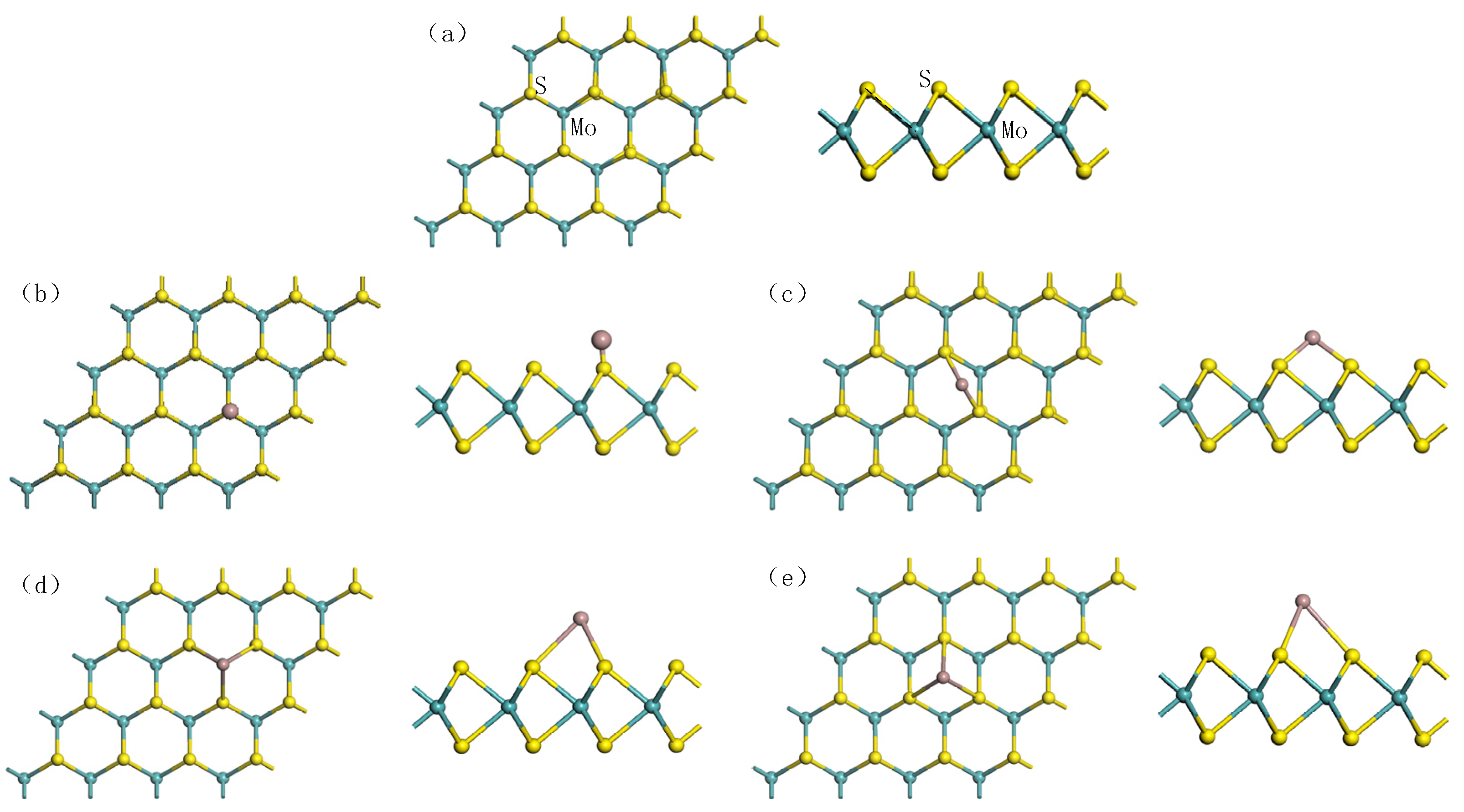
| Doping Sites | TS | BS-S | TMo | TH |
|---|---|---|---|---|
| (eV) | −1.70 | −1.74 | −1.75 | −1.60 |
| Gas Molecules | D (Å) | Structure | |||
|---|---|---|---|---|---|
| SO2 | 3.50 | 22.34 | −0.04 | ∠ O-S-O | 112.64 |
| SOF2 | 3.58 | 22.41 | −0.04 | ∠ O-S-F ∠ F-S-F | 104.10 95.73 |
| SO2F2 | 1.69 | 25.03 | −0.68 | ∠ O-S-O ∠ O-S-F ∠ F-S-F | 100.17 95.38 94.08 |
| Parameters | SO2 | SOF2 | SO2F2 | |||
|---|---|---|---|---|---|---|
| Approach | Mode 1 | Mode 2 | Mode 1 | Mode 2 | Mode 1 | Mode 2 |
| D (Å) | Ga-O:1.95 | Ga-S:2.86 | Ga-F:3.57 | Ga-O:3.21 | Ga-O:2.24 | Ga-F:1.91 |
| (eV) | −0.61 | −0.67 | 0.36 | 0.27 | −0.15 | −0.63 |
| (e) | −0.40 | −0.42 | −0.01 | 0.01 | −0.59 | −0.60 |
| Gas Molecule | D (Å) | Gas Structure | dGa-S (Å) | ∠ S-Ga-S (°) | |||
|---|---|---|---|---|---|---|---|
| SO2 | 2.30 | −0.67 | −0.42 | ∠ O-S-O | 105.53 | 3.32 | 52.80 |
| SOF2 | 3.21 | 0.27 | 0.01 | ∠ O-S-F | 106.76 | 2.66 | 71.19 |
| ∠ F-S-F | 93.543 | ||||||
| SO2F2 | 1.91 | −0.63 | −0.60 | ∠ O-S-O | 121.60 | 2.94 | 48.62 |
| ∠ O-S-F | 105.25 | ||||||
| ∠ F-S-F | 88.32 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, W.; Mi, H.; Peng, R.; Peng, S.; Zeng, W.; Zhou, Q. First-Principle Insight into Ga-Doped MoS2 for Sensing SO2, SOF2 and SO2F2. Nanomaterials 2021, 11, 314. https://doi.org/10.3390/nano11020314
Hou W, Mi H, Peng R, Peng S, Zeng W, Zhou Q. First-Principle Insight into Ga-Doped MoS2 for Sensing SO2, SOF2 and SO2F2. Nanomaterials. 2021; 11(2):314. https://doi.org/10.3390/nano11020314
Chicago/Turabian StyleHou, Wenjun, Hongwan Mi, Ruochen Peng, Shudi Peng, Wen Zeng, and Qu Zhou. 2021. "First-Principle Insight into Ga-Doped MoS2 for Sensing SO2, SOF2 and SO2F2" Nanomaterials 11, no. 2: 314. https://doi.org/10.3390/nano11020314
APA StyleHou, W., Mi, H., Peng, R., Peng, S., Zeng, W., & Zhou, Q. (2021). First-Principle Insight into Ga-Doped MoS2 for Sensing SO2, SOF2 and SO2F2. Nanomaterials, 11(2), 314. https://doi.org/10.3390/nano11020314








