Controllable Fabrication of SiC@C-Fe3O4 Hybrids and Their Excellent Electromagnetic Absorption Properties
Abstract
:1. Introduction
2. Experimental Section
2.1. Pristine Materials and Fabrication of SiC@C Nanowires
2.2. Fabrication of SiC@C-Fe3O4 Hybrids
2.3. Characterization and Measurement
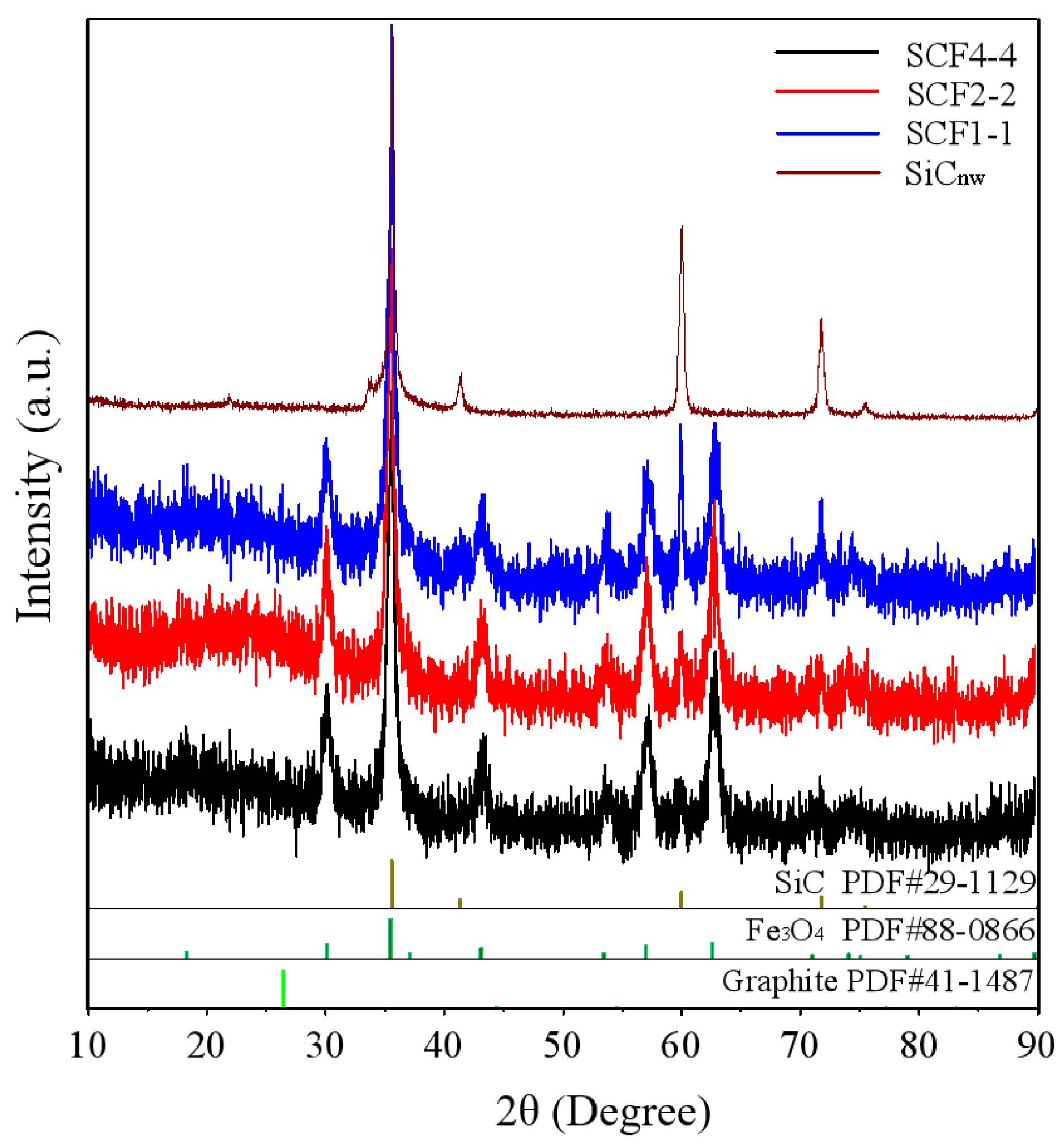
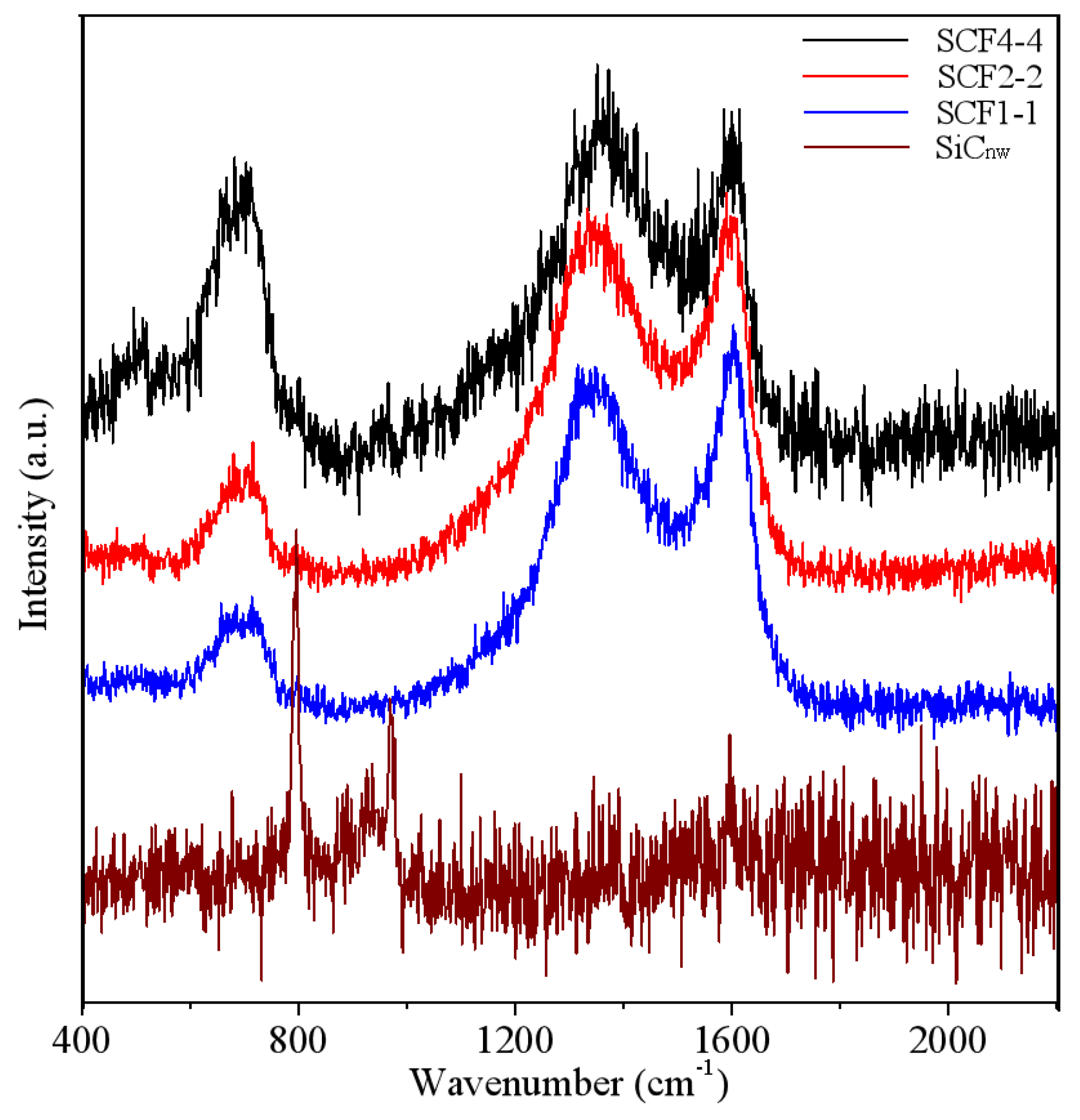
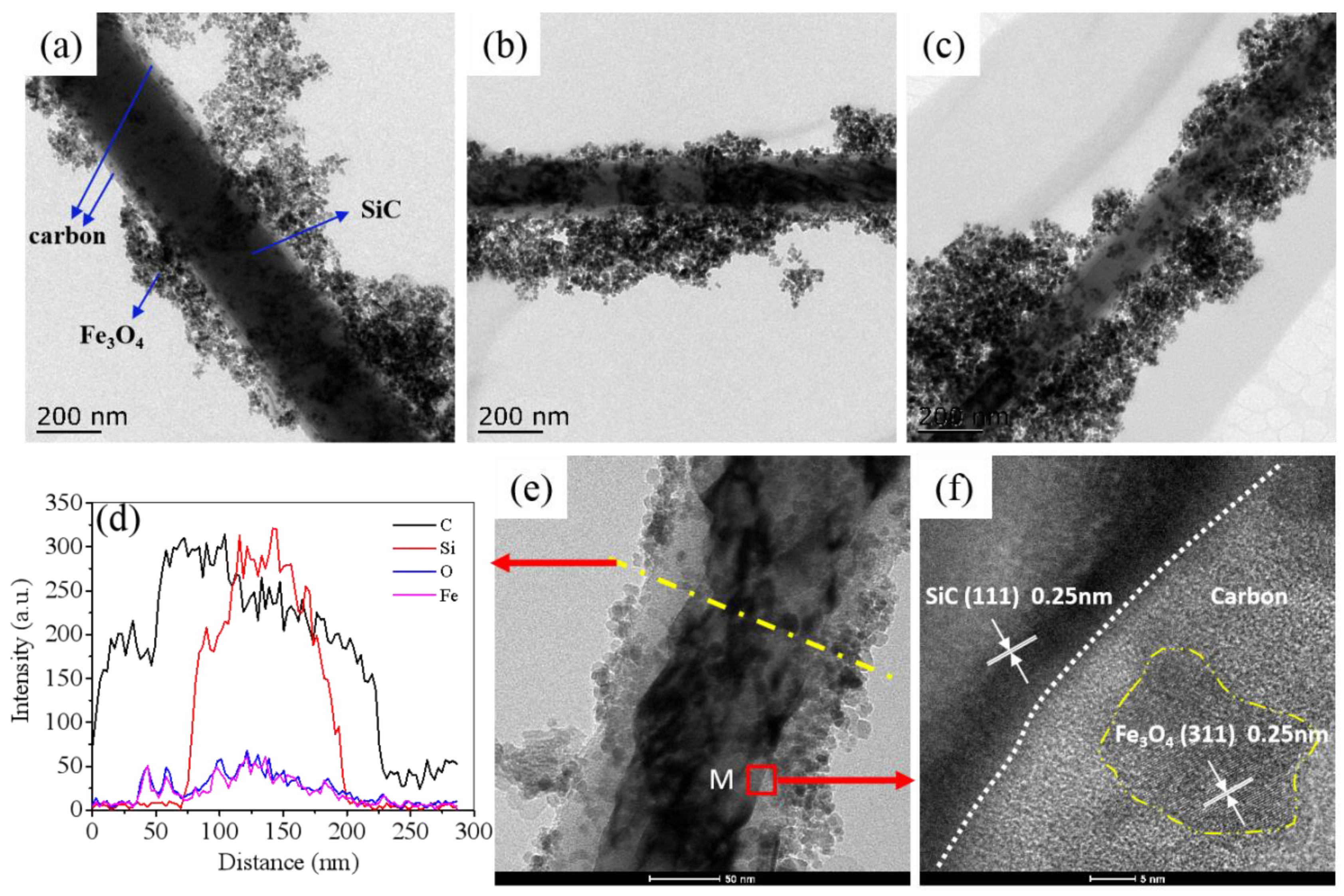
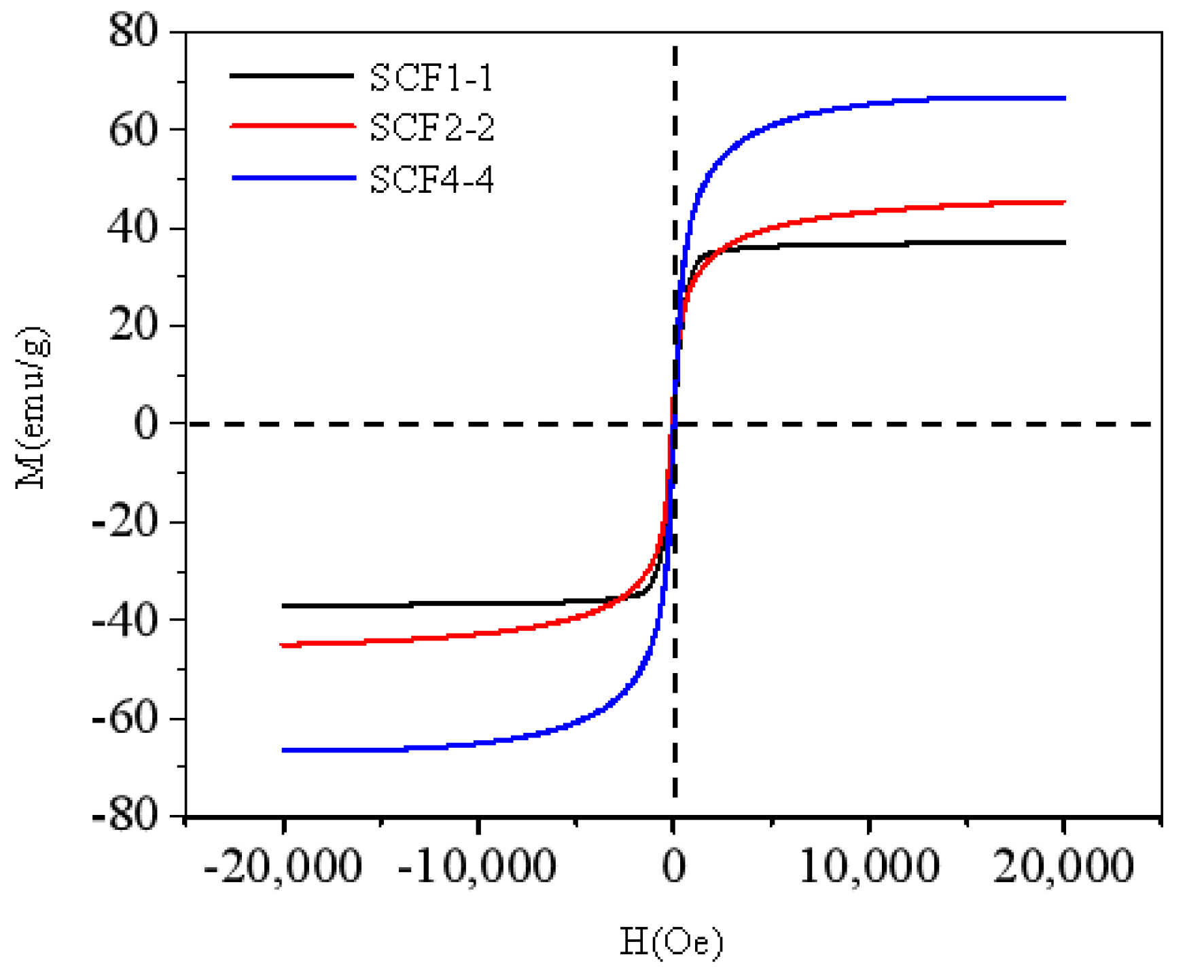
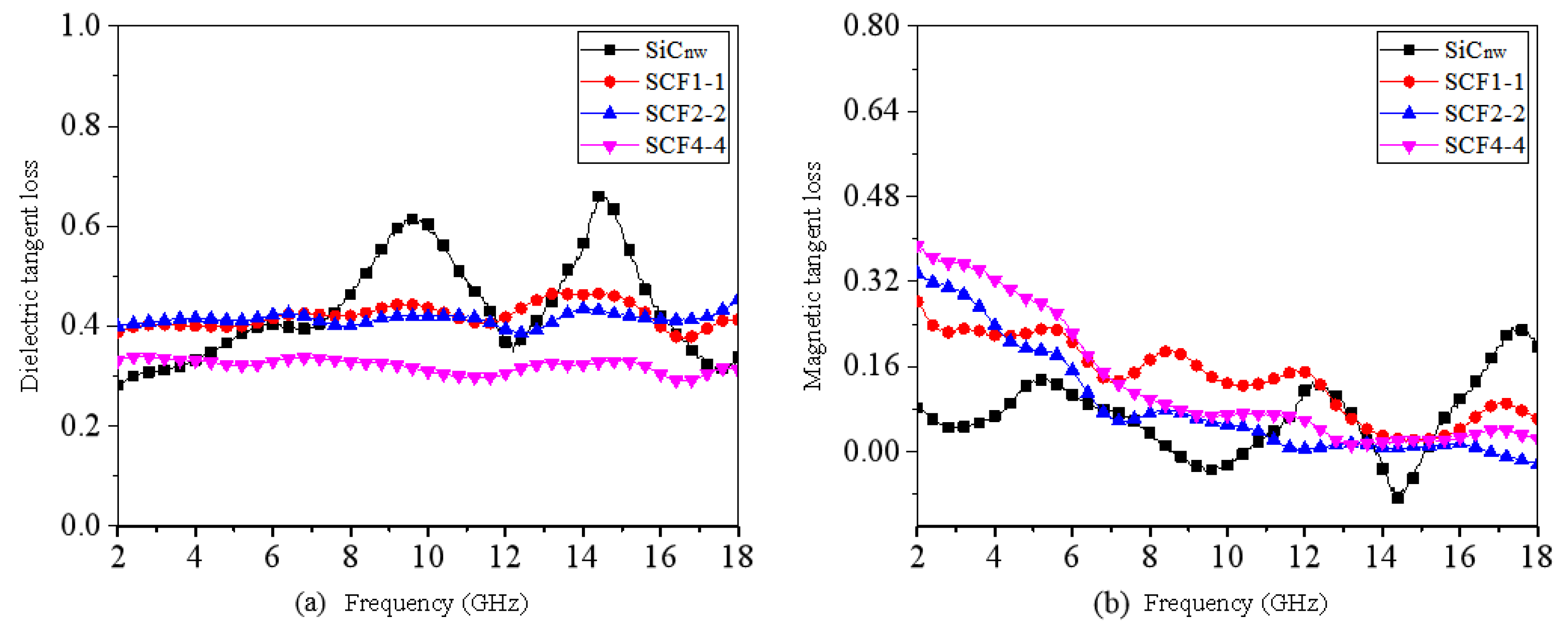
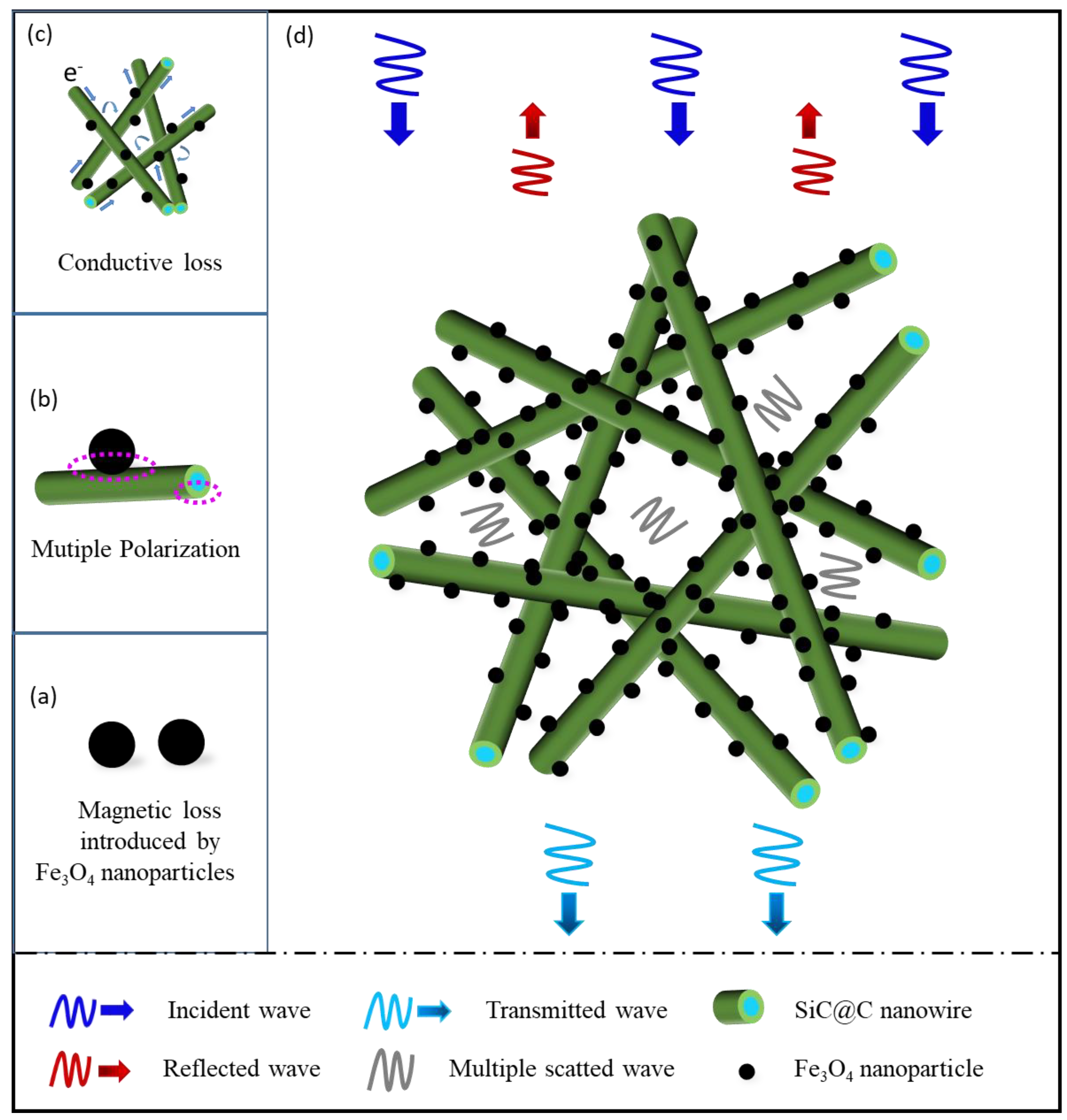
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yusoff, A.N.; Abdullah, M.H.; Ahmad, S.H.; Jusoh, S.F.; Mansor, A.A.; Hamid, S.A.A. Electromagnetic and absorption properties of some microwave absorbers. J. Appl. Phys. 2002, 92, 876–882. [Google Scholar] [CrossRef]
- Jiang, J.; Li, D.; Geng, D.; An, J.; He, J.; Liu, W.; Zhang, Z. Microwave absorption properties of core double-shell FeCo/C/BaTiO3 nanocomposites. Nanoscale 2014, 6, 3967–3971. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, L.; Zhao, R.; Ren, J.; Lu, G.; Wang, Y. Electromagnetic and microwave-absorbing properties of magnetic nickel ferrite nanocrystals. Nanoscale 2011, 3, 2862–2864. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Luo, R.; Cui, G.; Wang, L. Effect of SiC nanowires on the high-temperature microwave absorption properties of SiCf/SiC composites. J. Eur. Ceram. Soc. 2019, 39, 1743–1756. [Google Scholar] [CrossRef]
- Wu, R.; Zhou, K.; Yue, C.Y.; Wei, J.; Pan, Y. Recent progress in synthesis, properties and potential applications of SiC nanomaterials. Prog. Mater. Sci. 2015, 72, 1–60. [Google Scholar] [CrossRef]
- Chiu, S.-C.; Yu, H.-C.; Li, Y.-Y. High Electromagnetic Wave Absorption Performance of Silicon Carbide Nanowires in the Gigahertz Range. J. Phys. Chem. C 2010, 114, 1947–1952. [Google Scholar] [CrossRef]
- Yang, H.-J.; Yuan, J.; Li, Y.; Hou, Z.-L.; Jin, H.-B.; Fang, X.-Y.; Cao, M.-S. Silicon carbide powders: Temperature-dependent dielectric properties and enhanced microwave absorption at gigahertz range. Solid State Commun. 2013, 163, 1–6. [Google Scholar] [CrossRef]
- Liu, Z.; Kirihara, S.; Miyamoto, Y.; Zhang, D. Microwave Absorption in Photonic Crystals Composed of SiC/Resin with a Diamond Structure. J. Am. Ceram. Soc. 2006, 89, 2492–2495. [Google Scholar] [CrossRef]
- Kuang, J.; Cao, W. Stacking faults induced high dielectric permittivity of SiC wires. Appl. Phys. Lett. 2013, 103, 112906. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Xu, Y.; Zhou, J.; Jiao, J.; Chen, Y.; Wang, H.; Liu, C.; Jiang, Z.; Wang, Z. Stacking fault and unoccupied densities of state dependence of electromagnetic wave absorption in SiC nanowires. J. Mater. Chem. C 2015, 3, 4416–4423. [Google Scholar] [CrossRef]
- Kuang, J.; Xiao, T.; Hou, X.; Zheng, Q.; Wang, Q.; Jiang, P.; Cao, W. Microwave synthesis of worm-like SiC nanowires for thin electromagnetic wave absorbing materials. Ceram. Int. 2019, 45, 11660–11667. [Google Scholar] [CrossRef]
- Liu, C.; Yu, D.; Kirk, D.W.; Xu, Y. Porous silicon carbide derived from apple fruit with high electromagnetic absorption performance. J. Mater. Chem. C 2016, 4, 5349–5356. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.-L.; Bai, Y.-J.; Liu, R.; Lun, N.; Qi, Y.-X.; Han, F.-D.; Bi, J.-Q. In situ synthesis of one-dimensional MWCNT/SiC porous nanocomposites with excellent microwave absorption properties. J. Mater. Chem. 2011, 21, 13581–13587. [Google Scholar] [CrossRef]
- Han, M.; Yin, X.; Hou, Z.; Song, C.; Li, X.; Zhang, L.; Cheng, L. Flexible and Thermostable Graphene/SiC Nanowire Foam Composites with Tunable Electromagnetic Wave Absorption Properties. ACS Appl. Mater. Interfaces 2017, 9, 11803–11810. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, M.; Jiang, W.; Wang, Y.; Wang, D.; Wu, F.; Xie, A.; Dong, W. Core-shell polypyrrole@silicon carbide nanowire (PPy@SiC) nanocomposite for the broadband elimination of electromagnetic pollution. RSC Adv. 2016, 6, 43056–43059. [Google Scholar] [CrossRef]
- Su, X.; Jia, Y.; Wang, J.; Xu, J.; He, X.; Fu, C.; Liu, S. Combustion synthesis and microwave absorption property of SiC(Fe) solid solution powder under different reaction time. J. Mater. Sci. Mater. Electron. 2013, 24, 1905–1912. [Google Scholar] [CrossRef]
- Li, D.; Jin, H.-B.; Cao, M.-S.; Chen, T.; Dou, Y.-K.; Wen, B.; Agathopoulos, S. Production of Ni-Doped SiC Nanopowders and their Dielectric Properties. J. Am. Ceram. Soc. 2011, 94, 1523–1527. [Google Scholar] [CrossRef]
- Wang, H.; Wu, L.; Jiao, J.; Zhou, J.; Xu, Y.; Zhang, H.; Jiang, Z.; Shen, B.; Wang, Z. Covalent interaction enhanced electromagnetic wave absorption in SiC/Co hybrid nanowires. J. Mater. Chem. A 2015, 3, 6517–6525. [Google Scholar] [CrossRef]
- Jin, H.; Cao, M.; Zhou, W.; Agathopoulos, S. Microwave synthesis of Al-doped SiC powders and study of their dielectric properties. Mater. Res. Bull. 2010, 45, 247–250. [Google Scholar] [CrossRef]
- Singh, S.; Sinha, A.; Zunke, R.H.; Kumar, A.; Singh, D. Double layer microwave absorber based on Cu dispersed SiC composites. Adv. Powder Technol. 2018, 29, 2019–2026. [Google Scholar] [CrossRef]
- Liang, C.; Liu, C.; Wang, H.; Wu, L.; Jiang, Z.; Xu, Y.; Shen, B.; Wang, Z. SiC-Fe3O4 dielectric-magnetic hybrid nanowires: Controllable fabrication, characterization and electromagnetic wave absorption. J. Mater. Chem. A 2014, 2, 16397–16402. [Google Scholar] [CrossRef]
- Yang, H.-J.; Cao, W.-Q.; Zhang, D.-Q.; Su, T.-J.; Shi, H.-L.; Wang, W.-Z.; Yuan, J.; Cao, M.-S. NiO Hierarchical Nanorings on SiC: Enhancing Relaxation to Tune Microwave Absorption at Elevated Temperature. ACS Appl. Mater. Interfaces 2015, 7, 7073–7077. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Lv, X.; Xie, A.; Jiang, W.; Wu, F. Growing 3D ZnO nano-crystals on 1D SiC nanowires: Enhancement of dielectric properties and excellent electromagnetic absorption performance. J. Mater. Chem. C 2016, 4, 8897–8902. [Google Scholar] [CrossRef]
- Hou, Y.; Cheng, L.; Zhang, Y.; Yang, Y.; Deng, C.; Yang, Z.; Chen, Q.; Du, X.; Zhao, C.; Zheng, L. Enhanced Flexibility and Microwave Absorption Properties of HfC/SiC Nanofiber Mats. ACS Appl. Mater. Interfaces 2018, 10, 29876–29883. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Guo, X.N.; Jin, G.Q.; Guo, X.Y. Carbon coated Co-SiC nanocomposite with high-performance microwave absorption. Phys. Chem. Chem. Phys. 2013, 15, 16104–16110. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, J.; Liu, M.; Jiang, P.; Li, B.; Hou, X. Microwave absorption properties of SiC@SiO2@Fe3O4 hybrids in the 2–18 GHz range. Int. J. Miner. Metall. Mater. 2017, 24, 804–813. [Google Scholar] [CrossRef]
- Duan, L.-Q.; Xu, C.; Dai, X.-Q.; Xiong, Z.-M.; Zhang, B.; Zhang, Z.-W.; Cui, C.-A.; Xie, A.-M.; Wu, F. Nano-porous carbon wrapped SiC nanowires with tunable dielectric properties for electromagnetic applications. Mater. Des. 2020, 192, 108738. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kang, G.-H.; Kim, S.-H.; Park, S. Enhancement in Electromagnetic Wave Shielding Effectiveness through the Formation of Carbon Nanofiber Hybrids on Carbon-Based Nonwoven Fabrics. Nanomaterials 2021, 11, 2910. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Tselev, A.; Wang, J.; Yuan, D.; Chu, H.; McNicholas, T.P.; Li, Y.; Liu, J. Selective Growth of Well-Aligned Semiconducting Single-Walled Carbon Nanotubes. Nano Lett. 2009, 9, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Presser, V.; Heon, M.; Gogotsi, Y. Carbide-Derived Carbons-From Porous Networks to Nanotubes and Graphene Raman spectroscopy studies of carbide derived carbons. Adv. Funct. Mater. 2011, 21, 810–833. [Google Scholar] [CrossRef]
- Wesełucha-Birczyńska, A.; Kołodziej, A.; Świętek, M.; Skalniak, Ł.; Długoń, E.; Pajda, M.; Błażewicz, M. Early Recognition of the PCL/Fibrous Carbon Nanocomposites Interaction with Osteoblast-like Cells by Raman Spectroscopy. Nanomaterials 2021, 11, 2890. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-M.; Cao, M.-S.; Chen, Y.-H.; Cao, W.-Q.; Liu, J.; Shi, H.-L.; Zhang, D.-Q.; Wang, W.-Z.; Yuan, J. Multiscale Assembly of Grape-Like Ferroferric Oxide and Carbon Nanotubes: A Smart Absorber Prototype Varying Temperature to Tune Intensities. ACS Appl. Mater. Interfaces 2015, 7, 19408–19415. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Huang, Z.-H.; Lu, M.-M.; Cao, W.-Q.; Yuan, J.; Zhang, D.-Q.; Cao, M.-S. 3D Fe3O4 nanocrystals decorating carbon nanotubes to tune electromagnetic properties and enhance microwave absorption capacity. J. Mater. Chem. A 2015, 3, 12621–12625. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.L. Anomalous Increase in Carbon Capacitance at Pore Sizes Less Than 1 Nanometer. Science 2006, 313, 1760–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Yang, W.; Han, G.; Yu, Y.; Ma, S.; Liu, W.; Zhang, Z. Three-dimensional foam-like Fe3O4@C core-shell nanocomposites: Controllable synthesis and wideband electromagnetic wave absorption properties. J. Magn. Magn. Mater. 2020, 502, 166518. [Google Scholar] [CrossRef]
- Yang, L.; Lv, H.; Li, M.; Zhang, Y.; Liu, J.; Yang, Z. Multiple polarization effect of shell evolution on hierarchical hollow C@MnO2 composites and their wideband electromagnetic wave absorption properties. Chem. Eng. J. 2020, 392, 123666. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, L.; Dai, X.; Wu, F.; Xie, A.; Wu, J.-A.; Sun, M.; Xia, Y. Controllable Fabrication of SiC@C-Fe3O4 Hybrids and Their Excellent Electromagnetic Absorption Properties. Nanomaterials 2021, 11, 3438. https://doi.org/10.3390/nano11123438
Duan L, Dai X, Wu F, Xie A, Wu J-A, Sun M, Xia Y. Controllable Fabrication of SiC@C-Fe3O4 Hybrids and Their Excellent Electromagnetic Absorption Properties. Nanomaterials. 2021; 11(12):3438. https://doi.org/10.3390/nano11123438
Chicago/Turabian StyleDuan, Liqun, Xiaoqing Dai, Fan Wu, Aming Xie, Jian-An Wu, Minqian Sun, and Yilu Xia. 2021. "Controllable Fabrication of SiC@C-Fe3O4 Hybrids and Their Excellent Electromagnetic Absorption Properties" Nanomaterials 11, no. 12: 3438. https://doi.org/10.3390/nano11123438
APA StyleDuan, L., Dai, X., Wu, F., Xie, A., Wu, J.-A., Sun, M., & Xia, Y. (2021). Controllable Fabrication of SiC@C-Fe3O4 Hybrids and Their Excellent Electromagnetic Absorption Properties. Nanomaterials, 11(12), 3438. https://doi.org/10.3390/nano11123438





