Polymer Micro and Nanoparticles Containing B(III) Compounds as Emissive Soft Materials for Cargo Encapsulation and Temperature-Dependent Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Methods
2.3. Synthesis of Polymer Particles Doped with the B(III) Compounds
2.4. Preparation of Coumarin-6 Loaded Particles
3. Results
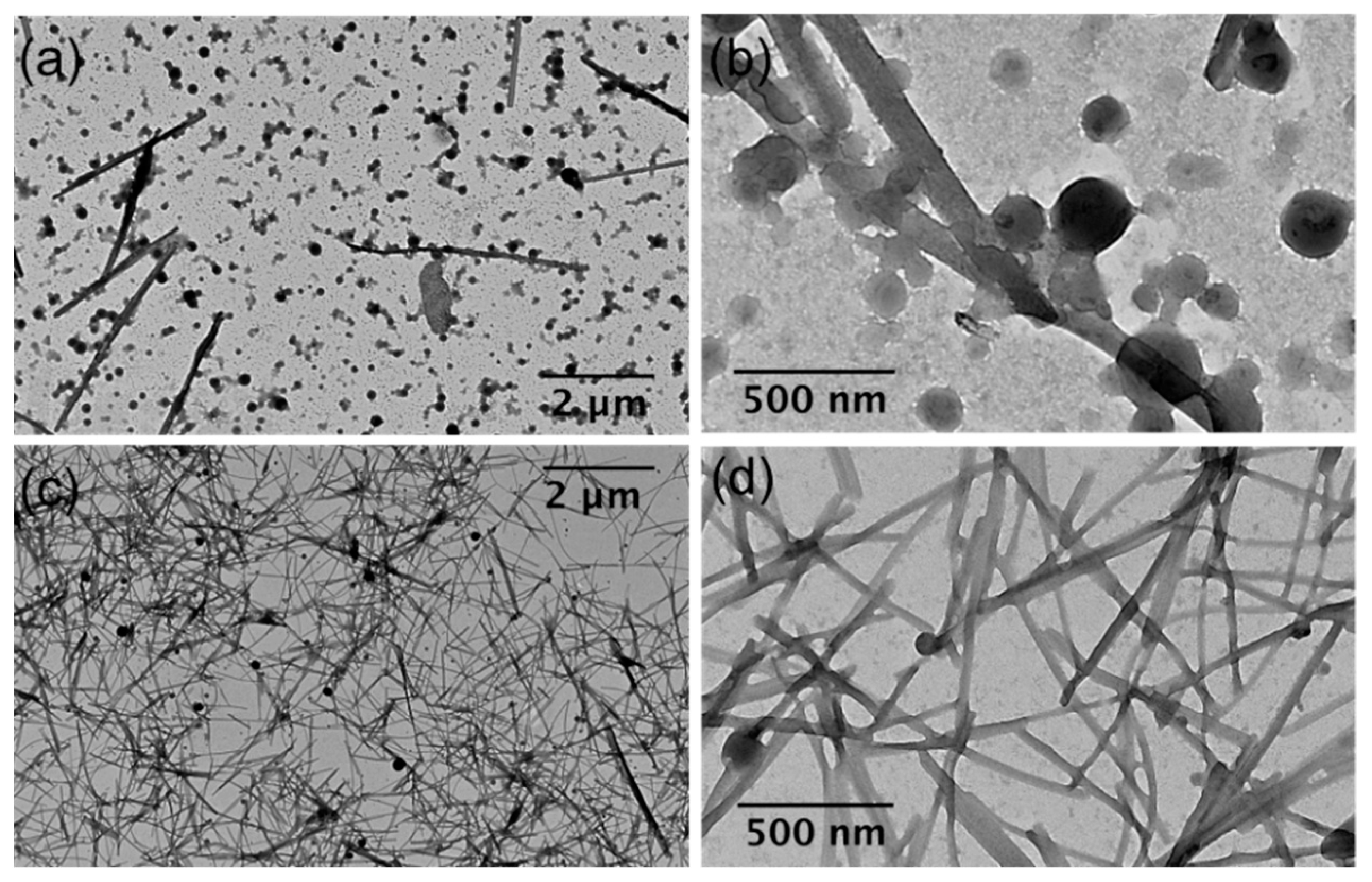
3.1. Preparation and Characterization of Micro- and Nanoparticles
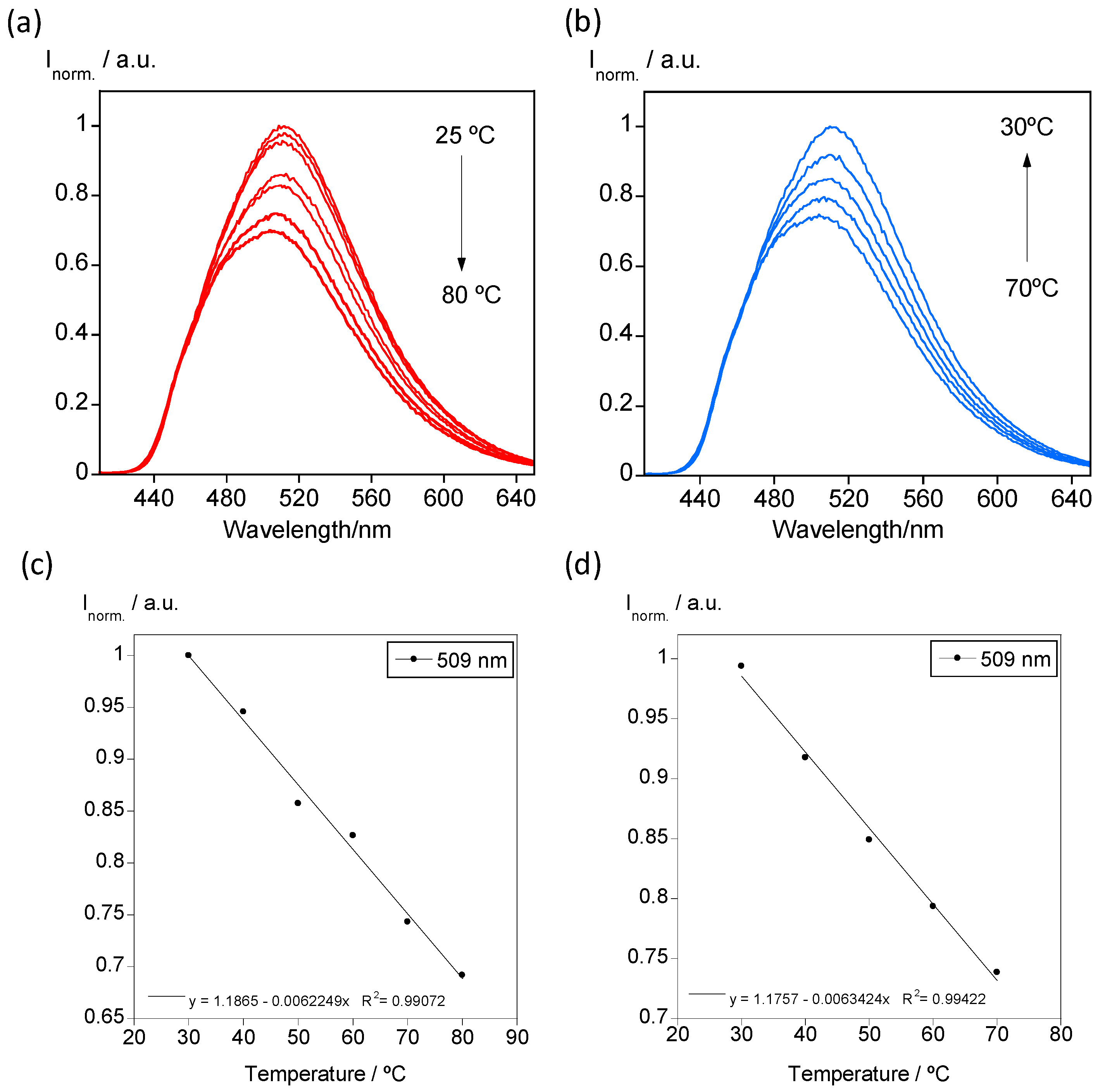
3.2. Temperature-Dependent Emission Properties
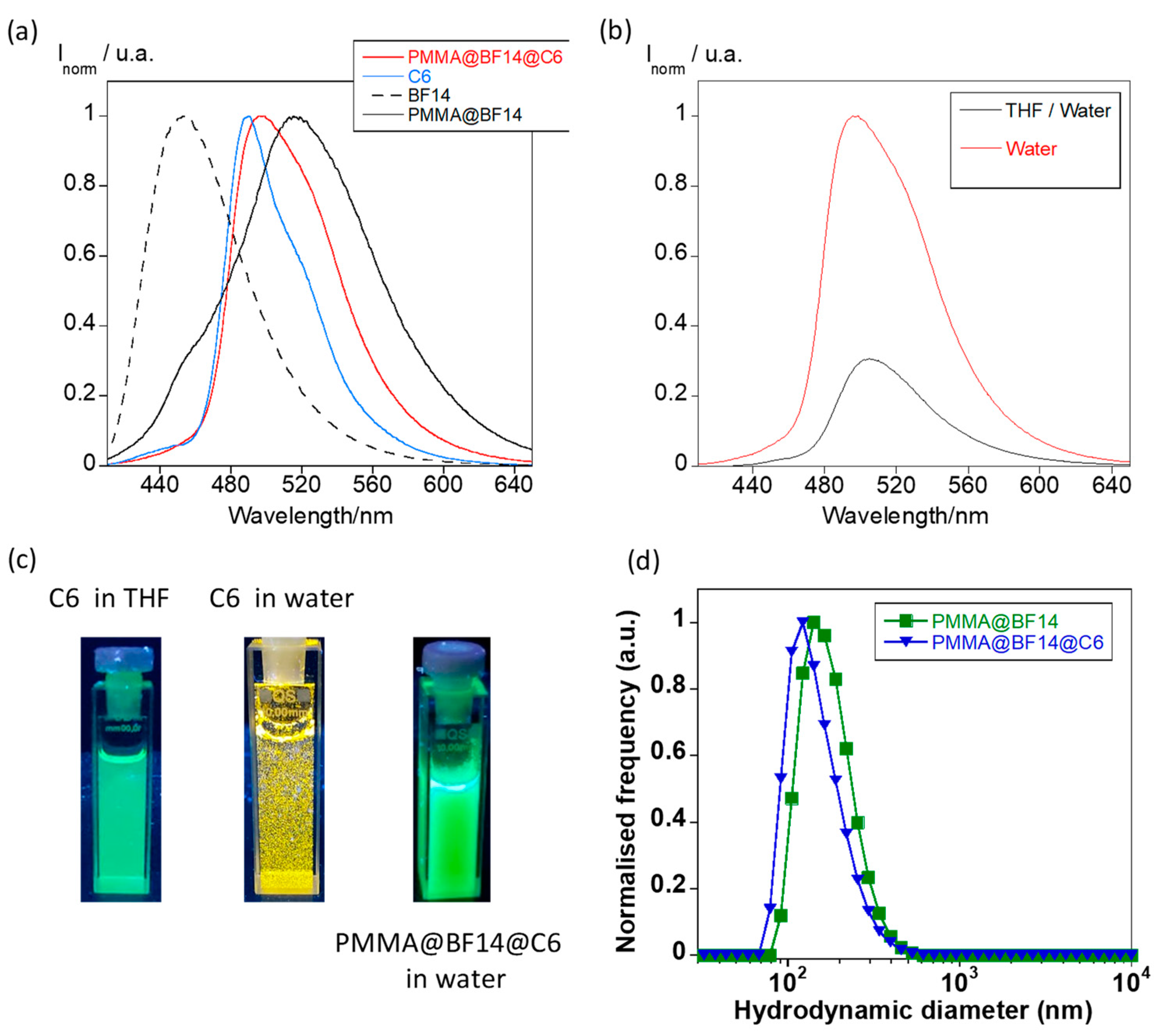
3.3. Hydrophobic Drug Entrapment into B(III)-Doped Microparticles
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, W.; Zhang, W.; Yu, X.; Zhang, G.; Su, Z. Synthesis and biomedical applications of fluorescent nanogels. Polym. Chem. 2016, 7, 5749–5762. [Google Scholar] [CrossRef]
- Montalti, M.; Prodi, L.; Rampazzo, E.; Zaccheroni, N. Dye-doped silica nanoparticles as luminescent organized systems for nanomedicine. Chem. Soc. Rev. 2014, 43, 4243–4268. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zou, X.; Su, Q.; Yuan, W.; Cao, C.; Wang, Q.; Zhu, X.; Feng, W.; Li, F. Ratiometric nanothermometer in vivo based on triplet sensitized upconversion. Nat. Commun. 2018, 9, 2698. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Li, Y. Fluorescent hybrid silica nanoparticles and their biomedical applications. WIREs Nanomed. Nanobiol. 2020, 12, e1603. [Google Scholar] [CrossRef]
- Sitia, L.; Ferrari, R.; Violatto, M.B.; Talamini, L.; Dragoni, L.; Colombo, C.; Colombo, L.; Lupi, M.; Ubezio, P.; D’Incalci, M.; et al. Fate of PLA and PCL-based polymeric nanocarriers in cellular and animal models of triplenegative breast cancer. Biomacromolecules 2016, 17, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bai, Y.; Zheng, N.; Liu, Y.; Vincil, G.A.; Pedretti, B.J.; Cheng, J.; Zimmerman, S.C. Crosslinked dendronized polyols as a general approach to brighter and more stable fluorophores. Chem. Commun. 2016, 52, 3781–3784. [Google Scholar] [CrossRef] [PubMed]
- Reisch, A.; Klymchenko, A.S. Fluorescent polymer nanoparticles based on dyes: Seeking brighter tools for bioimaging. Small 2016, 12, 1968–1992. [Google Scholar] [CrossRef] [Green Version]
- Trofymchuk, K.; Valanciunaite, J.; Andreiuk, B.; Reisch, A.; Collot, M.; Klymchenko, A.S. BODIPY-loaded polymer nanoparticles: Chemical structure of cargo defines leakage from nanocarrier in living cells. J. Mat. Chem. B 2019, 7, 5199–5210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visaveliya, N.; Hoffmann, C.; Groß, A.; Täuscher, E.; Ritter, U.; Koehler, J.M. Micro-flow assisted synthesis of fluorescent polymer nanoparticles with tuned size and surface properties. Nanotechnol. Rev. 2016, 5, 259–272. [Google Scholar] [CrossRef]
- Gubala, V.; Giovannini, G.; Kunc, F.; Monopoli, M.P.; Moore, C.J. Dye-doped silica nanoparticles: Synthesis, surface chemistry and bioapplications. Cancer Nanotechnol. 2020, 11, 1. [Google Scholar] [CrossRef]
- Desbiens, J.; Bergeron, B.; Patry, M.; Ritcey, A.M. Polystyrene nanoparticles doped with a luminescent europium complex. J. Colloid Interface Sci. 2012, 376, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Amela-Cortes, M.; Molard, Y.; Paofai, S.; Desert, A.; Duvail, J.-L.; Naumov, N.G.; Cordier, S. Versatility of the ionic assembling method to design highly luminescent PMMA nanocomposites containing [M6Qi8La6]n-octahedral nano-building blocks. Dalton Trans. 2016, 45, 237–245. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, K.; Liu, M.; Zhang, X.; Tao, L.; Chen, Y.; Wei, Y. Polymeric AIE-based nanoprobes for biomedical applications: Recent advances and perspectives. Nanoscale 2015, 7, 11486–11508. [Google Scholar] [CrossRef]
- Algar, W.R.; Massey, M.; Rees, K.; Higgins, R.; Krause, K.D.; Darwish, G.H.; Peveler, W.J.; Xiao, Z.; Tsai, H.-Y.; Gupta, R.; et al. Photoluminescent nanoparticles for chemical and biological analysis and imaging. Chem. Rev. 2021, 121, 9243–9358. [Google Scholar] [CrossRef]
- Moradi Kashkooli, F.; Soltani, M.; Souri, M.; Meaney, C.; Kohandel, M. Nexus between in silico and in vivo models to enhance clinical translation of nanomedicine. Nano Today 2021, 36, 101057. [Google Scholar] [CrossRef]
- Moradi Kashkooli, F.; Soltani, M.; Souri, M. Controlled anti-cancer drug release through advanced nano-drug delivery systems: Static and dynamic targeting strategies. J. Control Release 2020, 327, 316–349. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, H.; Han, R.; Huang, L.; Shi, H.; Sha, Y.; Jiang, Y. Extremely high brightness from polymer-encapsulated quantum dots for two-photon cellular and deep-tissue imaging. Sci. Rep. 2015, 5, 9908. [Google Scholar] [CrossRef] [PubMed]
- Alves Rico, S.R.; Abbasi, A.Z.; Ribeiro, G.; Ahmed, T.; Wu, X.Y.; De Oliveira Silva, D. Diruthenium(II,III) metallodrugs of ibuprofen and naproxen encapsulated in intravenously injectable polymer-lipid nanoparticles exhibit enhanced activity against breast and prostate cancer cells. Nanoscale 2017, 9, 10701–10714. [Google Scholar] [CrossRef] [PubMed]
- Kerr, C.; DeRosa, C.A.; Daly, M.L.; Zhang, H.; Palmer, G.M.; Fraser, C.L. Luminescent difluoroboron β-diketonate pla–peg nanoparticle. Biomacromolecules 2017, 18, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Yip, Y.J.; Lee, S.S.C.; Neo, M.L.; Teo, S.L.-M.; Valiyaveettil, S. A comparative investigation of toxicity of three polymer nanoparticles on acorn barnacle (Amphibalanus amphitrite). Sci. Total Environ. 2021, 806, 150965. [Google Scholar] [CrossRef]
- Sánchez, I.; Núñez, C.; Campo, J.A.; Torres, M.R.; Cano, M.; Lodeiro, C. Polycatenar unsymmetrical β-diketonate ligands as a useful tool to induce columnar mesomorphism on highly luminescent boron difluoride complexes. J. Mater. Chem. C 2014, 2, 9653–9665. [Google Scholar] [CrossRef]
- Jiménez, R.; Duarte, F.; Nuti, S.; Campo, J.A.; Lodeiro, C.; Cano, M.; Cuerva, C. Thermochromic and acidochromic properties of polymer films doped with pyridyl-β-diketonate boron(III) complexes. Dyes Pigments 2020, 177, 108272. [Google Scholar] [CrossRef]
- Louis, M.; Piñero García, C.; Brosseau, A.; Allain, C.; Métivier, R. Mechanofluorochromism of a Difluoroboron-β-Diketonate Derivative at the Nanoscale. J. Phys. Chem. Lett. 2019, 10, 4758–4762. [Google Scholar] [CrossRef] [PubMed]
- Zairov, R.R.; Dovzhenko, A.P.; Sapunova, A.S.; Voloshina, A.D.; Sarkanich, K.A.; Daminova, A.G.; Nizameev, I.R.; Lapaev, D.V.; Sudakova, S.N.; Podyachev, S.N.; et al. Terbium(III)-thiacalix[4]arene nanosensor for highly sensitive intracellular monitoring of temperature changes within the 303–313 K range. Sci. Rep. 2020, 10, 20541. [Google Scholar] [CrossRef] [PubMed]
- Zairov, R.R.; Yagodin, A.V.; Khrizanforov, M.; Martynov, A.G.; Nizameev, I.R.; Syakaev, V.V.; Gubaidullin, A.T.; Kornev, T.; Kaman, O.; Budnikova, Y.H.; et al. Unusual magnetic relaxation behavior of hydrophilic colloids based on gadolinium(III) octabutoxyphthalocyaninate. J. Nanoparticle Res. 2019, 21, 12. [Google Scholar] [CrossRef]
- Zhao, N.; Ma, C.; Yang, W.; Yin, W.; Wei, J.; Li, N. Facile construction of boranil complexes with aggregation-induced emission characteristics and their specific lipid droplet imaging applications. Chem. Commun. 2019, 55, 8494–8497. [Google Scholar] [CrossRef]
- Yabu, H. Self-organized precipitation: An emerging method for preparation of unique polymer particles. Polym. J. 2013, 45, 261–268. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Vivek, R.; Thangam, R.; NipunBabu, V.; Rejeeth, C.; Sivasubramanian, S.; Gunasekaran, P.; Muthuchelian, K.; Kannan, S. Multifunctional HER2-antibody conjugated polymeric nanocarrier-based drug delivery system for multi-drug-resistant breast cancer therapy. ACS Appl. Mat. Int. 2014, 6, 6469–6480. [Google Scholar] [CrossRef]
- Duong, H.D.; Shin, Y.; Rhee, J.I. Development of novel optical pH sensors based on coumarin 6 and nile blue A encapsulated in resin particles and specific support materials. Mat. Sci. Eng. C 2020, 107, 110323. [Google Scholar] [CrossRef]
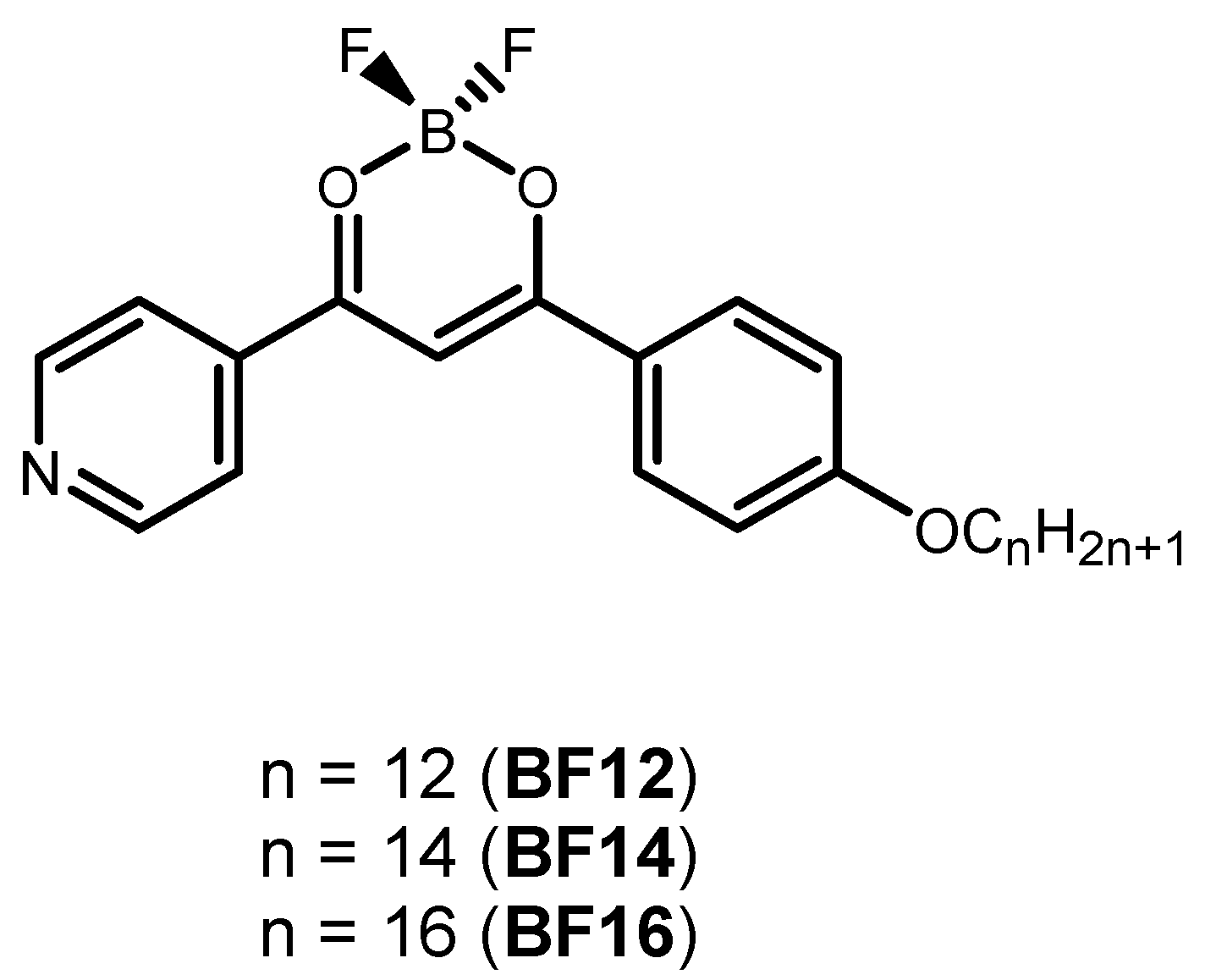

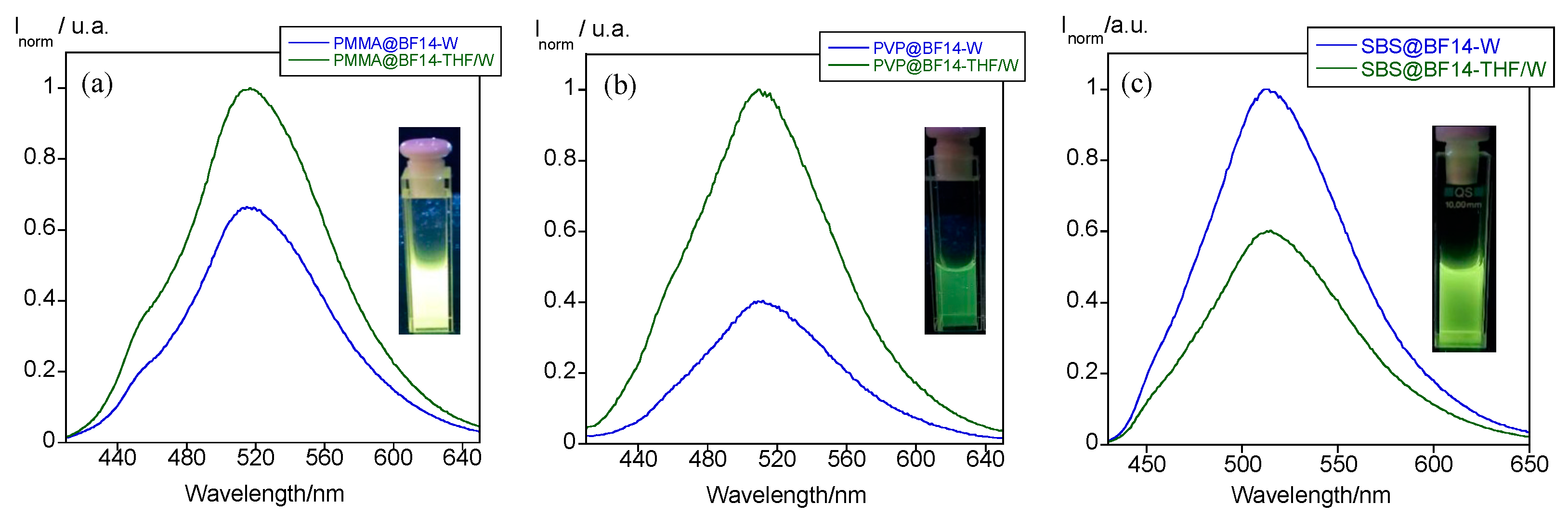
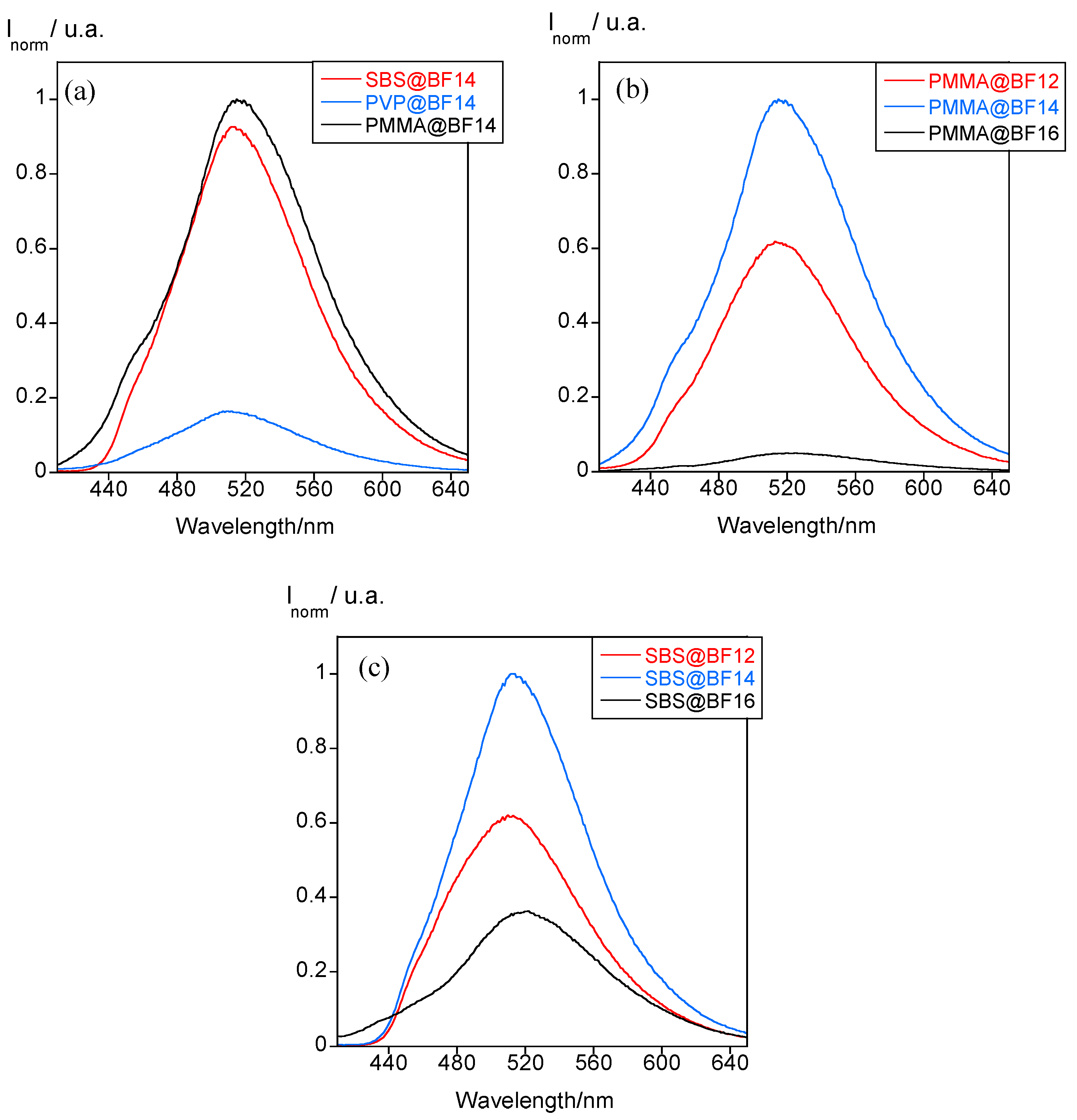
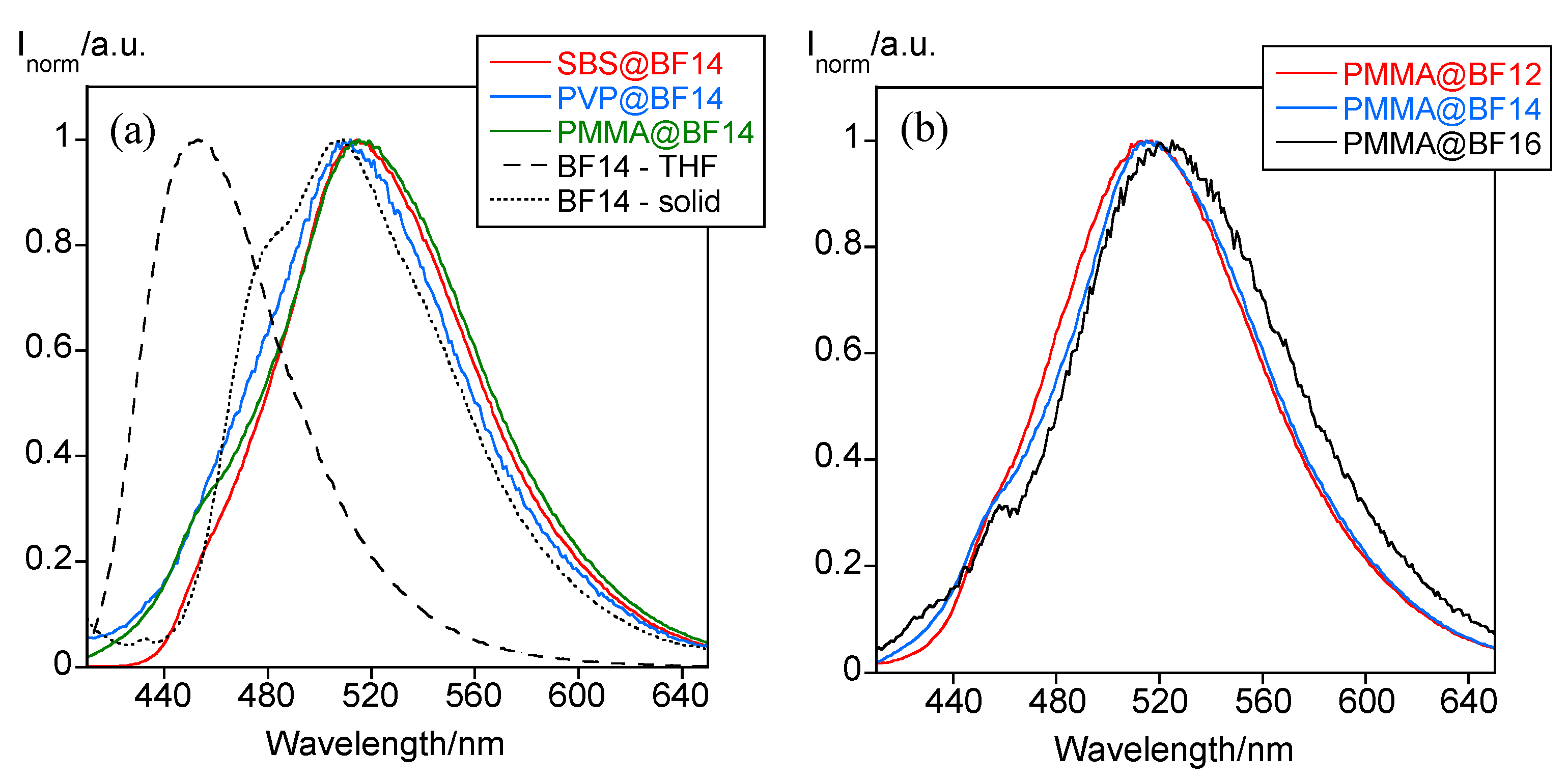
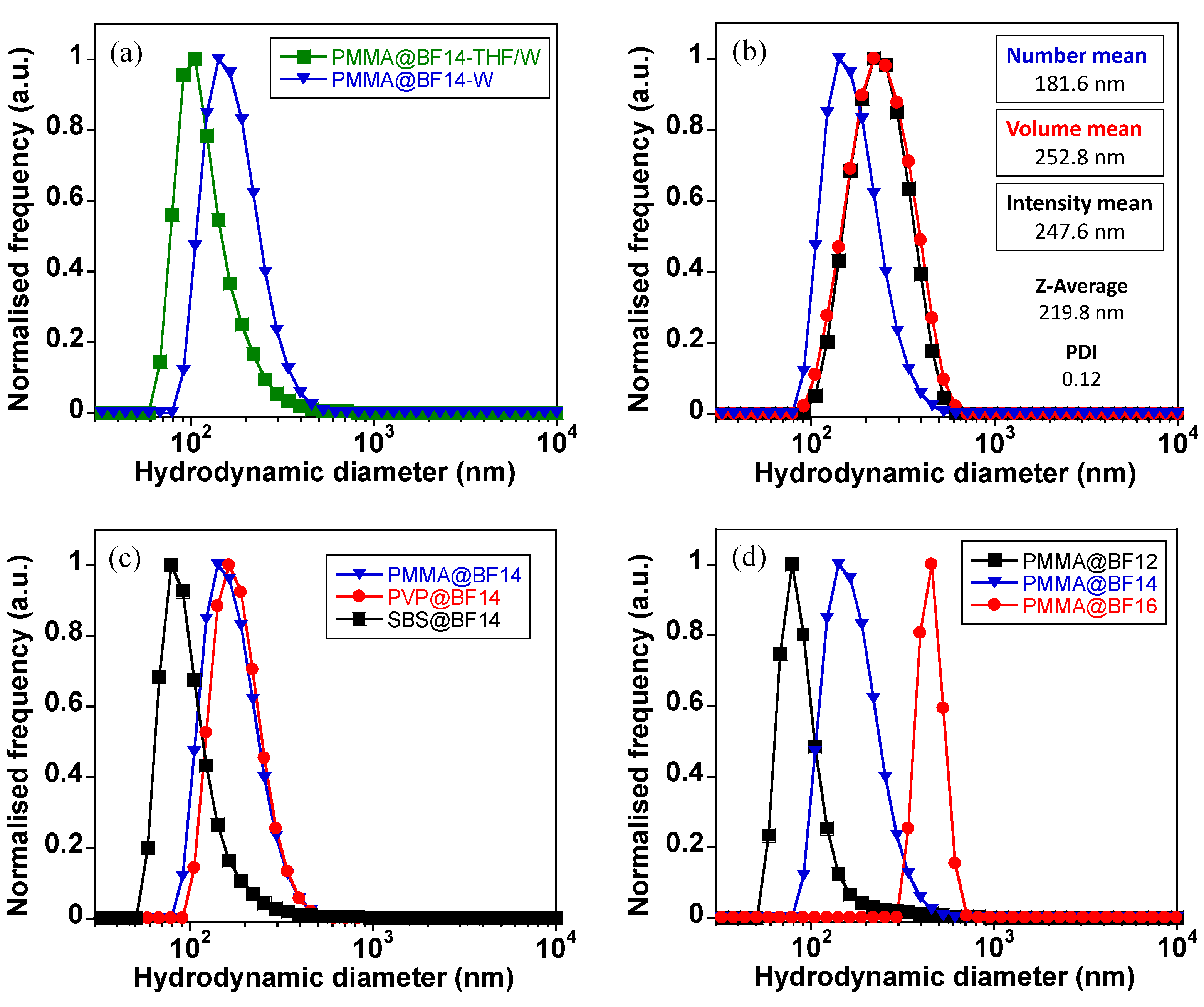


| Particles | Z-Average (nm) | PDI | ζ-Potential (mV) |
|---|---|---|---|
| PMMA@BF12 | 284.4 | 0.39 | −43.2 |
| PMMA@BF14 | 219.8 | 0.12 | −40.3 |
| PMMA@BF16 | 1120.0 | 0.76 | −23.4 |
| PVP@BF14 | 231.0 | 0.10 | −40.0 |
| SBS@BF12 | 380.1 | 0.33 | −41.3 |
| SBS@BF14 | 244.2 | 0.38 | −39.4 |
| SBS@BF16 | 190.0 | 0.21 | −60.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, F.; Cuerva, C.; Fernández-Lodeiro, C.; Fernández-Lodeiro, J.; Jiménez, R.; Cano, M.; Lodeiro, C. Polymer Micro and Nanoparticles Containing B(III) Compounds as Emissive Soft Materials for Cargo Encapsulation and Temperature-Dependent Applications. Nanomaterials 2021, 11, 3437. https://doi.org/10.3390/nano11123437
Duarte F, Cuerva C, Fernández-Lodeiro C, Fernández-Lodeiro J, Jiménez R, Cano M, Lodeiro C. Polymer Micro and Nanoparticles Containing B(III) Compounds as Emissive Soft Materials for Cargo Encapsulation and Temperature-Dependent Applications. Nanomaterials. 2021; 11(12):3437. https://doi.org/10.3390/nano11123437
Chicago/Turabian StyleDuarte, Frederico, Cristián Cuerva, Carlos Fernández-Lodeiro, Javier Fernández-Lodeiro, Raquel Jiménez, Mercedes Cano, and Carlos Lodeiro. 2021. "Polymer Micro and Nanoparticles Containing B(III) Compounds as Emissive Soft Materials for Cargo Encapsulation and Temperature-Dependent Applications" Nanomaterials 11, no. 12: 3437. https://doi.org/10.3390/nano11123437
APA StyleDuarte, F., Cuerva, C., Fernández-Lodeiro, C., Fernández-Lodeiro, J., Jiménez, R., Cano, M., & Lodeiro, C. (2021). Polymer Micro and Nanoparticles Containing B(III) Compounds as Emissive Soft Materials for Cargo Encapsulation and Temperature-Dependent Applications. Nanomaterials, 11(12), 3437. https://doi.org/10.3390/nano11123437







