Facile Synthesis Bi2Te3 Based Nanocomposites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis
2.2. Characterization
2.3. Techniques of Catalysis
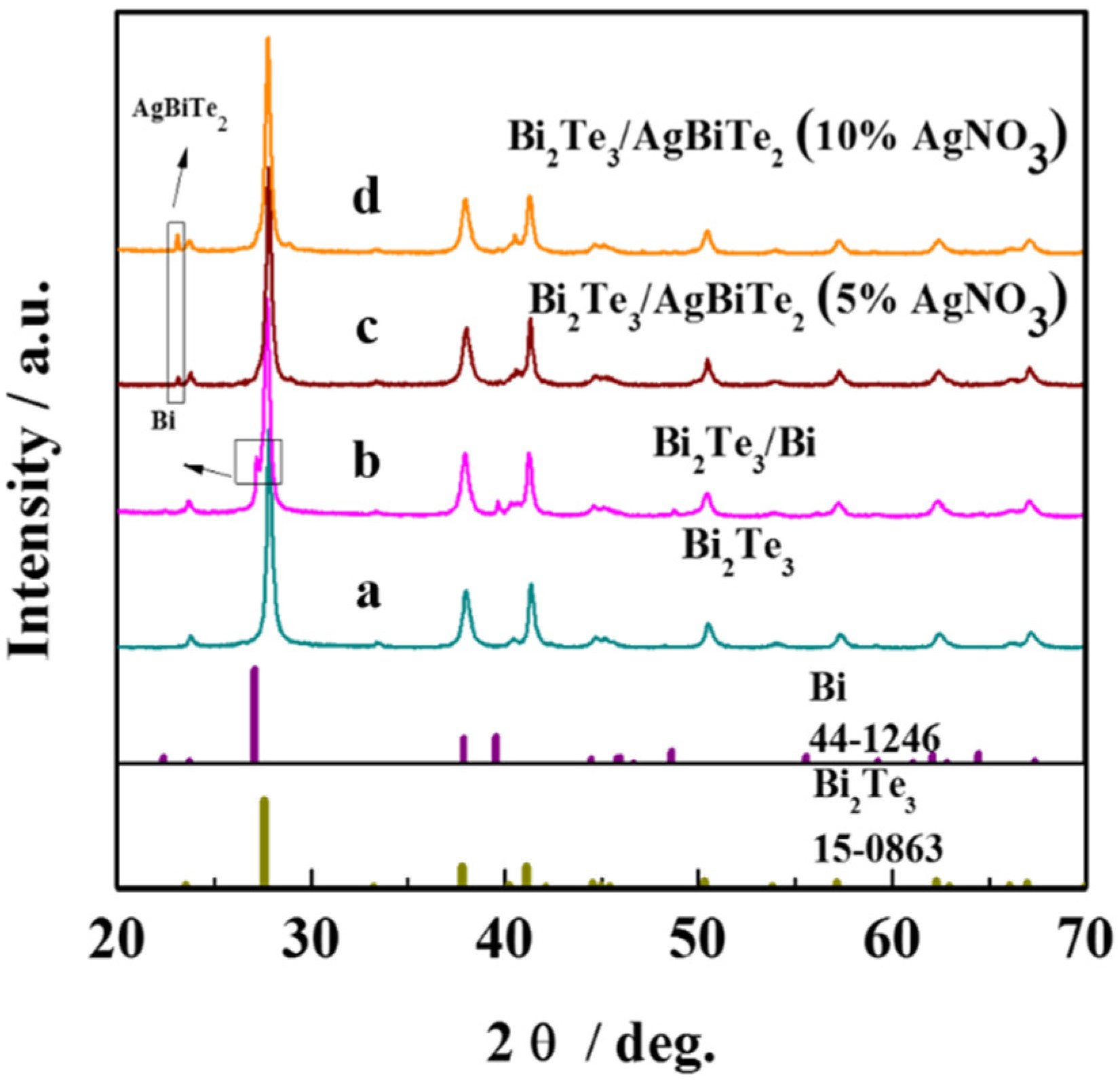
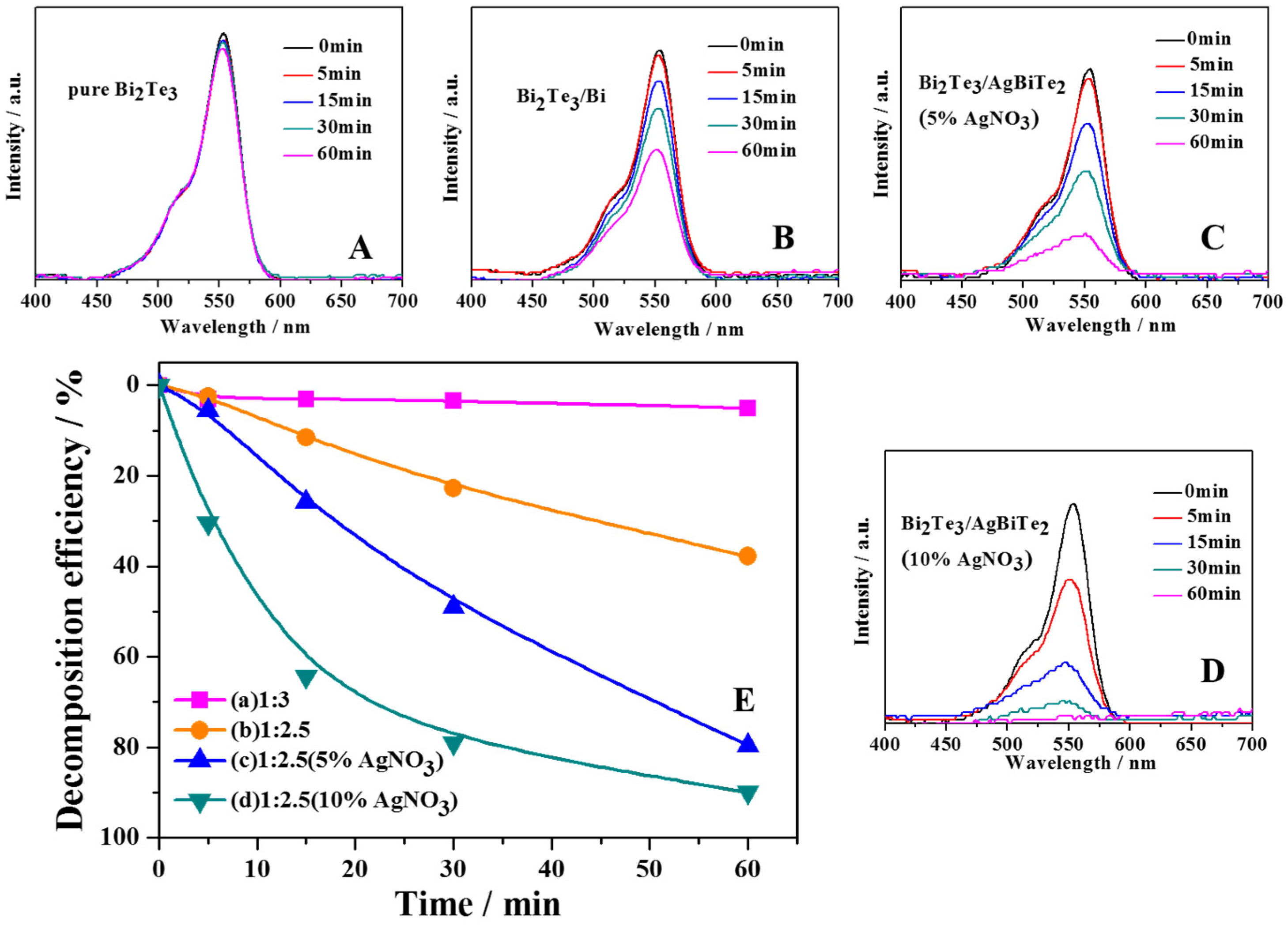
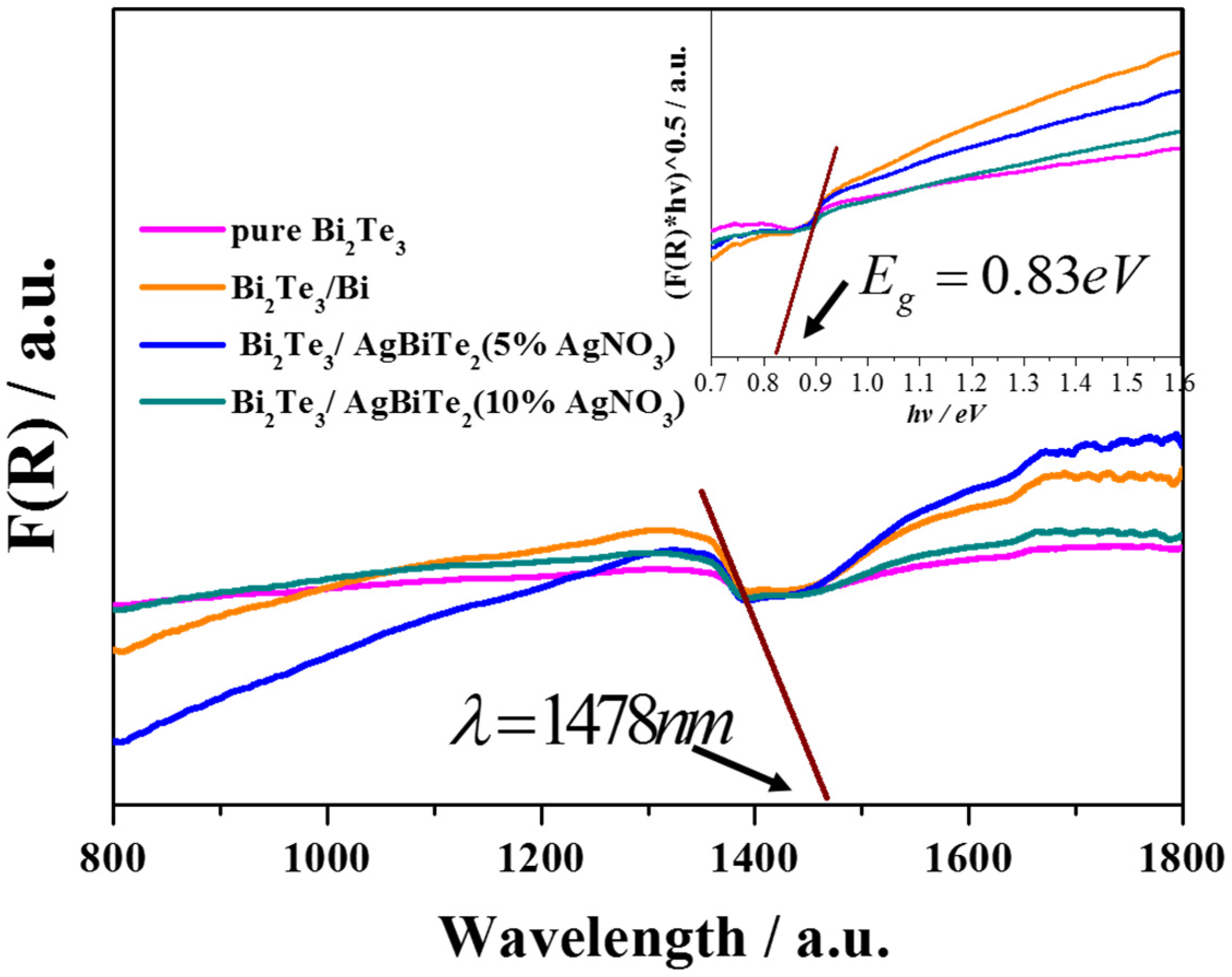
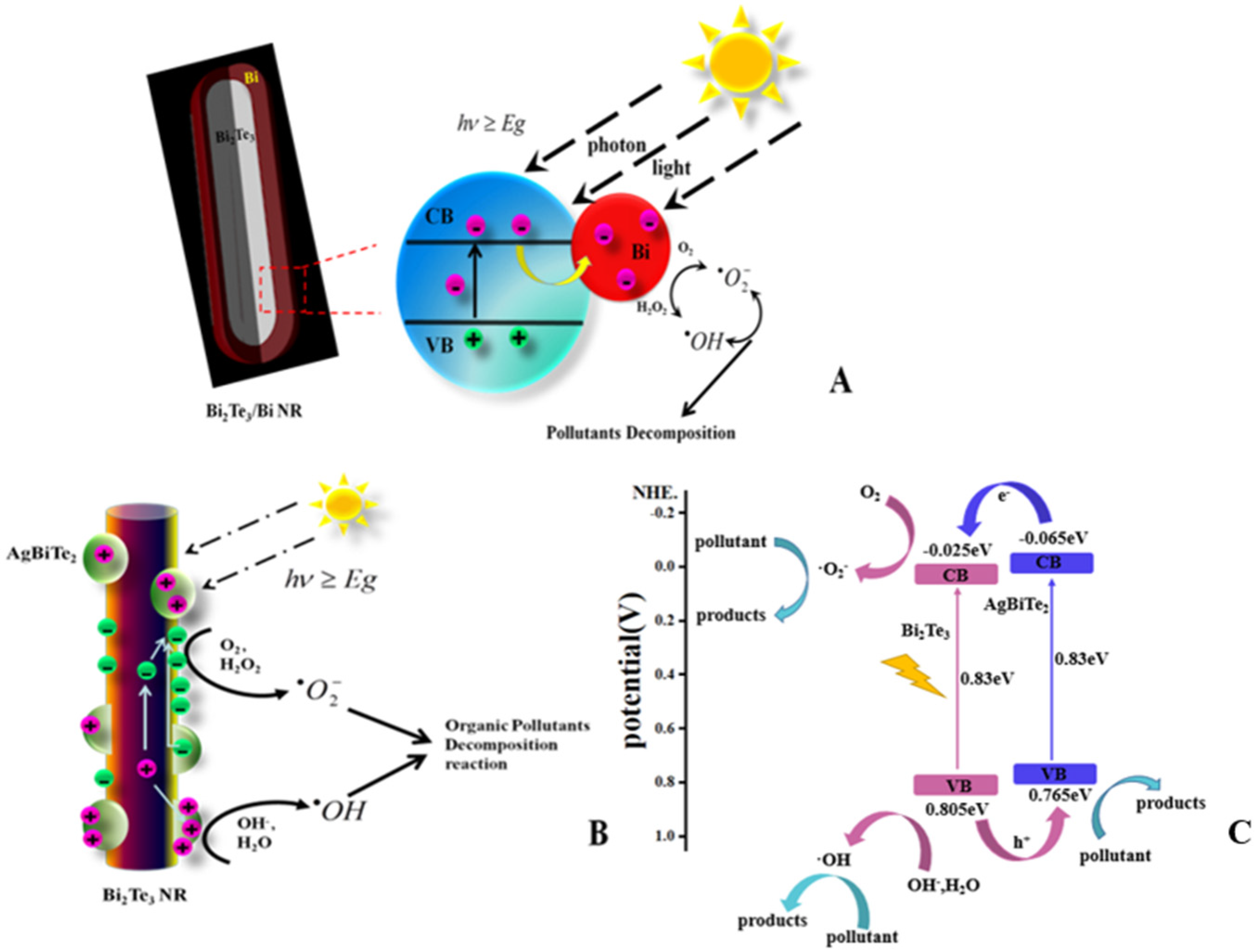
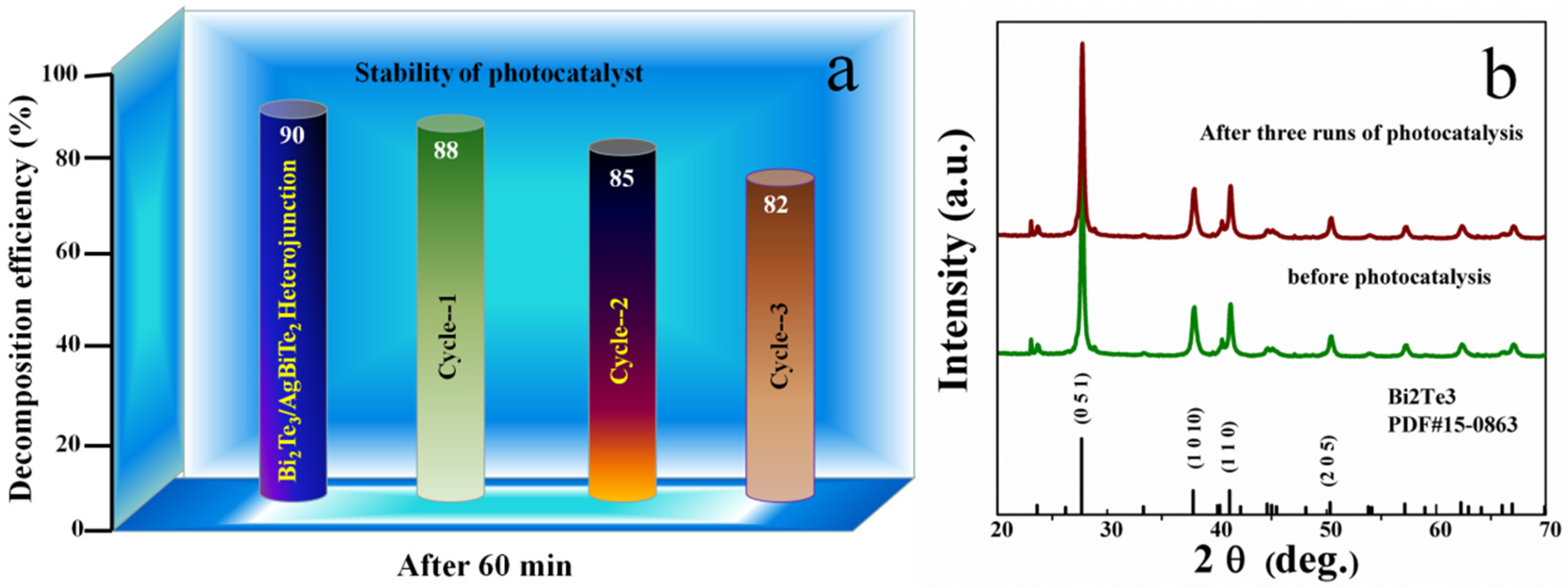
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Qu, J.G.; Li, N.N.; Liu, B.J.; He, J.X. Preparation of BiVO4/bentonite catalysts and their photocatalytic properties under simulated solar irradiation. Mater. Sci. Semicond. Process. 2013, 16, 99–105. [Google Scholar] [CrossRef]
- Chen, Y.; Li, F.; Cao, W.; Li, T. Preparation of recyclable CdS photocatalytic and superhydrophobic films with photostability by using a screen-printing technique. J. Mater. Chem. A 2015, 3, 16934–16940. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J.; Jaroniec, M. Carbon-based two-dimensional layered materials for photocatalytic CO2 reduction to solar fuels. Energy Storage Mater. 2016, 3, 24–35. [Google Scholar] [CrossRef]
- Xie, G.; Zhang, K.; Guo, B.; Liu, Q.; Fang, L.; Gong, J.R. Graphene-based materials for hydrogen generation from light-driven water splitting. Adv. Mater. 2013, 25, 3820–3839. [Google Scholar] [CrossRef]
- Tu, W.; Zhou, Y.; Zou, Z. Photocatalytic conversion of CO2 into renewable hydrocarbon fuels: State-of-the-art accomplishment, challenges, and prospects. Adv. Mater. 2014, 26, 4607–4626. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor Composites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting—A critical review. Energy Environ. Sci 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- He, S.; Wang, G.-S.; Lu, C.; Luo, X.; Wen, B.; Guo, L.; Cao, M.-S. Controllable Fabrication of CuS Hierarchical Nanostructures and Their Optical, Photocatalytic, and Wave Absorption Properties. Chem. Plus Chem. 2013, 78, 250–258. [Google Scholar] [CrossRef]
- Zhu, L.; Xie, Y.; Zheng, X.; Liu, X.; Zhou, G.E. Fabrication of novel urchin-like architecture and snowflake-like pattern CuS. J. Cryst. Growth. 2004, 260, 494–499. [Google Scholar] [CrossRef]
- Tan, C.; Lu, R.; Xue, P.; Bao, C.; Zhao, Y. Synthesis of CuS nanoribbons templated by hydrogel. Mater. Chem. Phys. 2008, 112, 500–503. [Google Scholar] [CrossRef]
- Mao, J.F.; Shu, Q.; Wen, Y.Q.; Yuan, H.Y.; Xiao, D.; Choi, M.M.F. Facile Fabrication of Porous CuS Nanotubes Using Well-Aligned [Cu(tu)]Cl·1/2H2O Nanowire Precursors as Self-Sacrificial Templates. Cryst. Growth Des. 2009, 9, 2546–2548. [Google Scholar] [CrossRef]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and Properties of Nanocrystals of Different Shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef]
- Liu, X.; Iocozzia, J.; Wang, Y.; Cui, X.; Chen, Y.; Zhao, S.; Li, Z.; Lin, Z. Noble metal–metal oxide nanohybrids with tailored nanostructures for efficient solar energy conversion, photocatalysis and environmental remediation. Energy Environ. Sci. 2017, 10, 402–434. [Google Scholar] [CrossRef]
- Lang, L.; Wu, D.; Xu, Z. Controllable fabrication of TiO2 1D-nano/micro structures: Solid, hollow, and tube-in-tube fibers by electrospinning and the photocatalytic performance. Chemistry 2012, 18, 10661–10668. [Google Scholar] [CrossRef]
- Chen, G.; Dresselhaus, M.S.; Dresselhaus, G.; Fleurial, J.P.; Caillat, T. Recent developments in thermoelectric materials. Int. Mater. Rev. 2013, 48, 45–66. [Google Scholar] [CrossRef]
- Dirmyer, M.R.; Martin, J.; Nolas, G.S.; Sen, A.; Badding, J.V. Thermal and electrical conductivity of size-tuned bismuth telluride nanoparticles. Small 2009, 5, 933–937. [Google Scholar] [CrossRef]
- Ge, Z.-H.; Zhang, B.-P.; Shang, P.-P.; Li, J.-F. Control of anisotropic electrical transport property of Bi2S3 thermoelectric polycrystals. J. Mater. Chem. 2011, 21, 9194–9200. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Wang, P.; Chen, L.; Chen, Y.-B.; Wu, L.-M. Syntheses, Growth Mechanism, and Optical Properties of [001] Growing Bi2S3 Nanorods. J. Phys. Chem. C 2009, 113, 16009–16014. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, H.; Wang, J.; Liu, D.; Du, G.; Cui, J. Ag2O/TiO2 nanobelts heterostructure with enhanced ultraviolet and visible photocatalytic activity. ACS Appl. Mater. Interfaces 2010, 2, 2385–2392. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, C.; Ji, J.; Wei, Y.; Zhang, P.; Wang, S.; Fan, X.; Gong, J. Reduced Graphene Oxide (rGO)/BiVO4 Composites with Maximized Interfacial Coupling for Visible Lght Photocatalysis. ACS Sustain. Chem. Eng. 2014, 2, 2253–2258. [Google Scholar] [CrossRef]
- Su, Y.; Zhu, B.; Guan, K.; Gao, S.; Lv, L.; Du, C.; Peng, L.; Hou, L.; Wang, X. Particle Size and Structural Control of ZnWO4 Nanocrystals via Sn2+ Doping for Tunable Optical and Visible Photocatalytic Properties. J. Phys. Chem. C 2012, 116, 18508–18517. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: A comparative study. J. Sol.-Gel. Sci. Technol. 2011, 61, 1–7. [Google Scholar] [CrossRef]
- Ge, Z.-H.; Nolas, G.S. Controllable Synthesis of Bismuth Chalcogenide Core–shell Nanorods. Cryst. Growth Des. 2014, 14, 533–536. [Google Scholar] [CrossRef]
- Jin, R.; Chen, G.; Pei, J.; Yan, C. Hydrothermal synthesis and thermoelectric transport property of PbS–PbTe core–shell heterostructures. New J. Chem. 2012, 36, 2574–2579. [Google Scholar] [CrossRef]
- Zhao, X.B.; Sun, T.; Zhu, T.J.; Tu, J.P. In-situ investigation and effect of additives on low temperature aqueous chemical synthesis of Bi2Te3 nanocapsules. J. Mater. Chem. 2005, 15, 1621–1625. [Google Scholar] [CrossRef]
- Li, Y.; Hwang, D.-S.; Lee, N.H.; Kim, S.-J. Synthesis and characterization of carbon-doped titania as an artificial solar light sensitive photocatalyst. Chem. Phys. Lett. 2005, 404, 25–29. [Google Scholar] [CrossRef]
- Emilio, C.A.; Litter, M.I.; Kunst, M.; Bouchard, M.; Colbeau-Justin, C. Phenol Photodegradation on Platinized-TiO2 Photocatalysts Related to Charge-Carrier Dynamic. Langmuir 2006, 22, 3606–3613. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Liqiang, J.; Yichun, Q.; Baiqi, W.; Shudan, L.; Baojiang, J.; Libin, Y.; Wei, F.; Honggang, F.; Jiazhong, S. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. Cells. 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Gunawan, C.; Teoh, W.Y.; Marquis, C.P.; Lifia, J.; Amal, R. Reversible antimicrobial photoswitching in nanosilver. Small 2009, 5, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Nolan, N.T.; Seery, M.K.; Hinder, S.J.; Healy, L.F.; Pillai, S.C. A Systematic Study of the Effect of Silver on the Chelation of Formic Acid to a Titanium Precursor and the Resulting Effect on the Anatase to Rutile Transformation of TiO2. J. Phys. Chem. C 2010, 114, 13026–13034. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Zhong, C.Y.; Ge, Z.H.; Feng, J. Realizing High Photocatalytic Performance of NaBiS2 Nanopowders via the Introduction of Rare-Earth Elements. Phys. Status Solidi A 2019, 216, 1900061. [Google Scholar] [CrossRef]
- Mu, J.; Chen, B.; Zhang, M.; Guo, Z.; Zhang, P.; Zhang, Z.; Sun, Y.; Shao, C.; Liu, Y. Enhancement of the Visible-Light Photocatalytic Activity of In2O3–TiO2 Nanofiber Heteroarchitectures. ACS Appl. Mater. Interfaces 2011, 4, 424–430. [Google Scholar] [CrossRef]
- Ge, Z.-H.; Zhang, B.-P.; Yu, Z.-X.; Jiang, B.-B. Controllable synthesis: Bi2S3 nanostructure powders and highly textured polycrystals. CrystEngComm 2012, 14, 2283–2288. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Guo, J.; Ge, Z.-H.; Feng, J. Facile Synthesis Bi2Te3 Based Nanocomposites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Nanomaterials 2021, 11, 3390. https://doi.org/10.3390/nano11123390
Wu D, Guo J, Ge Z-H, Feng J. Facile Synthesis Bi2Te3 Based Nanocomposites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Nanomaterials. 2021; 11(12):3390. https://doi.org/10.3390/nano11123390
Chicago/Turabian StyleWu, Di, Jun Guo, Zhen-Hua Ge, and Jing Feng. 2021. "Facile Synthesis Bi2Te3 Based Nanocomposites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity" Nanomaterials 11, no. 12: 3390. https://doi.org/10.3390/nano11123390
APA StyleWu, D., Guo, J., Ge, Z.-H., & Feng, J. (2021). Facile Synthesis Bi2Te3 Based Nanocomposites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Nanomaterials, 11(12), 3390. https://doi.org/10.3390/nano11123390




