Application of Selected Biomaterials and Stem Cells in the Regeneration of Hard Dental Tissue in Paediatric Dentistry—Based on the Current Literature
Abstract
1. Introduction
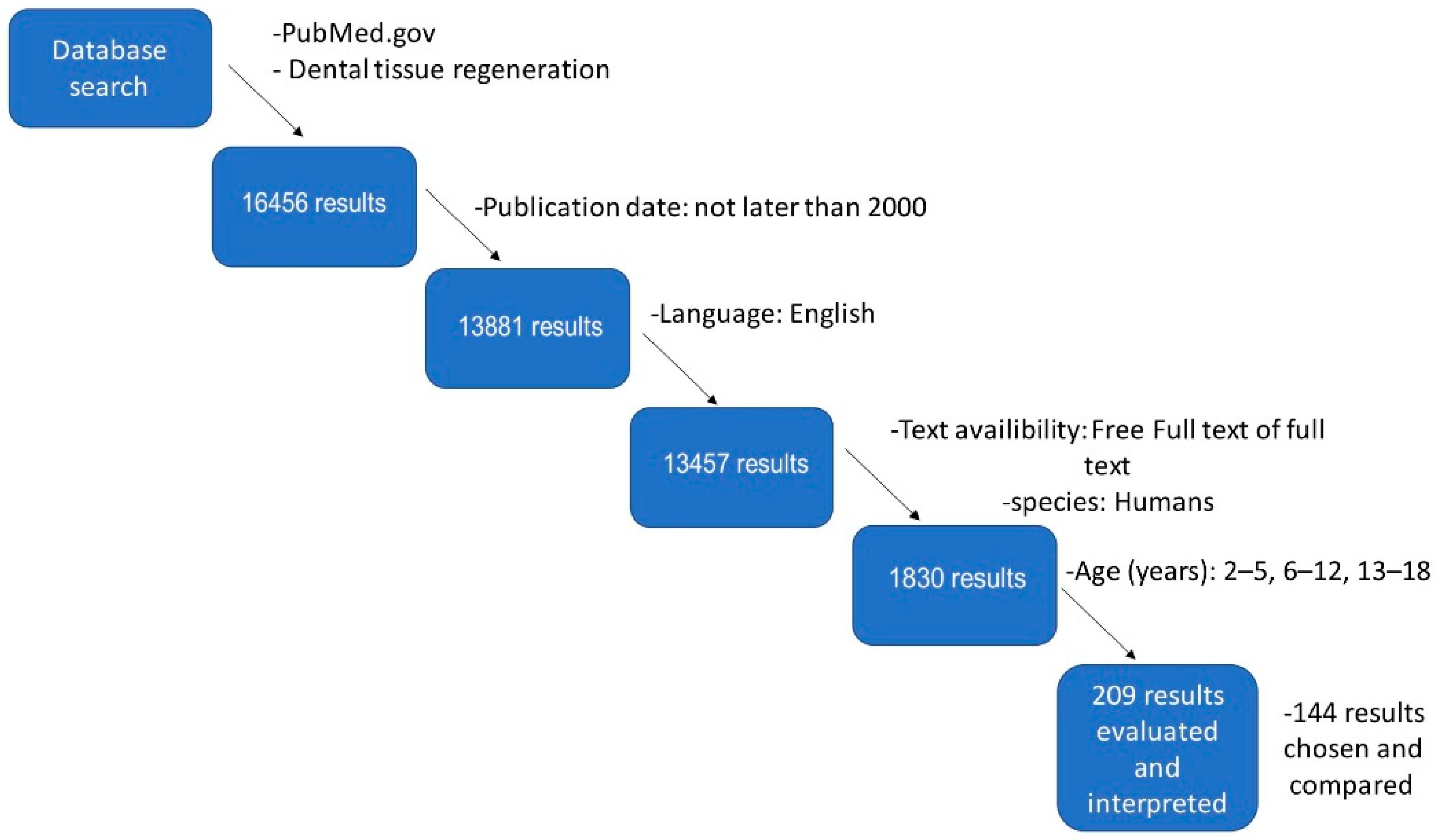
2. Materials and Methods
3. Methods of Treatment and Classification of Dental Caries
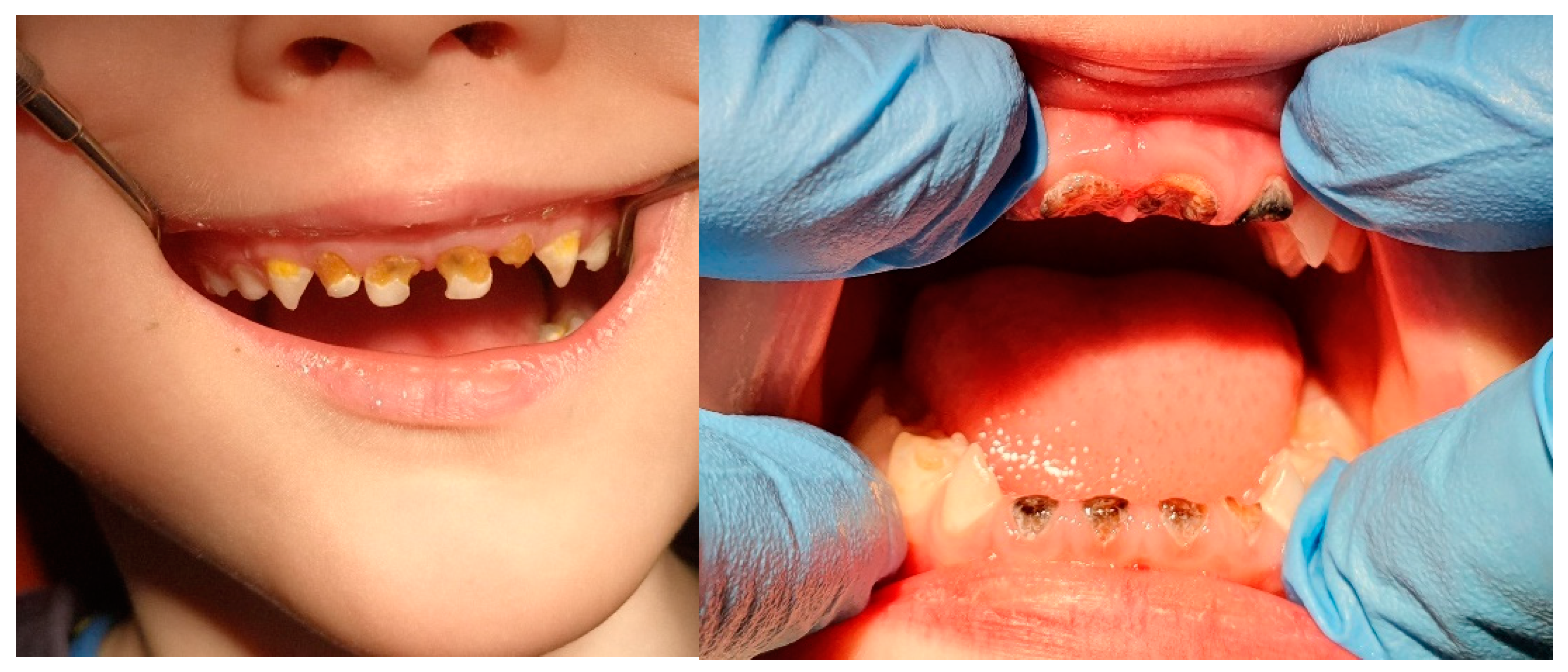
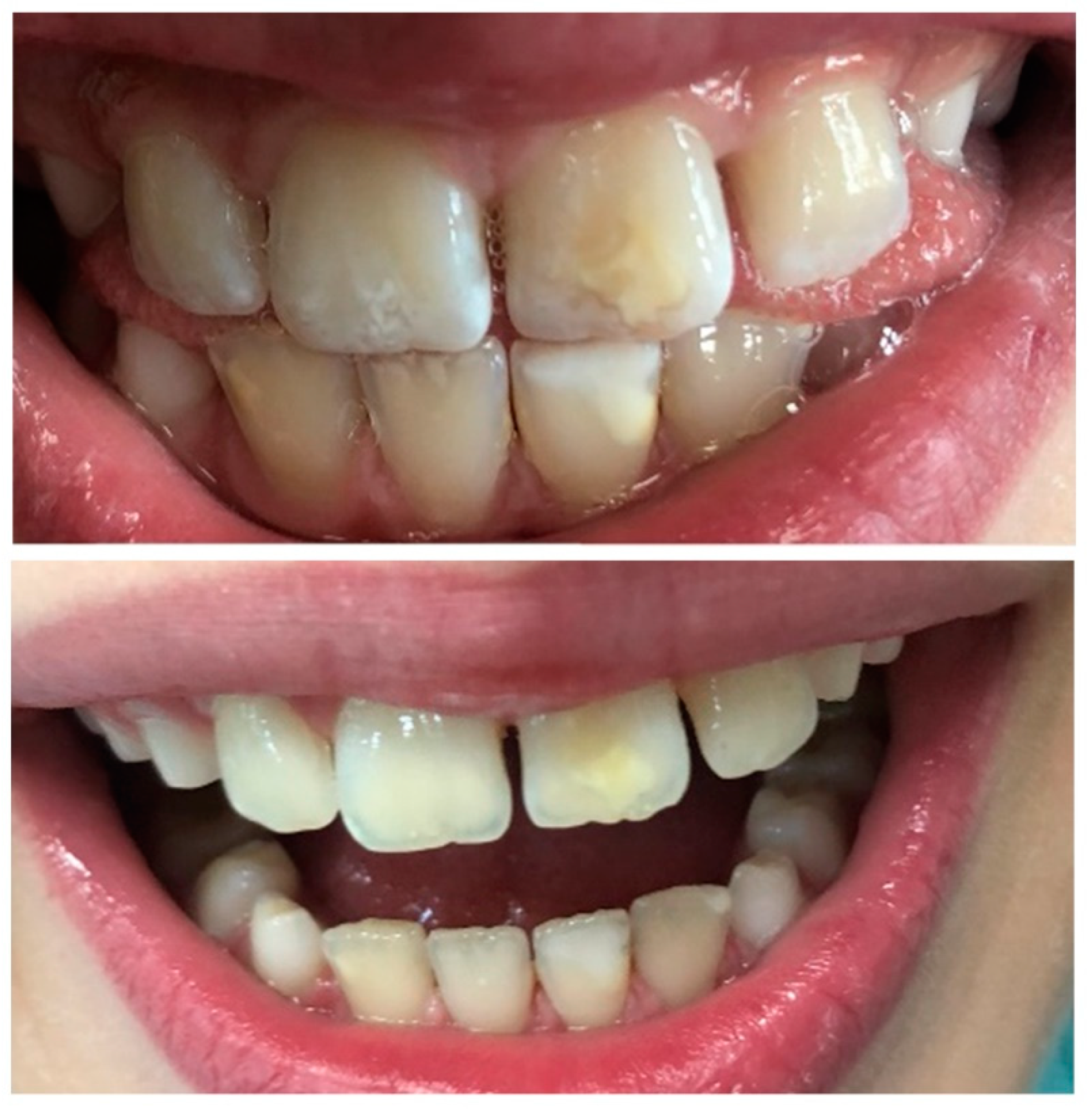
3.1. White Spot Lesion (WSL) and Remineralization
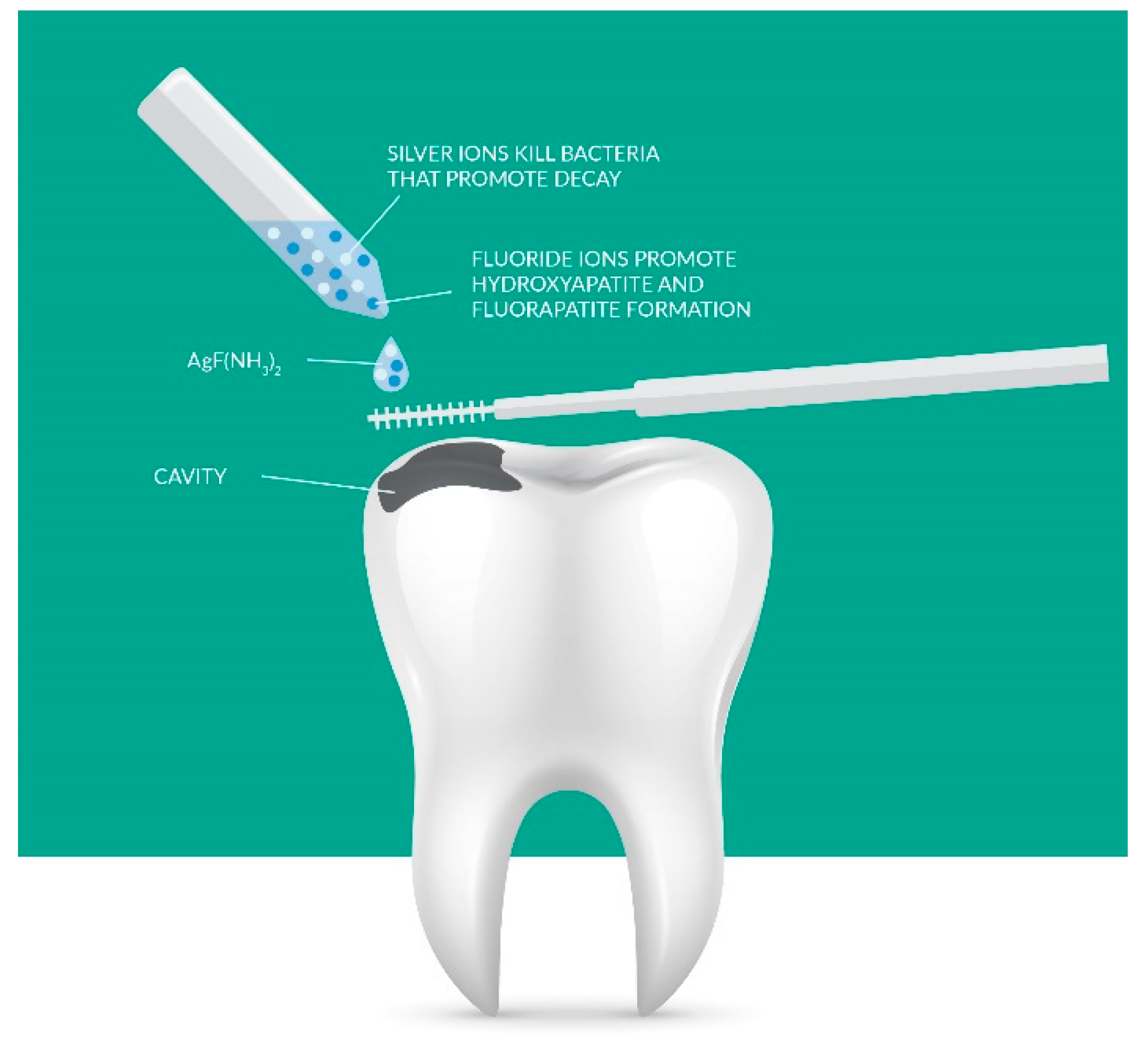
3.2. Nanohydroxyapatite and Remineralization
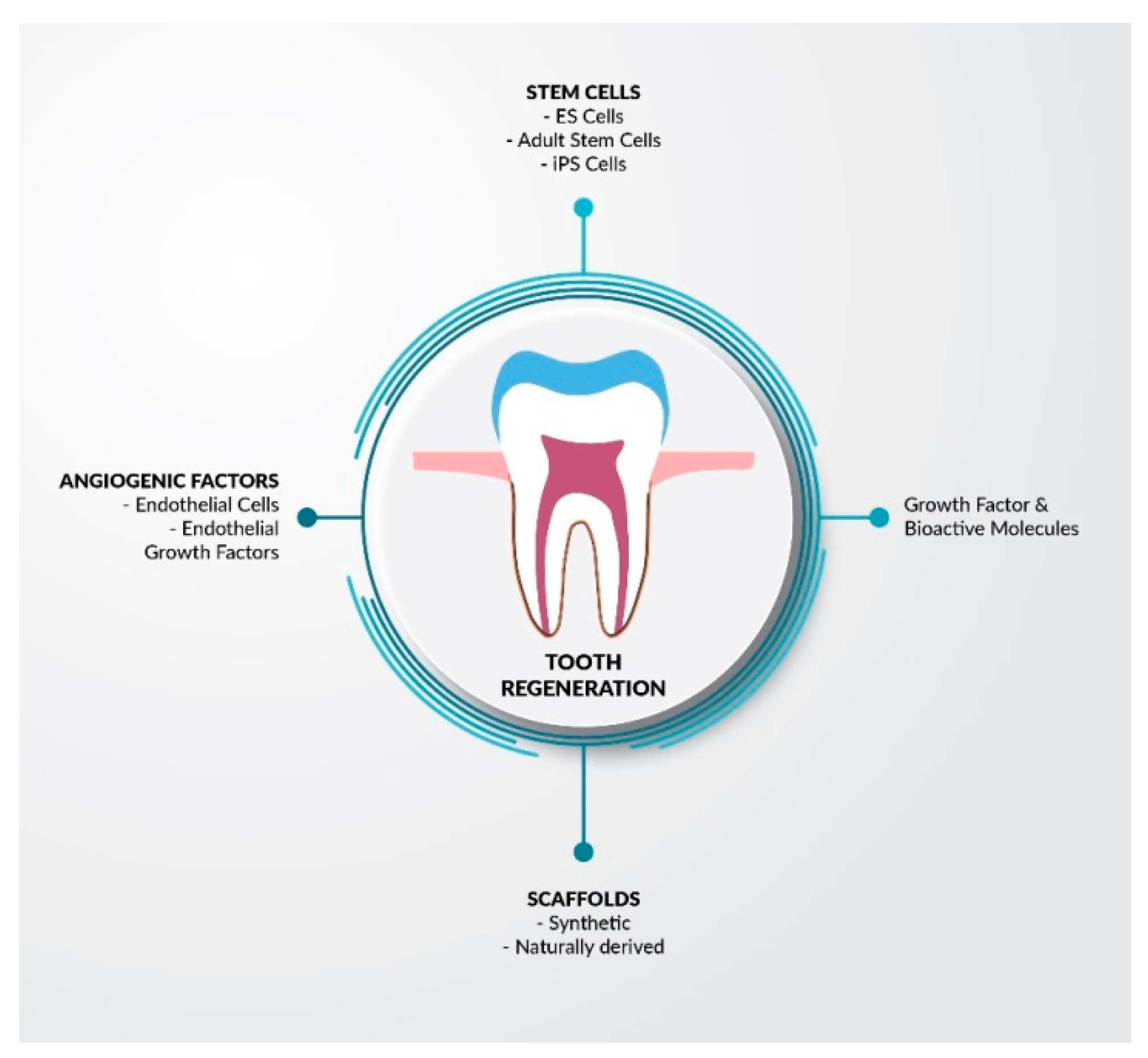
3.3. Stem Cells
3.4. Caries Infiltration
3.5. Fissure Sealants
3.6. Indirect Pulp Capping
3.7. Staged Caries Removal
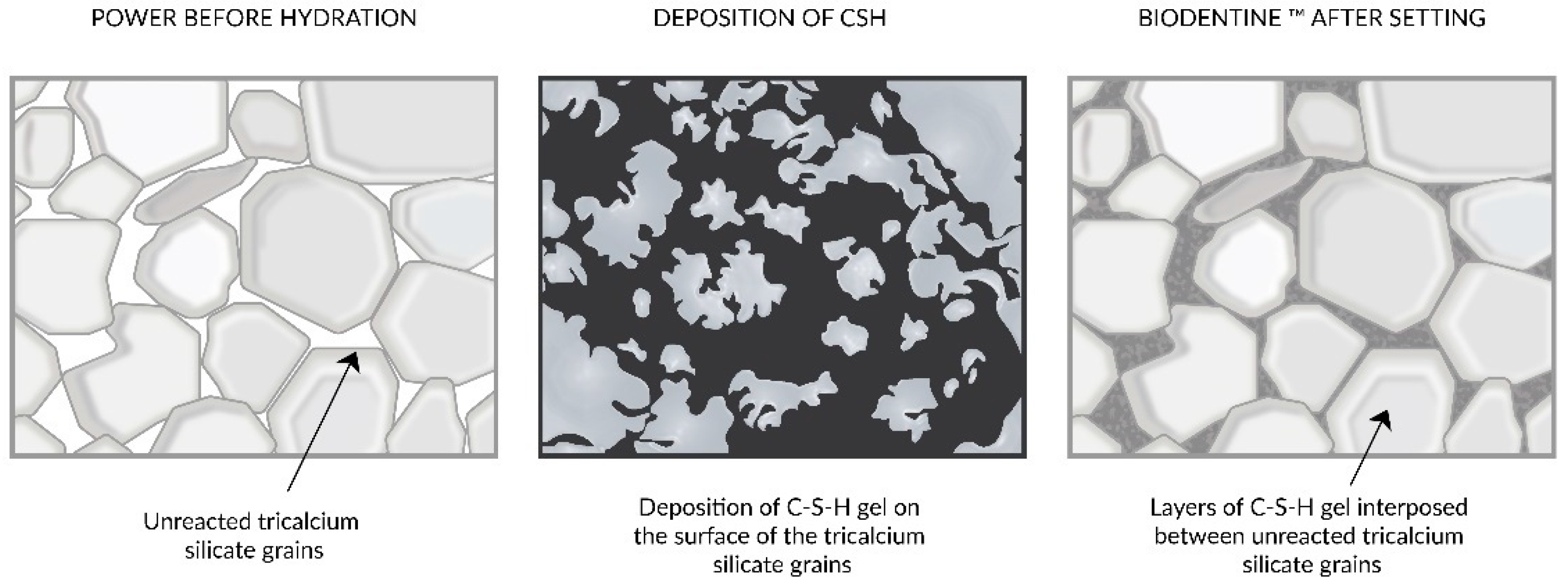
3.8. MTA, Biodentine, and Deep Caries
3.9. Glass Ionomer Cement
3.10. Composite Material
3.11. Atraumatic Restorative Techniques
4. Effect of Dental Biomaterials Application on the Progress of Oral Inflammatory Reactions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Righolt, A.J.; Jevdjevic, M.; Marcenes, W.; Listl, S. Global-, Regional-, and Country-Level Economic Impacts of Dental Diseases in 2015. J. Dent. Res. 2018, 97, 501–507. [Google Scholar] [CrossRef]
- Mack, F.; Dent, M.; Schwahn, C.; Nat, R.; Feine, J.S.; Mundt, T.; Bernhardt, O.; John, U.; Kocher, T.; Biffar, R. The Impact of Tooth Loss on General Health Related to Quality of Life Among Elderly Pomeranians: Results from the Study of Health in Pomerania (SHIP-0). Int. J. Prosthodont. 2005, 18, 414–419. [Google Scholar]
- Barboza-Solís, C.; Porras-Chaverri, M.; Fantin, R. Is tooth loss important when evaluating perceived general health? Findings from a nationally representative study of Costa Rican adults. Community Dent. Oral Epidemiol. 2019, 47, 358–365. [Google Scholar] [CrossRef]
- Petersen, P.E. The World Oral Health Report 2003: Continuous improvement of oral health in the 21st century—The approach of the WHO Global Oral Health Programme. Community Dent. Oral Epidemiol. 2003, 31, 3–24. [Google Scholar] [CrossRef]
- Farci, F.; Soni, A. Histology, Tooth; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Angelova Volponi, A.; Zaugg, L.K.; Neves, V.; Liu, Y.; Sharpe, P.T. Tooth Repair and Regeneration. Curr. Oral Health Rep. 2018, 5, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Young, C.S.; Terada, S.; Vacanti, J.P.; Honda, M.; Bartlett, J.D.; Yelick, P.C. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J. Dent. Res. 2002, 81, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Essentials of Oral Pathology and Oral Medicine Edisi 07 by dental.id—Issuu. Available online: https://issuu.com/dental.id/docs/essentials_of_oral_pathology_and_or (accessed on 11 November 2021).
- Fan, Y.; Sun, Z.; Moradian-Oldak, J. Controlled remineralization of enamel in the presence of amelogenin and fluoride. Biomaterials 2009, 30, 478–483. [Google Scholar] [CrossRef]
- Moradian-Oldak, J. The regeneration of tooth enamel. Dimens. Dent. Hyg. 2009, 7, 12–15. [Google Scholar] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Li, Z.Y.; Wang, H.M.; Bao, T.W. Tissue engineering teeth using bone marrow mesenchymal stem cells with odontogenic potential compounding collagen/nano-hydroxyapatite composite scaffold. Int. J. Oral Maxillofac. Surg. 2009, 38, 525–526. [Google Scholar] [CrossRef]
- Jones, F.H. Teeth and bones: Applications of surface science to dental materials and related biomaterials. Surf. Sci. Rep. 2001, 42, 75–205. [Google Scholar] [CrossRef]
- Shuai, Y.; Ma, Y.; Guo, T.; Zhang, L.; Yang, R.; Qi, M.; Liu, W.; Jin, Y. Dental stem cells and tooth regeneration. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2018; Volume 1107, pp. 41–52. [Google Scholar]
- Volponi, A.A.; Pang, Y.; Sharpe, P.T. Stem cell-based biological tooth repair and regeneration. Trends Cell Biol. 2010, 20, 715–722. [Google Scholar] [CrossRef]
- Mironov, V.; Visconti, R.P.; Markwald, R.R. What is regenerative medicine? Emergence of applied stem cell and developmental biology. Expert Opin. Biol. Ther. 2004, 4, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S.; Khurshid, Z.; Almas, K. Oral tissue engineering progress and challenges. Tissue Eng. Regen. Med. 2015, 12, 387–397. [Google Scholar] [CrossRef]
- Khurshid, Z.; Tariq, R.; Asiri, F.Y.; Abid, K.; Zafar, M.S. Literature search strategies in dental education and research. J. Taibah Univ. Med. Sci. 2021, 16, 799–806. [Google Scholar] [CrossRef]
- Çolak, H.; Dülgergil, Ç.; Dalli, M.; Hamidi, M. Early childhood caries update: A review of causes, diagnoses, and treatments. J. Nat. Sci. Biol. Med. 2013, 4, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Mendes, F.M.; Braga, M.M. Caries detection in primary teeth is less challenging than in permanent teeth. Dent. Hypotheses 2013, 4, 17. [Google Scholar] [CrossRef]
- Mejàre, I.; Stenlund, H.; Zelezny-Holmlund, C. Caries Incidence and Lesion Progression from Adolescence to Young Adulthood: A Prospective 15-Year Cohort Study in Sweden. Caries Res. 2004, 38, 130–141. [Google Scholar] [CrossRef]
- Skeie, M.S.; Raadal, M.; Strand, G.V.; Espelid, I. The relationship between caries in the primary dentition at 5 years of age and permanent dentition at 10 years of age—A longitudinal study. Int. J. Paediatr. Dent. 2006, 16, 152–160. [Google Scholar] [CrossRef]
- Chai, Y.; Slavkin, H.C. Prospects for tooth regeneration in the 21st century: A perspective. Microsc. Res. Tech. 2003, 60, 469–479. [Google Scholar] [CrossRef]
- Chai, Y.; Jiang, X.; Ito, Y.; Bringas, P.; Han, J.; Rowitch, D.H.; Soriano, P.; McMahon, A.P.; Sucov, H.M. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 2000, 127, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Borges, B.C.D.; de Souza Borges, J.; de Araujo, L.S.N.; Machado, C.T.; dos Santos, A.J.S.; de Assunçao Pinheiro, I.V. Update on Nonsurgical, Ultraconservative Approaches to Treat Effectively Non-Cavitated Caries Lesions in Permanent Teeth. Eur. J. Dent. 2011, 5, 229. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.H.; Tom, H.; Lee, R.C.; Kang, H.; Simon, J.C.; Staninec, M.; Darling, C.L.; Pelzner, R.B.; Fried, D. Clinical Monitoring of Smooth Surface Enamel Lesions Using CP-OCT During Nonsurgical Intervention. Lasers Surg. Med. 2016, 48, 915–923. [Google Scholar] [CrossRef] [PubMed]
- CHAPTER 1 Biological Apatites in Bone and Teeth. RSC Nanosci. Nanotechnol. 2015, 2016, 1–29. [CrossRef]
- Gomez, J. Detection and diagnosis of the early caries lesion. BMC Oral Health 2014, 15, S1–S3. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, N.J.; Cai, F.; Huq, N.L.; Burrow, M.F.; Reynolds, E.C. New approaches to enhanced remineralization of tooth enamel. J. Dent. Res. 2010, 89, 1187–1197. [Google Scholar] [CrossRef]
- Badr, S.; Ragab, H. The Effectiveness of a Nano-Hydroxyapatite Paste and a Tri-Calcium Phosphate Fluoride Varnish in White Spot Lesions Remineralization (Randomized Clinical Trial). Egypt. Dent. J. 2018, 64, 2757–2765. [Google Scholar] [CrossRef]
- Wasiluk, A. Fluoride compounds in dental caries prophylaxis in children and adolescents—Review of Polish literature. Epidemiol. Rev. 2017, 71, 603–611. [Google Scholar]
- Deveci, C.; Çınar, Ç.; Tirali, R.E. Management of White Spot Lesions. Dent. Caries Diagn. Prev. Manag. 2018. [Google Scholar] [CrossRef]
- Khalaf, K. Factors Affecting the Formation, Severity and Location of WhiteSpot Lesions during Orthodontic Treatment with Fixed Appliances. J. Oral Maxillofac. Res. 2014, 5, e4. [Google Scholar] [CrossRef]
- Szpringer-Nodzak, M.; Wochna-Sobańska, M.; Bruzda-Zwiech, A. Stomatologia Wieku Rozwojowego; PZWL: Warsaw, Poland, 2010. [Google Scholar]
- Mielczarek, A.; Michalik, J.; Kujawa, M. The effect of selected fluoride products on microstructure of early caries lesions. Nowa Stomatol. 2013, 3, 120–124. [Google Scholar]
- Cury, J.A.; Tenuta, L.M. How to maintain a cariostatic fluoride concentration in the oral environment. Adv. Dent. Res. 2008, 20, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Whitford, G.M.; Wasdin, J.L.; Schafer, T.E.; Adair, S.M. Plaque Fluoride Concentrations Are Dependent on Plaque Calcium Concentrations. Caries Res. 2002, 36, 256–265. [Google Scholar] [CrossRef]
- Damle, S.; Nagpal, D. Comparison of salivary fluoride levels following use of dentifrices containing different concentrations of fluoride. J. Indian Soc. Pedod. Prev. Dent. 2007, 25, 20. [Google Scholar] [CrossRef]
- Patel, P.M.; Hugar, S.M.; Halikerimath, S.; Badakar, C.M.; Gokhale, N.S.; Thakkar, P.J.; Kohli, D.; Shah, S. Comparison of the Effect of Fluoride Varnish, Chlorhexidine Varnish and Casein Phosphopeptide- Amorphous Calcium Phosphate (CPP-ACP) Varnish on Salivary Streptococcus mutans Level: A Six Month Clinical Study. J. Clin. Diagn. Res. 2017, 11, ZC53–ZC59. [Google Scholar] [CrossRef]
- Naumova, E.A.; Niemann, N.; Aretz, L.; Arnold, W.H. Effects of different amine fluoride concentrations on enamel remineralization. J. Dent. 2012, 40, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Turska-Szybka, A.; Olczak-Kowalczyk, D. The use of prophylactic resources with high level of fluoride in children and youth. Capabilities and limitations. Nowa Stomatol. 2012, 17, 102–107. [Google Scholar]
- Bhat, S.; Kumar, A. Biomaterials and bioengineering tomorrow’s healthcare. Biomatter 2013, 3, e24717. [Google Scholar] [CrossRef] [PubMed]
- Koliniotou-Koumpia, E.; Tziafas, D. Pulpal responses following direct pulp capping of healthy dog teeth with dentine adhesive systems. J. Dent. 2005, 33, 639–647. [Google Scholar] [CrossRef]
- Andelin, W.E.; Shabahang, S.; Wright, K.; Torabinejad, M. Identification of hard tissue after experimental pulp capping using dentin sialoprotein (DSP) as a marker. J. Endod. 2003, 29, 646–650. [Google Scholar] [CrossRef]
- Camilleri, J.; Montesin, F.E.; Papaioannou, S.; McDonald, F.; Pitt Ford, T.R. Biocompatibility of two commercial forms of mineral trioxide aggregate. Int. Endod. J. 2004, 37, 699–704. [Google Scholar] [CrossRef]
- Laurent, P.; Camps, J.; About, I. Biodentine TM induces TGF-β1 release from human pulp cells and early dental pulp mineralization. Int. Endod. J. 2012, 45, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, G.G.; McArdle, A.; Tevlin, R.; Momeni, A.; Atashroo, D.; Hu, M.S.; Feroze, A.H.; Wong, V.W.; Lorenz, P.H.; Longaker, M.T.; et al. Nanotechnology in bone tissue engineering. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzynski, M.; Rybak, Z.; Szymonowicz, M.; Wiglusz, R.J. Selected Nanomaterials’ Application Enhanced with the Use of Stem Cells in Acceleration of Alveolar Bone Regeneration during Augmentation Process. Nanomaterials 2020, 10, 1216. [Google Scholar] [CrossRef]
- Vandiver, J.; Dean, D.; Patel, N.; Bonfield, W.; Ortiz, C. Nanoscale variation in surface charge of synthetic hydroxyapatite detected by chemically and spatially specific high-resolution force spectroscopy. Biomaterials 2005, 26, 271–283. [Google Scholar] [CrossRef]
- Pan, S.; Yu, H.; Yang, X.; Yang, X.; Wang, Y.; Liu, Q.; Jin, L.; Yang, Y. Application of Nanomaterials in Stem Cell Regenerative Medicine of Orthopedic Surgery. J. Nanomater. 2017, 2017. [Google Scholar] [CrossRef]
- Marc, S. Reisch New materials take a bite out of tooth decay. C&EN Glob. Enterp. 2016, 94, 16–19. [Google Scholar] [CrossRef]
- Herman, K.; Wujczyk, M.; Dobrzynski, M.; Diakowska, D.; Wiglusz, K.; Wiglusz, R.J. In Vitro Assessment of Long-Term Fluoride Ion Release from Nanofluorapatite. Materials 2021, 14, 3747. [Google Scholar] [CrossRef] [PubMed]
- Daas, I.; Badr, S.; Osman, E. Comparison between fluoride and nano-hydroxyapatite in remineralizing initial enamel lesion: An in vitro study. J. Contemp. Dent. Pract. 2018, 19, 306–312. [Google Scholar] [CrossRef]
- Coelho, C.C.; Grenho, L.; Gomes, P.S.; Quadros, P.A.; Fernandes, M.H. Nano-hydroxyapatite in oral care cosmetics: Characterization and cytotoxicity assessment. Sci. Rep. 2019, 9, 11050. [Google Scholar] [CrossRef]
- Al-Hazmi, F.; Alnowaiser, F.; Al-Ghamdi, A.A.; Al-Ghamdi, A.A.; Aly, M.M.; Al-Tuwirqi, R.M.; El-Tantawy, F. A new large—Scale synthesis of magnesium oxide nanowires: Structural and antibacterial properties. Superlattices Microstruct. 2012, 52, 200–209. [Google Scholar] [CrossRef]
- Kantharia, N.; Naik, S.; Apte, S.; Kheur, M.; Kheur, S.; Kale, B. Nano-hydroxyapatite and its contemporary applications. J. Dent. Res. Sci. Dev. 2014, 1, 15. [Google Scholar] [CrossRef]
- Ahmed, R.; Faisal, N.H.; Paradowska, A.M.; Fitzpatrick, M.E.; Khor, K.A. Neutron diffraction residual strain measurements in nanostructured hydroxyapatite coatings for orthopaedic implants. J. Mech. Behav. Biomed. Mater. 2011, 4, 2043–2054. [Google Scholar] [CrossRef]
- Chen, L.; Al-Bayatee, S.; Khurshid, Z.; Shavandi, A.; Brunton, P.; Ratnayake, J. Hydroxyapatite in Oral Care Products—A Review. Materials 2021, 14, 4865. [Google Scholar] [CrossRef]
- Juntavee, A.; Juntavee, N.; Hirunmoon, P. Remineralization potential of nanohydroxyapatite toothpaste compared with tricalcium phosphate and fluoride toothpaste on artificial carious lesions. Int. J. Dent. 2021, 2021, 5588832. [Google Scholar] [CrossRef]
- Burwell, A.K.; Litkowski, L.J.; Greenspan, D.C. Calcium sodium phosphosilicate (NovaMin): Remineralization potential. Adv. Dent. Res. 2009, 21, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Manchery, N.; John, J.; Nagappan, N.; Subbiah, G.K.; Premnath, P. Remineralization potential of dentifrice containing nanohydroxyapatite on artificial carious lesions of enamel: A comparative in vitro study. Dent. Res. J. 2019, 16, 310–317. [Google Scholar] [CrossRef]
- Juntavee, N.; Juntavee, A.; Plongniras, P. Remineralization potential of nano-hydroxyapatite on enamel and cementum surrounding margin of computer-aided design and computer-aided manufacturing ceramic restoration. Int. J. Nanomed. 2018, 13, 2755–2765. [Google Scholar] [CrossRef]
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, composition and mineralization. Front. Biosci. 2011, 3E, 711–735. [Google Scholar] [CrossRef] [PubMed]
- Tschoppe, P.; Zandim, D.L.; Martus, P.; Kielbassa, A.M. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J. Dent. 2011, 39, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Lubarsky, G.V.; Lemoine, P.; Meenan, B.J.; Deb, S.; Mutreja, I.; Carolan, P.; Petkov, N. Enamel proteins mitigate mechanical and structural degradations in mature human enamel during acid attack. Mater. Res. Express 2014, 1, 025404. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, Z.; Yang, J.; Lu, D.; Kishen, A.; Li, Y.; Chen, Z.; Que, K.; Zhang, Q.; Deng, X.; et al. Oriented and Ordered Biomimetic Remineralization of the Surface of Demineralized Dental Enamel Using HAP@ACP Nanoparticles Guided by Glycine. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Pepla, E.; Besharat, L.K.; Palaia, G.; Tenore, G.; Migliau, G. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: A review of literature. Ann. Stomatol. 2014, 5, 108–114. [Google Scholar] [CrossRef]
- Horst, O.V.; Chavez, M.G.; Jheon, A.H.; Desai, T.; Klein, O.D. Stem Cell and Biomaterials Research in Dental Tissue Engineering and Regeneration. Dent. Clin. N. Am. 2012, 56, 495–520. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef]
- Duailibi, M.T.; Duailibi, S.E.; Young, C.S.; Bartlett, J.D.; Vacanti, J.P.; Yelick, P.C. Bioengineered teeth from cultured rat tooth bud cells. J. Dent. Res. 2004, 83, 523–528. [Google Scholar] [CrossRef]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef]
- Gazalah, H.; Mantash, S.; Ramadan, N.; Al Lafi, S.; El Sitt, S.; Darwish, H.; Azari, H.; Fawaz, L.; Ghanem, N.; Zibara, K.; et al. Postnatal neural stem cells in treating traumatic brain injury. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2016; Volume 1462, pp. 689–710. [Google Scholar]
- Liu, H.; Gronthos, S.; Shi, S. Dental Pulp Stem Cells. In Methods in Enzymology; Academic Press Inc.: Cambridge, MA, USA, 2006; Volume 419, pp. 99–113. [Google Scholar]
- Hu, B.; Unda, F.; Bopp-Kuchler, S.; Jimenez, L.; Wang, X.J.; Haïkel, Y.; Wang, S.L.; Lesot, H. Bone marrow cells can give rise to ameloblast-like cells. J. Dent. Res. 2006, 85, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Telles, P.D.; Machado, M.A.D.A.M.; Sakai, V.T.; Nör, J.E. Pulp tissue from primary teeth: New source of stem cells. J. Appl. Oral Sci. 2011, 19, 189–194. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzynski, M.; Nowicka, J.; Pajaczkowska, M.; Szymonowicz, M.; Targonska, S.; Sobierajska, P.; Wiglusz, K.; Dobrzynski, W.; Lubojanski, A.; et al. The Influence of Ozonated Olive Oil-Loaded and Copper-Doped Nanohydroxyapatites on Planktonic Forms of Microorganisms. Nanomaterials 2020, 10, 1997. [Google Scholar] [CrossRef] [PubMed]
- Coppe, C.; Zhang, Y.; Den Besten, P.K. Characterization of primary dental pulp cells in vitro. Pediatr. Dent. 2009, 31, 467–471. [Google Scholar] [PubMed]
- Tran-Hung, L.; Mathieu, S.; About, I. Role of human pulp fibroblasts in angiogenesis. J. Dent. Res. 2006, 85, 819–823. [Google Scholar] [CrossRef]
- Pejcic, A.; Kojovic, D.; Mirkovic, D.; Minic, I. Stem cells for periodontal regeneration. Balk. J. Med. Genet. 2013, 16, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Albandar, J.M. Epidemiology and risk factors of periodontal diseases. Dent. Clin. N. Am. 2005, 49, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H.; Hirachi, A.; Hasegawa, N.; Iwata, T.; Hamaguchi, H.; Shiba, H.; Takata, T.; Kato, Y.; Kurihara, H. Enhancement of Periodontal Tissue Regeneration by Transplantation of Bone Marrow Mesenchymal Stem Cells. J. Periodontol. 2004, 75, 1281–1287. [Google Scholar] [CrossRef]
- Ding, G.; Liu, Y.; Wang, W.; Wei, F.; Liu, D.; Fan, Z.; An, Y.; Zhang, C.; Wang, S. Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem Cells 2010, 28, 1829–1838. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Ding, G.; Fang, D.; Zhang, C.; Bartold, P.M.; Gronthos, S.; Shi, S.; Wang, S. Periodontal Ligament Stem Cell-Mediated Treatment for Periodontitis in Miniature Swine. Stem Cells 2008, 26, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry—Part II: Clinical applications. J. Prosthodont. Res. 2012, 56, 229–248. [Google Scholar] [CrossRef] [PubMed]
- Tobita, M.; Uysal, A.C.; Ogawa, R.; Hyakusoku, H.; Mizuno, H. Periodontal tissue regeneration with adipose-derived stem cells. Tissue Eng. Part A 2008, 14, 945–953. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Rahiotis, C.; Zinelis, S.; Eliades, G.; Eliades, T. Setting characteristics of a resin infiltration system for incipient caries treatment. J. Dent. 2015, 43, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Paris, S.; Lausch, J.; Selje, T.; Dörfer, C.E.; Meyer-Lueckel, H. Comparison of sealant and infiltrant penetration into pit and fissure caries lesions in vitro. J. Dent. 2014, 42, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Askar, H.; Lausch, J.; Dörfer, C.E.; Meyer-Lueckel, H.; Paris, S. Penetration of micro-filled infiltrant resins into artificial caries lesions. J. Dent. 2015, 43, 832–838. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taher, N.M.; Alkhamis, H.A.; Dowaidi, S.M.L. The influence of resin infiltration system on enamel microhardness and surface roughness: An in vitro study. Saudi Dent. J. 2012, 24, 79–84. [Google Scholar] [CrossRef]
- Meyer-Lueckel, H.; Paris, S. Infiltration of natural caries lesions with experimental resins differing in penetration coefficients and ethanol addition. Caries Res. 2010, 44, 408–414. [Google Scholar] [CrossRef]
- Călin, D.; Mitrea, M. Assessment of aesthetic improvement of buccal white spot lesions through infiltration method. Rom. J. Funct. Clin. Macro- Microsc. Anat. Anthropol. Româna Anat. Funct. Clin. Macro Microsc. Antropol. 2015, 14, 91–99. [Google Scholar]
- Paris, S.; Meyer-Lueckel, H. Inhibition of caries progression by resin infiltration in situ. Caries Res. 2010, 44, 47–54. [Google Scholar] [CrossRef]
- Memarpour, M.; Shafiei, F.; Rafiee, A.; Soltani, M.; Dashti, M.H. Effect of hydroxyapatite nanoparticles on enamel remineralization and estimation of fissure sealant bond strength to remineralized tooth surfaces: An in vitro study. BMC Oral Health 2019, 19, 92. [Google Scholar] [CrossRef]
- Simonsen, R.J.; Neal, R.C. A review of the clinical application and performance of pit and fissure sealants. Aust. Dent. J. 2011, 56, 45–58. [Google Scholar] [CrossRef]
- Korporowicz, E.; Jasiński, P. Indications for pit and fissure sealing in view of the current literature. Nowa Stomatol. 2013, 4, 175–179. [Google Scholar]
- Alsadat, F.A.; El-Housseiny, A.A.; Alamoudi, N.M.; Alnowaiser, A.M. Conservative treatment for deep carious lesions in primary and young permanent teeth. Niger. J. Clin. Pract. 2018, 21, 1549–1556. [Google Scholar]
- Dhar, V.; Marghalani, A. Use of Vital Pulp Therapies in Primary Teeth with Deep Caries Lesions—PubMed. Pediat. Dent. 2017, 15, 146–159. [Google Scholar]
- Pulp Therapy for Primary and Immature Permanent Teeth: An Overview—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30444704/ (accessed on 17 October 2021).
- Goldberg, M. Central Indirect and Direct Pulp Capping: Reactionary vs. Reparative Dentins. JSM Dent 2020, 8, 1119. [Google Scholar]
- Opal, S.; Garg, S.; Dhindsa, A.; Taluja, T. Minimally invasive clinical approach in indirect pulp therapy and healing of deep carious lesions. J. Clin. Pediatr. Dent. 2014, 38, 185–192. [Google Scholar] [CrossRef]
- Orhan, A. Pulp Exposure Occurrence and Outcomes after 1-or 2-visit Indirect Pulp Therapy Vs Complete Caries Removal in Primary and Permanent Molars. Pediatr. Dent. 2009, 32, 347–355. [Google Scholar]
- Gruythuysen, R.; Van Strijp, G.; Wu, M.K. Long-term survival of indirect pulp treatment performed in primary and permanent teeth with clinically diagnosed deep carious lesions. J. Endod. 2010, 36, 1490–1493. [Google Scholar] [CrossRef]
- Pinto, A.S.; De Araújo, F.B.; Franzon, R.; Figueiredo, M.C.; Henz, S.; García-Godoy, F.; Maltz, M. Clinical and microbiological effect of calcium hydroxide protection in indirect pulp capping in primary teeth. Am. J. Dent. 2006, 19, 382–386. [Google Scholar]
- Schwendicke, F.; Dörfer, C.E.; Paris, S. Incomplete Caries Removal: A Systematic Review and Meta-analysis. J. Dent. Res. 2013, 92, 306–314. [Google Scholar] [CrossRef]
- Bergenholtz, G.; Axelsson, S.; Davidson, T.; Frisk, F.; Hakeberg, M.; Kvist, T.; Norlund, A.; Petersson, A.; Portenier, I.; Sandberg, H.; et al. Treatment of pulps in teeth affected by deep caries—A systematic review of the literature. Singap. Dent. J. 2013, 34, 1–12. [Google Scholar] [CrossRef]
- Ricketts, D.; Lamont, T.; Innes, N.P.; Kidd, E.; Clarkson, J.E. Operative caries management in adults and children. Cochrane Database Syst. Rev. 2013, 2013, CD003808. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, D.; Kidd, E.; Innes, N.P.T.; Clarkson, J.E. Complete or ultraconservative removal of decayed tissue in unfilled teeth. In Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Aminoshariae, A.; Hartwell, G.R.; Moon, P.C. Placement of mineral trioxide aggregate using two different techniques. J. Endod. 2003, 29, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Łuczaj-Cepowicz, E.; Marczuk-Kolada, G.; Waszkiel, D. Application of mineral trioxide aggregate (MTA) in direct pulp capping of Permanent teeth—One year observations. Nowa Stomatol. 2006, 4, 165–169. [Google Scholar]
- Ćwiklak, K.; Szczepańska, J. The use of MTA in immature permanent teeth—A review of the literature. Nowa Stomatol. 2012, 1, 19–23. [Google Scholar]
- Porenczuk, A. Application of the MTA in the management of pulpal floor perforation—A case report. Nowa Stomatol. 2018, 23, 126–131. [Google Scholar] [CrossRef]
- Menezes, R.; Monteiro Bramante, C.; Letra, A.; Graciela Gomes Carvalho, V.; Brandão Garcia, R. Histologic evaluation of pulpotomies in dog using two types of mineral trioxide aggregate and regular and white Portland cements as wound dressings. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 98, 376–379. [Google Scholar] [CrossRef]
- Gomes, A.C.; Gomes-Filho, J.E.; Oliveira, S.H.P. Mineral trioxide aggregate stimulates macrophages and mast cells to release neutrophil chemotactic factors: Role of IL-1β, MIP-2 and LTB4. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, e135–e142. [Google Scholar] [CrossRef]
- About, I. Biodentine: From biochemical and bioactive properties to clinical applications ScienceDirect. G. Ital. Endod. 2016, 30, 81–88. [Google Scholar] [CrossRef]
- Tran, X.V.; Gorin, C.; Willig, C.; Baroukh, B.; Pellat, B.; Decup, F.; Opsahl Vital, S.; Chaussain, C.; Boukpessi, T. Effect of a calcium-silicate-based restorative cement on pulp repair. J. Dent. Res. 2012, 91, 1166–1171. [Google Scholar] [CrossRef]
- Bachoo, I.K.; Seymour, D.; Brunton, P. A biocompatible and bioactive replacement for dentine: Is this a reality? The properties and uses of a novel calcium-based cement. Br. Dent. J. 2013, 214, E5. [Google Scholar] [CrossRef]
- Patel, N. Comparing Gray and White Mineral Trioxide Aggregate as a Repair Material for Furcation Perforation: An in Vitro Dye Extraction Study. J. Clin. Diagn. Res. 2014, 8, ZC70–ZC73. [Google Scholar] [CrossRef]
- Hakki, S.S.; Bozkurt, S.B.; Ozcopur, B.; Purali, N.; Belli, S. Periodontal ligament fibroblast response to root perforations restored with different materials—A laboratory study. Int. Endod. J. 2012, 45, 240–248. [Google Scholar] [CrossRef]
- Mnyusiwalla, A.; Daar, A.S.; Singer, P.A. “Mind the gap”: Science and ethics in nanotechnology. Nanotechnology 2003, 14, R9. [Google Scholar] [CrossRef]
- Najeeb, S.; Khurshid, Z.; Zafar, M.S.; Khan, A.S.; Zohaib, S.; Martí, J.M.N.; Sauro, S.; Matinlinna, J.P.; Rehman, I.U. Modifications in glass ionomer cements: Nano-sized fillers and bioactive nanoceramics. Int. J. Mol. Sci. 2016, 17, 1134. [Google Scholar] [CrossRef]
- Amin, F.; Rahman, S.; Khurshid, Z.; Zafar, M.S.; Sefat, F.; Kumar, N. Effect of Nanostructures on the Properties of Glass Ionomer Dental Restoratives/Cements: A Comprehensive Narrative Review. Materials 2021, 14, 6260. [Google Scholar] [CrossRef] [PubMed]
- Lyapina, M.G.; Tzekova, M.; Dencheva, M.; Krasteva, A.; Yaneva-Deliverska, M.; Kisselova, A. Nano-glass-ionomer cements in modern restorative dentistry. J. IMAB Annu. Proceed. 2016, 22, 1160–1165. [Google Scholar] [CrossRef][Green Version]
- Chen, M.-H. Update on Dental Nanocomposites. J. Dent. Res. 2010, 89, 549–560. [Google Scholar] [CrossRef]
- Hannig, M.; Hannig, C. Nanomaterials in preventive dentistry. Nat. Nanotechnol. 2010, 5, 565–569. [Google Scholar] [CrossRef]
- Huang, S.; Gao, S.; Cheng, L.; Yu, H. Remineralization Potential of Nano-Hydroxyapatite on Initial Enamel Lesions: An in vitro Study. Caries Res. 2011, 45, 460–468. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Ansari, S.; Moshaverinia, M.; Roohpour, N.; Darr, J.A.; Rehman, I. Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional glass ionomer cements (GIC). Acta Biomater. 2008, 4, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.L.; Xu, H.H.K. Fluoride releasing restorative materials: Effects of pH on mechanical properties and ion release. Dent. Mater. 2010, 26, e227–e235. [Google Scholar] [CrossRef]
- Paschoal, M.A.B.; Gurgel, C.V.; Rios, D.; Magalhães, A.C.; Buzalaf, M.A.R.; Machado, M.A.D.A.M. Fluoride release profile of a nanofilled resin-modified glass ionomer cement. Braz. Dent. J. 2011, 22, 275–279. [Google Scholar] [CrossRef] [PubMed]
- De Caluwé, T.; Vercruysse, C.W.J.; Ladik, I.; Convents, R.; Declercq, H.; Martens, L.C.; Verbeeck, R.M.H. Addition of bioactive glass to glass ionomer cements: Effect on the physico-chemical properties and biocompatibility. Dent. Mater. 2017, 33, e186–e203. [Google Scholar] [CrossRef]
- Gölz, L.; Simonis, R.A.; Reichelt, J.; Stark, H.; Frentzen, M.; Allam, J.P.; Probstmeier, R.; Winter, J.; Kraus, D. In vitro biocompatibility of ICON® and TEGDMA on human dental pulp stem cells. Dent. Mater. 2016, 32, 1052–1064. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Leyhausen, G.; Volk, J.; Tsiftsoglou, A.; Garefis, P.; Koidis, P.; Geurtsen, W. Effects of HEMA and TEDGMA on the in vitro odontogenic differentiation potential of human pulp stem/progenitor cells derived from deciduous teeth. Dent. Mater. 2011, 6, 608–617. [Google Scholar] [CrossRef] [PubMed]
- AlKahtani, R.N. The implications and applications of nanotechnology in dentistry: A review. Saudi Dent. J. 2018, 30, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wang, L.; Xing, D.; Arola, D.D.; Weir, M.D.; Bai, Y.; Xu, H.H.K. Protein-repellent and antibacterial functions of a calcium phosphate rechargeable nanocomposite. J. Dent. 2016, 52, 15–22. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, H.; Weir, M.D.; Melo, M.A.S.; Levine, E.D.; Xu, H.H.K. Effect of dimethylaminohexadecyl methacrylate mass fraction on fracture toughness and antibacterial properties of CaP nanocomposite. J. Dent. 2015, 43, 1539–1546. [Google Scholar] [CrossRef]
- Ionescu, A.C.; Brambilla, E.; Travan, A.; Marsich, E.; Donati, I.; Gobbi, P.; Turco, G.; Di Lenarda, R.; Cadenaro, M.; Paoletti, S.; et al. Silver-polysaccharide antimicrobial nanocomposite coating for methacrylic surfaces reduces Streptococcus mutans biofilm formation in vitro. J. Dent. 2015, 43, 1483–1490. [Google Scholar] [CrossRef]
- Cianetti, S.; Abraha, I.; Pagano, S.; Lupatelli, E.; Lombardo, G. Sonic and ultrasonic oscillating devices for the management of pain and dental fear in children or adolescents that require caries removal: A systematic review. BMJ Open 2018, 8, e020840. [Google Scholar] [CrossRef]
- Wu, L.; Gao, X. Children’s dental fear and anxiety: Exploring family related factors. BMC Oral Health 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Olegário, I.C.; Hesse, D.; Bönecker, M.; Imparato, J.C.P.; Braga, M.M.; Mendes, F.M.; Raggio, D.P. Effectiveness of conventional treatment using bulk-fill composite resin versus Atraumatic Restorative Treatments in primary and permanent dentition: A pragmatic randomized clinical trial. BMC Oral Health 2016, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, D.N.J.; Pitts, N.B. Novel operative treatment options. Monogr. Oral Sci. 2009, 21, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Velickovic, M.; Arsenijevic, A.; Acovic, A.; Arsenijevic, D.; Milovanovic, J.; Dimitrijevic, J.; Todorovic, Z.; Milovanovic, M.; Kanjevac, T.; Arsenijevic, N. Galectin-3, Possible Role in Pathogenesis of Periodontal Diseases and Potential Therapeutic Target. Front. Pharmacol. 2021, 12, 378. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.G.; Lira Junior, R.; Retamal-Valdes, B.; Figueiredo, L.C.D.; Malheiros, Z.; Stewart, B.; Feres, M. Periodontal disease and its impact on general health in Latin America. Section V: Treatment of periodontitis. Braz. Oral Res. 2020, 34, e026. [Google Scholar] [CrossRef]
- Aoyama, N.; Kobayashi, N.; Hanatani, T.; Ashigaki, N.; Yoshida, A.; Shiheido, Y.; Sato, H.; Takamura, C.; Yoshikawa, S.; Matsuo, K.; et al. Periodontal condition in Japanese coronary heart disease patients: A comparison between coronary and non-coronary heart diseases. J. Periodontal Res. 2019, 54, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhen, Z.; Liu, H.N.; Lai, I.; Pelekos, G.; Tse, H.; Yiu, K.; Jin, L. Periodontitis links to exacerbation of myocardial dysfunction in subjects with type 2 diabetes. J. Periodontal Res. 2019, 54, 339–348. [Google Scholar] [CrossRef]
- Agnello, L.; Bivona, G.; Lo Sasso, B.; Scazzone, C.; Bazan, V.; Bellia, C.; Ciaccio, M. Galectin-3 in acute coronary syndrome. Clin. Biochem. 2017, 50, 797–803. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Alibrandi, A.; Williams, R.C.; Lo Giudice, A. Analysis of galectin-3 levels as a source of coronary heart disease risk during periodontitis. J. Periodontal Res. 2021, 56, 597–605. [Google Scholar] [CrossRef]
- Strauss, F.-J.; Espinoza, I.; Stähli, A.; Baeza, M.; Cortés, R.; Morales, A.; Gamonal, J. Dental caries is associated with severe periodontitis in Chilean adults: A cross-sectional study. BMC Oral Health 2019, 19, 1–8. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wrzyszcz-Kowalczyk, A.; Dobrzynski, M.; Grzesiak-Gasek, I.; Zakrzewski, W.; Mysiak-Debska, M.; Nowak, P.; Zimolag, M.; Wiglusz, R.J. Application of Selected Biomaterials and Stem Cells in the Regeneration of Hard Dental Tissue in Paediatric Dentistry—Based on the Current Literature. Nanomaterials 2021, 11, 3374. https://doi.org/10.3390/nano11123374
Wrzyszcz-Kowalczyk A, Dobrzynski M, Grzesiak-Gasek I, Zakrzewski W, Mysiak-Debska M, Nowak P, Zimolag M, Wiglusz RJ. Application of Selected Biomaterials and Stem Cells in the Regeneration of Hard Dental Tissue in Paediatric Dentistry—Based on the Current Literature. Nanomaterials. 2021; 11(12):3374. https://doi.org/10.3390/nano11123374
Chicago/Turabian StyleWrzyszcz-Kowalczyk, Alina, Maciej Dobrzynski, Iwona Grzesiak-Gasek, Wojciech Zakrzewski, Monika Mysiak-Debska, Patrycja Nowak, Malgorzata Zimolag, and Rafal J. Wiglusz. 2021. "Application of Selected Biomaterials and Stem Cells in the Regeneration of Hard Dental Tissue in Paediatric Dentistry—Based on the Current Literature" Nanomaterials 11, no. 12: 3374. https://doi.org/10.3390/nano11123374
APA StyleWrzyszcz-Kowalczyk, A., Dobrzynski, M., Grzesiak-Gasek, I., Zakrzewski, W., Mysiak-Debska, M., Nowak, P., Zimolag, M., & Wiglusz, R. J. (2021). Application of Selected Biomaterials and Stem Cells in the Regeneration of Hard Dental Tissue in Paediatric Dentistry—Based on the Current Literature. Nanomaterials, 11(12), 3374. https://doi.org/10.3390/nano11123374







