Heat-Up Colloidal Synthesis of Shape-Controlled Cu-Se-S Nanostructures—Role of Precursor and Surfactant Reactivity and Performance in N2 Electroreduction
Abstract
:1. Introduction
2. Results and Discussion
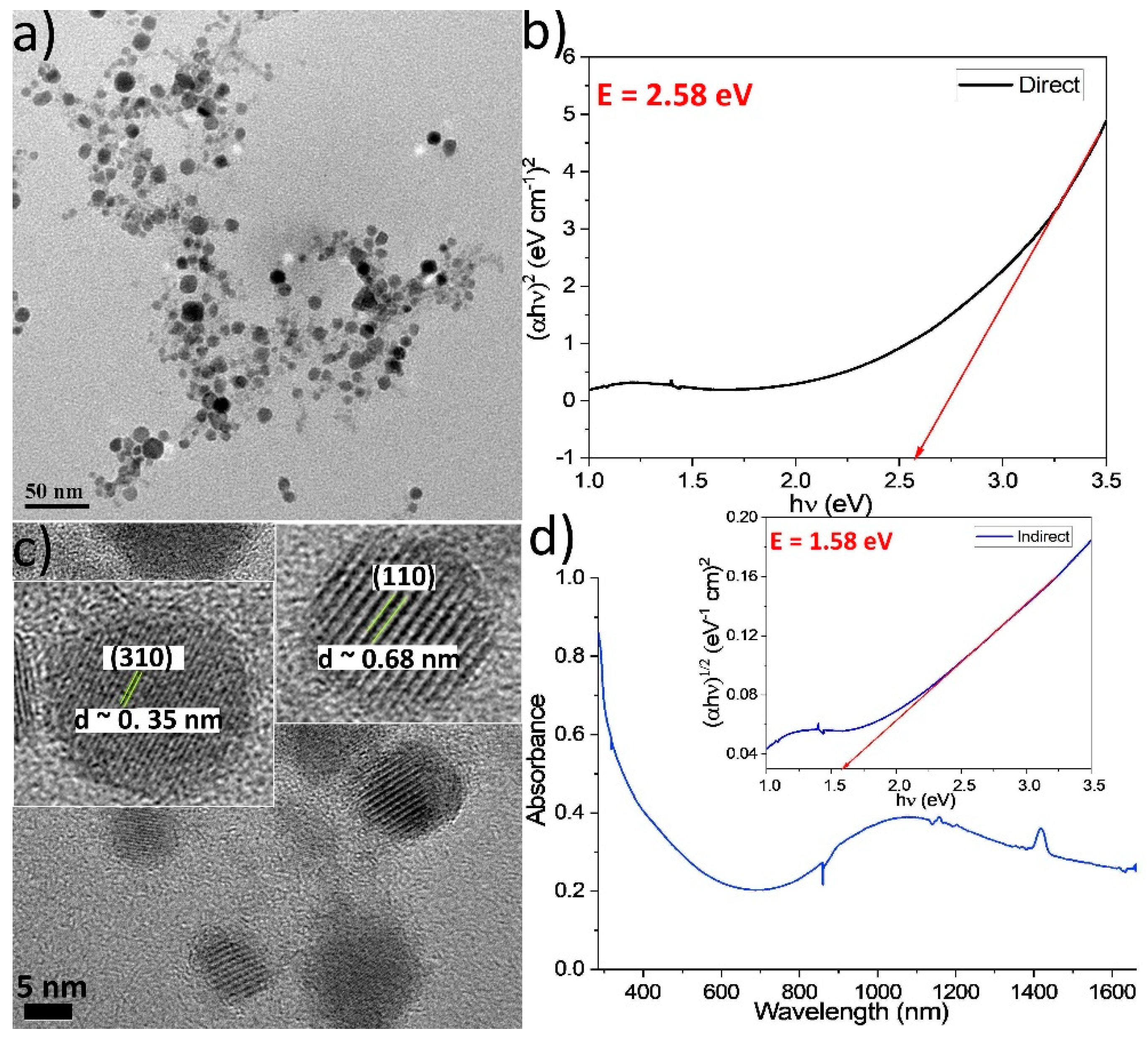
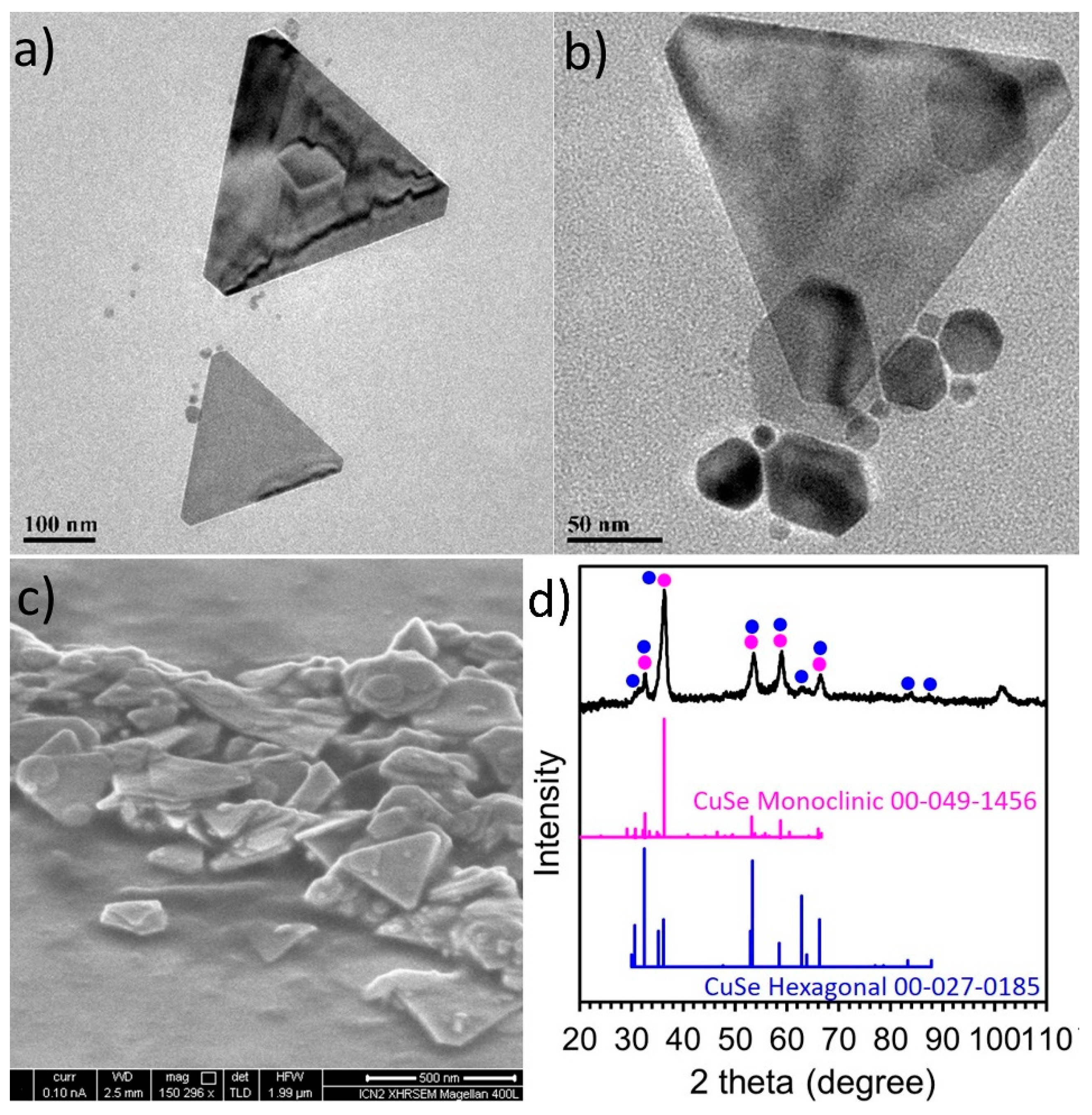
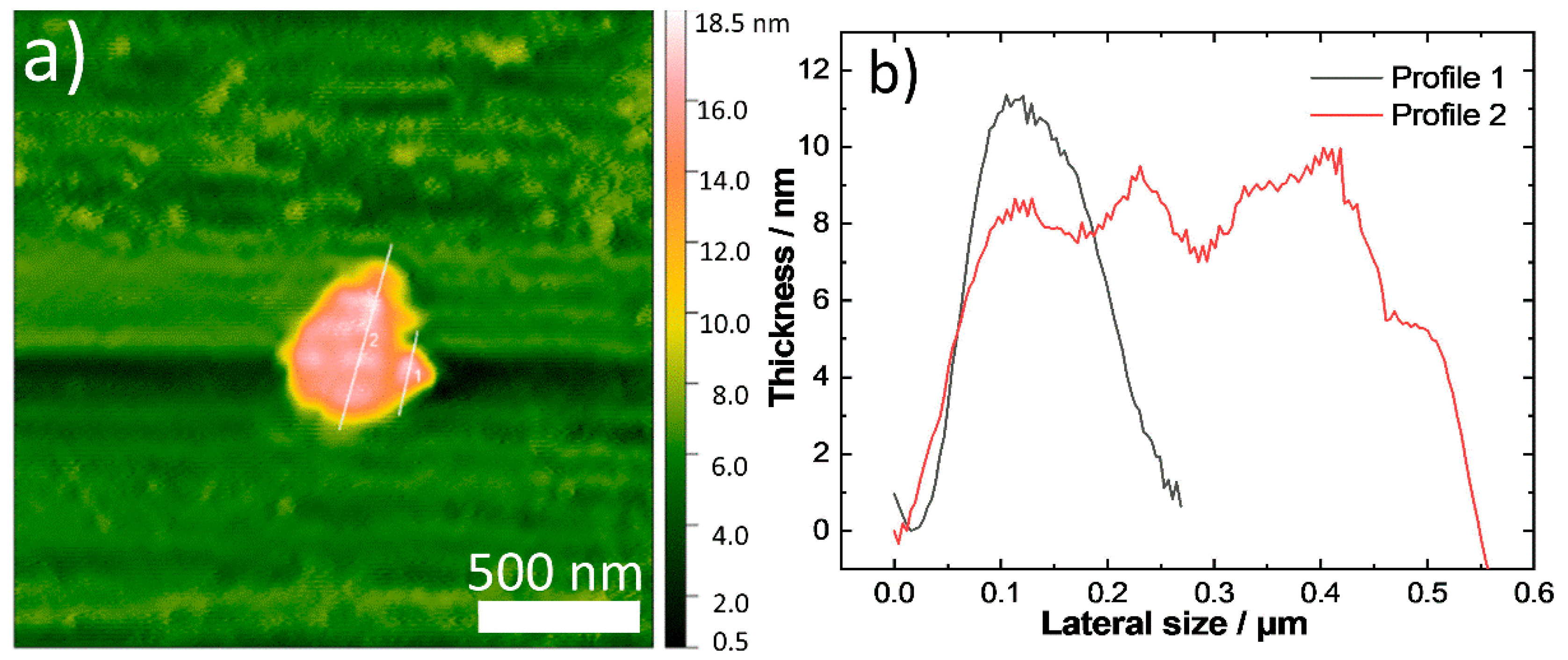
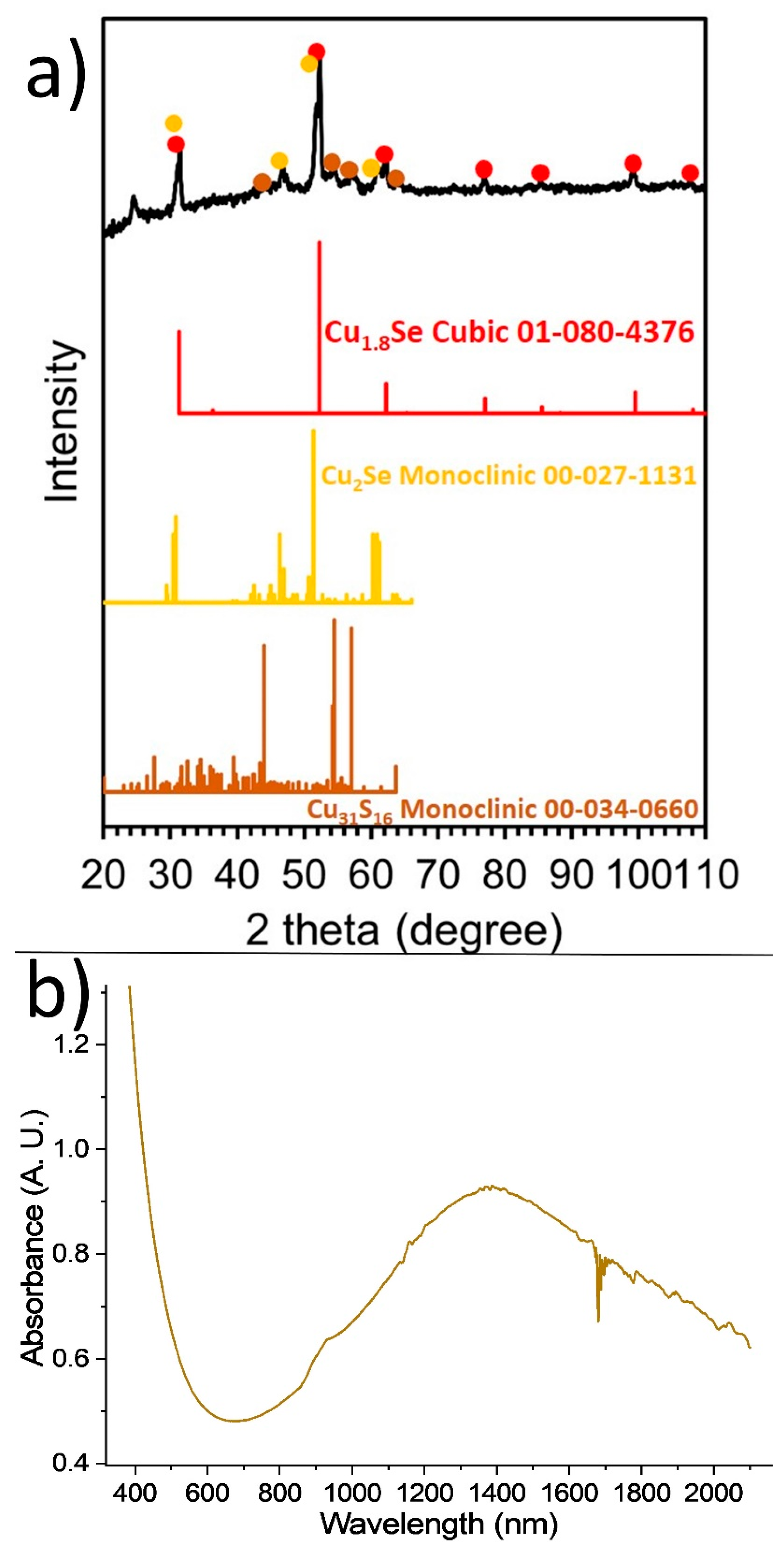
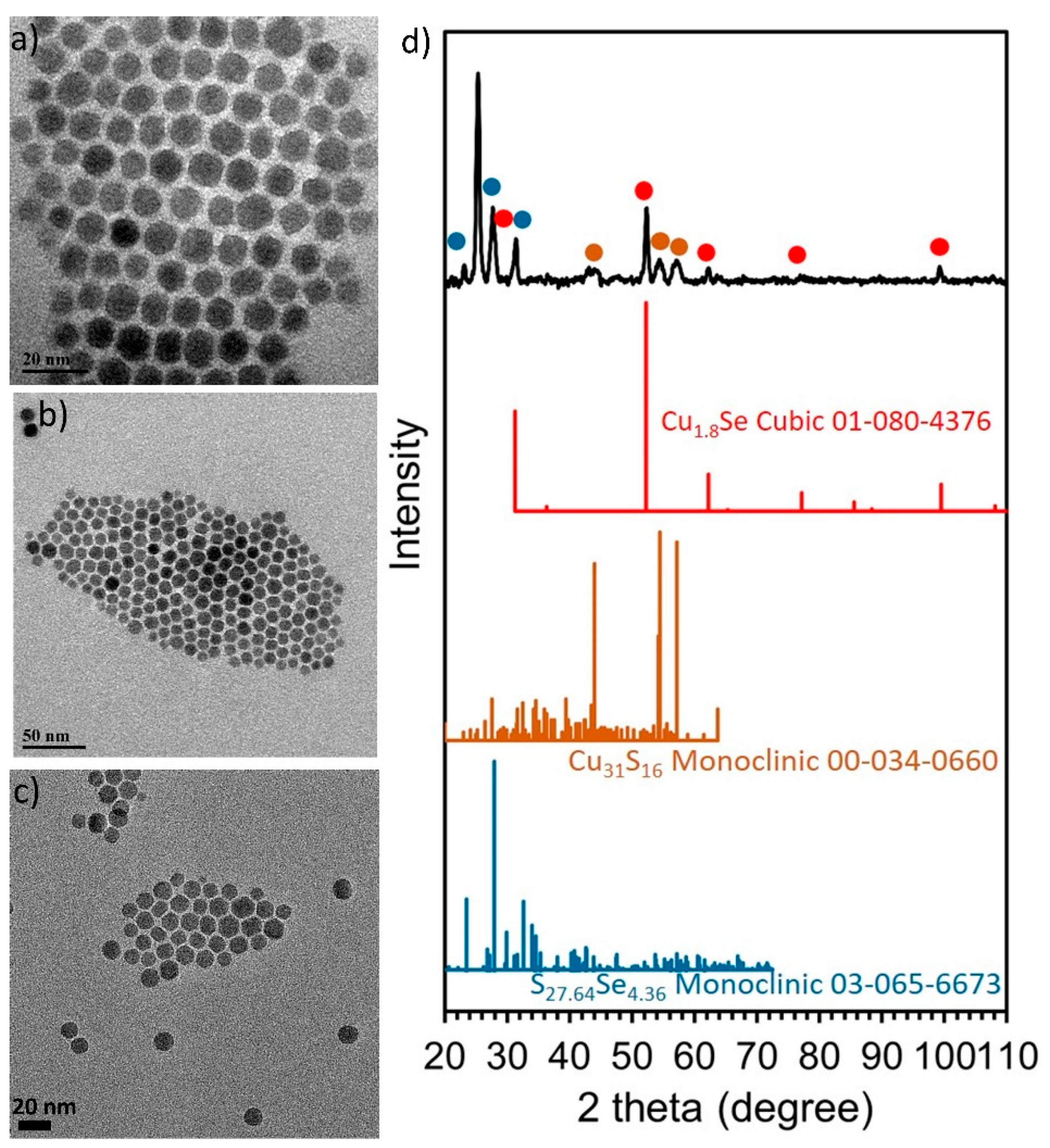
2.1. Synthesis, Composition and Morphology
2.2. Tauc Plots—Band Gaps
2.3. Nitrogen Reduction Reaction
3. Conclusions
4. Materials and Methods
4.1. Synthesis of CuSe Nanostructures
4.2. Characterisation Instruments
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willhammar, T.; Sentosun, K.; Mourdikoudis, S.; Goris, B.; Kurttepeli, M.; Bercx, M.; Lamoen, D.; Partoens, B.; Pastoriza-Santos, I.; Perez-Juste, J.; et al. Structure and vacancy distribution in copper telluride nanoparticles influence plasmonic activity in the near-infrared. Nat. Commun. 2017, 8, 14925. [Google Scholar] [CrossRef]
- Yang, D.; Zhu, Q.; Chen, C.; Liu, H.; Liu, Z.; Zhao, H.; Zhang, X.; Liu, S.; Han, B. Selective electroreduction of carbon dioxide to methanol on copper selenide nanocatalysts. Nat. Commun. 2019, 10, 677. [Google Scholar] [CrossRef]
- Hessel, C.M.; Pattani, V.P.; Rasch, M.; Panthani, M.G.; Koo, B.; Tunnell, J.W.; Korgel, B.A. Copper selenide nanocrystals for photothermal therapy. Nano Lett. 2011, 11, 2560. [Google Scholar] [CrossRef] [Green Version]
- Riha, S.C.; Johnson, D.C.; Prieto, A.L. Cu2Se nanoparticles with tunable electronic properties due to a controlled solid-state phase transition driven by copper oxidation and cationic conduction. J. Am. Chem. Soc. 2011, 133, 1383. [Google Scholar] [CrossRef]
- Chen, X.Q.; Li, Z.; Dou, S.X. Ambient facile synthesis of gram-scale selenide nanostructures from commercial copper and selenium powder. ACS Appl. Mater. Interfaces 2015, 7, 13295. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.-Z.; Zhou, Y.-N.; Zhang, B.; Yu, L.; Zhang, H.; Fu, Z.-W. Fabrication and electrochemical characterization of copper selenide thin films by pulsed laser deposition. J. Electrochem. Soc. 2006, 153, A2262. [Google Scholar] [CrossRef]
- Sibokoza, S.B.; Moloto, M.J.; Mtunzi, F.; Moloto, N. Diphenyldiselenide mediated synthesis of copper selenide nanoparticles and their poly(methyl methacrylate) nanofibers. Asian J. Chem. 2018, 30, 1455. [Google Scholar] [CrossRef]
- Balitskii, O.A.; Sytnyk, M.; Stangl, J.; Primetzhofer, D.; Groiss, H.; Heiss, W. Tuning the localized surface plasmon resonance in Cu2−xSe nanocrystals by postsynthetic ligand exchange. ACS Appl. Mater. Interfaces 2014, 6, 17770. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, L.; Liu, Z.; Tang, A. Effects of surface ligands on localized surface plasmon resonance and stabilization of Cu2−xSe nanocrystals. Appl. Surf. Sci. 2020, 509, 145327. [Google Scholar] [CrossRef]
- Polavarapu, L.; Mourdikoudis, S.; Pastoriza-Santos, I.; Perez-Juste, J. Nanocrystal engineering of noble metals and metal chalcogenides: Controlling the morphology, composition and crystallinity. CrystEngComm 2015, 17, 3727. [Google Scholar] [CrossRef]
- Hardtdegen, H.; Mikulics, M.; Rieß, S.; Schuck, M.; Saltzmann, T.; Simon, U.; Longo, M. Modern chemical synthesis methods towards low-dimensional phase change structures in the Ge-Sb-Te material system. Prog. Cryst. Growth Charact. Mater. 2015, 61, 27. [Google Scholar] [CrossRef]
- Mikulics, M.; Hardtdegen, H.H. Fully photon operated transmistor/all-optical switch based on a layered Ge1Sb2Te4 phase change medium. FlatChem 2020, 23, 100186. [Google Scholar] [CrossRef]
- Chen, X.; Dai, W.; Qin, F.; Xu, K.; Xu, H.; Wu, T.; Li, J.; Luo, W.; Yang, J. Low-dimensional copper selenide nanostructures: Controllable morphology and its dependence on electrocatalytic performance. ChemElectroChem 2019, 6, 574. [Google Scholar] [CrossRef]
- Zhu, L.; Gao, F.; Lv, P.; Zheng, Y.; Wang, W.; Zheng, W. Facile synthesis of 3D flower-like Cu2−xSe nanostructures via a sacrificing template method and their excellent antibacterial activities. CrystEngComm 2017, 19, 7253. [Google Scholar] [CrossRef]
- Chen, H.; Zou, B.; Wang, N.; Chen, H.; Zhang, Z.; Sun, Y.; Yu, L.; Tian, Q.; Chen, Z.; Hu, J. Morphology-selective synthesis and wettability properties of well-aligned Cu2−xSe nanostructures on a copper substrate. J. Mater. Chem. 2011, 21, 3053. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, D.; Hu, H.; Swihart, M.T.; Wei, W. Controlled synthesis of Cu2−xSe nanoparticles as near-infrared photothermal agents and irradiation wavelength dependence of their photothermal conversion efficiency. Langmuir 2018, 34, 13905. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, T.; Liu, H.; Zhang, S.; Zhang, H.; Li, M.; Sun, Q.; Li, Z. The release and detection of copper ions from ultrasmall theranostic Cu2−xSe nanoparticles. Nanoscale 2019, 11, 11819. [Google Scholar]
- Saldanha, P.L.; Brescia, R.; Prato, M.; Li, H.; Povia, M.; Manna, L.; Lesnyak, V. Generalized one-pot synthesis of copper sulfide, selenide-sulfide and telluride-sulfide nanoparticles. Chem. Mater. 2014, 26, 1442. [Google Scholar] [CrossRef]
- Zhao, T.; Oh, N.; Jishkariani, D.; Zhang, M.; Wang, H.; Li, N.; Lee, J.D.; Zeng, C.; Muduli, M.; Choi, H.-J.; et al. General synthetic route to high-quality colloidal III-V semiconductor quantum dots based on pnictogen chlorides. J. Am. Chem. Soc. 2019, 141, 15145. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, K.; Ye, Y.; Zhang, S.; Liu, Y.; Wang, G.; Liang, C.; Zhang, H.; Zhao, H. Efficient electrocatalytic nitrogen reduction to ammonia with aqueous silver nanodots. Commun. Chem. 2021, 4, 10. [Google Scholar] [CrossRef]
- Xiao, G.; Ning, J.; Liu, Z.; Sui, Y.; Wang, Y.; Dong, Q.; Tian, W.; Liu, B.; Zou, G.; Zou, B. Solution synthesis of copper selenide nanocrystals and their electrical transport properties. CrystEngComm 2012, 14, 2139. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Liz-Marzan, L.M. Oleylamine in nanoparticle synthesis. Chem. Mater. 2013, 25, 1465. [Google Scholar]
- White, S.L.; Banerjee, P.; Jain, P.K. Liquid-like cationic sub-lattice in copper selenide clusters. Nat. Commun. 2017, 8, 14514. [Google Scholar]
- Zobac, O.; Kroupa, A.; Zemanova, A.; Richter, K.W. Experimental description of the Al-Cu binary phase diagram. Metal. Mater. Trans. A 2019, 50, 3805. [Google Scholar] [CrossRef] [Green Version]
- Dorfs, D.; Hartling, T.; Miszta, K.; Bigall, N.C.; Kim, M.R.; Genovese, A.; Falqui, A.; Povia, M.; Manna, L. Reversible tenability of the near-infrared valence band plasmon resonance in Cu2−xSe nanocrystals. J. Am. Chem. Soc. 2011, 133, 11175. [Google Scholar] [CrossRef] [PubMed]
- Kriegel, I.; Jiang, C.; Rodriguez-Fernandez, J.; Schaller, R.D.; Talapin, D.V.; da Como, E.; Feldmann, J. Tuning the excitonic and plasmonic properties of copper chalcogenide nanocrystals. J. Am. Chem. Soc. 2012, 134, 1583. [Google Scholar] [CrossRef]
- Coughlan, C.; Ibanez, M.; Dobrozhan, O.; Singh, A.; Cabo, A.; Ryan, K.M. Compound copper chalcogenide nanocrystals. Chem. Rev. 2017, 117, 5865. [Google Scholar] [CrossRef] [PubMed]
- Webber, D.H.; Buckley, J.J.; Antunez, P.D.; Brutchey, R.L. Facile dissolution of selenium and tellurium in a thiol-amine solvent mixture under ambient conditions. Chem. Sci. 2014, 5, 2498. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, D.; Shen, L.; Zhang, H.; Zhang, X.; Yang, B. Alkythiol-enabled Se powder dissolution in oleylamine at room temperature for the phosphine-free synthesis of copper-based quaternary selenide nanocrystals. J. Am. Chem. Soc. 2012, 134, 7207. [Google Scholar] [CrossRef]
- Shao, H.; Huang, Y.; Lee, H.S.; Suh, Y.J.; Kim, C.O. Effect of surfactants on the size and shape of cobalt nanoparticles synthesized by thermal decomposition. J. Appl. Phys. 2006, 99, 08N702. [Google Scholar] [CrossRef]
- Lesyuk, R.; Klein, E.; Yaremchuk, I.; Klinke, C. Copper sulfide nanosheets with shape-tunable plasmonic properties in the NIR region. Nanoscale 2018, 10, 20640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gargioni, C.; Borzenkov, M.; D’Alfonso, L.; Sperandeo, P.; Polissi, A.; Cucca, L.; Dacarro, G.; Grisoli, P.; Pallavicini, P.; D’Agostino, A.; et al. Self-assembled monolayers of copper sulfide nanoparticles on glass as antibacterial coatings. Nanomaterials 2020, 10, 352. [Google Scholar] [CrossRef] [Green Version]
- Materials Science and Metallurgy Course A: Metals and Alloys; Lecture Notes; D’Agostino, A. (Ed.) University of Cambridge: Cambridge, UK.
- Marin, R.; Lifante, J.; Besteiro, L.V.; Wang, Z.; Govorov, A.O.; Rivero, F.; Alfonso, F.; Sanz-Rodriguez, F.; Jaque, D. Plasmonic copper sulfide nanoparticles enable dark contrast in optical coherence tomography. Adv. Healthc. Mater. 2020, 9, 1901627. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, G. Functions of 1-dodecanethiol in the synthesis and post-treatment of copper sulfide nanoparticles relevant to their photocatalytic applications. ACS Appl. Nano Mater. 2018, 1, 4587. [Google Scholar] [CrossRef]
- Green, M. The nature of quantum dot capping ligands. J. Mater. Chem. 2010, 20, 5797. [Google Scholar] [CrossRef]
- Becerra, L.R.; Murray, C.B.; Griffin, R.G.; Bawendi, M.G. Investigation of the surface morphology of capped CdSe nanocrystallites by 31P nuclear magnetic resonance. J. Chem. Phys. 1994, 100, 3297. [Google Scholar] [CrossRef]
- Lorenz, J.K.; Ellis, A.B. Surfactant-semiconductor interfaces: Perturbation of the photoluminescence of bulk cadmium selenide by adsorption of tri-n-octylphosphine oxide as a probe of solution aggregation with relevance to nanocrystal stabilization. J. Am. Chem. Soc. 1998, 120, 10970. [Google Scholar] [CrossRef]
- Abutbul, R.E.; Golan, Y. ‘Beneficial impurities’ in colloidal synthesis of surfactant coated inorganic nanoparticles. Nanotechnology 2021, 32, 102001. [Google Scholar] [CrossRef]
- Liz-Marzan, L.M.; Kagan, C.R.; Millstone, J.E. Reproducibility in nanocrystal synthesis? Watch out for impurities. ACS Nano 2020, 14, 6359. [Google Scholar] [CrossRef]
- Peng, X.; Manna, L.; Wang, W.; Wickham, J.; Scher, E.; Kadanavich, A.; Alivisatos, A.P. Shape control of CdSe nanocrystals. Nature 2020, 404, 59. [Google Scholar] [CrossRef]
- Petrovic, M.; Gilic, M.; Cirkovic, J.; Romcevic, M.; Romcevic, N.; Trajic, J.; Yahia, I. Optical properties of CuSe thin films—Band gap determination. Sci. Sinter. 2017, 49, 167. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Li, P.; Liang, S.; Yang, Z. Diversified copper sulfide (Cu2−xS) micro-/nanostructures: A comprehensive review on synthesis, modifications and applications. Nanoscale 2017, 9, 11357. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.A.; Panda, E. Copper deficiency induced varying electronic structure and optoelectronic properties of Cu2−xS thin films. Appl. Surf. Sci. 2019, 488, 477. [Google Scholar] [CrossRef]
- Gupta, K.; Singh, M.; Mohan, P.; Mott, D.; Maenosono, S. Synthesis and Characterization of copper sulfide-manganese sulfide nanoparticles with chestnut morphology and study on the semiconducting properties. ChemistrySelect 2019, 4, 3898. [Google Scholar] [CrossRef]
- Itoh, K.; Kuzuya, T.; Sumiyama, K. Morphology and composition-controls of CuxS nancrystals using alkylamine ligands. Mater. Trans. 2006, 47, 1953. [Google Scholar] [CrossRef] [Green Version]
- Shitu, I.G.; Talib, Z.A.; Chi, J.L.Y.; Kechlick, M.M.A.; Baqiah, H. Influence of tartaric acid concentration on structural and optical properties of CuSe nanoparticles synthesized via microwave assisted method. Results Phys. 2020, 17, 103041. [Google Scholar] [CrossRef]
- Wang, X.; Miao, Z.; Ma, Y.; Chen, H.; Qian, H.; Zha, Z. One-pot solution synthesis of shape-controlled copper selenide nanostructures and their potential applications in photocatalysis and photothermal therapy. Nanoscale 2017, 9, 14512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Xiong, B.; Chen, L.; Shi, J. Modulation strategies of Cu-based electrocatalysts for efficient nitrogen reduction. J. Mater. Chem. A 2020, 8, 20286. [Google Scholar] [CrossRef]
- Ma, L.; Li, Y.; Xu, Y.; Sun, J.; Liu, J.; Wu, T.; Ding, X.; Niu, Y. Two-dimensional transition metal dichalcogenides for electrocatalytic nitrogen fixation to ammonia: Advance, challenges and perspectives. A mini review. Electrochem. Commun. 2021, 125, 107002. [Google Scholar] [CrossRef]
- Ciglenecki, I.; Krznaric, D.; Helz, G. Voltammetry of copper sulfide particles and nanoparticles: Investigation of the cluster hypothesis. Environ. Sci. Technol. 2005, 39, 7492. [Google Scholar] [CrossRef]
- Singh, S.C.; Li, H.; Yao, C.; Zhan, Z.; Yu, W.; Yu, Z.; Guo, C. Structural and compositional control in copper selenide nanocrystals for light-induced self-repairable electrodes. Nano Energy 2018, 51, 774. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.D.; Opallo, M.; Revaprasadu, N. Colloidal synthesis of metal chalcogenide nanomaterials from metal-organic precursors and capping ligand effect on electrocatalytic performance: Progress, challenges and future perpsectives. Dalton Trans. 2021, 50, 11347. [Google Scholar] [CrossRef] [PubMed]
- Giuffredi, G.; Asset, T.; Liu, Y.; Atanassov, P.; Di Fonzo, F. Transition metal chalcogenides as a versatile and tunable platform for catalytic CO2 and N2 electroreduction. ACS Mater. Au 2021, 1, 6. [Google Scholar] [CrossRef]
- Antonatos, N.; Kovalska, E.; Mazanek, V.; Vesely, M.; Sedmidubsky, D.; Wu, B.; Sofer, Z. Electrochemical exfoliation of Janus-like BiTeI nanosheets for electrocatalytic nitrogen reduction. ACS Appl. Nano Mater. 2021, 4, 590. [Google Scholar] [CrossRef]
- Connor, S.; Schuch, J.; Kaiser, B.; Jaegermann, W. The determination of electrochemical active surface are and specific capacity revisited for the system MnOx as an oxygen evolution catalyst. Z. Phys. Chem. 2020, 234, 979. [Google Scholar] [CrossRef] [Green Version]




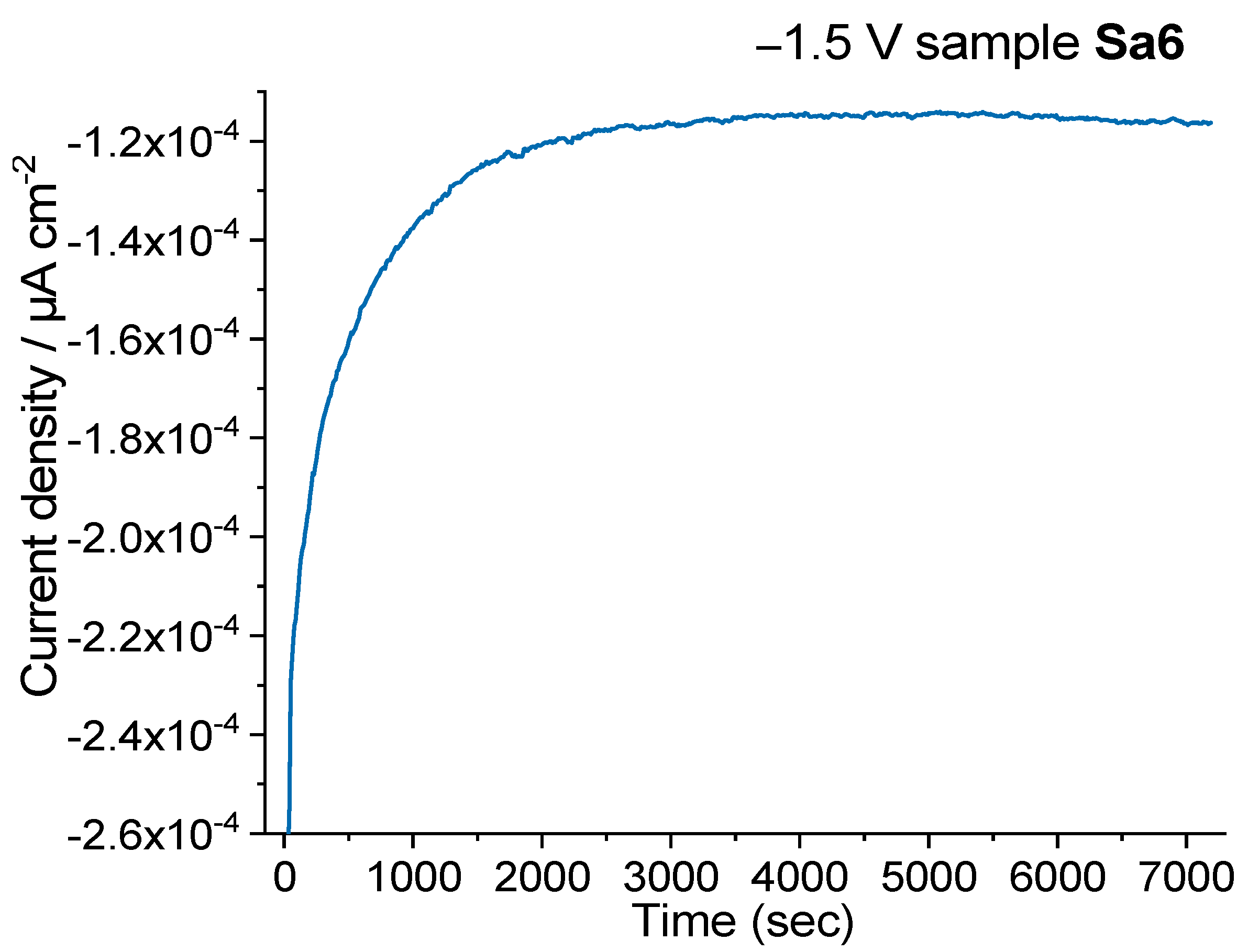
| −1.5 V | −1.7 V | −1.8 V | −1.9 V | |
|---|---|---|---|---|
| Sample Sa6 | 99/8.8 | 174/1.29 | 167/0.34 | 190/0.1 |
| Sample Sa7 | 88/1.97 | 115/0.38 | ||
| Sample Sa8 | 112/2.1 | 112/1.03 | 205/0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourdikoudis, S.; Antonaropoulos, G.; Antonatos, N.; Rosado, M.; Storozhuk, L.; Takahashi, M.; Maenosono, S.; Luxa, J.; Sofer, Z.; Ballesteros, B.; et al. Heat-Up Colloidal Synthesis of Shape-Controlled Cu-Se-S Nanostructures—Role of Precursor and Surfactant Reactivity and Performance in N2 Electroreduction. Nanomaterials 2021, 11, 3369. https://doi.org/10.3390/nano11123369
Mourdikoudis S, Antonaropoulos G, Antonatos N, Rosado M, Storozhuk L, Takahashi M, Maenosono S, Luxa J, Sofer Z, Ballesteros B, et al. Heat-Up Colloidal Synthesis of Shape-Controlled Cu-Se-S Nanostructures—Role of Precursor and Surfactant Reactivity and Performance in N2 Electroreduction. Nanomaterials. 2021; 11(12):3369. https://doi.org/10.3390/nano11123369
Chicago/Turabian StyleMourdikoudis, Stefanos, George Antonaropoulos, Nikolas Antonatos, Marcos Rosado, Liudmyla Storozhuk, Mari Takahashi, Shinya Maenosono, Jan Luxa, Zdeněk Sofer, Belén Ballesteros, and et al. 2021. "Heat-Up Colloidal Synthesis of Shape-Controlled Cu-Se-S Nanostructures—Role of Precursor and Surfactant Reactivity and Performance in N2 Electroreduction" Nanomaterials 11, no. 12: 3369. https://doi.org/10.3390/nano11123369
APA StyleMourdikoudis, S., Antonaropoulos, G., Antonatos, N., Rosado, M., Storozhuk, L., Takahashi, M., Maenosono, S., Luxa, J., Sofer, Z., Ballesteros, B., Thanh, N. T. K., & Lappas, A. (2021). Heat-Up Colloidal Synthesis of Shape-Controlled Cu-Se-S Nanostructures—Role of Precursor and Surfactant Reactivity and Performance in N2 Electroreduction. Nanomaterials, 11(12), 3369. https://doi.org/10.3390/nano11123369









