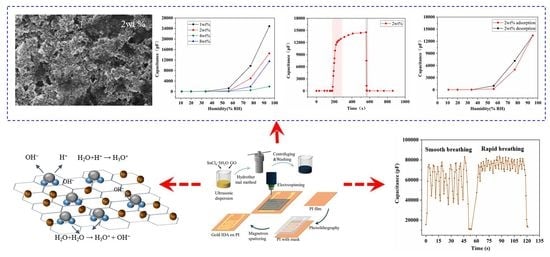
The Effect of rGO-Doping on the Performance of SnO2/rGO Flexible Humidity Sensor
Abstract
:1. Introduction
2. Materials and Methods
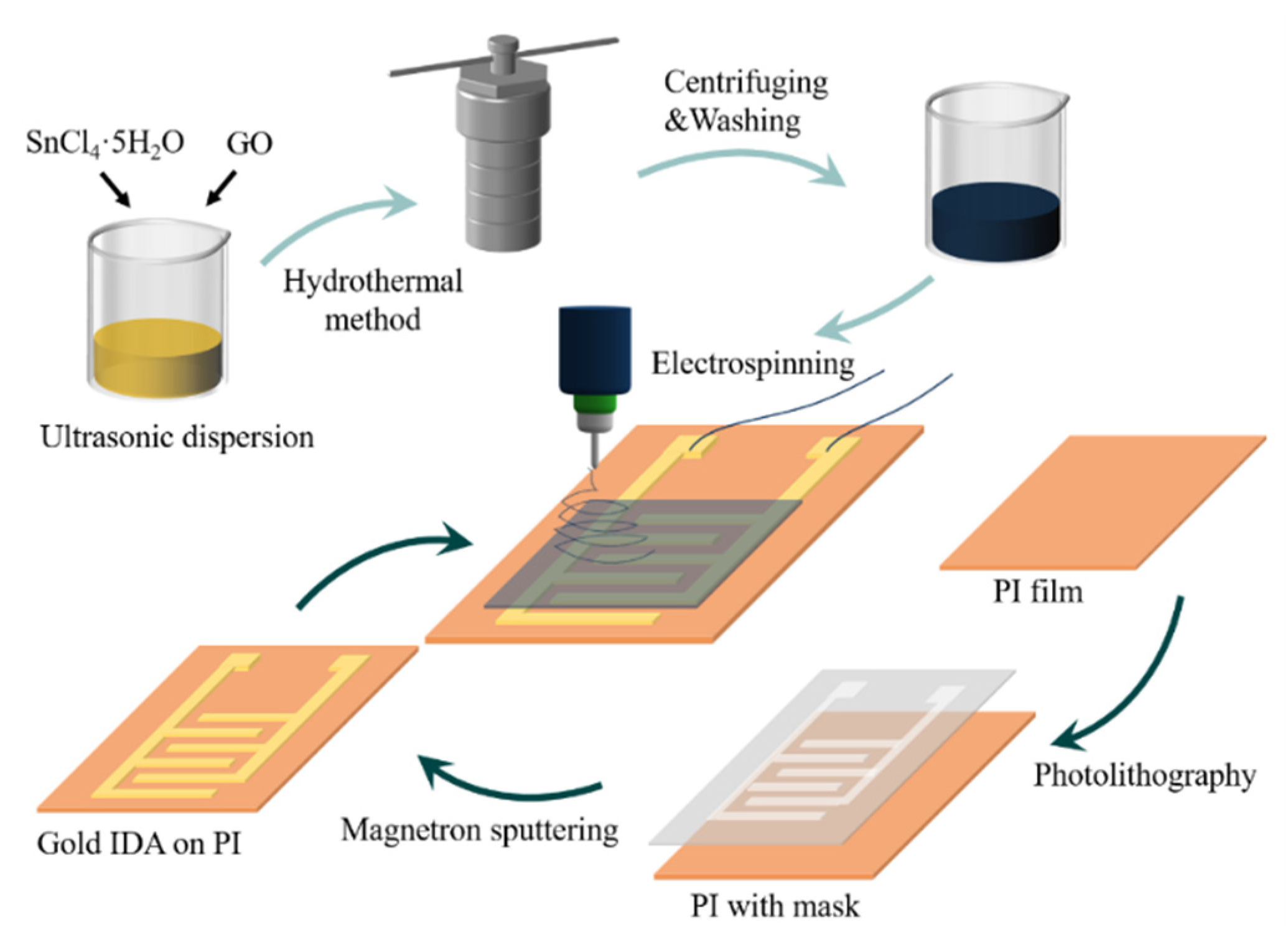
2.1. Preparation of SnO2/rGO
2.2. Fabrication of a Flexible Humidity Sensor
2.3. Characterization and Humidity Performance Test
3. Results
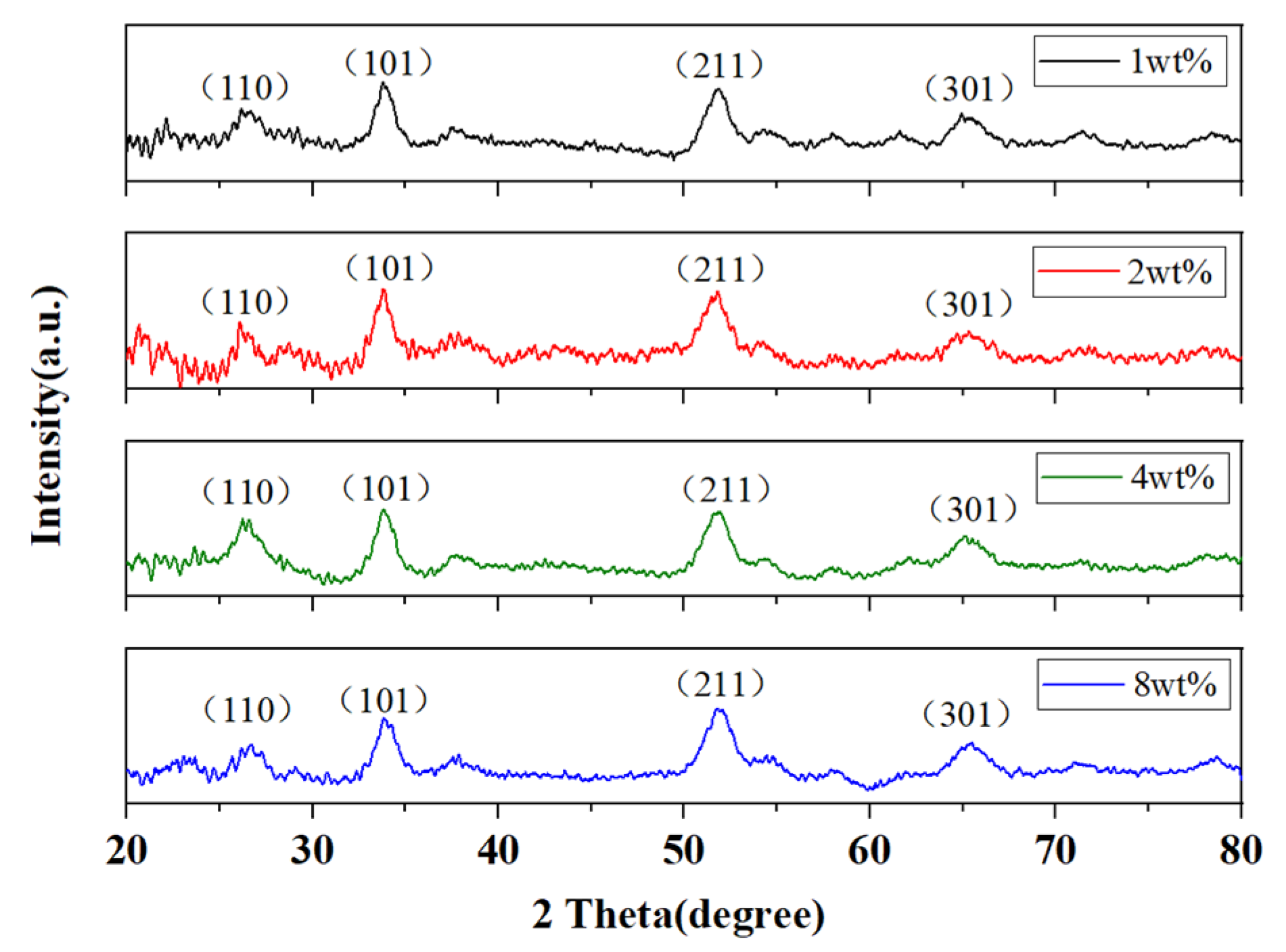
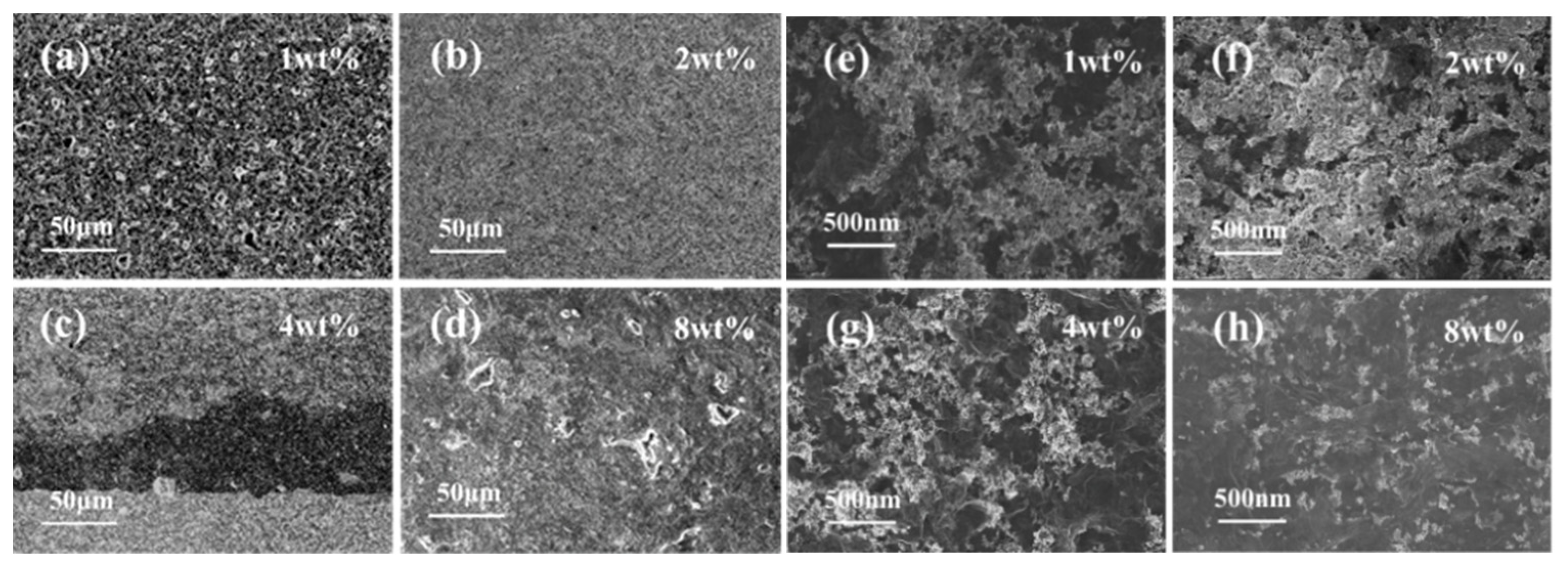
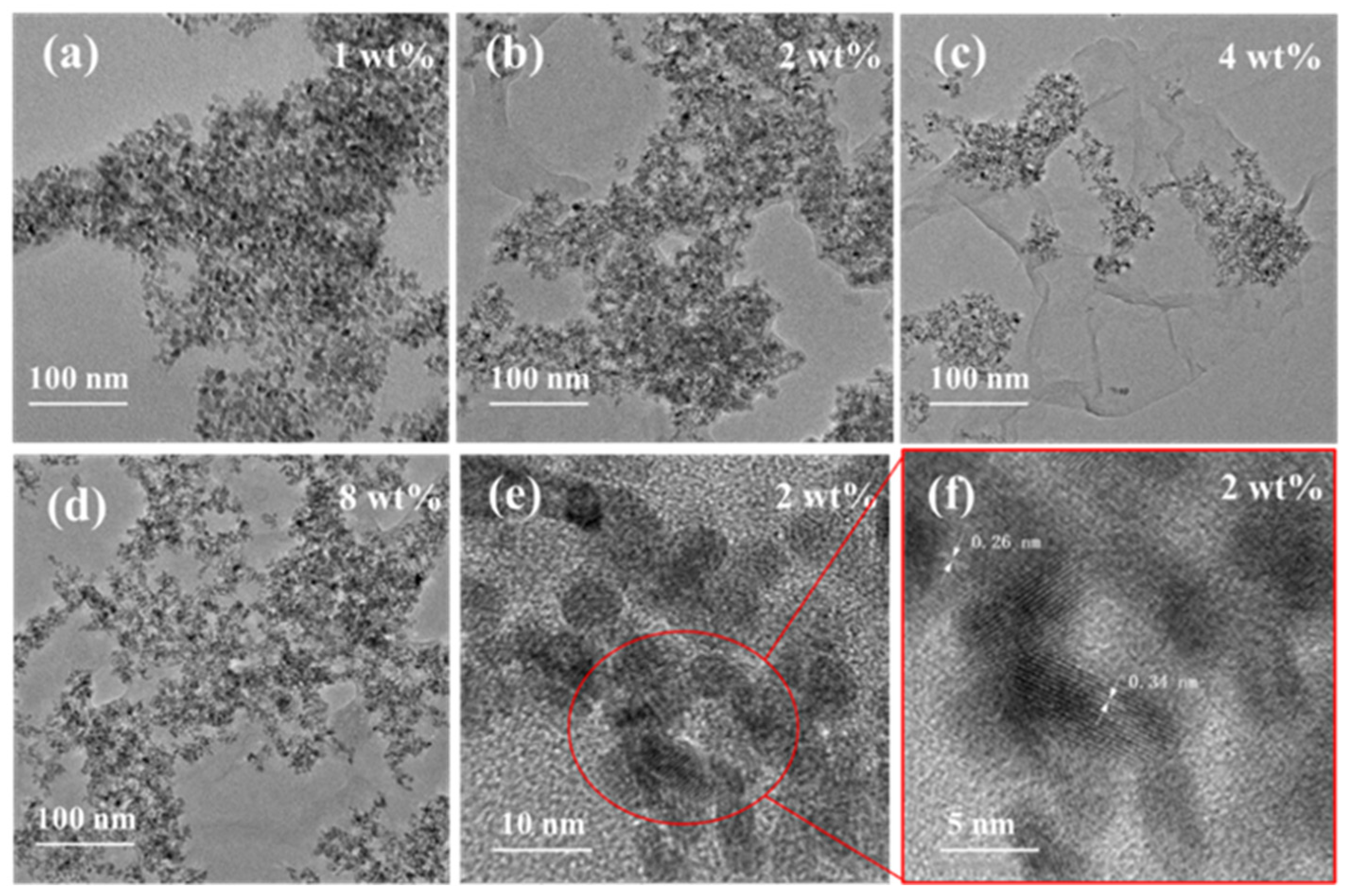
3.1. Material Characterizations
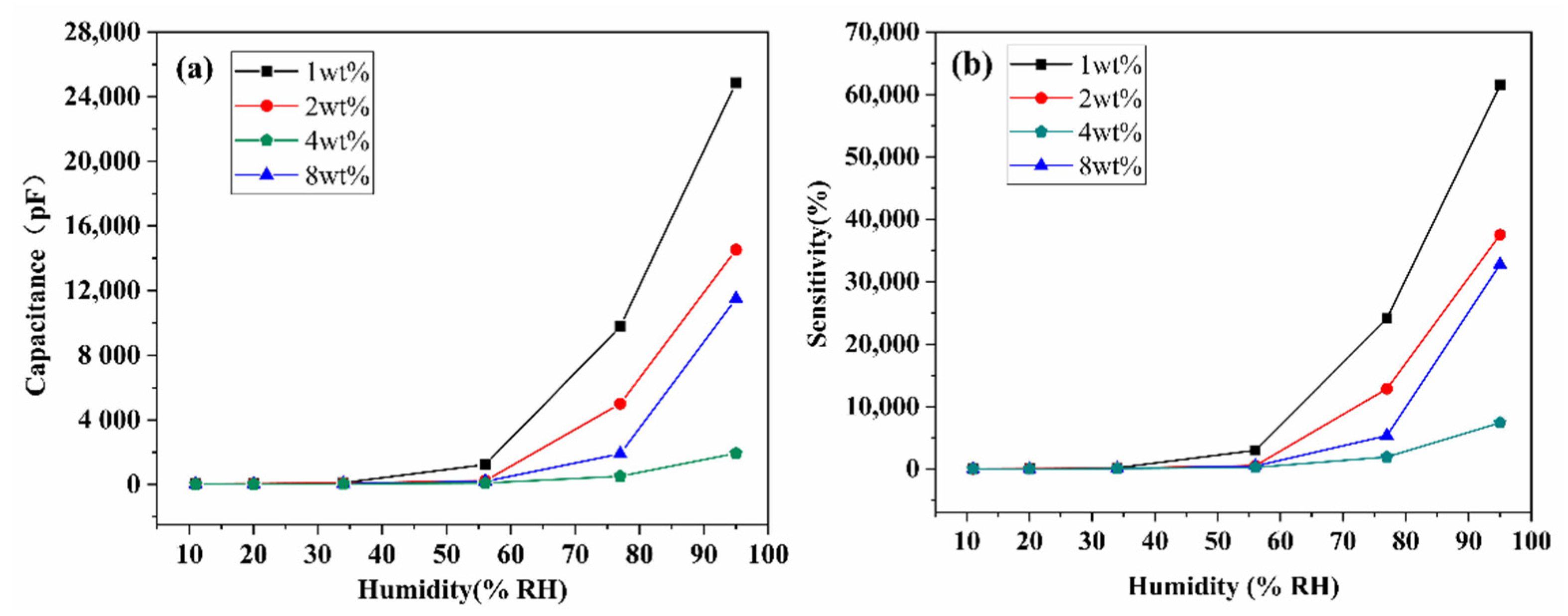
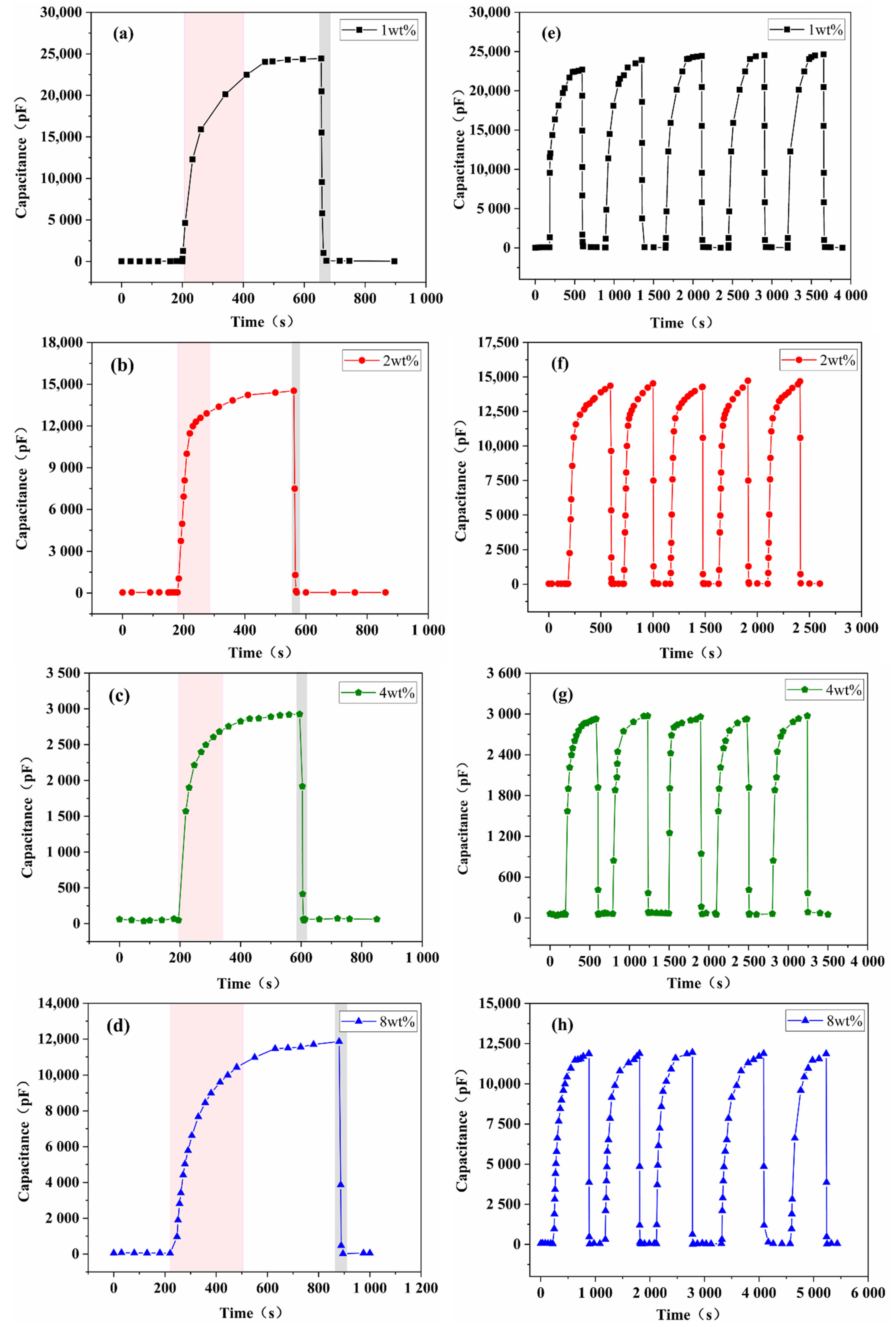
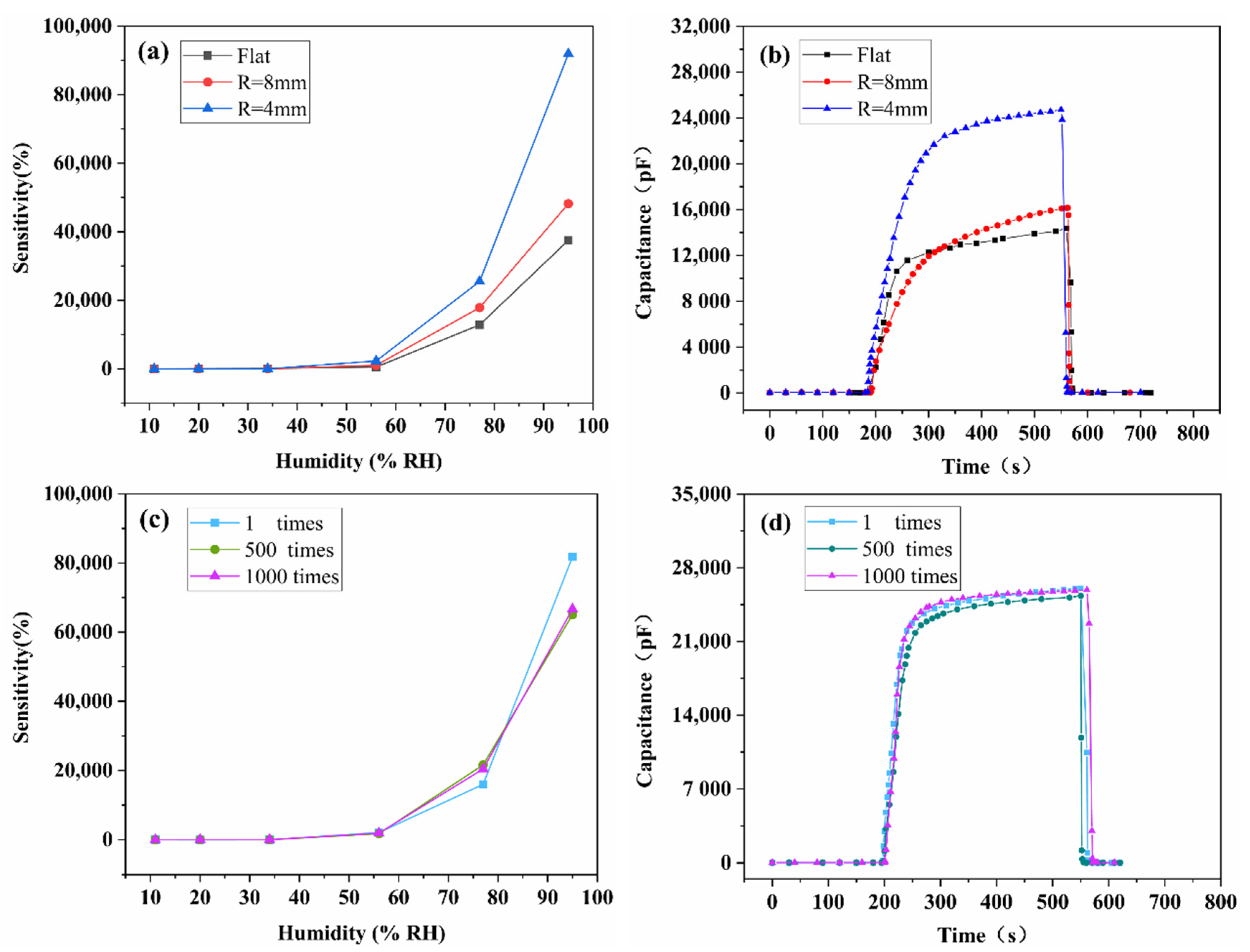
3.2. Humidity Sensing Properties
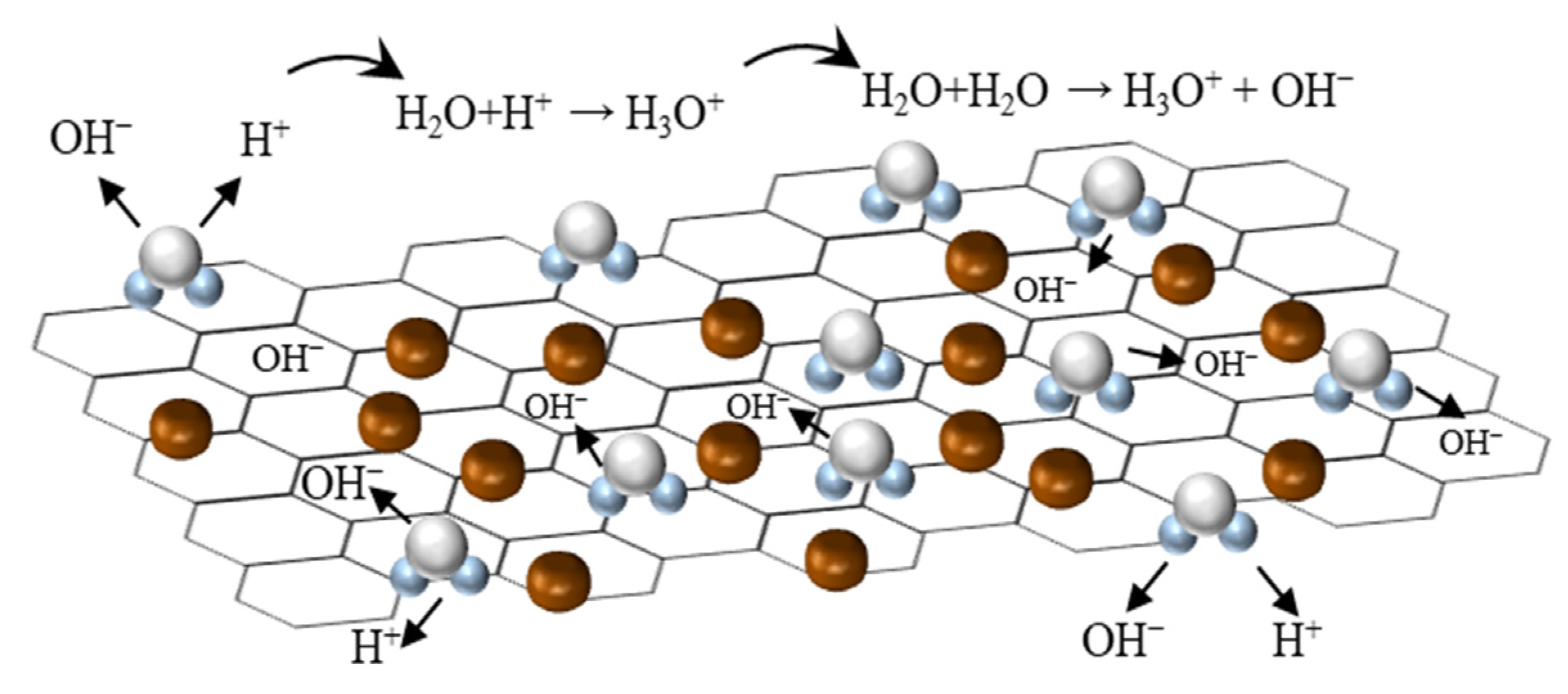
3.3. Humidity Sensing Mechanism
3.4. Bending Test
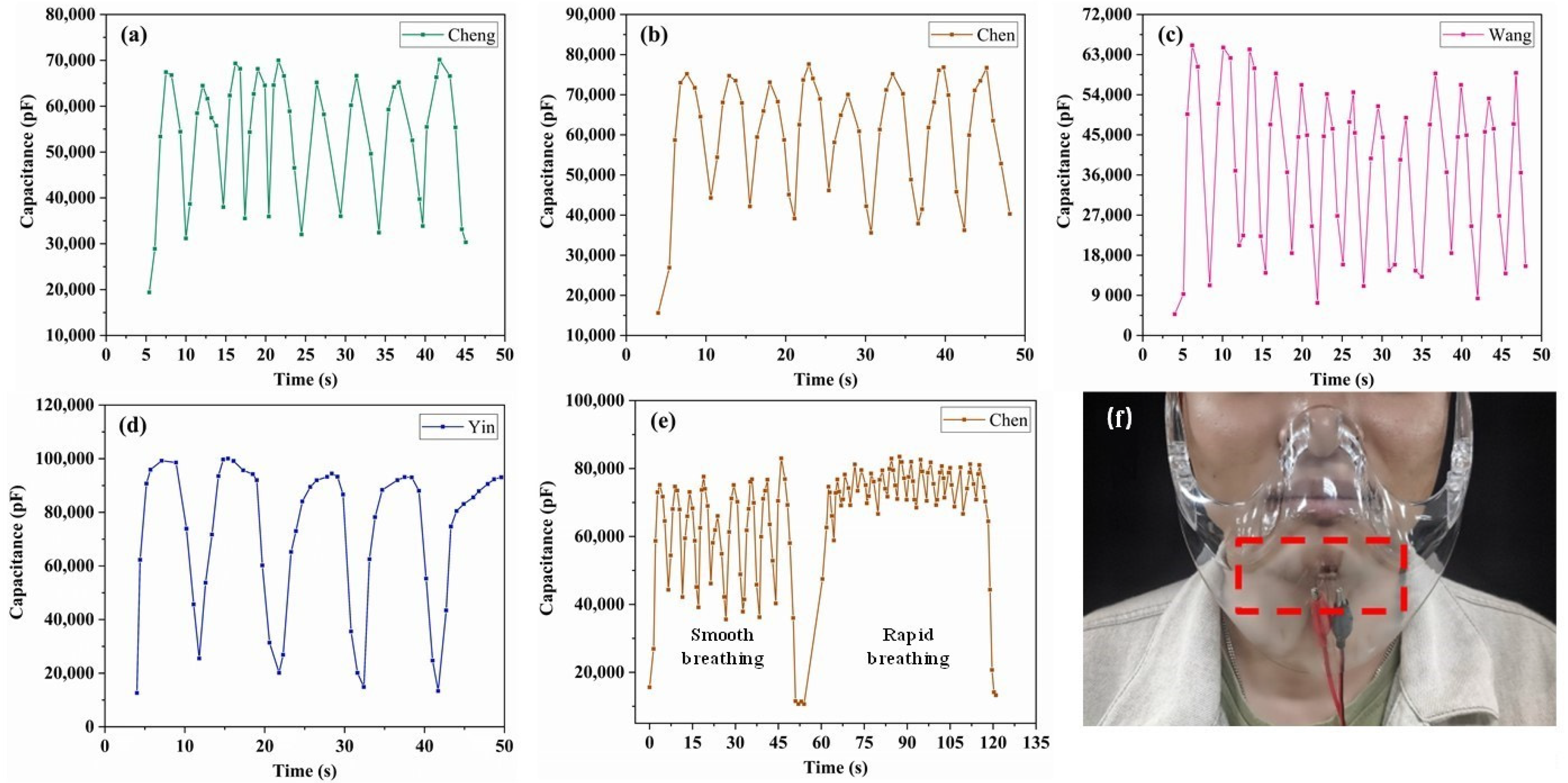
3.5. Monitoring Human Respiration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, Y.H.; Wang, J.G.; Qiu, Z.J.; Zheng, X.Y.; Leung, N.L.C.; Lam, J.W.Y.; Tang, B.Z. Multiscale humidity visualization by environmentally sensitive fluorescent molecular rotors. Adv. Mater. 2017, 29, 1703900.1–1703900.7. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Y.-M.; Wu, Z.; Li, X.; Wang, N.; Tao, K.; Wang, G.P. Carbon Nanocoil-Based Fast-Response and Flexible Humidity Sensor for Multifunctional Applications. ACS Appl. Mater. Interfaces 2019, 11, 4242–4251. [Google Scholar] [CrossRef]
- Blank, T.A.; Eksperiandova, L.P.; Belikov, K.N. Recent trends of ceramic humidity sensors development: A review. Sens. Actuators B Chem. 2016, 228, 416–442. [Google Scholar] [CrossRef]
- Xiao, S.; Nie, J.; Tan, R.; Duan, X.; Ma, J.; Li, Q.; Wang, T. Fast-response ionogel humidity sensor for real-time monitoring of breathing rate. Mater. Chem. Front. 2019, 3, 484–491. [Google Scholar] [CrossRef]
- Kano, S.; Fujii, M. All-Painting Process To Produce Respiration Sensor Using Humidity-Sensitive Nanoparticle Film and Graphite Trace. ACS Sustain. Chem. Eng. 2018, 6, 12217–12223. [Google Scholar] [CrossRef] [Green Version]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Nyein, H.Y.Y.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z.; et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, L.; Zhou, J.; Lu, A. Flexible and Transparent Cellulose-Based Ionic Film as a Humidity Sensor. ACS Appl. Mater. Interfaces 2020, 12, 7631–7638. [Google Scholar] [CrossRef]
- Bian, C.; Wang, J.; Bai, X.; Hu, M.; Gang, T. Optical fiber based on humidity sensor with improved sensitivity for monitoring applications. Opt. Laser Technol. 2020, 130, 106342. [Google Scholar] [CrossRef]
- Zhang, D.; Zong, X.; Wu, Z.; Zhang, Y. Hierarchical Self-Assembled SnS2 Nanoflower/Zn2SnO4 Hollow Sphere Nanohybrid for Humidity-Sensing Applications. ACS Appl. Mater. Interfaces 2018, 10, 32631–32639. [Google Scholar] [CrossRef]
- Torres Alonso, E.; Shin, D.W.; Rajan, G.; Neves, A.I.S.; Russo, S.; Craciun, M.F. Water-based solution processing and wafer-scale integration of all-graphene humidity sensors. Adv. Sci. 2019, 6, 1802318. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.F.; Xiao, S.H.; Feng, C.Y.; Li, H.Y.; Li, X.J. Resistive humidity sensitivity of arrayed multi-wall carbon nanotube nests grown on arrayed nanoporous silicon pillars. Sens. Actuators B Chem. 2007, 125, 651–655. [Google Scholar] [CrossRef]
- Kumar, R.; Yadav, B. Humidity sensing investigation on nanostructured polyaniline synthesized via chemical polymerization method. Mater. Lett. 2016, 167, 300–302. [Google Scholar] [CrossRef]
- Wang, L.L.; Kang, L.P.; Wang, H.Y.; Chen, Z.P.; Li, X.J. Capacitive humidity sensitivity of SnO2:Sn thin film grown on silicon nanoporous pillar array. Sens. Actuators B Chem. 2016, 229, 513–519. [Google Scholar] [CrossRef]
- Kuang, Q.; Lao, C.; Wang, Z.L.; Xie, Z.; Zheng, L. High-Sensitivity Humidity Sensor Based on a Single SnO2 Nanowire. J. Am. Chem. Soc. 2007, 129, 6070–6071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comini, E.; Faglia, G.; Sberveglieri, G.; Pan, Z.; Wang, Z.L. Stable and highly sensitive gas sensors based on semiconducting oxide nanobelts. Appl. Phys. Lett. 2002, 81, 1869–1871. [Google Scholar] [CrossRef]
- Tomer, V.K.; Duhan, S. A facile nanocasting synthesis of mesoporous Ag-doped SnO 2 nanostructures with enhanced humidity sensing performance. Sens. Actuators B Chem. 2016, 223, 750–760. [Google Scholar] [CrossRef]
- Parthibavarman, M.; Hariharan, V.; Sekar, C. High-sensitivity humidity sensor based on SnO2 nanoparticles synthesized by microwave irradiation method. Mater. Sci. Eng. C 2011, 31, 840–844. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, C. Humidity Sensors: A Review of Materials and Mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Tao, L.; Chen, Z.; Fang, H.; Li, X.; Wang, X.; Xu, J.-B.; Zhu, H. Graphene and related two-dimensional materials: Structure-property relationships for electronics and optoelectronics. Appl. Phys. Rev. 2017, 4, 021306. [Google Scholar] [CrossRef]
- Zhao, X.; Long, Y.; Yang, T.; Li, J.; Zhu, H. Simultaneous High Sensitivity Sensing of Temperature and Humidity with Graphene Woven Fabrics. ACS Appl. Mater. Interfaces 2017, 9, 30171–30176. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, C.; Li, L.; Liu, Y.; Li, X.; Xu, X.; Xia, F.; Wang, W.; Gao, J. Sn Powder as Reducing Agents and SnO2 Precursors for the Synthesis of SnO2-Reduced Graphene Oxide Hybrid Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 13333–13339. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gu, S.; Lu, B. Graphene and graphene oxide double decorated SnO2 nanofibers with enhanced humidity sensing performance. RSC Adv. 2015, 5, 72046–72050. [Google Scholar] [CrossRef]
- Toloman, D.; Popa, A.; Stan, M.; Socaci, C.; Biris, A.; Katona, G.; Tudorache, F.; Petrila, I.; Iacomi, F. Reduced graphene oxide decorated with Fe doped SnO2 nanoparticles for humidity sensor. Appl. Surf. Sci. 2017, 402, 410–417. [Google Scholar] [CrossRef]
- Zhang, D.; Chang, H.; Li, P.; Liu, R.; Xue, Q. Fabrication and characterization of an ultrasensitive humidity sensor based on metal oxide/graphene hybrid nanocomposite. Sens. Actuators B Chem. 2016, 225, 233–240. [Google Scholar] [CrossRef]
- Ganiger, S.K.; Chaluvaraju, B.V.; Ananda, S.R.; Murugendrappa, M.V. A feasibility study of polypyrrole/zinc tungstate (ceramics) nanocomposites for D. C. conductivity and as a humidity sensor. Mater. Today-Proc. 2018, 5, 2803–2810. [Google Scholar] [CrossRef]
- Su, Y.; Li, C.; Li, M.; Li, H.; Xu, S.; Qian, L.; Yang, B. Surface acoustic wave humidity sensor based on three-dimensional architecture graphene/PVA/SiO2 and its application for respiration monitoring. Sens. Actuators B Chem. 2020, 308, 127693. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, J.; Zhang, J.; Li, C.; Li, Y.; Wang, X. Capacitance-type MWCNTs/SiO2 humidity sensor based on capillary condensation and percolation theory. Sens. Actuators A Phys. 2017, 263, 648–653. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Su, I.-L.; Hsueh, T.-J. Tunable Schottky contact humidity sensor based on S-doped ZnO nanowires on flexible PET substrate with piezotronic effect. J. Alloy. Compd. 2017, 705, 722–733. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, Y.; Gan, W.; Yang, M. Optical fiber Fabry–Perot humidity sensor based on polyimide membrane: Sensitivity and adsorption kinetics. Sens. Actuators A Phys. 2018, 281, 48–54. [Google Scholar] [CrossRef]
- Chai, J.; Liu, Q.; Liu, J.; Zhang, D. Optical fiber sensors based on novel polyimide for humidity monitoring of building materials. Opt. Fiber Technol. 2018, 41, 40–47. [Google Scholar] [CrossRef]
- Khalifa, M.; Wuzella, G.; Lammer, H.; Mahendran, A.R. Smart paper from graphene coated cellulose for high-performance humidity and piezoresistive force sensor. Synth. Met. 2020, 266, 116420. [Google Scholar] [CrossRef]
- Liu, H.; Xiang, H.; Wang, Y.; Li, Z.; Qian, L.; Li, P.; Ma, Y.; Zhou, H.; Huang, W. A Flexible Multimodal Sensor That Detects Strain, Humidity, Temperature, and Pressure with Carbon Black and Reduced Graphene Oxide Hierarchical Composite on Paper. ACS Appl. Mater. Interfaces 2019, 11, 40613–40619. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yao, Y.; Duan, X.; Liu, T. Force and humidity dual sensors fabricated by laser writing on polyimide/paper bilayer structure for pulse and respiration monitoring. J. Mater. Chem. C 2018, 6, 4727–4736. [Google Scholar] [CrossRef]
- Liao, X.; Liao, Q.; Zhang, Z.; Yan, X.; Liang, Q.; Wang, Q.; Li, M.-H.; Zhang, Y. A highly stretchable ZnO@fiber-based multifunctional nanosensor for strain/temperature/UV detection. Adv. Funct. Mater. 2016, 26, 3074–3081. [Google Scholar] [CrossRef]
- Li, T.; Li, L.H.; Sun, H.W.; Xu, Y.; Wang, X.W.; Luo, H.; Liu, Z.; Zhang, T. Porous ionic membrane based flexible humidity sensor and its multifunctional applications. Adv. Sci. 2017, 4, 1600404. [Google Scholar] [CrossRef]
- Kano, S.; Kim, K.; Fujii, M. Fast-Response and Flexible Nanocrystal-Based Humidity Sensor for Monitoring Human Respiration and Water Evaporation on Skin. ACS Sens. 2017, 2, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Sun, Y.; Li, P.; Zhang, Y. Facile Fabrication of MoS2-Modified SnO2 Hybrid Nanocomposite for Ultrasensitive Humidity Sensing. ACS Appl. Mater. Interfaces 2016, 8, 14142–14149. [Google Scholar] [CrossRef]
- Li, L.; He, S.; Liu, M.; Zhang, C.; Chen, W. Three-Dimensional Mesoporous Graphene Aerogel-Supported SnO2 Nanocrystals for High-Performance NO2 Gas Sensing at Low Temperature. Anal. Chem. 2015, 87, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Song, H.; Ma, L.; Chen, X. Magnetite/graphene nanosheet composites: Interfacial interaction and its impact on the durable high-rate performance in lithium-ion batteries. RSC Adv. 2011, 1, 782–791. [Google Scholar] [CrossRef]
- He, Y.-D.; Zhang, Z.-L.; Xue, J.; Wang, X.-H.; Song, F.; Wang, X.-L.; Zhu, L.-L.; Wang, Y.-Z. Biomimetic Optical Cellulose Nanocrystal Films with Controllable Iridescent Color and Environmental Stimuli-Responsive Chromism. ACS Appl. Mater. Interfaces 2018, 10, 5805–5811. [Google Scholar] [CrossRef]
- Duan, Z.; Jiang, Y.; Yan, M.; Wang, S.; Yuan, Z.; Zhao, Q.; Sun, P.; Xie, G.; Du, X.; Tai, H. Facile, Flexible, Cost-Saving, and Environment-Friendly Paper-Based Humidity Sensor for Multifunctional Applications. ACS Appl. Mater. Interfaces 2019, 11, 21840–21849. [Google Scholar] [CrossRef] [PubMed]
- Bergese, S.D.; Mestek, M.L.; Kelley, S.D.; McIntyre, R., Jr.; Uribe, A.A.; Sethi, R.; Watson, J.N.; Addison, P.S. Multicenter study validating accuracy of a continuous respiratory rate measurement derived from pulse oximetry: A comparison with capnography. Anesth. Analg. 2017, 124, 1153–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Chen, Z.; Zeng, L.; Wang, Z.; Zheng, G.; Zhou, R. The Effect of rGO-Doping on the Performance of SnO2/rGO Flexible Humidity Sensor. Nanomaterials 2021, 11, 3368. https://doi.org/10.3390/nano11123368
Yan H, Chen Z, Zeng L, Wang Z, Zheng G, Zhou R. The Effect of rGO-Doping on the Performance of SnO2/rGO Flexible Humidity Sensor. Nanomaterials. 2021; 11(12):3368. https://doi.org/10.3390/nano11123368
Chicago/Turabian StyleYan, Huangping, Zilu Chen, Linyuan Zeng, Zijun Wang, Gaofeng Zheng, and Rui Zhou. 2021. "The Effect of rGO-Doping on the Performance of SnO2/rGO Flexible Humidity Sensor" Nanomaterials 11, no. 12: 3368. https://doi.org/10.3390/nano11123368
APA StyleYan, H., Chen, Z., Zeng, L., Wang, Z., Zheng, G., & Zhou, R. (2021). The Effect of rGO-Doping on the Performance of SnO2/rGO Flexible Humidity Sensor. Nanomaterials, 11(12), 3368. https://doi.org/10.3390/nano11123368