LDH Nanocubes Synthesized with Zeolite Templates and Their High Performance as Adsorbents
Abstract
1. Introduction
2. Experimental Details
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Zn-Fe LDH
2.2.2. Preparation of Zeolite
2.2.3. Preparation of Zeolite/Zn-Fe LDH Composite
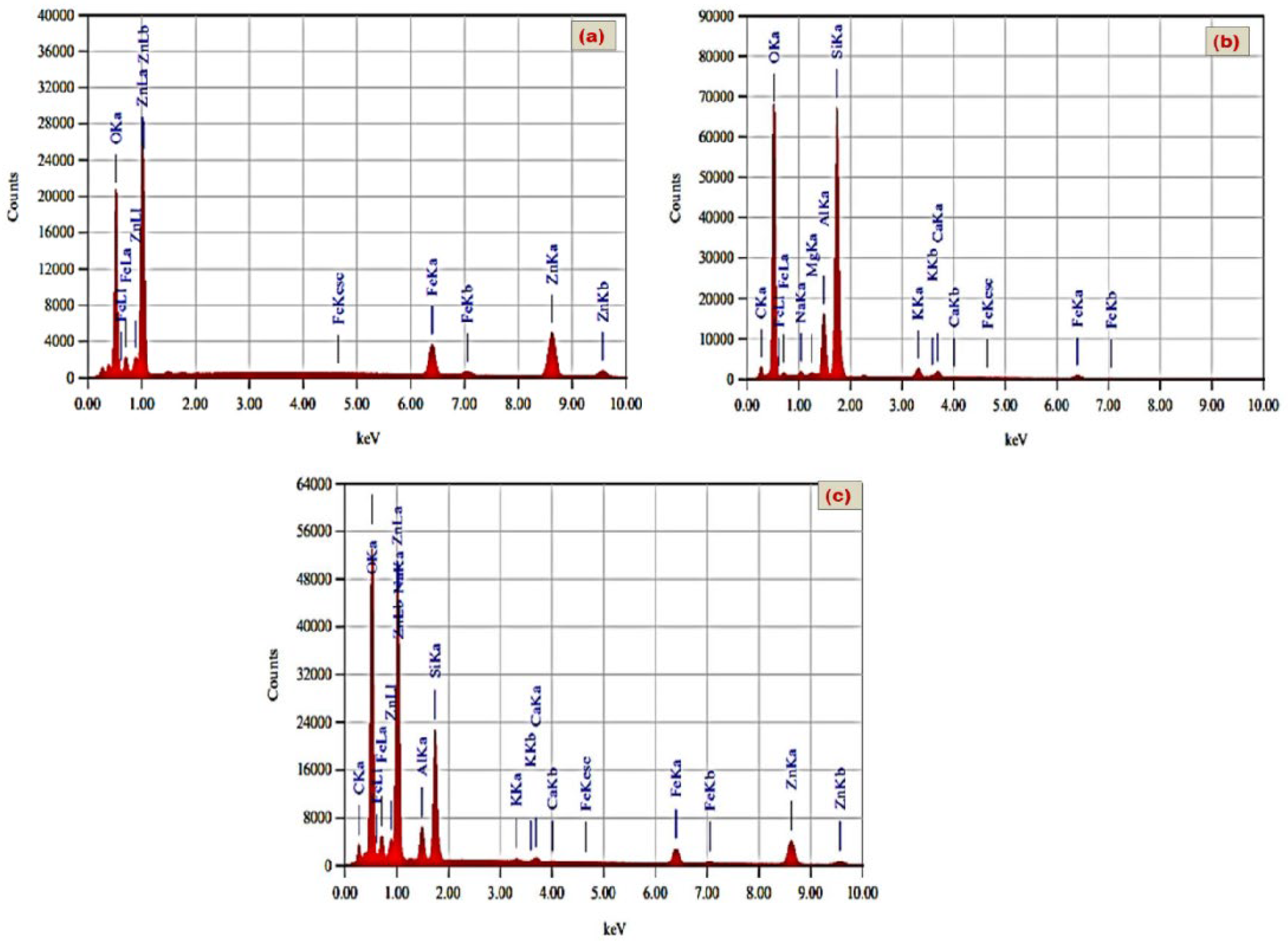
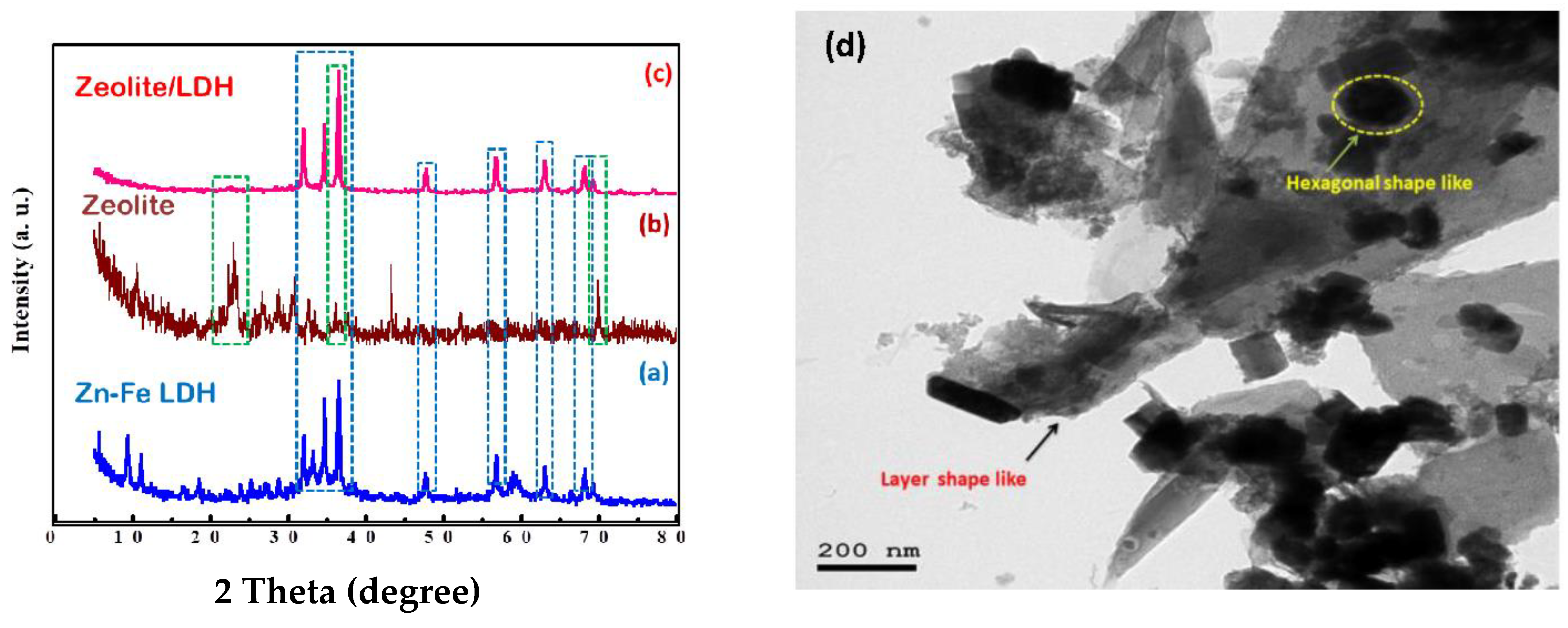
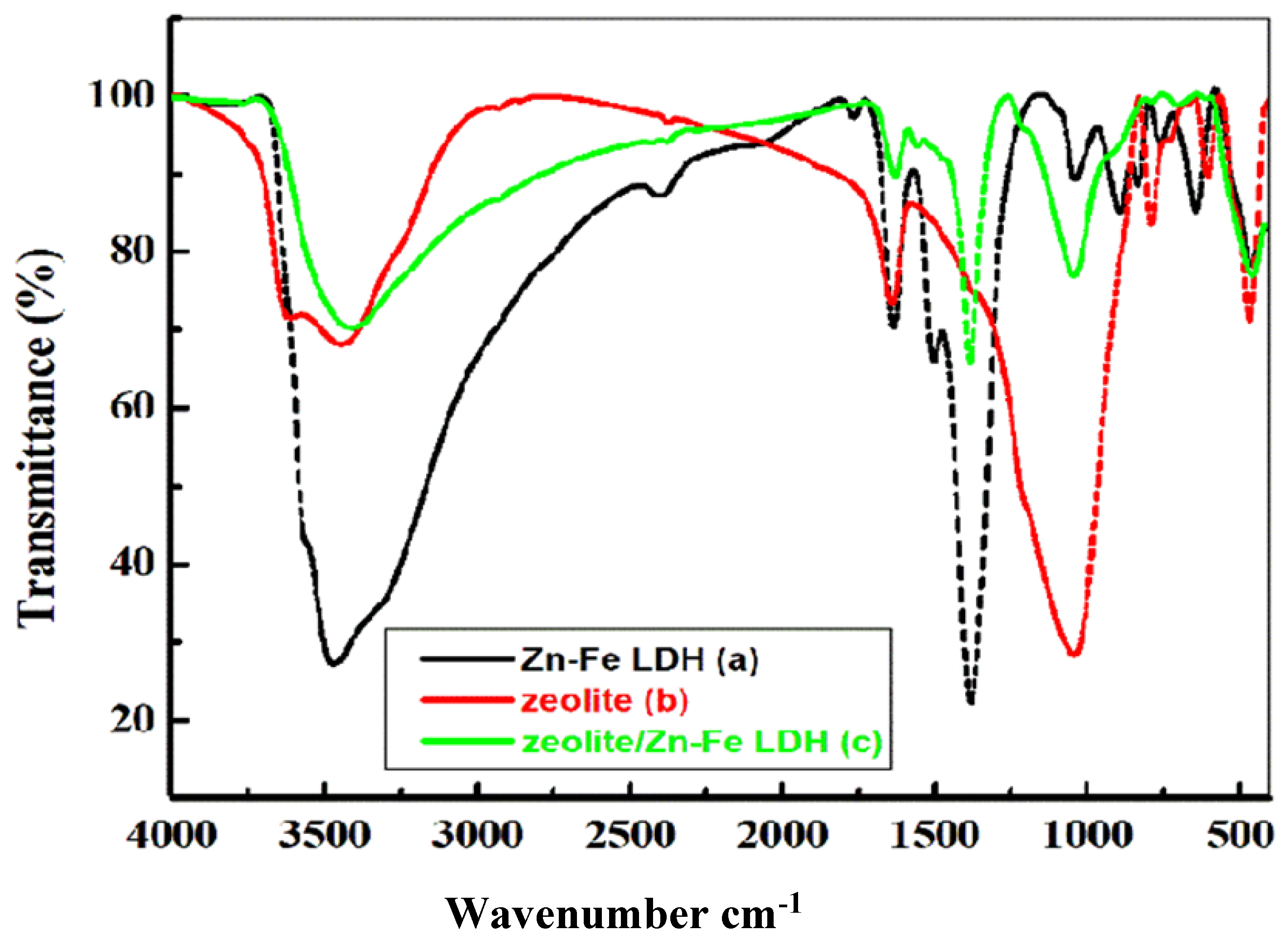
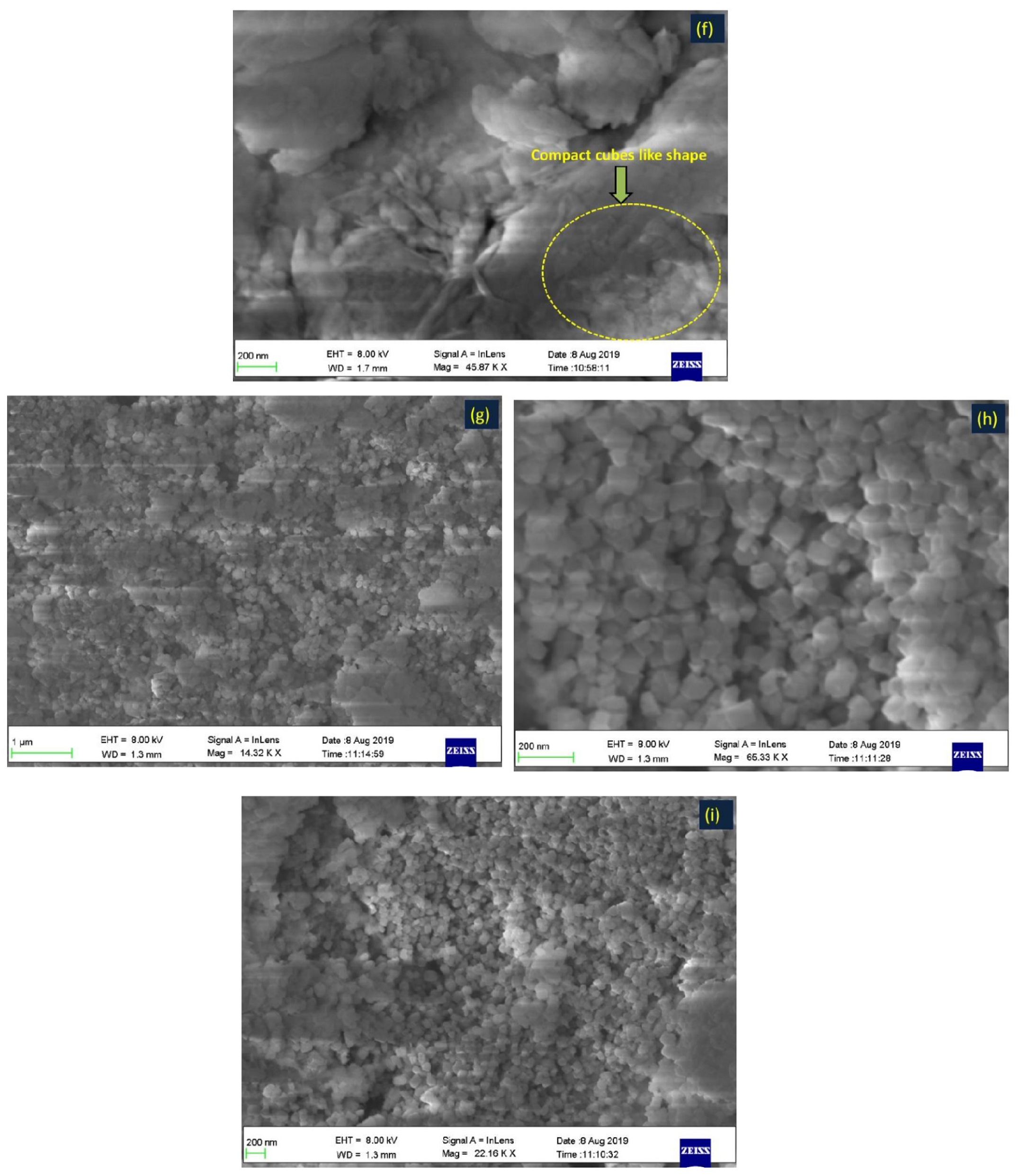
2.3. Characterizations of the Prepared Materials
2.4. Adsorption Arrays
2.5. Quality Assurance and Results Reliability
3. Results and Discussion
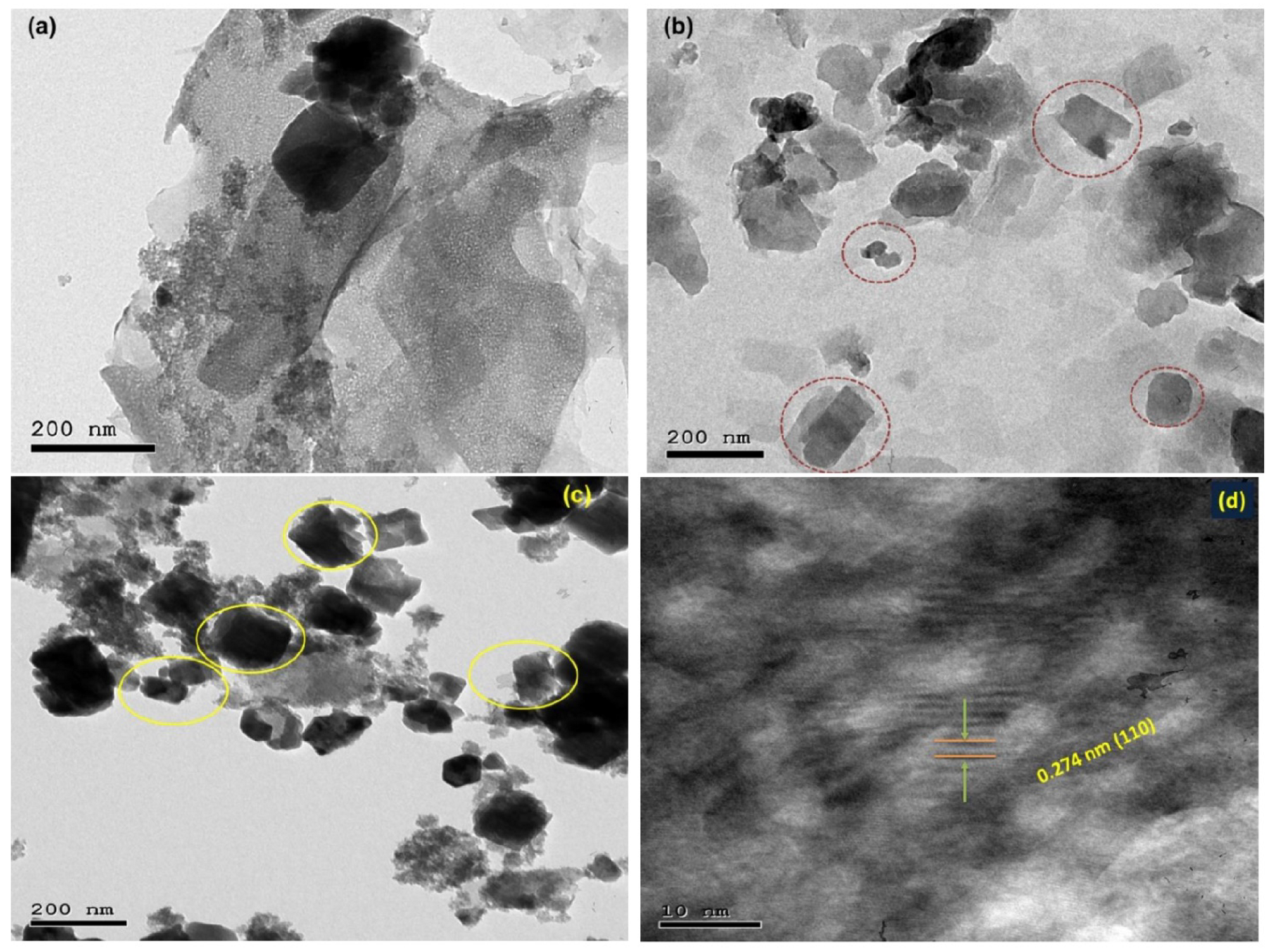
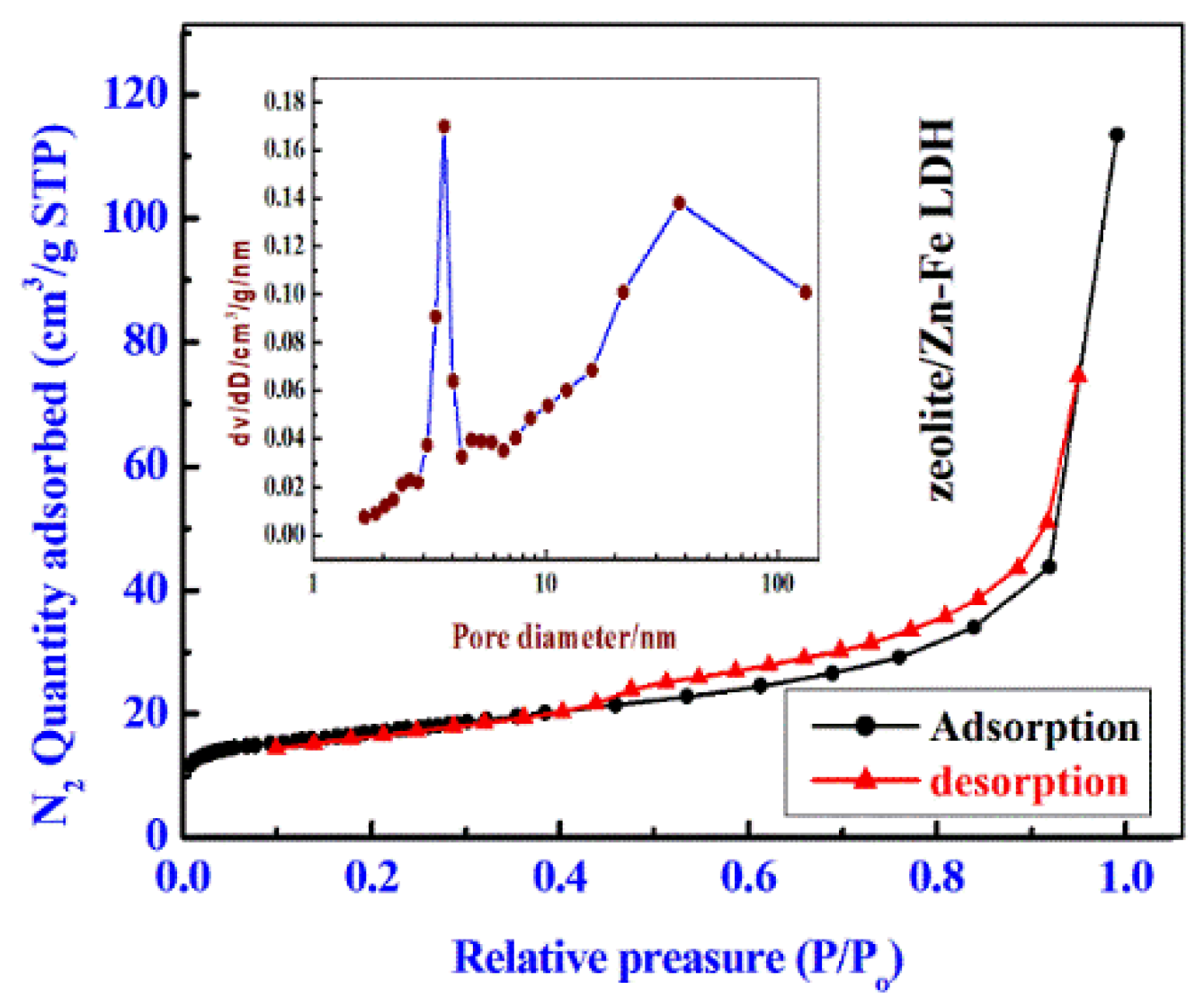
3.1. Discussion of Characterization Results
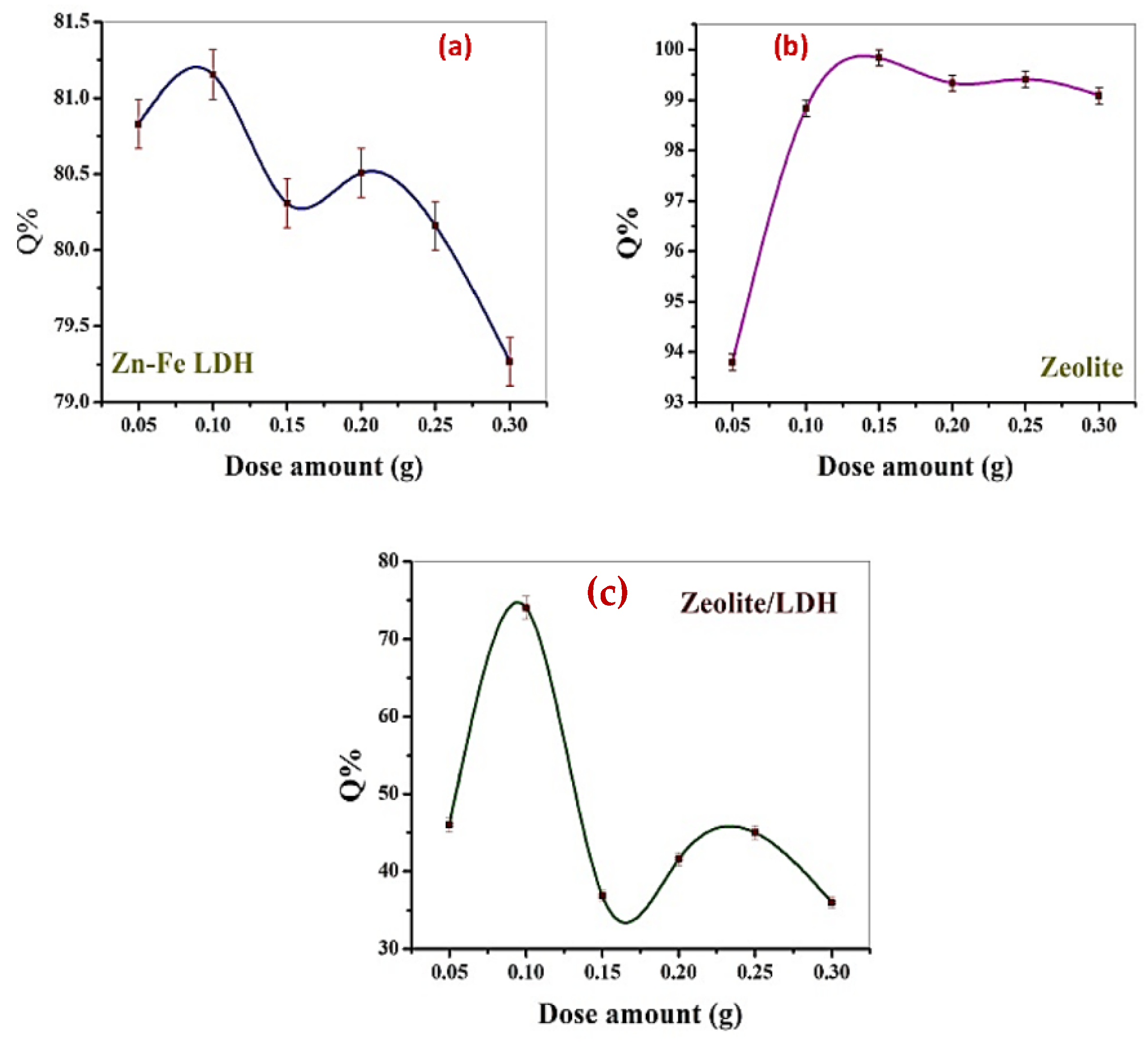
3.2. Discussion of Adsorption Studies
3.3. Adsorption Isotherms
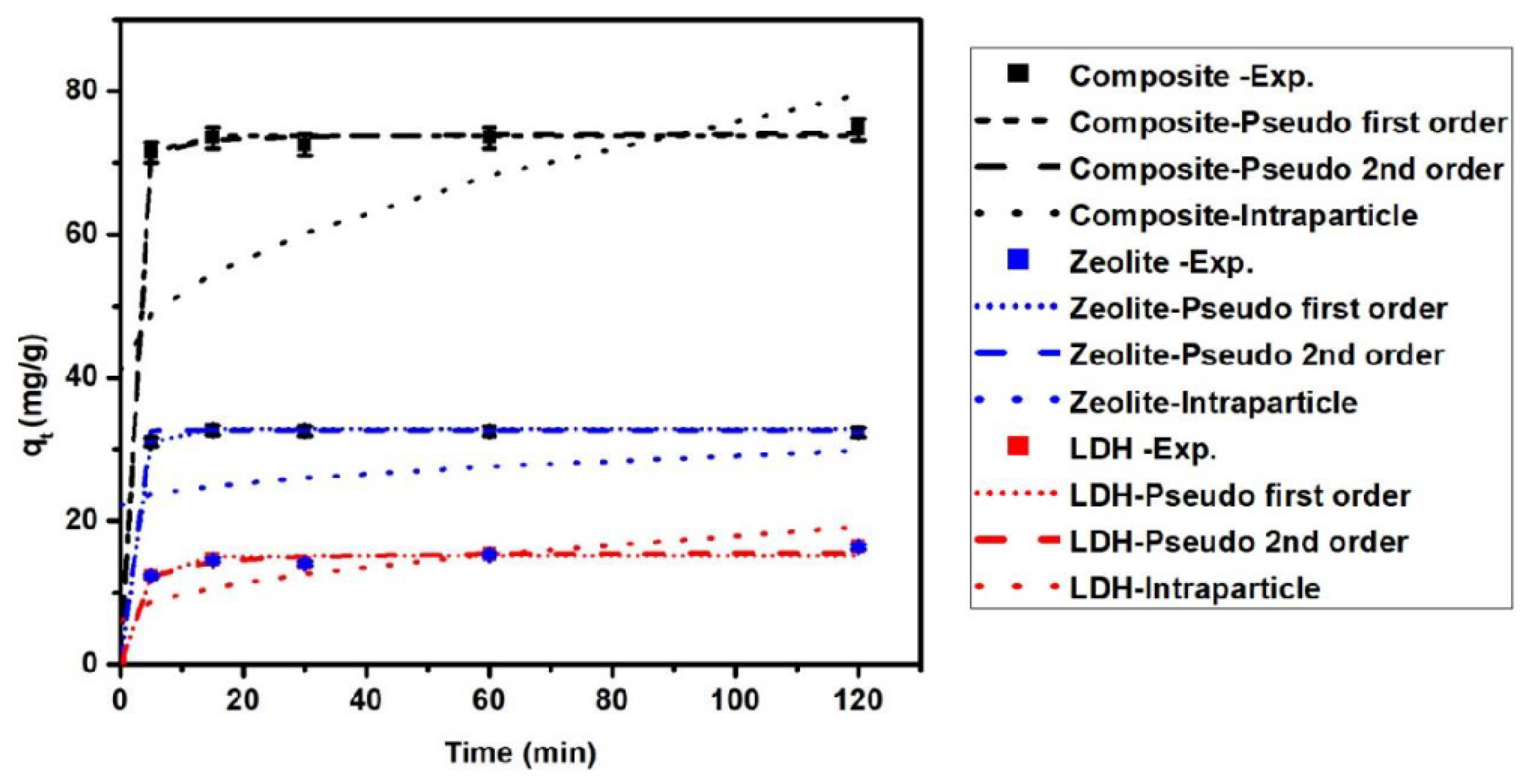
3.4. Adsorption Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LDH | Layer double hydroxide |
| Co | The initial concentration |
| Ct | The dye concentration (mg/L) |
| V | The volume of the dye solution (L) |
| W | The mass of the sorbent (g) |
| qe | Equilibrium adsorption capacity of adsorbent (mg/g) |
| Ce | Equilibrium concentration of the adsorbate (mg/L) |
| Q% | Removal percent |
| 2 | Chi square |
| qexp | Experimental adsorption capacity of adsorbent at equilibrium (mg/g) |
| qcal | Calculated adsorption capacity of adsorbent at equilibrium (mg/g) |
| Determination coefficient | |
| Adjusted correlation coefficient | |
| Kf | Freundlich constant (L/g) |
| n | The sample size |
| k | The number of independent variables in the regression equation |
| 1/nf | Freundlich adsorption intensity |
| KLF | Equilibrium constant for heterogeneous solid |
| k1 | Rate constant of pseudo first-order model (1/min) |
| k2 | Rate constant of pseudo second-order model (g/mg min) |
| kip | Measure of diffusion coefficient (mg g−1 min−1(1/2)) |
| Cip | Intraparticle diffusion constant mg/g |
References
- Bayomie, O.S.; Kandeel, H.; Shoeib, T.; Yang, H.; Youssef, N.; El-Sayed, M.M. Novel approach for effective removal of methylene blue dye from water using fava bean peel waste. Sci. Rep. 2020, 10, 7824. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kim, J.H.; Park, H.; Kwak, C.; Yang, J.; Kim, J.; Ryu, S.Y.; Lee, J. Role of electrostatic interactions in the adsorption of dye molecules by Ti3C2-MXenes. RSC Adv. 2021, 11, 6201–6211. [Google Scholar] [CrossRef]
- Soniya, M.; Muthuraman, G. Comparative study between liquid-liquid extraction and bulk liquid membrane for the removal and recovery of methylene blue from wastewater. J. Ind. Eng. Chem. 2015, 30, 266–273. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Cui, J.; Cui, J.; Wang, F.; Zhang, F. High-efficiency adsorption and regeneration of methylene blue and aniline onto activated carbon from waste edible fungus residue and its possible mechanism. RSC Adv. 2020, 10, 14262–14273. [Google Scholar] [CrossRef]
- Pang, X.; Sellaoui, L.; Franco, D.; Netto, M.S.; Georgin, J.; Luiz Dotto, G.; Abu Shayeb, M.K.; Belmabrouk, H.; Bonilla-Petriciolet, A.; Li, Z. Preparation and characterization of a novel mountain soursop seeds powder adsorbent and its application for the removal of crystal violet and methylene blue from aqueous solutions. Chem. Eng. J. 2020, 391, 123617. [Google Scholar] [CrossRef]
- Li, W.H.; Yue, Q.Y.; Gao, B.Y.; Ma, Z.H.; Li, Y.J.; Zhao, H.X. Preparation and utilization of sludge-based activated carbon for the adsorption of dyes from aqueous solutions. Chem. Eng. J. 2011, 171, 320–327. [Google Scholar] [CrossRef]
- Xia, Y.; Yao, Q.; Zhang, W.; Zhang, Y.; Zhao, M. Comparative adsorption of methylene blue by magnetic baker’s yeast and EDTAD-modified magnetic baker’s yeast: Equilibrium and kinetic study. Arab. J. Chem. 2019, 12, 2448–2456. [Google Scholar] [CrossRef]
- Zaher, A. Photocatalytic degradation of phenol wastewater: Optimization of photo fenton process. Egypt. J. Chem. 2020, 63, 4439–4445. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Ma, X.; Du, Q.; Sui, K.; Wang, D.; Wang, C.; Li, H.; Xia, Y. Filtration and adsorption properties of porous calcium alginate membrane for methylene blue removal from water. Chem. Eng. J. 2017, 316, 623–630. [Google Scholar] [CrossRef]
- Vescovi, T.; Coleman, H.M.; Amal, R. The effect of pH on UV-based advanced oxidation technologies—1,4-Dioxane degradation. J. Hazard. Mater. 2010, 182, 75–79. [Google Scholar] [CrossRef]
- Santoso, E.; Ediati, R.; Kusumawati, Y.; Bahruji, H.; Sulistiono, D.O.; Prasetyoko, D. Review on recent advances of carbon based adsorbent for methylene blue removal from waste water. Mater. Today Chem. 2020, 16, 100233. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, F.; Zou, H.; Yuan, Y.; Wang, H.; Xia, J.; Wang, Z. Preparation of a Pt/NiFe layered double hydroxide/reduced graphene oxide composite as an electrocatalyst for methanol oxidation. J. Electroanal. Chem. 2018, 818, 198–203. [Google Scholar] [CrossRef]
- Yu, J.; Cao, Q.; Qiu, C.; Chen, L.; Delaunay, J.J. Modulating ni/ce ratio in niyce100-yox electrocatalysts for enhanced water oxidation. Nanomaterials 2021, 11, 437. [Google Scholar] [CrossRef]
- Awes, H.; Zaki, Z.; Abbas, S.; Dessoukii, H.; Zaher, A.; Abd-El Moaty, S.A.; Shehata, N.; Farghali, A.; Mahmoud, R.K. Removal of Cu2+ metal ions from water using Mg-Fe layered double hydroxide and Mg-Fe LDH/5-(3-nitrophenyllazo)-6-aminouracil nanocomposite for enhancing adsorption properties. Environ. Sci. Pollut. Res. 2021, 28, 47651–47667. [Google Scholar] [CrossRef]
- Cuautli, C.; Valente, J.S.; Conesa, J.C.; Ganduglia-Pirovano, M.V.; Ireta, J. Theoretical Study of the Catalytic Performance of Activated Layered Double Hydroxides in the Cyanoethylation of Alcohols. J. Phys. Chem. C 2019, 123, 8777–8784. [Google Scholar] [CrossRef]
- Zaher, A.; El Rouby, W.M.; Barakat, N.A. Influences of tungsten incorporation, morphology and calcination temperature on the electrocatalytic activity of Ni/C nanostructures toward urea elimination from wastewaters. Int. J. Hydrog. Energy 2020, 45, 8082–8093. [Google Scholar] [CrossRef]
- Li, L.; Gu, W.; Chen, J.; Chen, W.; Xu, Z.P. Co-delivery of siRNAs and anti-cancer drugs using layered double hydroxide nanoparticles. Biomaterials 2014, 35, 3331–3339. [Google Scholar] [CrossRef]
- Hanif, A.; Sun, M.; Shang, S.; Tian, Y.; Yip, A.C.; Ok, Y.S.; Iris, K.M.; Tsang, D.C.; Gu, Q.; Shang, J. Exfoliated Ni-Al LDH 2D nanosheets for intermediate temperature CO2 capture. J. Hazard. Mater. 2019, 374, 365–371. [Google Scholar] [CrossRef]
- Abd Elhaleem, M.B.; Farghali, A.A.; El-Shahawy, A.A.; Abo El-Ela, F.I.; Eldine, Z.E.; Mahmoud, R.K. Chemisorption and sustained release of cefotaxime between a layered double hydroxide and polyvinyl alcohol nanofibers for enhanced efficacy against second degree burn wound infection. RSC Adv. 2020, 10, 13196–13214. [Google Scholar] [CrossRef]
- Daud, M.; Hai, A.; Banat, F.; Wazir, M.B.; Habib, M.; Bharath, G.; Al-Harthi, M.A. A review on the recent advances, challenges and future aspect of layered double hydroxides (LDH)—Containing hybrids as promising adsorbents for dyes removal. J. Mol. Liq. 2019, 288, 110989. [Google Scholar] [CrossRef]
- Karadag, D.; Akgul, E.; Tok, S.; Erturk, F.; Kaya, M.A.; Turan, M. Basic and reactive dye removal using natural and modified zeolites. J. Chem. Eng. Data 2007, 52, 2436–2441. [Google Scholar] [CrossRef]
- Torres-Rodríguez, A.; Enrique, L.; Jaime, S.V.; Heriberto, P. CO2 Capture at Low Temperatures (3080 C) and in the Presence of Water Vapor over a Thermally Activated MgAl Layered Double Hydroxide Daniela. J. Phys. Chem. A 2011, 115, 12243–12250. [Google Scholar] [CrossRef]
- Majid, Z.; AbdulRazak, A.A.; Noori, W.A. Modification of Zeolite by Magnetic Nanoparticles for Organic Dye Removal. Arab. J. Sci. Eng. 2019, 44, 5457–5474. [Google Scholar] [CrossRef]
- Korkuna, O.; Vrublevs, T.Õ. Structural and physicochemical properties of natural zeolites: Clinoptilolite and mordenite. Microporous Mesoporous Mater. 2006, 87, 243–254. [Google Scholar] [CrossRef]
- Zaher, A.; Shehata, N. Recent Advances and Challenges in Management of Urea Wastewater: A Mini Review. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021; Volume 1046, p. 012021. [Google Scholar] [CrossRef]
- Belachew, N. Preparation of Zeolite 4A for Adsorptive Removal of Methylene Blue: Optimization, Kinetics, Isotherm, and Mechanism Study. Silicon 2021, 1–13. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Parameswaranpillai, J. Removal of anionic dye Congo red from aqueous environment using polyvinyl alcohol/sodium alginate/ZSM-5 zeolite membrane. Sci. Rep. 2020, 10, 15452. [Google Scholar] [CrossRef] [PubMed]
- Isawi, H. Using Zeolite/Polyvinyl alcohol/sodium alginate nanocomposite beads for removal of some heavy metals from wastewater. Arab. J. Chem. 2020, 13, 5691–5716. [Google Scholar] [CrossRef]
- Radoor, S.; Karayil, J.; Parameswaranpillai, J.; Siengchin, S. Adsorption Study of Anionic Dye, Eriochrome Black T from Aqueous Medium Using Polyvinyl Alcohol/Starch/ZSM-5 Zeolite Membrane. J. Polym. Environ. 2020, 28, 2631–2643. [Google Scholar] [CrossRef]
- Gao, Y.; Ru, Y.; Zhou, L.; Wang, X.; Wang, J. Preparation and Characterization of Chitosan-Zeolite Molecular Sieve Composite for Ammonia and Nitrate Removal. Adv. Compos. Lett. 2018, 27, 5. [Google Scholar] [CrossRef]
- Liu, M.; Tong, H.; Liu, Y.; Li, C.; Wu, X.; Li, M.; Li, X.; Tang, Y. Genetic progress in grain yield and the associated physiological traits of popular wheat in southwestern China from 1969 to 2012. Crop Sci. 2021, 61, 1971–1986. [Google Scholar] [CrossRef]
- Meili, L.; Lins, P.V.; Zanta, C.L.; Soletti, J.I.; Ribeiro, L.M.; Dornelas, C.B.; Silva, T.L.; Vieira, M.G. MgAl-LDH/Biochar composites for methylene blue removal by adsorption. Appl. Clay Sci. 2019, 168, 11–20. [Google Scholar] [CrossRef]
- Nazir, M.A.; Khan, N.A.; Cheng, C.; Shah, S.S.; Najam, T.; Arshad, M.; Sharif, A.; Akhtar, S.; Rehman, A.U. Surface induced growth of ZIF-67 at Co-layered double hydroxide: Removal of methylene blue and methyl orange from water. Appl. Clay Sci. 2020, 190, 105564. [Google Scholar] [CrossRef]
- Sharifi-Bonab, M.; Aber, S.; Salari, D.; Khodam, F. Synthesis of CoZnAl-layered double hydroxide/graphene oxide nanocomposite for the removal of methylene blue: Kinetic, thermodynamic, and isotherm studies. Environ. Prog. Sustain. Energy 2020, 39, e13316. [Google Scholar] [CrossRef]
- Lesbani, A.; Asri, F.; Palapa, N.R.; Taher, T.; Rachmat, A. Efficient removal of methylene blue by adsorption using composite based Ca/Al layered double hydroxide-biochar. Global NEST J. 2020, 22, 250–257. [Google Scholar]
- Zhu, S.; Jiao, S.; Liu, Z.; Pang, G.; Feng, S. High adsorption capacity for dye removal by CuZn hydroxyl double salts. Environ. Sci. Nano 2014, 1, 172–180. [Google Scholar] [CrossRef]
- Mahmoud, R.K.; Taha, M.; Zaher, A.; Amin, R.M. Understanding the physicochemical properties of Zn–Fe LDH nanostructure as sorbent material for removing of anionic and cationic dyes mixture. Sci. Rep. 2021, 11, 21365. [Google Scholar] [CrossRef]
- Nekouei, F.; Kargarzadeh, H.; Nekouei, S.; Keshtpour, F.; Makhlouf, A.S. Efficient method for determination of methylene blue dye in water samples based on a combined dispersive solid phase and cloud point extraction using Cu(OH)2 nanoflakes: Central composite design optimization. Anal. Bioanal. Chem. 2017, 409, 1079–1092. [Google Scholar] [CrossRef]
- Zaher, A.; Taha, M.; Farghali, A.A.; Mahmoud, R.K. Zn/Fe LDH as a clay-like adsorbent for the removal of oxytetracycline from water: Combining experimental results and molecular simulations to understand the removal mechanism. Environ. Sci. Pollut. Res. 2020, 27, 12256–12269. [Google Scholar] [CrossRef]
- Gupta, N.K.; Saifuddin, M.; Kim, S.; Kim, K.S. Microscopic, spectroscopic, and experimental approach towards understanding the phosphate adsorption onto Zn–Fe layered double hydroxide. J. Mol. Liq. 2020, 297, 111935. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Babaei, M.; Anbia, M.; Kazemipour, M. Synthesis of zeolite/carbon nanotube composite for gas separation. Can. J. Chem. 2017, 95, 162–168. [Google Scholar] [CrossRef]
- Saleh, R.; Zaki, A.H.; El-Ela, F.I.; Farghali, A.A.; Taha, M.; Mahmoud, R. Consecutive removal of heavy metals and dyes by a fascinating method using titanate nanotubes. J. Environ. Chem. Eng. 2021, 9, 104726. [Google Scholar] [CrossRef]
- Saraya, M.E.; Sayed, M. Characterization and Evaluation of Natural Zeolite As a Pozzolanic Material. Al-Azhar Bull. Sci. 2018, 29, 17–34. [Google Scholar] [CrossRef][Green Version]
- Prasetyo, T.A.; Soegijono, B. Characterization of sonicated natural zeolite/ferric chloride hexahydrate by infrared spectroscopy. J. Phys. Conf. Ser. 2018, 985, 012022. [Google Scholar] [CrossRef]
- Lu, H.; Zhu, Z.; Zhang, H.; Zhu, J.; Qiu, Y. Simultaneous removal of arsenate and antimonate in simulated and practical water samples by adsorption onto Zn/Fe layered double hydroxide. Chem. Eng. J. 2015, 276, 365–375. [Google Scholar] [CrossRef]
- Anggraini, M.; Kurniawan, A.; Ong, L.K.; Martin, M.A.; Liu, J.C.; Soetaredjo, F.E.; Indraswati, N.; Ismadji, S. Antibiotic detoxification from synthetic and real effluents using a novel MTAB surfactant-montmorillonite (organoclay) sorbent. RSC Adv. 2014, 4, 16298–16311. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Neimark, A.V. Relations between structural parameters and adsorption characterization of templated nanoporous materials with cubic symmetry. Langmuir 2000, 16, 2419–2423. [Google Scholar] [CrossRef]
- Liu, W.; Sun, W.; Borthwick, A.G.; Ni, J. Comparison on aggregation and sedimentation of titanium dioxide, titanate nanotubes and titanate nanotubes-TiO2: Influence of pH, ionic strength and natural organic matter. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 434, 319–328. [Google Scholar] [CrossRef]
- Padmavathy, K.S.; Madhu, G.; Haseena, P.V. A study on Effects of pH, Adsorbent Dosage, Time, Initial Concentration and Adsorption Isotherm Study for the Removal of Hexavalent Chromium (Cr (VI)) from Wastewater by Magnetite Nanoparticles. Procedia Technol. 2016, 24, 585–594. [Google Scholar] [CrossRef]
- Mahgoub, S.M.; Shehata, M.R.; Abo El-Ela, F.I.; Farghali, A.; Zaher, A.; Mahmoud, R.K. Erratum: Sustainable waste management and recycling of Zn-Al layered double hydroxide after adsorption of levofloxacin as a safe anti-inflammatory nanomaterial. RSC Adv. 2020, 10, 29128. [Google Scholar] [CrossRef]
- Zaher, A.; Taha, M.; Mahmoud, R.K. Possible adsorption mechanisms of the removal of tetracycline from water by La-doped Zn-Fe-layered double hydroxide. J. Mol. Liq. 2021, 322, 114546. [Google Scholar] [CrossRef]
- Guarín, J.R.; Moreno-Pirajan, J.C.; Giraldo, L. Kinetic Study of the Bioadsorption of Methylene Blue on the Surface of the Biomass Obtained from the Algae D. antarctica. J. Chem. 2018, 1–12. [Google Scholar] [CrossRef]



| Adsorbent | Adsorption Capacity | Ref. |
|---|---|---|
| MgAl-LDH/Biochar composites | 406.47 mg/g | [32] |
| ZIF-67@CoAl-LDH composites | 57.14 mg/g | [33] |
| Zn/Al LDH/Rice Husk Biochar | 62.39mg/g | |
| CoZnAl-LDH/GO nanocomposite | 169.49 mg/g | [34] |
| Ca/Al LDH/biochar composites | 32.535 mg/g | [35] |
| LDH | 37.58 mg/g | This study |
| zeolite | 749.99 mg/g | This study |
| zeolite/LDH composite | 932.31mg/g | This study |
| Process Condition | Description |
|---|---|
| Balls diameters | Range from 1.5 to 1.8 cm |
| Vessel diameter | 7.5 cm |
| Materials of vessel | Stain steel |
| Materials of used balls | Porcelain |
| Ball/natural-zeolite mass ratio | 10:1 |
| Speed | 200 rpm |
| Time | 10 h |
| Sample | Surface Area (m2/g) | PV (cm3/g) | ADP (nm) |
|---|---|---|---|
| Zn-Fe LDH | 16.85 | 0.07 | 3.6 |
| zeolite | 59.83 | 0.15 | 3.71 |
| zeolite/Zn-Fe LDH nanocomposite | 55.94 | 0.018 | 3.33 |
| Isotherm Models | Parameter | Values | R2 | χ2 | |
|---|---|---|---|---|---|
| Zn-Fe LDH | |||||
| Langmuir | qmax (mg/g) | 37.58 | 0.89 | 0.74 | 0.002521 |
| Kad (L/mg) | 0.055 | ||||
| Freundlich | Kf (mg/g) | 4.38 | 0.84 | 0.70 | 0.003877 |
| 1/nF (-) | 0.48 | ||||
| Langmuir-Freundlich | qmax (mg/g) | 51.56 | 0.86 | 0.86 | 0.003388 |
| KLF (L/mg) | 0.027 | ||||
| βLF (-) | 0.81 | ||||
| zeolite | |||||
| Langmuir | qmax (mg/g) | 749.99 | 0.95 | 0.97 | 0.00272 |
| Kad (L/mg) | 0.0069 | ||||
| Freundlich | Kf (mg/g) | 3.50 | 0.96 | 0.80 | 0.00629 |
| 1/nF (-) | 1.02 | ||||
| Langmuir-Freundlich | qmax (mg/g) | 401.85 | 0.93 | 0.93 | 0.010704 |
| KLF (L/mg) | 0.023 | ||||
| βLF (-) | 1.37 | ||||
| zeolite/LDH composite | |||||
| Langmuir | qmax (mg/g) | 932.31 | 0.99 | 0.83 | 0.00146 |
| Kad (L/mg) | 0.002 | ||||
| Freundlich | Kf (mg/g) | 3.33 | 0.99 | 0.83 | 0.000815 |
| 1/nF (-) | 0.83 | ||||
| Langmuir-Freundlich | qmax (mg/g) | 433.91 | 0. 88 | 0.88 | 0.001097 |
| KLF (L/mg) | 0.008 | ||||
| βLF (-) | 0.83 |
| Model | LDH | Zeolite | Composite | |||
|---|---|---|---|---|---|---|
| Pseudo-first-order | Co, 50 mg/L | Co, 100 mg/L | Co, 50 mg/L | Co, 100 mg/L | Co, 50 mg/L | Co, 100 mg/L |
| k1 (min−1) | 0.336 | 97.4 | 0.58 | 108.8 | 0.69 | 140.11 |
| qt,cal (mg/g) | 15.11 | 48.15 | 32.79 | 125.18 | 73.77 | 145.68 |
| R2 | 0.98 | 0.98 | 0.99 | 0.99 | 0.99 | 0.99 |
| Pseudo-second-order | ||||||
| k2 (g/mg min) | 0.043 | 0.021 | 0.101 | 4.92 | 0.067 | 0.047 |
| qe,cal (mg/g) | 15.77 | 50.8 | 32.57 | 125.19 | 74.17 | 146.68 |
| R2 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 |
| Intraparticle diffusion | ||||||
| Kip (mg/g min (1/2)) | 1.195 | 3.54 | 0.69 | 2.67 | 3.51 | 6.837 |
| Cip (mg/g) | 6.07 | 22.2 | 22.169 | 85.27 | 40.94 | 82.18 |
| R2 | 0.59 | 0.49 | 0.25 | 0.25 | 0.37 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkartehi, M.E.; Mahmoud, R.; Shehata, N.; Farghali, A.; Gamil, S.; Zaher, A. LDH Nanocubes Synthesized with Zeolite Templates and Their High Performance as Adsorbents. Nanomaterials 2021, 11, 3315. https://doi.org/10.3390/nano11123315
Elkartehi ME, Mahmoud R, Shehata N, Farghali A, Gamil S, Zaher A. LDH Nanocubes Synthesized with Zeolite Templates and Their High Performance as Adsorbents. Nanomaterials. 2021; 11(12):3315. https://doi.org/10.3390/nano11123315
Chicago/Turabian StyleElkartehi, Moftah Essa, Rehab Mahmoud, Nabila Shehata, Ahmed Farghali, Shimaa Gamil, and Amal Zaher. 2021. "LDH Nanocubes Synthesized with Zeolite Templates and Their High Performance as Adsorbents" Nanomaterials 11, no. 12: 3315. https://doi.org/10.3390/nano11123315
APA StyleElkartehi, M. E., Mahmoud, R., Shehata, N., Farghali, A., Gamil, S., & Zaher, A. (2021). LDH Nanocubes Synthesized with Zeolite Templates and Their High Performance as Adsorbents. Nanomaterials, 11(12), 3315. https://doi.org/10.3390/nano11123315





