Smart Mn7+ Sensing via Quenching on Dual Fluorescence of Eu3+ Complex-Modified TiO2 Nanoparticles
Abstract
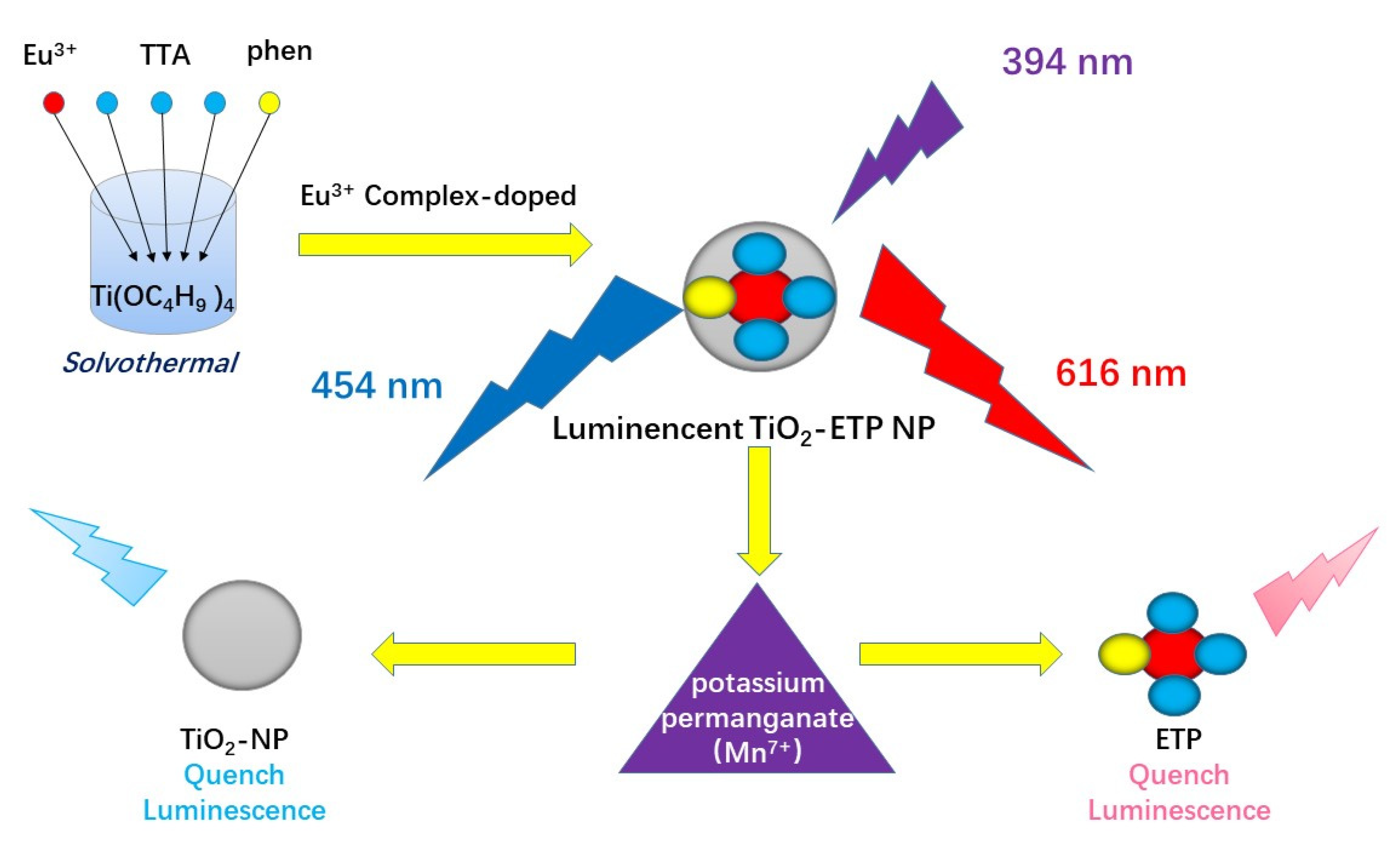
:1. Introduction
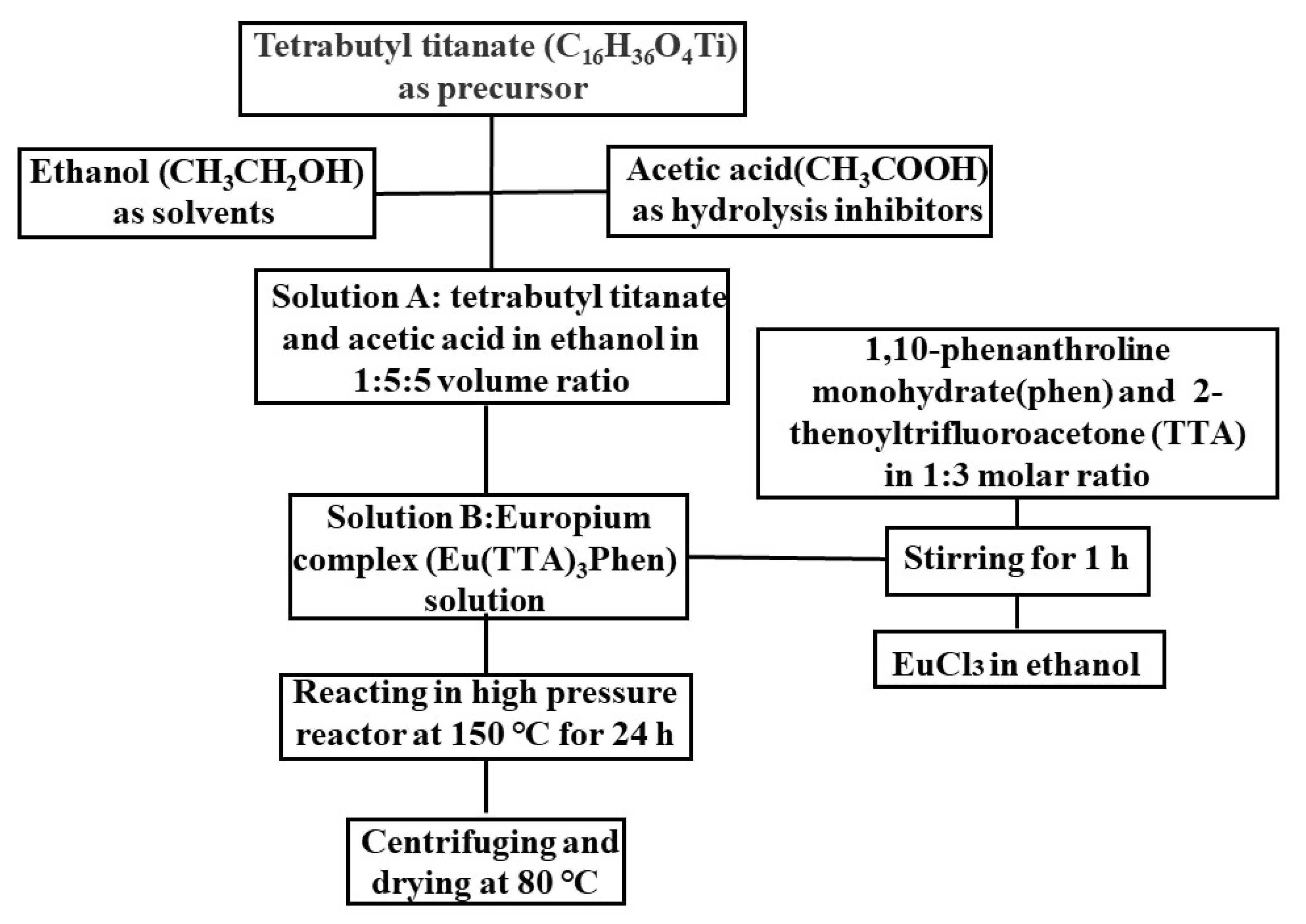
2. Experimental Details
3. Results and Discussion
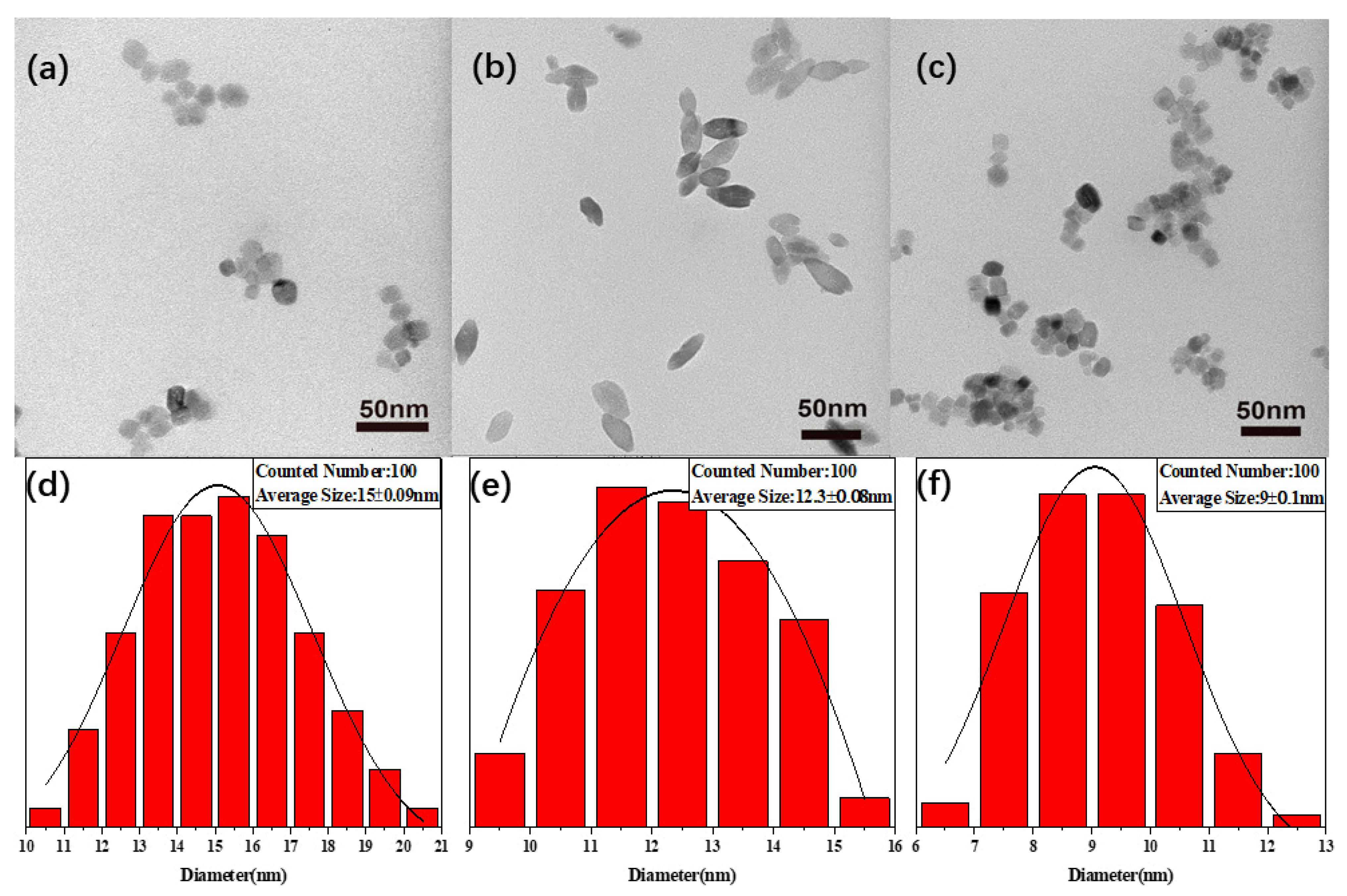
3.1. Morphological Structures
3.2. Crystalline Structure
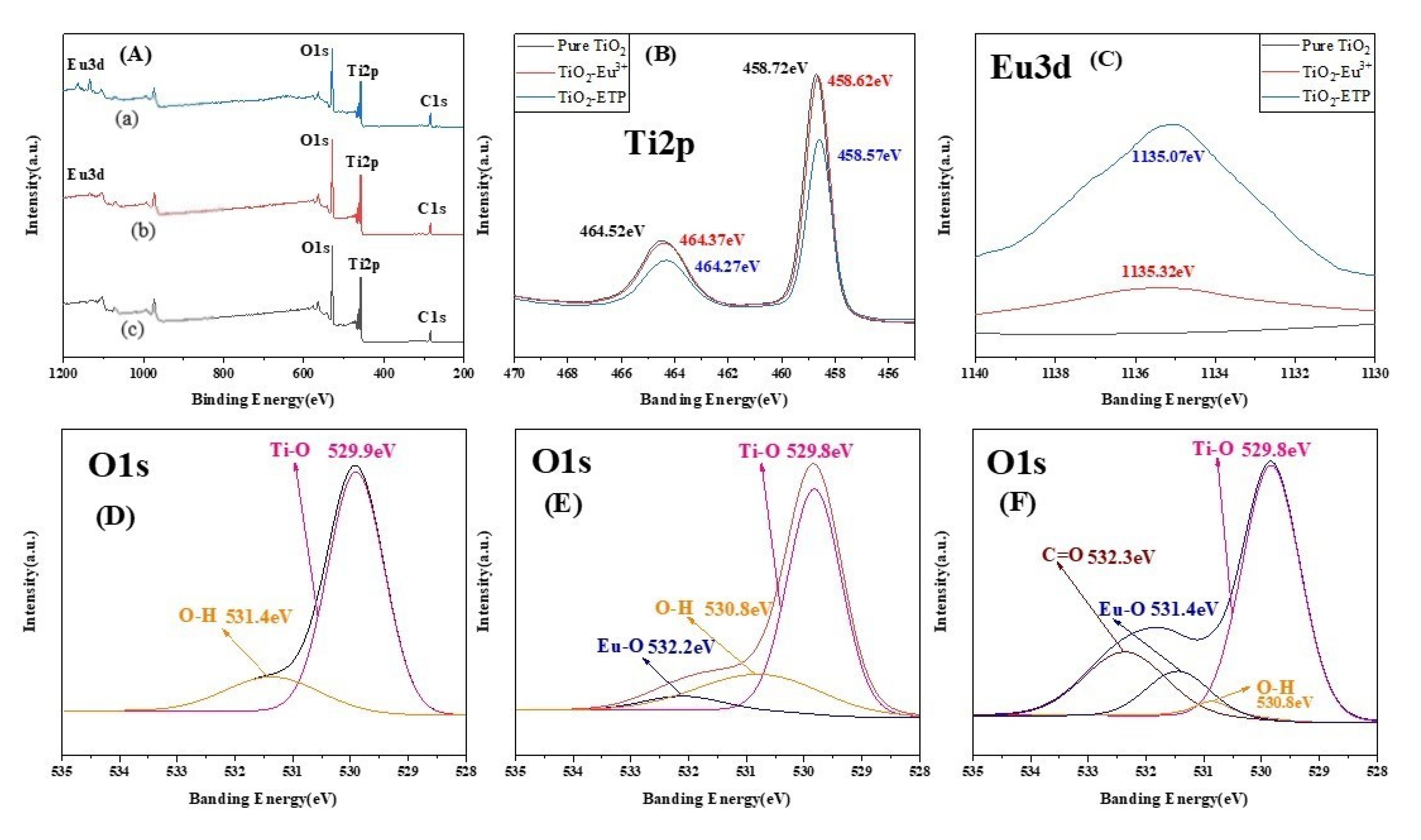
3.3. Confirmation of Eu3+ in TiO2
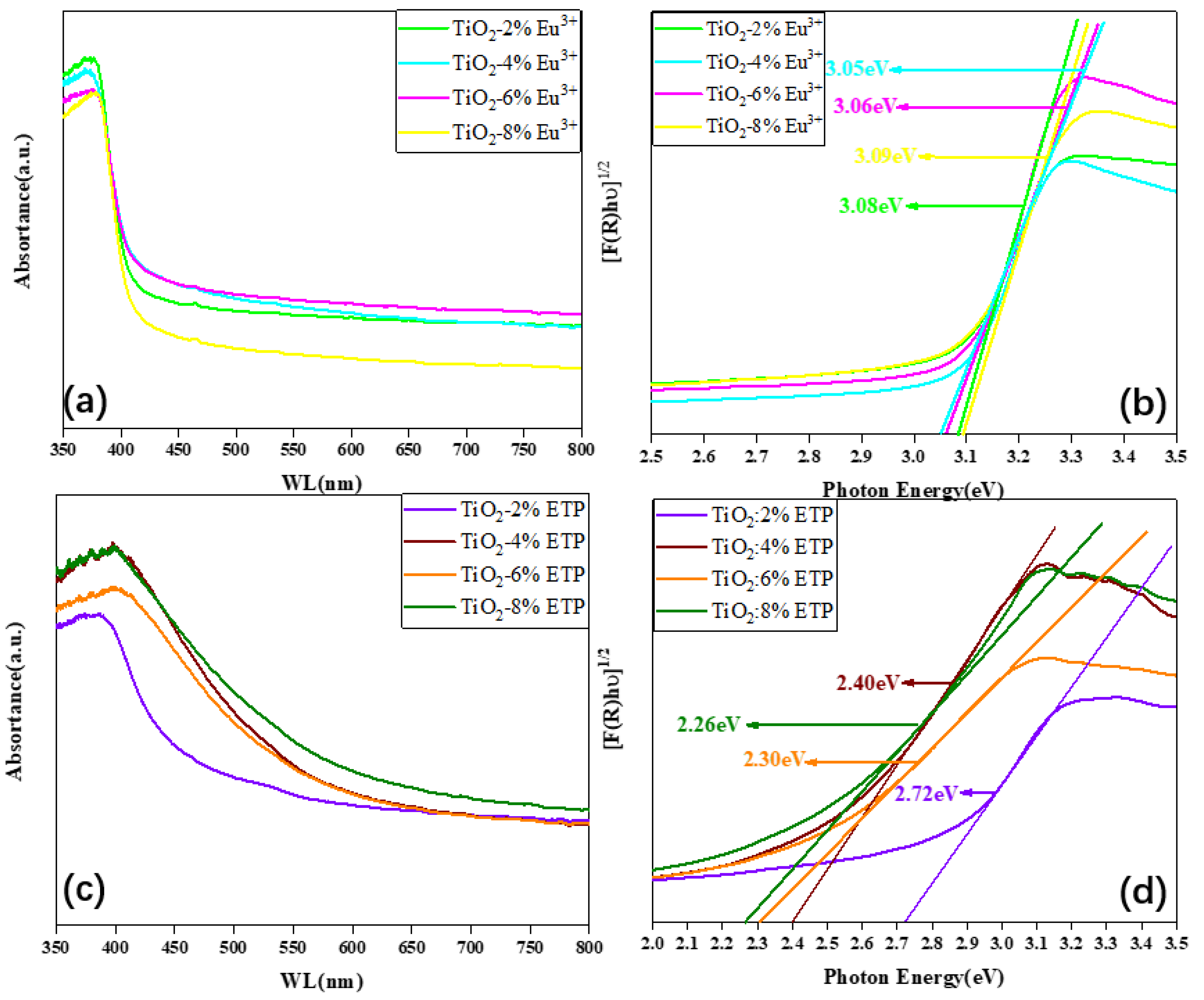
3.4. UV Absorption and Bandgap of TiO2
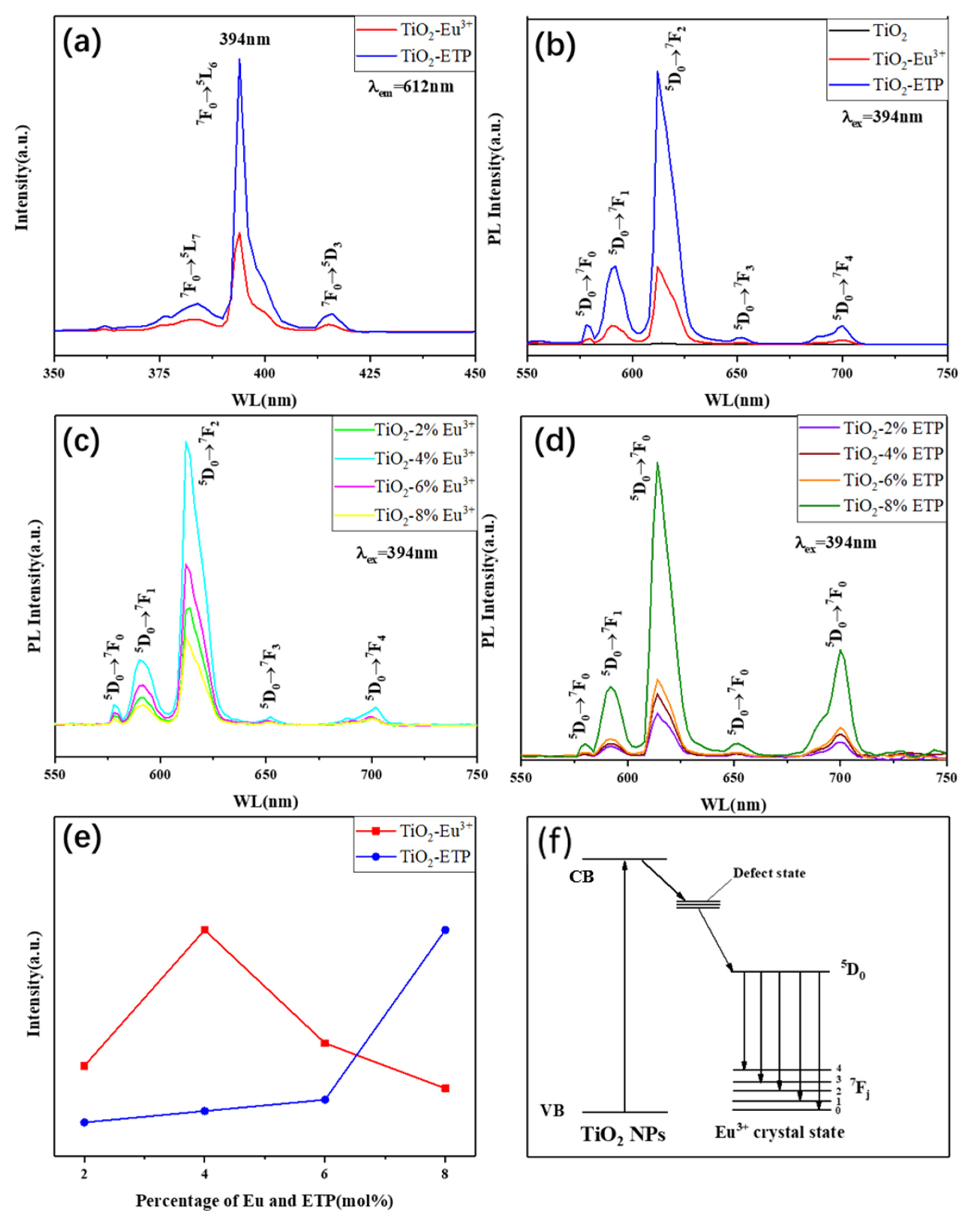
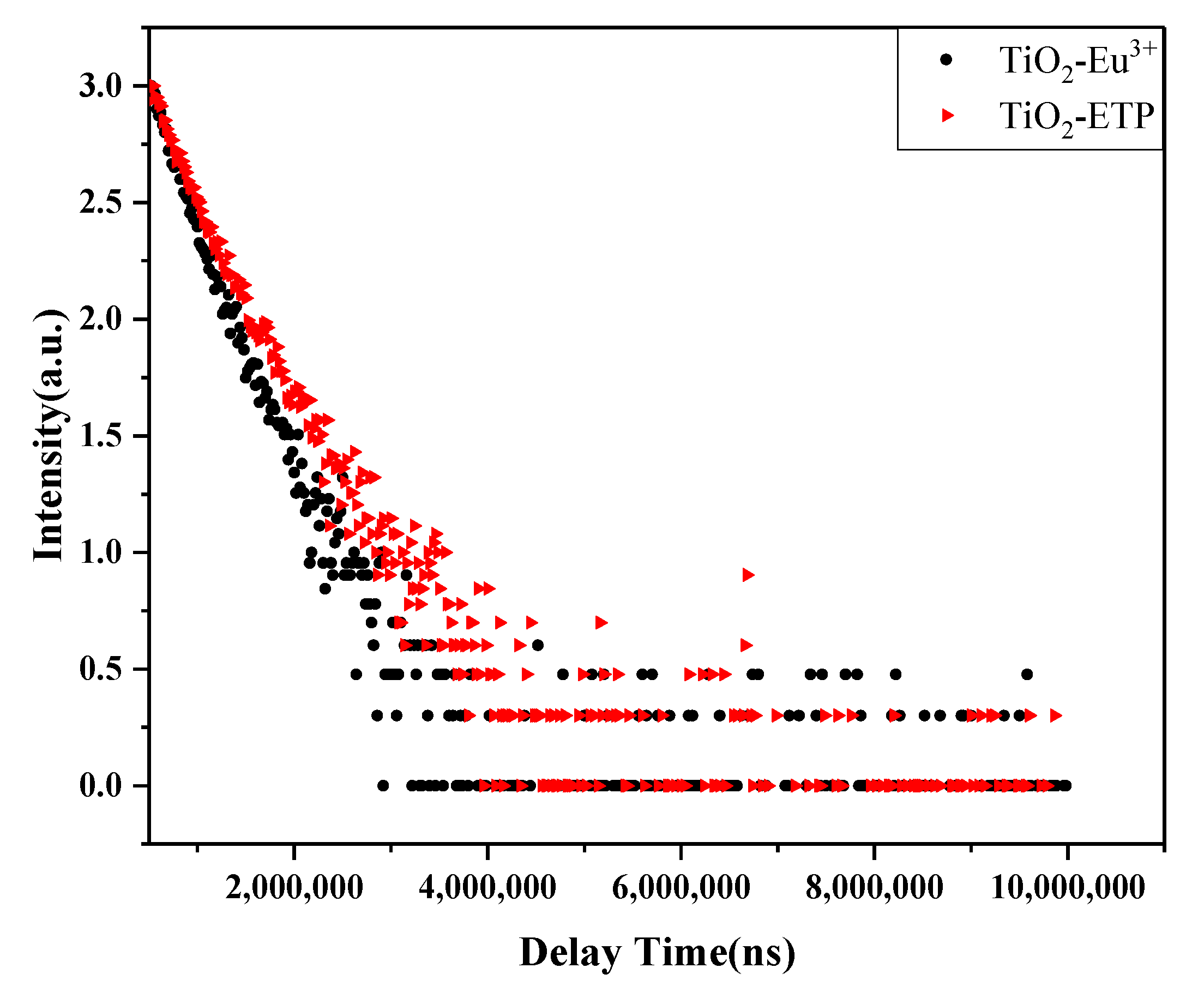
3.5. Photoluminescence Analysis
3.6. Fluorescence Spectra of TiO2-ETP in the Presence of Metal Ions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brown, S.; Taylor, N.L. Could mitochondrial dysfunction play a role in manganese toxicity? Environ. Toxicol. Pharmacol. 1999, 7, 49–57. [Google Scholar] [CrossRef]
- Gerber, G.B.; Léonard, A.; Hantson, P. Carcinogenicity, mutagenicity and teratogenicity of manganese compounds. Crit. Rev. Oncol. Hematol. 2002, 42, 25–34. [Google Scholar] [CrossRef]
- Crossgrove, J.; Wei, Z. Manganese toxicity upon overexposure. NMR Biomed. 2004, 17, 544–553. [Google Scholar] [CrossRef]
- Zhong, Q.; Liao, D.X.; Ming, L.X. Review of Research in the Treatment of Electrolytic Manganese Waste Water. China’s Manganese Ind. 2005, 4, 7–9. [Google Scholar]
- Horiguchi, T. Mechanism of Manganese Toxicity and Tolerance of Plants. J. Plant Nutr. 1988, 11, 235–246. [Google Scholar] [CrossRef]
- Talasek, T. Ion Chromatography—ScienceDirect. Charact. Integr. Circuit Packag. Mater. 1993, 379, 240–242. [Google Scholar]
- Porento, M.; Sutinen, V.; Julku, T.; Oikari, R. Detection of copper in water using on-line plasma-excited atomic absorption spectroscopy (AAS). Appl. Spectrosc. 2011, 65, 678–683. [Google Scholar] [CrossRef]
- Nardi, E.P.; Evangelista, F.S.; Tormen, L.; Saint, T.D.; Curtius, A.J.; de Souza, S.S.; Barbosa, F., Jr. The use of inductively coupled plasma mass spectrometry (ICP-MS) for the determination of toxic and essential elements in different types of food samples. Food Chem. 2009, 112, 727–732. [Google Scholar] [CrossRef]
- Groves, J.T.; Lee, J.; Marla, S.S. Detection and Characterization of an Oxomanganese(V) Porphyrin Complex by Rapid-Mixing Stopped-Flow Spectrophotometry. J. Am. Chem. Soc. 1997, 119, 6269–6273. [Google Scholar] [CrossRef]
- Citak, D.; Tuzen, M.; Soylak, M. Speciation of Mn(II), Mn(VII) and total manganese in water and food samples by coprecipitation-atomic absorption spectrometry combination. J. Hazard. Mater. 2010, 173, 773–777. [Google Scholar] [CrossRef]
- Qian, A.X.; He, G.H.; Han, X. Separation and preconcentration of MnVII/MnII speciation on crosslinked chitosan and determination by flame atomic absorption spectrometry. Analyst 2001, 126, 239–241. [Google Scholar] [CrossRef]
- Pandya, S.; Yu, J.; Parker, D. Engineering emissive europium and terbium complexes for molecular imaging and sensing. Dalton Trans. 2006, 2757–2766. [Google Scholar] [CrossRef] [PubMed]
- Potts, P.J.; Webb, P.C. X-ray fluorescence spectrometry. J. Geochem. Explor. 2012, 44, 251–296. [Google Scholar] [CrossRef]
- Ferreira, V.J.; Almeida, J.S.; Lemos, V.A.; de Oliveira, O.M.C.; Garcia, K.S.; Teixeira, L.S.G. Determination of Cu, Ni, Mn, and Pb in diesel oil samples using reversed-phase vortex-assisted liquid-liquid microextraction associated with energy dispersive X-ray fluorescence spectrometry. Talanta 2021, 222, 121514. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.B. Fluorescence Sensors and Biosensors. Fluoresc. Sens. Biosens. 2006, 23, 2757–2766. [Google Scholar]
- Silva, A.P.D.; Gunaratne, H.Q.N.; Gunnlaugsson, T.; Huxley, A.J.M.; Rice, T.E. ChemInform Abstract: Signaling Recognition Events with Fluorescent Sensors and Switches. Chem. Rev. 1997, 97, 1515–1566. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Hou, X.; Xu, J.J.; Chen, H.Y. Ratiometric fluorescence, electrochemiluminescence, and photoelectrochemical chemo/biosensing based on semiconductor quantum dots. Nanoscale 2016, 8, 8427–8442. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Zhao, Z.M.; Miao, J.Y.; Zhao, B.X. A ratiometric fluorescence probe based on a novel FRET platform for imaging endogenous HOCl in the living cells. Sens. Actuators B Chem. 2016, 229, 408–413. [Google Scholar] [CrossRef]
- Liu, Z.M.; Feng, L.; Hou, J.; Lv, X.; Ning, J.; Ge, G.B.; Wang, K.W.; Cui, J.N.; Yang, L. A ratiometric fluorescent sensor for highly selective detection of human carboxylesterase 2 and its application in living cells. Sens. Actuators B Chem. 2014, 205, 151–157. [Google Scholar] [CrossRef]
- Long, L.; Lin, W.; Chen, B.; Gao, W.; Yuan, L. Construction of a FRET-based ratiometric fluorescent thiol probe. Chem. Commun. 2010, 47, 893. [Google Scholar] [CrossRef]
- Wang, K.; Qian, J.; Jiang, D.; Yang, Z.; Du, X.; Wang, K. Onsite naked eye determination of cysteine and homocysteine using quencher displacement-induced fluorescence recovery of the dual-emission hybrid probes with desired intensity ratio. Biosens. Bioelectron. 2015, 65, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.K. A novel core-satellite CdTe/Silica/Au \{NCs\} hybrid sphere as dual-emission ratiometric fluorescent probe for Cu2+. Biosens. Bioelectron. 2014, 51, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; He, J.; Zeng, M.; Hao, J.; Guo, Q.; Song, Y.; Wang, L. Cu–hemin metal-organic frameworks with peroxidase-like activity as peroxidase mimics for colorimetric sensing of glucose. J. Nanopart. Res. 2016, 18, 106. [Google Scholar] [CrossRef]
- Dong, Y.; Cai, J.; Fang, Q.; You, X.; Chi, Y. Dual-Emission of Lanthanide Metal–Organic Frameworks Encapsulating Carbon-Based Dots for Ratiometric Detection of Water in Organic Solvents. Anal. Chem. 2016, 88, 1748. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Gong, Q.; Li, Z.; Li, J. MOFs for CO2 capture and separation from flue gas mixtures: The effect of multifunctional sites on their adsorption capacity and selectivity. Chem. Commun. 2012, 49, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent Functional Metal-Organic Frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef]
- Rocha, J.; Carlos, L.; Paz, F.A.; Ananias, D. Luminescent multifunctional lanthanides-based metal-organic frameworks. Chem. Soc. Rev. 2011, 40, 926–940. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, D.; Zeng, J.; Gan, N.; Cuan, J. A luminescent Lanthanide-free MOF nanohybrid for highly sensitive ratiometric temperature sensing in physiological range. Talanta 2018, 181, 410–415. [Google Scholar] [CrossRef]
- Kaczmarek, A.M.; Kristof, V.H.; Rik, V.D. Enhanced luminescence in Ln3?-doped Y?WO? (Sm, Eu, Dy) 3D microstructures through Gd3? Codoping. Inorg. Chem. 2014, 53, 9498–9508. [Google Scholar] [CrossRef]
- Dorenbos, P. The 4fn4fn-15d transitions of the trivalent lanthanides in halogenides and chalcogenides. J. Lumin. 2000, 91, 91–106. [Google Scholar] [CrossRef]
- Werts, M.H.V.; Jukes, R.T.F.; Verhoeven, J.W. The emission spectrum and the radiative lifetime of Eu3+ in luminescent lanthanide complexes. Phys. Chem. Chem. Phys. 2002, 4, 1542–1548. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Zhai, D.; Zhang, K.; Yong, Z.; Sun, H.; Fan, G. Mesoporous silica equipped with europium-based chemosensor for mercury ion detection: Synthesis, characterization, and sensing performance. Inorg. Chim. Acta 2012, 387, 396–400. [Google Scholar] [CrossRef]
- Zhang, M.; Zhan, G.; Chen, Z. Iodometric Amplification Method for the Determinations of Microgram Amounts of Manganese(II), Manganese(VII), Chromium(III) and Chromium(VI) in Aqueous Solution. Anal. Ences 2005, 14, 1077–1083. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Xu, X.; Zhao, H.; Song, Y.; Sheng, Y.; Huo, Q.; Zou, H. Facile synthesis of TiO2:Eu3+ spindle shaped nanoparticles from titanate nanobelt precursors. Powder Technol. 2012, 228, 277–283. [Google Scholar]
- Lazzeri, M.; Vittadini, A.; Selloni, A. Erratum, Structure and energetics of stoichiometric TiO2 anatase surfaces. Phys. Rev. B 2001, 65, 011990. [Google Scholar]
- Wang, H.; Wang, Y.; Yang, Y.; Li, X.; Wang, C. Photoluminescence properties of the rare-earth ions in the TiO2 host nanofibers prepared via electrospinning. Mater. Res. Bull. 2009, 44, 408–414. [Google Scholar] [CrossRef]
- Vranješ, M.; Kuljanin-Jakovljević, J.; Radetić, T.; Stoiljković, M.; Mitrić, M.; Šaponjić, Z.V.; Nedeljković, J. Structure and luminescence properties of Eu3+ doped TiO2 nanocrystals and prolate nanospheroids synthesized by the hydrothermal processing. Ceram. Int. 2012, 38, 5629–5636. [Google Scholar] [CrossRef]
- Qi, X.; Zou, H.; Song, Y.; Zhang, H.; Zhao, H.; Shi, Z.; Sheng, Y. Hydrothermal synthesis and luminescence properties of TiO2: Eu3+ submicrospheres. Ceram. Int. 2014, 40, 12993–12997. [Google Scholar] [CrossRef]
- Hsiao, R.C.; Arul, N.S.; Mangalaraj, D.; Juang, R.S. Influence of Eu3+ doping on the degradation property of TiO2 nanostructures. J. Optoelectron. Adv. Mater. 2010, 12, 193–198. [Google Scholar]
- Pal, M.; Pal, U.; Jiménez, J.M.G.Y.; Pérez-Rodríguez, F. Effects of crystallization and dopant concentration on the emission behavior of TiO2:Eu nanophosphors. Nanoscale Res. Lett. 2012, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.F.; He, Y.L.; Zhang, M.S.; Yin, Z.; Chen, Q.Y.Z. Raman scattering study on anatase TiO2 nanocrystals. J. Phys. D Appl. Phys. 2000, 33, 912. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Feng, Z.; Chen, J.; Li, C. UV Raman spectroscopic study on TiO2. I. Phase transformation at the surface and in the bulk. J. Phys. Chem. B 2006, 110, 927–935. [Google Scholar] [CrossRef]
- Bersani, D.; Lottici, P.P.; Ding, X.Z. Phonon confinement effects in the Raman scattering by TiO2 nanocrystals. Appl. Phys. Lett. 1998, 72, 73–75. [Google Scholar] [CrossRef]
- Su, B.; Wang, S.; Yang, W.; Wang, Y.; Huang, L.; Popat, K.C.; Kipper, M.J.; Belfiore, L.A.; Tang, J. Synthesis of Eu-modified luminescent Titania nanotube arrays and effect of voltage on morphological, structural and spectroscopic properties. Mater. Sci. Semicond. Process. 2020, 113, 105026. [Google Scholar] [CrossRef]
- Tan, X.; Fan, Q.; Wang, X.; Grambow, B. Eu(III) sorption to TiO2 (anatase and rutile): Batch, XPS, and EXAFS studies. Environ. Sci. Technol. 2009, 43, 3115–3121. [Google Scholar] [CrossRef]
- Diebold, U. TiO2 by XPS. Surf. Sci. Spectra 1996, 4, 227–231. [Google Scholar] [CrossRef]
- Feng, X.; Yang, L.; Zhang, N.; Liu, Y. A facile one-pot hydrothermal method to prepare europium-doped titania hollow phosphors and their sensitized luminescence properties. J. Alloy. Compd. 2010, 506, 728–733. [Google Scholar] [CrossRef]
- Khan, S.B.; Rahman, M.M.; Asiri, A.M.; Marwani, H.M.; Bawaked, S.M.; Alamry, K.A. Co3O4 co-doped TiO2 nanoparticles as a selective marker of lead in aqueous solution. New J. Chem. 2013, 37, 2888–2893. [Google Scholar] [CrossRef]
- Yu, J.; Xiang, Q.; Zhou, M. Preparation, characterization and visible-light-driven photocatalytic activity of Fe-doped titania nanorods and first-principles study for electronic structures. Appl. Catal. B: Environ. 2009, 90, 595–602. [Google Scholar] [CrossRef]
- Krasil’Nikov, V.N.; Zhukov, V.P.; Perelyaeva, L.A.; Baklanova, I.V.; Shein, I.R. Electronic band structure, optical absorption, and photocatalytic activity of iron-doped anatase. Phys. Solid State 2013, 55, 1903–1912. [Google Scholar] [CrossRef]
- Yamashita, H.; Harada, M.; Misaka, J.; Takeuchi, M.; Anpo, M. Degradation of propanol diluted in water under visible light irradiation using metal ion-implanted titanium dioxide photocatalysts. J. Photochem. Photobiol. A Chem. 2002, 148, 257–261. [Google Scholar] [CrossRef]
- Komaraiah, D.; Radha, E.; Kalarikkal, N.; Sivakumar, J.; Reddy, M.R.; Sayanna, R. Structural, optical and photoluminescence studies of sol-gel synthesized pure and iron doped TiO2 photocatalysts. Ceram. Int. 2019, 45, 25060–25068. [Google Scholar] [CrossRef]
- Singh, D.; Singh, N.; Sharma, S.D.; Kant, C.; Sharma, C.P.; Pandey, R.R.; Saini, K.K. Bandgap modification of TiO2 sol–gel films by Fe and Ni doping. J. Sol.-Ge.l Sci. Technol. 2011, 58, 269–276. [Google Scholar] [CrossRef]
- Qi, X.; Song, Y.; Sheng, Y.; Zhang, H.; Zhao, H.; Shi, Z.; Zou, H. Controllable synthesis and luminescence properties of TiO2:Eu3+ nanorods, nanoparticles and submicrospheres by hydrothermal method. Opt. Mater. 2014, 38, 193–197. [Google Scholar] [CrossRef]
- Shahi, P.K.; Singh, A.K.; Singh, S.K.; Rai, S.B.; Ullrich, B. Revelation of the Technological Versatility of the Eu(TTA)3Phen Complex by Demonstrating Energy Harvesting, Ultraviolet Light Detection, Temperature Sensing, and Laser Applications. Acs. Appl. Mater. Interfaces 2015, 7, 18231–18239. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Li, Y. Luminescent ternary inorganic-organic mesoporous hybrids Eu(TTASi-SBA-15)phen: Covalent linkage in TTA directly functionalized SBA-15. Dalton Trans. 2010, 39, 1480–1487. [Google Scholar] [CrossRef]
- Coban, M.B.; Kocak, C.; Kara, H.; Aygun, M.; Amjad, A. Magnetic properties and sensitized visible and NIR luminescence of DyIII and EuIII coordination polymers by energy transfer antenna ligands. Mol. Cryst. Liq. Cryst. 2017, 648, 202–215. [Google Scholar] [CrossRef]
- Frindell, K.L.; Bartl, M.H.; Robinson, M.R.; Bazan, G.C.; Popitsch, A.; Stucky, G.D. Visible and near-IR luminescence via energy transfer in rare earth doped mesoporous titania thin films with nanocrystalline walls. J. Solid State Chem. 2003, 172, 81–88. [Google Scholar] [CrossRef]
- Knyazev, A.A.; Karyakin, M.E.; Krupin, A.S.; Romanova, K.A.; Galyametdinov, Y.G. Influence of Eu(III) Complexes Structural Anisotropy on Luminescence of Doped Conjugated Polymer Blends. Inorg. Chem. 2017, 56, 6067–6075. [Google Scholar] [CrossRef]
- Rino, L.; Simes, W.; Santos, G.; Fonseca, F.J.; Andrade, A.M.; Deichmann, V.A.F.; Akcelrud, L.; Pereira, L. Photo and electroluminescence behavior of Tb(ACAC)3phen complex used as emissive layer on organic light emitting diodes. J. Non.-Cryst. Solids 2008, 354, 5326–5327. [Google Scholar] [CrossRef]
- Song, Z.; Wang, J.; Liu, J.; Wang, X.; Tang, J. Eu3+-Induced Polysaccharide Nano-Dumbbell Aggregates (PNDA) as Drug Carriers to Smartly Report Drug Concentration through Variable Fluorescence. Sens. Actuators B Chem. 2021, 336, 129724. [Google Scholar] [CrossRef]
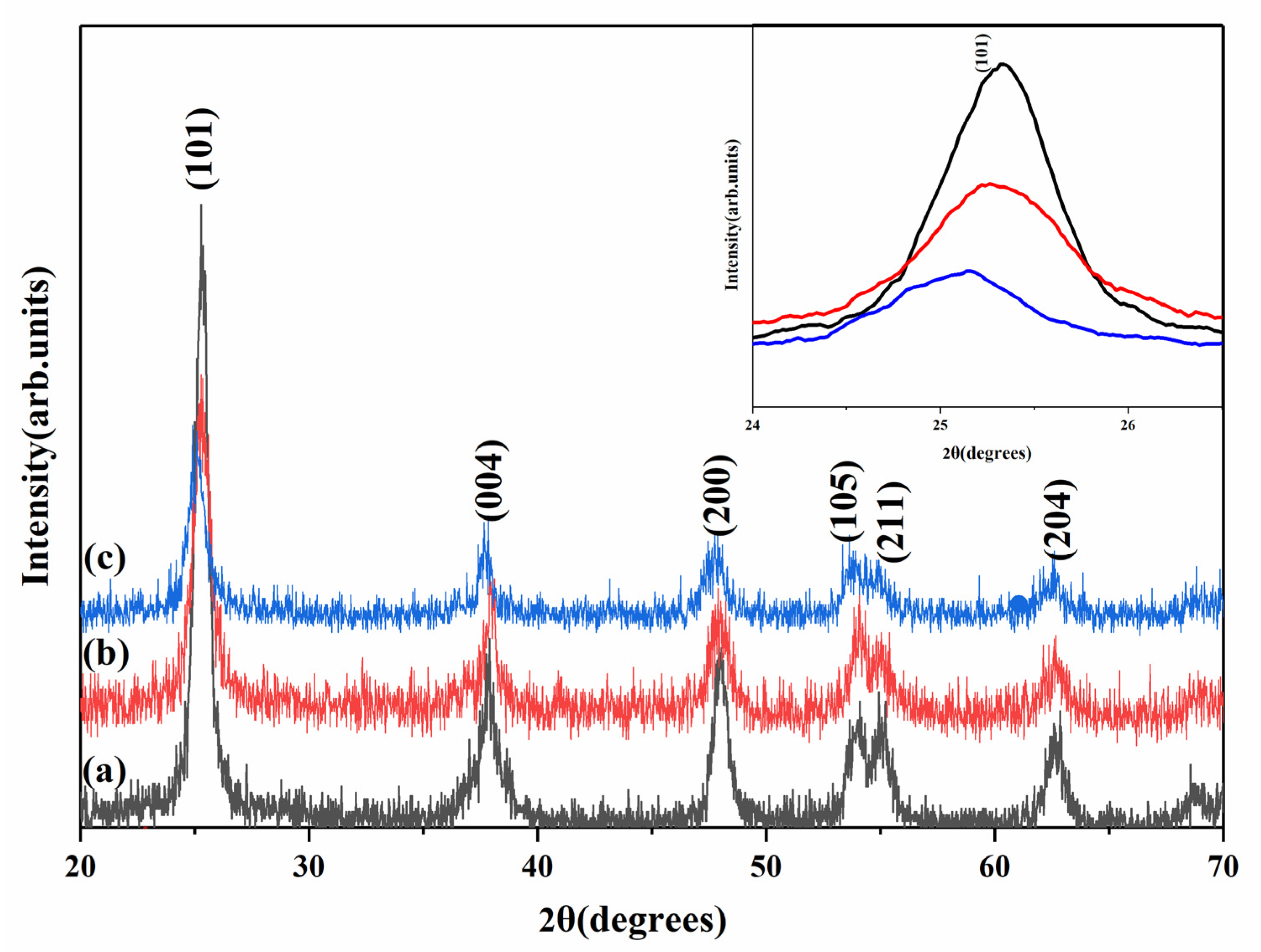



| Sample | hkl | 2θ (deg) | D (Å) | FWHM (deg) | Mean Grain Size (nm) | Crystal Structure |
|---|---|---|---|---|---|---|
| TiO2 | 101 | 25.34 | 3.51 | 0.671 | 15.1 | tetragonal |
| 004 | 37.84 | 2.37 | 0.846 | 15.0 | tetragonal | |
| 200 | 48.07 | 1.89 | 0.710 | 15.1 | tetragonal | |
| TiO2-Eu3+ | 101 | 25.31 | 3.50 | 1.115 | 12.2 | tetragonal |
| 004 | 37.66 | 2.38 | 0.786 | 12.4 | tetragonal | |
| 200 | 47.88 | 1.89 | 0.825 | 12.1 | tetragonal | |
| TiO2-ETP | 101 | 25.06 | 3.55 | 0.966 | 9.0 | tetragonal |
| 004 | 37.48 | 2.39 | 0.847 | 9.1 | tetragonal | |
| 200 | 47.76 | 1.90 | 0.971 | 8.9 | tetragonal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Niu, S.; Wang, Y.; Huang, L.; Wang, S.; Popat, K.C.; Kipper, M.J.; Belfiore, L.A.; Tang, J. Smart Mn7+ Sensing via Quenching on Dual Fluorescence of Eu3+ Complex-Modified TiO2 Nanoparticles. Nanomaterials 2021, 11, 3283. https://doi.org/10.3390/nano11123283
Yang W, Niu S, Wang Y, Huang L, Wang S, Popat KC, Kipper MJ, Belfiore LA, Tang J. Smart Mn7+ Sensing via Quenching on Dual Fluorescence of Eu3+ Complex-Modified TiO2 Nanoparticles. Nanomaterials. 2021; 11(12):3283. https://doi.org/10.3390/nano11123283
Chicago/Turabian StyleYang, Wenbin, Siqi Niu, Yao Wang, Linjun Huang, Shichao Wang, Ketul C. Popat, Matt J. Kipper, Laurence A. Belfiore, and Jianguo Tang. 2021. "Smart Mn7+ Sensing via Quenching on Dual Fluorescence of Eu3+ Complex-Modified TiO2 Nanoparticles" Nanomaterials 11, no. 12: 3283. https://doi.org/10.3390/nano11123283
APA StyleYang, W., Niu, S., Wang, Y., Huang, L., Wang, S., Popat, K. C., Kipper, M. J., Belfiore, L. A., & Tang, J. (2021). Smart Mn7+ Sensing via Quenching on Dual Fluorescence of Eu3+ Complex-Modified TiO2 Nanoparticles. Nanomaterials, 11(12), 3283. https://doi.org/10.3390/nano11123283








