Impacts of the Catalyst Structures on CO2 Activation on Catalyst Surfaces
Abstract
:1. Introduction
1.1. CO2 Conversion to Chemicals: Challenges, Thermodynamics, and Kinetics
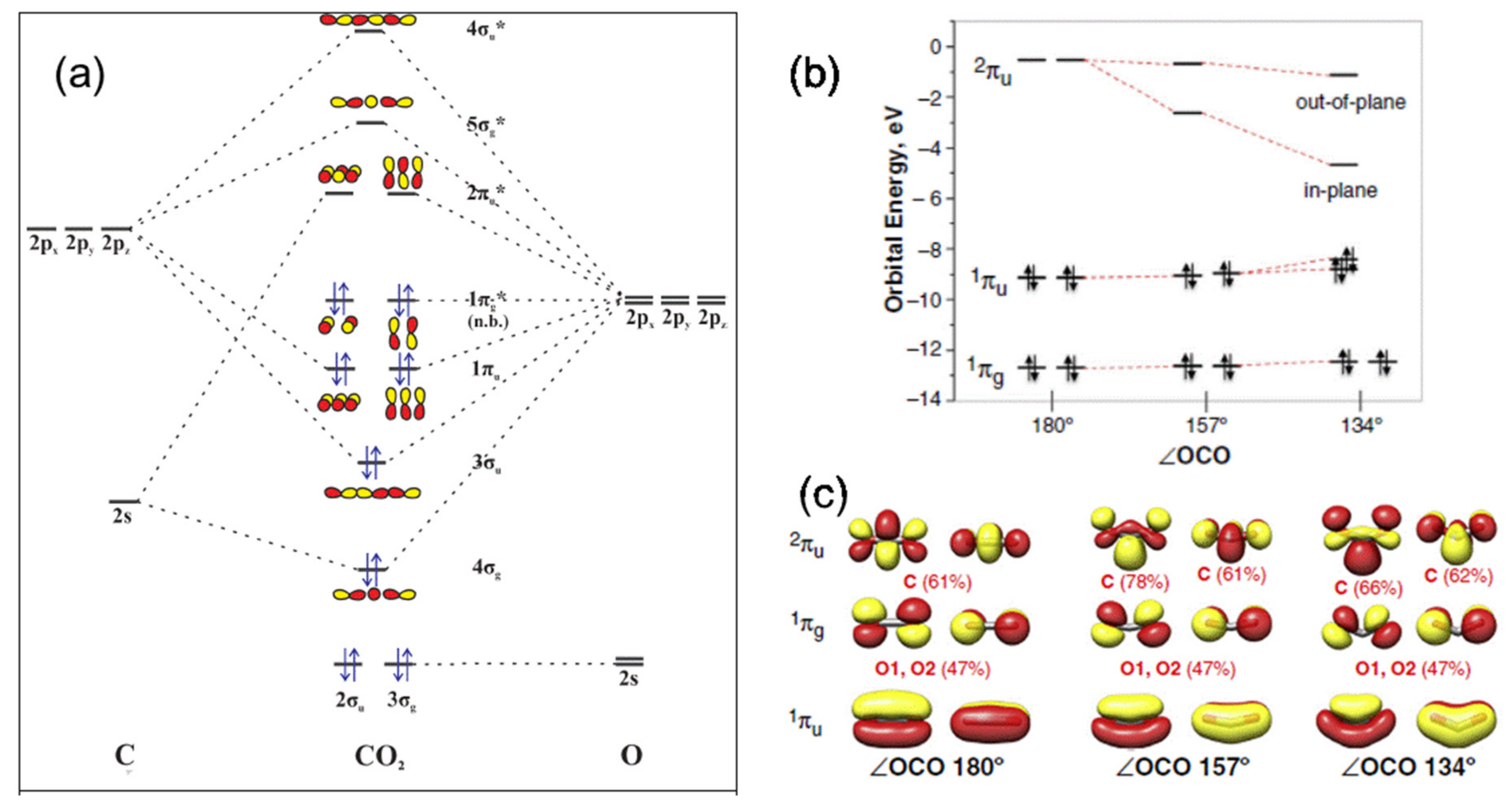
1.2. General Properties of CO2 Molecule: Molecular Structure and Bonding Properties
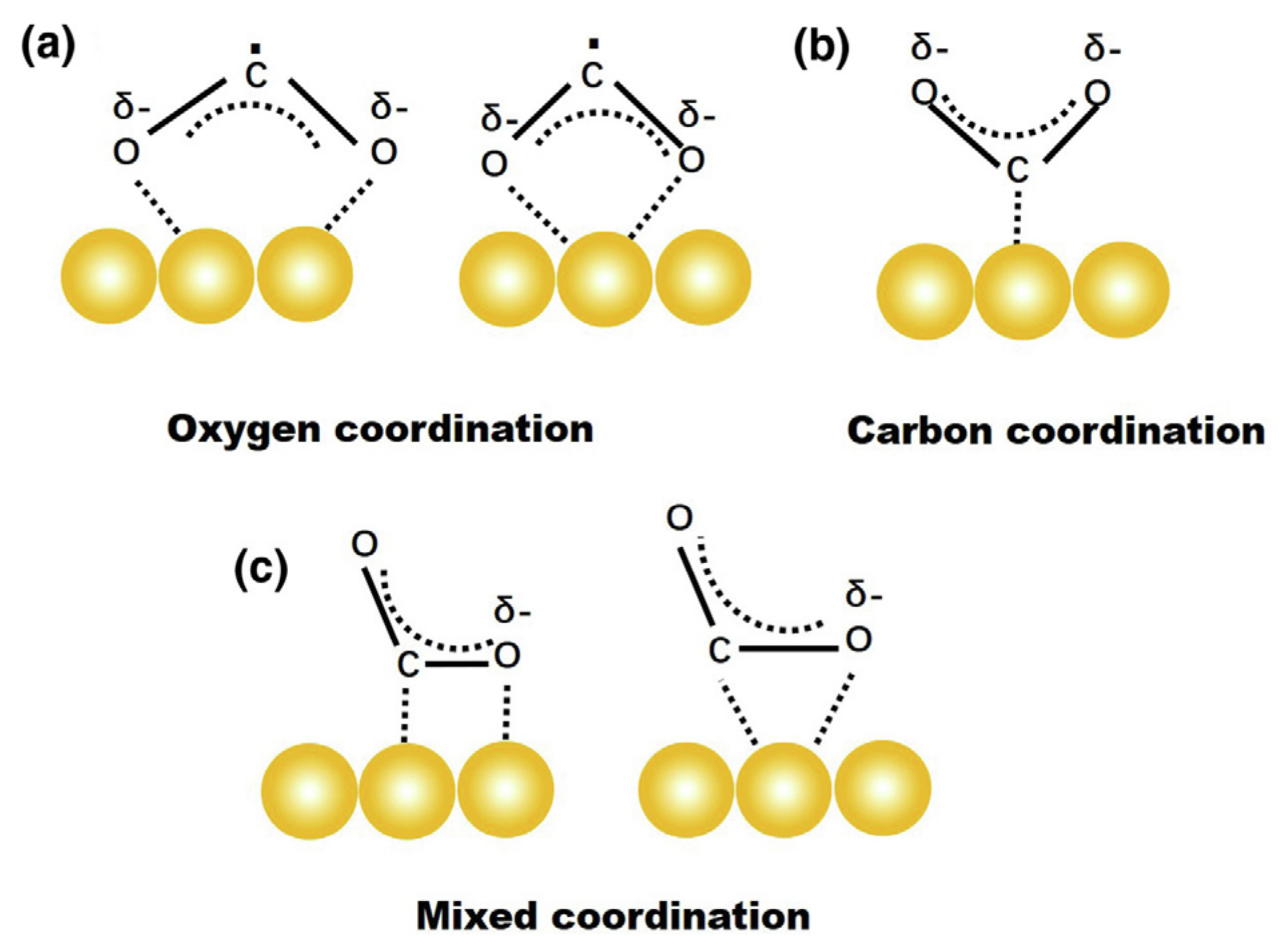
2. CO2 Activation and Different Modes
- bending of the O–C–O angle from 180 degrees,
- elongation of at least one of the two C–O bonds,
- polarization of the charges on C and O, leading to the transfer of charge/electron to CO2,
- hydride transfer, or
- redistribution of charges.
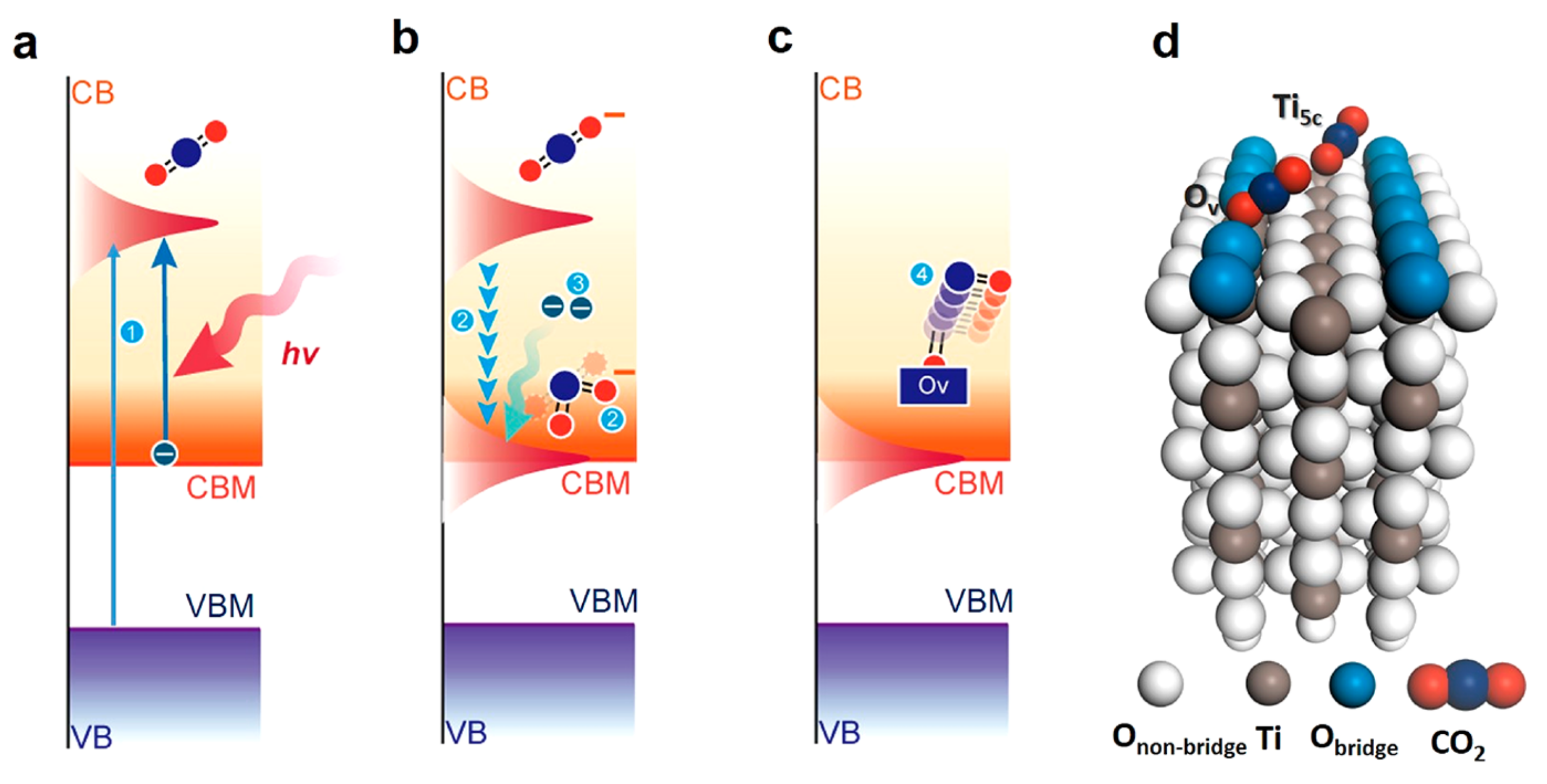
2.1. Photocatalytic Activation of CO2
- Photon absorption and excited carrier generation.
- Activation of CO2 to form an anion radical, CO2•−, or other intermediates by the photoexcited electrons.
- Dissociation of the C–O bond, involving the participation of protons and electron transfer, generating different products.
- the one-electron transfer to form CO2− is thermodynamically unfavorable as it requires a very negative reduction potential of −1.9 V NHE;
- the photoexcited holes (or OH radicals) easily oxidize water to oxygen, or oxidize the intermediates and products converted from CO2 to undesirable products (reverse reactions); and
- the electron–hole pairs recombination rate is much faster.
2.2. Electrocatalytic Activation of CO2
2.3. Activation of CO2 by Homogeneous Catalysts
3. Activation of CO2 on Heterogeneous Catalyst Surface
3.1. CO2 Activation on Representative Pure Metals
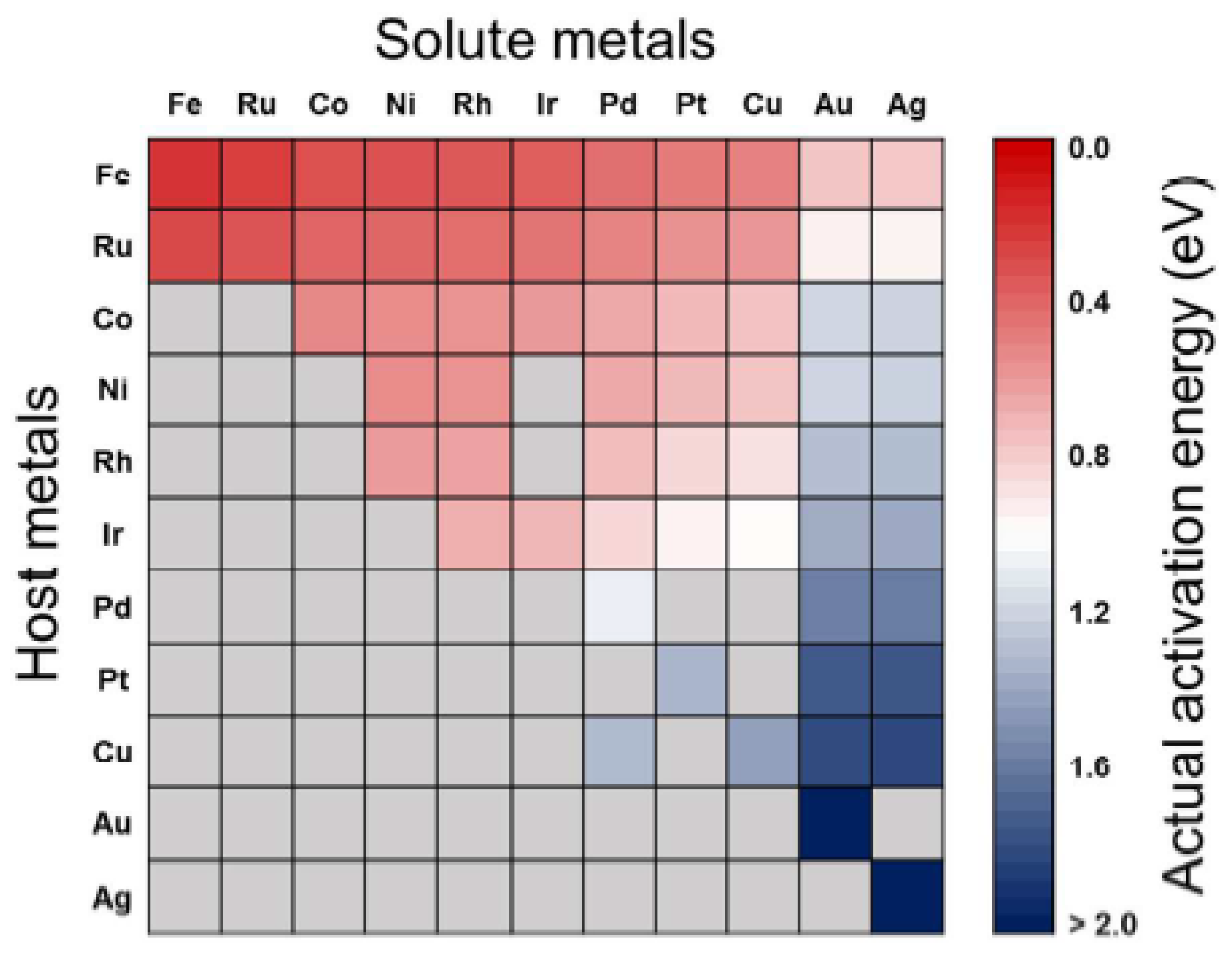
3.2. CO2 Activation on Bimetallic/Alloyed Catalyst Surfaces
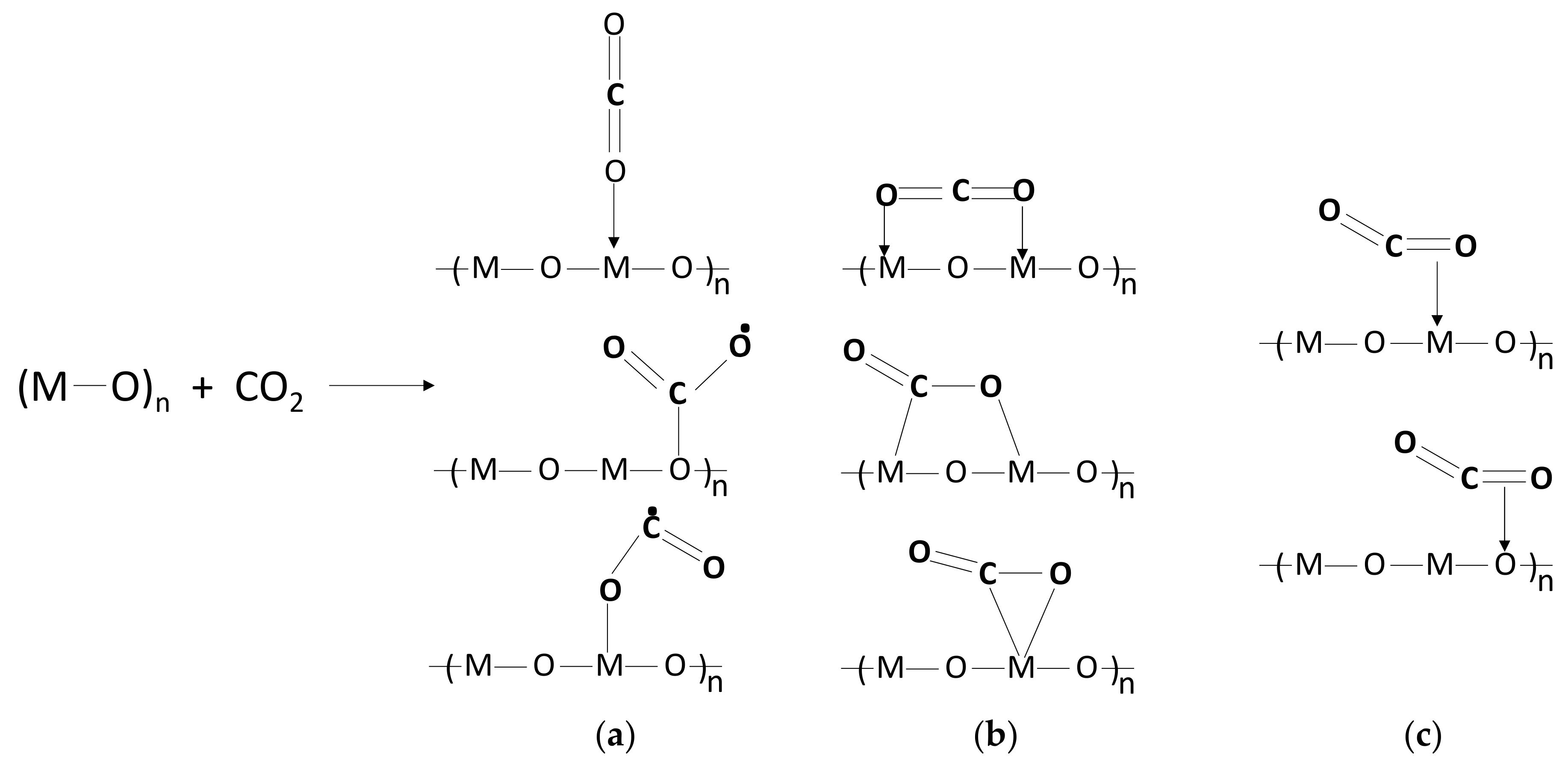
3.3. CO2 Adsorption and Activation on Metal Oxide Surfaces
3.3.1. Metal Oxide
3.3.2. Characteristic Adsorption of Representative Metal Oxides
3.3.3. Oxygen Vacancies and Their Roles in CO2 Activation
How to Generate Oxygen Vacancies
The Roles of Oxygen Vacancies
- (1)
- CO2 adsorption and dissociation, creation of binding and active sites
- (2)
- Selection of reaction pathway, stabilization of key intermediates and electronic structure modification
3.3.4. The Roles of Solvent in CO2 Transformation
3.3.5. General Methods for Modifying Metal Oxide Surface Structure for CO2 Transformation
- (i)
- Insertion of acidic and basic surface sites. The basic sites on the catalyst surface can facilitate CO2 adsorption because CO2 is an acidic compound whose interaction with the catalyst surface will be enhanced with a basic surface. The basic sites of metal oxide catalysts can be generated by pre-treatment with basic promoters such as La2O3, K2O, Na2O, and MgO [183]. Tian et al. [182] reported that introducing potassium into iron oxide catalyst increased the surface basicity. Thus, alkali metals can be incorporated into the metal oxides to tune the concentration of surface basic sites. The basic sites enhance the activation of acidic CO2 and also help to limit carbon deposition. The overall benefit is improvement in catalytic activity and stability. For example, in the CO2 dry reforming of methane, the catalytic reaction was initiated by an acid–base interaction [183]. On the In2O3−x(OH)y surface, comprising frustrated Lewis pairs (FLP) (A frustrated Lewis pairs consists of both Lewis acid and Lewis base that cannot combine to form an adduct due to steric hindrance.), the surface hydroxide site acts as Lewis base and the coordinately unsaturated In surface site acts as a Lewis acid to activate CO2 [180]. The Lewis acid and Lewis base synergistically interact to heterolytically dissociate H2 adsorbed on the In2O3−x(OH)y surface, forming protonic surface FLP sites that can capture and convert CO2 to CO and H2O. The surface Lewis basicity can be tuned by the nature of the metal site, which can be controlled by the size, charge, coordination number, geometry, and electronegativity of the metal in a particular oxidation state [114]. Surface basic sites can be characterized using CO2-TPD. Typically, desorption at high temperatures signifies the presence of strong basic sites. The CO2 adsorption strength on metal oxides has been related to the basicity, improving as the concentration increases. However, too strong adsorption may result in the formation of undesirable intermediate species such as surface carbonate and bicarbonate species.
- (ii)
- Metal oxide doping. Doping metal oxides with metallic elements can optimize their electronic and geometric structures and enhance catalytic performance. Alkali metals doping can modify the electronic structure by increasing the concentration of electron-withdrawing groups such as surface hydroxyl groups or reducing the kinetic barrier for CO2 dissociation [184]. Moreover, oxides with electron-rich defect centers may interact readily with CO2 by donating an electron. These, in turn, will facilitate surface reaction activity, boosting catalytic efficiency for CO2 activation. The list is not exclusive to alkali metals, as some transition metals have the potentials to tune the electronic characteristics of the doped oxide. Doping transition metal oxide to the based oxide can as well tune the concentration of oxygen vacancies
- (iii)
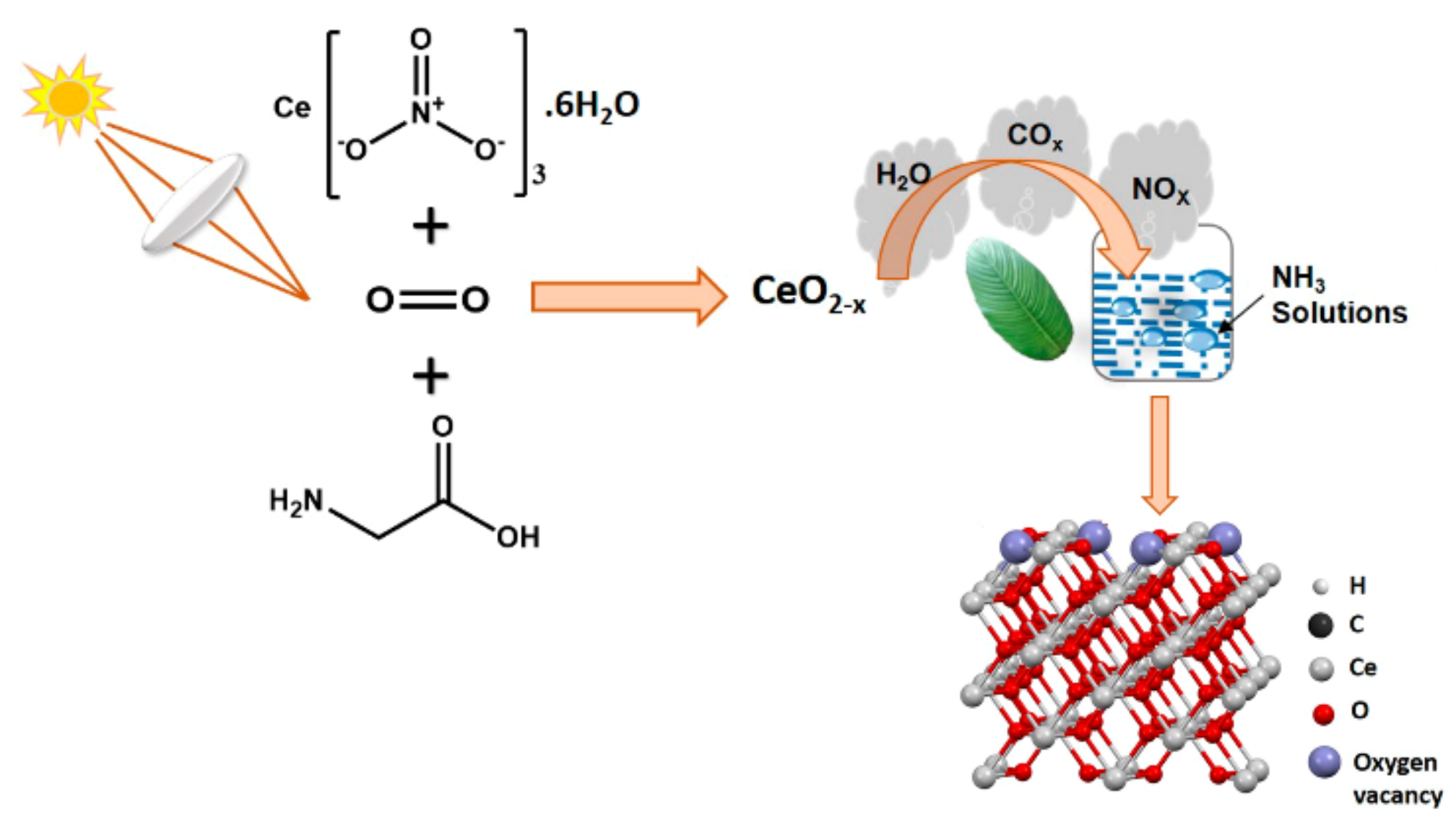
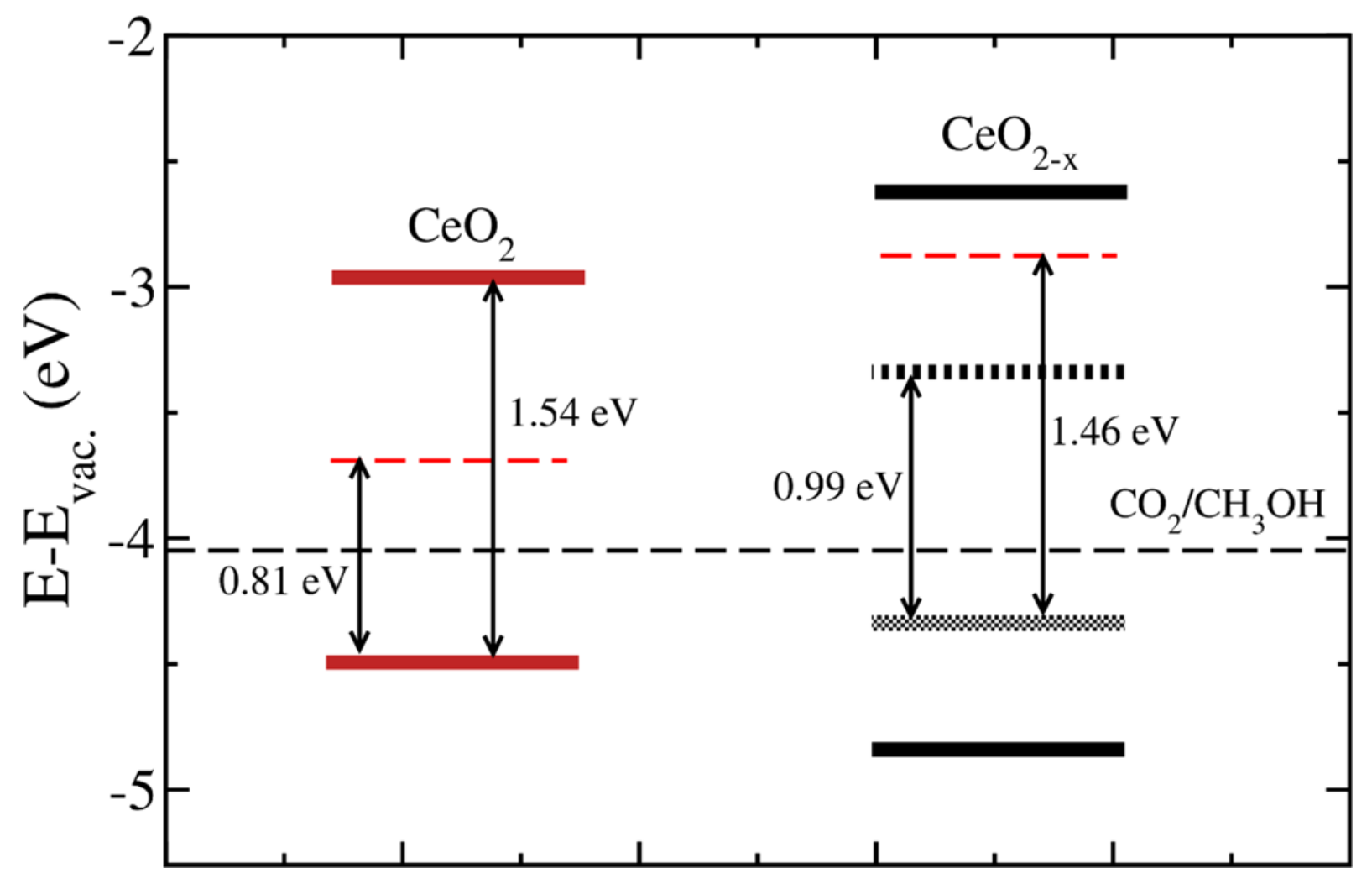
- Defect engineering. It involves creating or adjusting surface oxygen vacancies or hydroxyl groups in an oxide. The presence of oxygen defective sites can similarly impact the surface electronic property. Evidence has linked the activity of an oxide catalyst with surface defects. For example, it was demonstrated that the activity of MnO2 in HCHO oxidation correlated with the concentration of surface defects [185]. CeO2 nanoparticles with a high concentration of oxygen vacancies had high photocatalytic performance in converting CO2 to methanol (0.702 μmol h−1 g−1) [119]. Furthermore, oxygen vacancies are sites for adsorption and anchoring of CO2 molecules. Consequently, surface adsorption and reactivity of adsorbates, including H2 and H2O, would be facilitated in the presence of extra electrons in the vicinity of oxygen vacancies. The amount of defective sites in a metal oxide can be controlled by oxidative treatment during the catalyst synthesis and catalytic reactions.
- (iv)
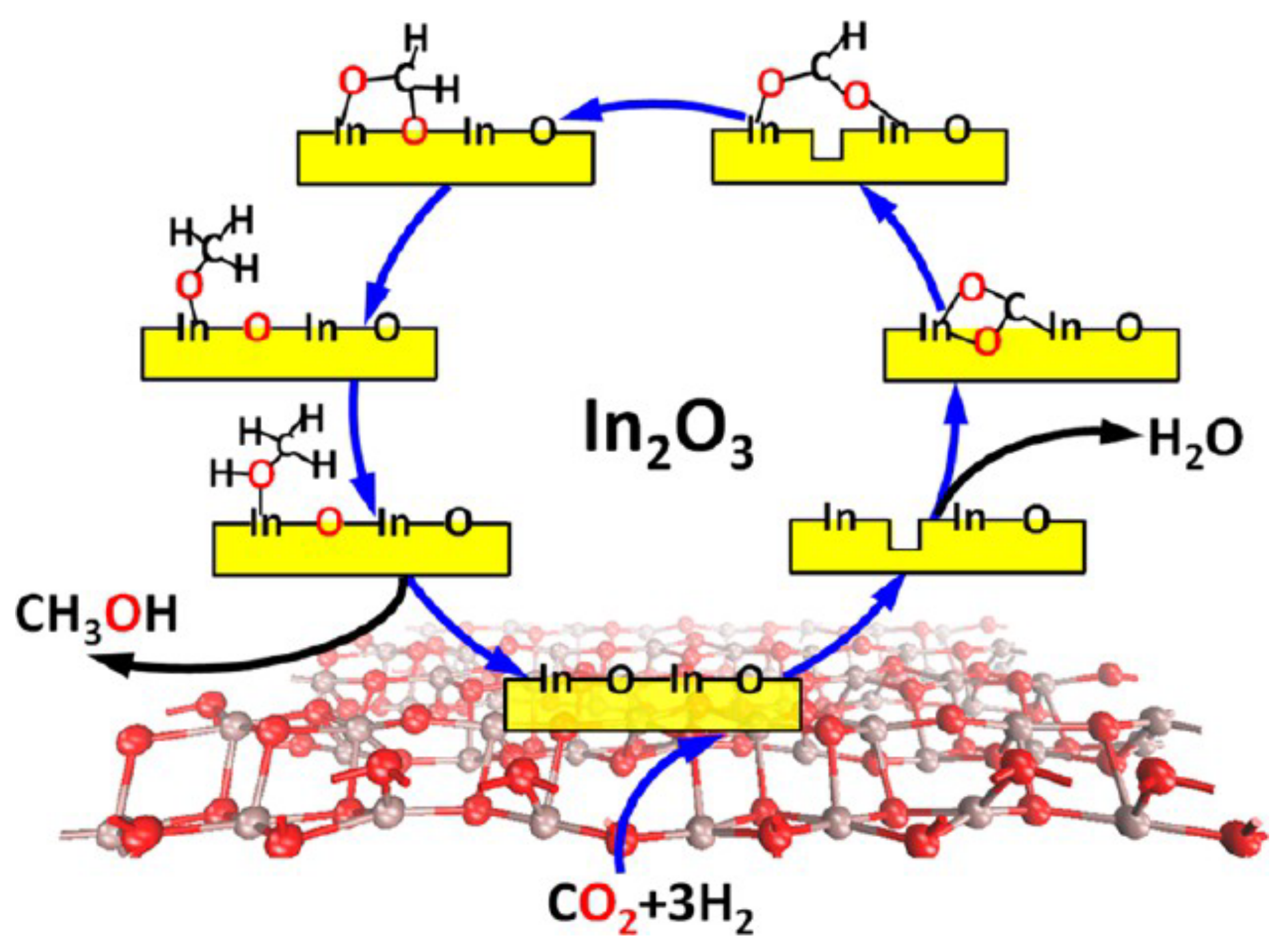
- Generation of surface hydroxyls. The surface hydroxyl groups participate in CO2 hydrogenation by incorporating hydrogen atom into CO2, facilitating CO2 activation and accelerating reaction rate. Mechanistic studies evidenced the surface hydroxyl species on SiC quantum dots (QDs) to directly participate in CO2 hydrogenation through the addition of H atoms of hydroxyl groups into CO2 to form HCOO* [186]. The surface hydroxide site on the In2O3−x(OH)y surface acted as a Lewis base that interacted with CO2 [180].
- (v)
- Mixed metal oxides or composites catalysts can yield good catalytic performances. Introducing a second oxide component can improve adsorption, increase the effective oxygen vacancies, and present an interfacial surface for reactions (adsorption/activation and desorption). When preparing a mixed-metal oxide catalyst, consideration should be given to factors that can improve catalytic performance, such as elemental composition since catalytic activity can be a function of how elements in the composite are combined. The deposition of sub-monolayer amounts of a second oxide over a host oxide can create nanostructures that can enhance the overall catalytic properties of the composite system [114,187].
- (vi)
- Forming metallic–non-metallic hybrid catalysts. These kinds of catalysts have high surface areas and improved adsorption properties. Carbon materials, such as carbon nanotubes (CNTs) and graphene oxides (GOs), and metal oxides (e.g., TiO2, CeO2, Al2O3, ZrO2, In2O3, Ga2O3, and NiO) can be grafted to form hybrid nanostructured materials [154,188,189,190]. Carbon materials possess very high surface areas, whereas metal oxides are endowed with rich oxygen vacancies. Combining materials with these features can result in a unique hybrid material with superior catalytic performance toward CO2 reduction reactions. The high surface area will enhance adsorption complemented by oxygen vacancies, which are also sites for CO2 activation on oxide catalysts. Modification of CuO–ZnO–ZrO2 with GO increased the adsorption capacities of both CO2 and H2, resulting in an improved catalytic activity (methanol selectivity) in methanol synthesis than the GO-free catalyst [188]. The electronic structure of the traditional methanol synthesis catalyst (Cu–ZnO–Al2O3) was altered by adding the N-doped graphene, which provided a synergistic effect for methanol synthesis [189]. Incorporating metal oxides to single-wall CNT electrode can form a hybrid structure with an increase in specific surface area, which also improved electrical conductivity and charge transfer [191,192]. Moreover, doping carbon into photocatalysts can enhance light absorption and improve the photothermal conversion efficiency by reducing the energy for oxygen vacancy formation, thus generating a high concentration of active sites [190]. Therefore, hybrid nanostructured materials with good electronic and charge transfer properties can be explored for both thermochemical and electrochemical reduction of CO2.
- (vii)
- Stabilization of metal oxide surface. Reduced oxides are more susceptible to react with CO2 [118,193]. Stabilizing the reduced surface can be achieved by creating a conducive environment for reactions such as forming oxide–metal or oxide–oxide interfaces [128,187,194]. The synergistic properties associated with these interfaces can improve the surface chemistry of metal oxides and could be beneficial for synthesizing efficient catalysts for CO2 transformation.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, R.; Liao, Y.; Bai, S.-T.; Zheng, M.; Zhou, C.; Zhang, T.; Sels, B.F. Heterogeneous catalysts for CO2 hydrogenation to formic acid/formate: From nanoscale to single atom. Energy Environ. Sci. 2021, 14, 1247–1285. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Li, Z.; Tang, C.; Feng, Z.; An, H.; Liu, H.; Liu, T.; Li, C. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol. Sci. Adv. 2017, 3, e1701290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, R.-P.; Li, Q.; Gong, W.; Wang, T.; Razink, J.J.; Lin, L.; Qin, Y.-Y.; Zhou, Z.; Adidharma, H.; Tang, J. High-performance of nanostructured Ni/CeO2 catalyst on CO2 methanation. Appl. Catal. B Environ. 2020, 268, 118474. [Google Scholar] [CrossRef]
- Lim, E.; Heo, J.; Zhang, X.; Bowen, K.H.; Lee, S.H.; Kim, S.K. Anionic Activation of CO2 via (Mn–CO2)–Complex on Magic-Numbered Anionic Coinage Metal Clusters Mn–(M= Cu, Ag, Au). J. Phy. Chem. A 2021, 125, 2243–2248. [Google Scholar] [CrossRef]
- Alvarez-Garcia, A.; Flórez, E.; Moreno, A.; Jimenez-Orozco, C. CO2 activation on small Cu-Ni and Cu-Pd bimetallic clusters. Mol. Catal. 2020, 484, 110733. [Google Scholar] [CrossRef]
- Álvarez, A.; Borges, M.; Corral-Pérez, J.J.; Olcina, J.G.; Hu, L.; Cornu, D.; Huang, R.; Stoian, D.; Urakawa, A. CO2 activation over catalytic surfaces. ChemPhysChem 2017, 18, 3135–3141. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, S.; Hatakeyama, M.; Wang, Y.; Ogata, K.; Fujii, K. A basic quantum chemical review on the activation of CO2. In Advances in CO2 Capture, Sequestration, and Conversion; ACS Publications: Washington, DC, USA, 2015; pp. 123–134. [Google Scholar]
- Mendes, P.C.; Verga, L.G.; Da Silva, J.L. Ab initio screening of Pt-based transition-metal nanoalloys using descriptors derived from the adsorption and activation of CO2. Phys. Chem. Chem. Phys. 2021, 23, 6029–6041. [Google Scholar] [CrossRef]
- Koppenol, W.; Rush, J. Reduction potential of the carbon dioxide/carbon dioxide radical anion: A comparison with other C1 radicals. J. Phys. Chem. 1987, 91, 4429–4430. [Google Scholar] [CrossRef]
- Das, S.; Pérez-Ramírez, J.; Gong, J.; Dewangan, N.; Hidajat, K.; Gates, B.C.; Kawi, S. Core–shell structured catalysts for thermocatalytic, photocatalytic, and electrocatalytic conversion of CO2. Chem. Soc. Rev. 2020, 49, 2937–3004. [Google Scholar] [CrossRef]
- Modak, A.; Bhanja, P.; Dutta, S.; Chowdhury, B.; Bhaumik, A. Catalytic reduction of CO2 into fuels and fine chemicals. Green Chem. 2020, 22, 4002–4033. [Google Scholar] [CrossRef]
- Etim, U.J.; Semiat, R.; Zhong, Z. CO2 Valorization Reactions over Cu-Based Catalysts: Characterization and the Nature of Active Sites. Am. J. Chem. Eng. 2021, 9, 53–78. [Google Scholar] [CrossRef]
- Green, A.E.; Justen, J.; Schöllkopf, W.; Gentleman, A.S.; Fielicke, A.; Mackenzie, S.R. IR Signature of Size-Selective CO2 Activation on Small Platinum Cluster Anions, Ptn−(n= 4–7). Angew. Chem. 2018, 130, 15038–15042. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Q.; Liu, G.; Wang, Y. Photocatalytic and photoelectrocatalytic reduction of CO2 using heterogeneous catalysts with controlled nanostructures. Chem. Commun. 2016, 52, 35–59. [Google Scholar] [CrossRef]
- Kumar, B.; Llorente, M.; Froehlich, J.; Dang, T.; Sathrum, A.; Kubiak, C.P. Photochemical and photoelectrochemical reduction of CO2. Annu. Rev. Phys. Chem. 2012, 63, 541–569. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Alvarez, F.J.; Oro, L.A. Homogeneous Catalytic Reduction of CO2 with Silicon-Hydrides, State of the Art. ChemCatChem 2018, 10, 4783–4796. [Google Scholar] [CrossRef]
- Jia, C.; Gao, J.; Dai, Y.; Zhang, J.; Yang, Y. The thermodynamics analysis and experimental validation for complicated systems in CO2 hydrogenation process. J. Energy Chem. 2016, 25, 1027–1037. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Quaranta, E. State of the art and perspectives in catalytic processes for CO2 conversion into chemicals and fuels: The distinctive contribution of chemical catalysis and biotechnology. J. Catal. 2016, 343, 2–45. [Google Scholar] [CrossRef]
- Ronda-Lloret, M.; Rothenberg, G.; Shiju, N.R. A critical look at direct catalytic hydrogenation of carbon dioxide to olefins. ChemSusChem 2019, 12, 3896–3914. [Google Scholar] [CrossRef]
- Radhakrishnan, S.G.; Roduner, E. Carbon dioxide activation. In Carbon Dioxide Utilization; Michael, N., Peter, S., Eds.; De Gruyter: Berlin, Germany, 2019; pp. 227–248. [Google Scholar]
- Niu, J.; Ran, J.; Ou, Z.; Du, X.; Wang, R.; Qi, W.; Zhang, P. CO2 dissociation over PtxNi4− x bimetallic clusters with and without hydrogen sources: A density functional theory study. J. CO2 Util. 2016, 16, 431–441. [Google Scholar] [CrossRef]
- Grice, K.A. Carbon dioxide reduction with homogenous early transition metal complexes: Opportunities and challenges for developing CO2 catalysis. Coord. Chem. Rev. 2017, 336, 78–95. [Google Scholar] [CrossRef]
- Taifan, W.; Boily, J.-F.; Baltrusaitis, J. Surface chemistry of carbon dioxide revisited. Surf. Sci. Rep. 2016, 71, 595–671. [Google Scholar] [CrossRef] [Green Version]
- Freund, H.-J.; Roberts, M.W. Surface chemistry of carbon dioxide. Surf. Sci. Rep. 1996, 25, 225–273. [Google Scholar] [CrossRef] [Green Version]
- Mondal, B.; Song, J.; Neese, F.; Ye, S. Bio-inspired mechanistic insights into CO2 reduction. Curr. Opin. Chem. Biol. 2015, 25, 103–109. [Google Scholar] [CrossRef]
- Nolan, M.; Fronzi, M. Activation of CO2 at chromia-nanocluster-modified rutile and anatase TiO2. Catal. Today 2019, 326, 68–74. [Google Scholar] [CrossRef]
- Mondal, K.; Banerjee, A.; Ghanty, T.K. Adsorption and activation of C on Zr n (n= 2–7) clusters. Phys. Chem. Chem. Phys. 2020, 22, 16877–16886. [Google Scholar]
- Weber, J.M. The interaction of negative charge with carbon dioxide–insight into solvation, speciation and reductive activation from cluster studies. Int. Rev. Phys. Chem. 2014, 33, 489–519. [Google Scholar] [CrossRef]
- Park, J.; Cho, M.; Rhee, Y.M.; Jung, Y. Theoretical Study on the Degree of CO2 Activation in CO2-Coordinated Ni (0) Complexes. ACS Omega 2021, 6, 7646–7654. [Google Scholar] [CrossRef]
- Mazheika, A.; Wang, Y.; Valero, R.; Ghiringhelli, L.M.; Vines, F.; Illas, F.; Levchenko, S.V.; Scheffler, M. Ab initio data-analytics study of carbon-dioxide activation on semiconductor oxide surfaces. arXiv 2019, arXiv:1912.06515. [Google Scholar]
- Chang, X.; Wang, T.; Gong, J. CO2 photo-reduction: Insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ. Sci 2016, 9, 2177–2196. [Google Scholar] [CrossRef]
- Fu, J.; Jiang, K.; Qiu, X.; Yu, J.; Liu, M. Product selectivity of photocatalytic CO2 reduction reactions. Mater. Today 2020, 32, 222–243. [Google Scholar] [CrossRef]
- Yang, B.; Wang, L.; Hua, Z.; Guo, L. How CO2 Chemisorption States Affect Hydrogenation Activity. Ind. Eng. Chem. Res. 2019, 58, 9838–9843. [Google Scholar] [CrossRef]
- Megha; Mondal, K.; Ghanty, T.K.; Banerjee, A. Adsorption and Activation of CO2 on Small-Sized Cu–Zr Bimetallic Clusters. J. Phys. Chem. A 2021, 125, 2558–2572. [Google Scholar] [CrossRef]
- Megha; Banerjee, A.; Ghanty, T.K. Role of metcar on the adsorption and activation of carbon dioxide: A DFT study. Phys. Chem. Chem. Phys. 2021, 23, 5559–5570. [Google Scholar] [CrossRef]
- Liu, X.; Kunkel, C.; Ramirez de la Piscina, P.; Homs, N.; Vines, F.; Illas, F. Effective and highly selective CO generation from CO2 using a polycrystalline α-Mo2C catalyst. ACS Catal. 2017, 7, 4323–4335. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Porosoff, M.D. Development of tandem catalysts for CO2 hydrogenation to olefins. ACS Catal. 2019, 9, 2639–2656. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, Y.; Zhu, Z.; Guo, H.; Ma, X. Highly selective conversion of CO2 into ethanol on Cu/ZnO/Al2O3 catalyst with the assistance of plasma. J. CO2 Util. 2018, 24, 34–39. [Google Scholar] [CrossRef]
- Li, Y.; Hui, D.; Sun, Y.; Wang, Y.; Wu, Z.; Wang, C.; Zhao, J. Boosting thermo-photocatalytic CO2 conversion activity by using photosynthesis-inspired electron-proton-transfer mediators. Nat. Commun. 2021, 12, 123. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y. Understanding the reaction mechanism of photocatalytic reduction of CO2 with H2O on TiO2-based photocatalysts: A review. Aerosol Air Qual. Res. 2014, 14, 453–469. [Google Scholar] [CrossRef]
- Montoya, J.H.; Seitz, L.C.; Chakthranont, P.; Vojvodic, A.; Jaramillo, T.F.; Nørskov, J.K. Materials for solar fuels and chemicals. Nat. Mater. 2017, 16, 70–81. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Kang, Z.; Jin, S.; Zhang, X.; Zheng, X.; Qi, Z.; Zhu, J.; Pan, B.; Xie, Y. Oxygen vacancy associated single-electron transfer for photofixation of CO2 to long-chain chemicals. Nat. Commun. 2019, 10, 788. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Das, T.; Kumar, S.; Bhosale, R.; Kabir, M.; Ogale, S. Photocatalytic activation and reduction of CO2 to CH4 over single phase nano Cu3SnS4: A combined experimental and theoretical study. ACS Appl. Energy Mater. 2019, 2, 5677–5685. [Google Scholar] [CrossRef]
- Chu, W.; Zheng, Q.; Prezhdo, O.V.; Zhao, J. CO2 photoreduction on metal oxide surface is driven by transient capture of hot electrons: Ab initio quantum dynamics simulation. J. Am. Chem. Soc. 2020, 142, 3214–3221. [Google Scholar] [CrossRef]
- Indrakanti, V.P.; Kubicki, J.D.; Schobert, H.H. Photoinduced activation of CO2 on Ti-based heterogeneous catalysts: Current state, chemical physics-based insights and outlook. Energy Environ. Sci. 2009, 2, 745–758. [Google Scholar] [CrossRef]
- Yuan, L.; Xu, Y.-J. Photocatalytic conversion of CO2 into value-added and renewable fuels. Appl. Surf. Sci. 2015, 342, 154–167. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, C.; Li, Y. Spontaneous Dissociation of CO2 to CO on Defective Surface of Cu(I)/TiO2–x Nanoparticles at Room Temperature. J. Phys. Chem. C 2012, 116, 7904–7912. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, H.; Andino, J.M.; Li, Y. Photocatalytic CO2 reduction with H2O on TiO2 nanocrystals: Comparison of anatase, rutile, and brookite polymorphs and exploration of surface chemistry. ACS Catal. 2012, 2, 1817–1828. [Google Scholar] [CrossRef]
- Rodriguez, M.M.; Peng, X.; Liu, L.; Li, Y.; Andino, J.M. A density functional theory and experimental study of CO2 interaction with brookite TiO2. J. Phys. Chem. C 2012, 116, 19755–19764. [Google Scholar] [CrossRef]
- He, H.; Zapol, P.; Curtiss, L.A. A theoretical study of CO2 anions on anatase (101) surface. J. Phys. Chem. C 2010, 114, 21474–21481. [Google Scholar] [CrossRef]
- Pan, H.; Gu, B.; Zhang, Z. Phase-dependent photocatalytic ability of TiO2: A first-principles study. J. Chem. Theory Comput. 2009, 5, 3074–3078. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, Y.; Wu, X.; Zheng, H.; Zhao, Z.; Liu, J.; Li, J. Graphene-wrapped Pt/TiO2 photocatalysts with enhanced photogenerated charges separation and reactant adsorption for high selective photoreduction of CO2 to CH4. Appl. Catal. B Environ. 2018, 226, 360–372. [Google Scholar] [CrossRef]
- Lu, L.; Wang, B.; Wang, S.; Shi, Z.; Yan, S.; Zou, Z. La2O3-Modified LaTiO2N Photocatalyst with Spatially Separated Active Sites Achieving Enhanced CO2 Reduction. Adv. Funct. Mater. 2017, 27, 1702447. [Google Scholar] [CrossRef]
- Xu, F.; Meng, K.; Cheng, B.; Yu, J.; Ho, W. Enhanced Photocatalytic Activity and Selectivity for CO2 Reduction over a TiO2 Nanofibre Mat Using Ag and MgO as Bi-Cocatalyst. ChemCatChem 2019, 11, 465–472. [Google Scholar] [CrossRef]
- Xie, S.; Wang, Y.; Zhang, Q.; Deng, W.; Wang, Y. MgO-and Pt-promoted TiO2 as an efficient photocatalyst for the preferential reduction of carbon dioxide in the presence of water. ACS Catal. 2014, 4, 3644–3653. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, G.; Bian, T.; Zhou, C.; Waterhouse, G.I.; Wu, L.Z.; Tung, C.H.; Smith, L.J.; O’Hare, D.; Zhang, T. Defect-rich ultrathin ZnAl-layered double hydroxide nanosheets for efficient photoreduction of CO2 to CO with water. Adv. Mater. 2015, 27, 7824–7831. [Google Scholar] [CrossRef]
- Xiong, Z.; Lei, Z.; Kuang, C.-C.; Chen, X.; Gong, B.; Zhao, Y.; Zhang, J.; Zheng, C.; Wu, J.C. Selective photocatalytic reduction of CO2 into CH4 over Pt-Cu2O TiO2 nanocrystals: The interaction between Pt and Cu2O cocatalysts. Appl. Catal. B Environ. 2017, 202, 695–703. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Lu, L.; Zhou, C.; Xin, Z.; Wang, J.; Ke, X.-K.; Sheng, G.; Yan, S.; Zou, Z. Oxygen-vacancy-activated CO2 splitting over amorphous oxide semiconductor photocatalyst. ACS Catal. 2018, 8, 516–525. [Google Scholar] [CrossRef]
- Kim, C.; Hyeon, S.; Lee, J.; Kim, W.D.; Lee, D.C.; Kim, J.; Lee, H. Energy-efficient CO2 hydrogenation with fast response using photoexcitation of CO2 adsorbed on metal catalysts. Nat. Commun. 2018, 9, 3027. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Huang, Y.; Wu, M.; Wang, Y. Electrochemical carbon dioxide splitting. ChemElectroChem 2019, 6, 1587–1604. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.; Chan, K.; Hahn, C. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef] [Green Version]
- Morris, A.J.; McGibbon, R.T.; Bocarsly, A.B. Electrocatalytic Carbon Dioxide Activation: The Rate-Determining Step of Pyridinium-Catalyzed CO2 Reduction. ChemSusChem 2011, 4, 191–196. [Google Scholar] [CrossRef]
- Yang, P.; Zhao, Z.J.; Chang, X.; Mu, R.; Zha, S.; Zhang, G.; Gong, J. The functionality of surface hydroxy groups on the selectivity and activity of carbon dioxide reduction over cuprous oxide in aqueous solutions. Angew. Chem. 2018, 130, 7850–7854. [Google Scholar] [CrossRef]
- Huang, Q.; Li, Q.; Liu, J.; Wang, R.; Lan, Y.-Q. Disclosing CO2 activation mechanism by hydroxyl-induced crystalline structure transformation in electrocatalytic process. Matter 2019, 1, 1656–1668. [Google Scholar] [CrossRef]
- Benson, E.E.; Kubiak, C.P.; Sathrum, A.J.; Smieja, J.M. Electrocatalytic and homogeneous approaches to conversion of CO 2 to liquid fuels. Chem. Soc. Rev. 2009, 38, 89–99. [Google Scholar] [CrossRef]
- Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide. Adv. Mater. 2016, 28, 3423–3452. [Google Scholar] [CrossRef]
- Peterson, A.A.; Abild-Pedersen, F.; Studt, F.; Rossmeisl, J.; Nørskov, J.K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci 2010, 3, 1311–1315. [Google Scholar] [CrossRef]
- Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci 2012, 5, 7050–7059. [Google Scholar] [CrossRef]
- Xiao, H.; Goddard, W.A.; Cheng, T.; Liu, Y. Cu metal embedded in oxidized matrix catalyst to promote CO2 activation and CO dimerization for electrochemical reduction of CO2. Proc. Natl. Acad. Sci. USA 2017, 114, 6685–6688. [Google Scholar] [PubMed] [Green Version]
- Favaro, M.; Xiao, H.; Cheng, T.; Goddard, W.A.; Yano, J.; Crumlin, E.J. Subsurface oxide plays a critical role in CO2 activation by Cu (111) surfaces to form chemisorbed CO2, the first step in reduction of CO2. Proc. Natl. Acad. Sci. USA 2017, 114, 6706–6711. [Google Scholar] [PubMed] [Green Version]
- Li, C.W.; Kanan, M.W. CO2 reduction at low overpotential on Cu electrodes resulting from the reduction of thick Cu2O films. J. Am. Chem. Soc. 2012, 134, 7231–7234. [Google Scholar] [CrossRef] [PubMed]
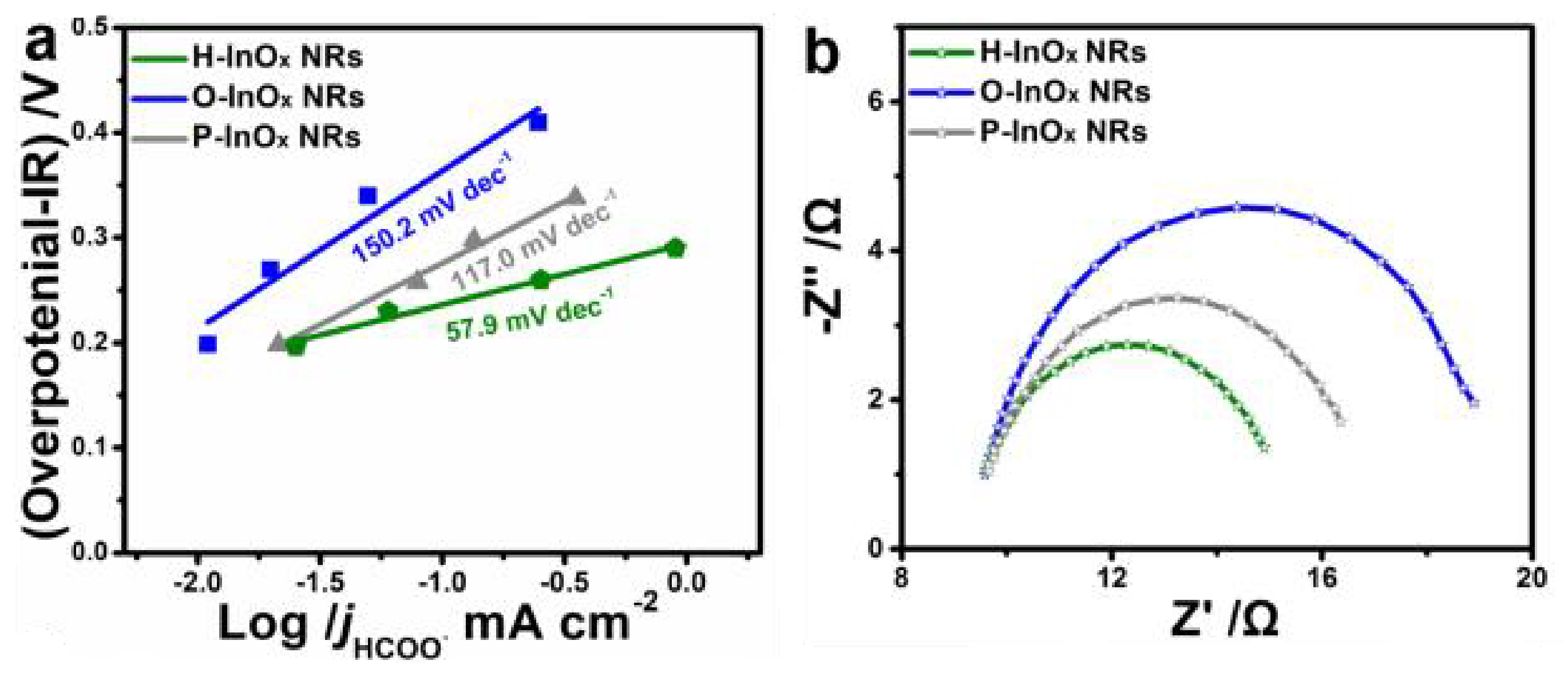
- Zhang, J.; Yin, R.; Shao, Q.; Zhu, T.; Huang, X. Oxygen vacancies in amorphous InOx nanoribbons enhance CO2 adsorption and activation for CO2 electroreduction. Angew. Chem. Int. Ed. 2019, 58, 5609–5613. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Yang, N.; Han, P.; Kuang, M.; Mei, B.; Jiang, Z.; Zhong, J.; Li, L.; Zheng, G. Oxygen vacancy tuning toward efficient electrocatalytic CO2 reduction to C2H4. Small Methods 2019, 3, 1800449. [Google Scholar]
- Geng, Z.; Kong, X.; Chen, W.; Su, H.; Liu, Y.; Cai, F.; Wang, G.; Zeng, J. Oxygen vacancies in ZnO nanosheets enhance CO2 electrochemical reduction to CO. Angew. Chem. 2018, 130, 6162–6167. [Google Scholar] [CrossRef]
- Baruch, M.F.; Pander III, J.E.; White, J.L.; Bocarsly, A.B. Mechanistic insights into the reduction of CO2 on tin electrodes using in situ ATR-IR spectroscopy. ACS Catal. 2015, 5, 3148–3156. [Google Scholar] [CrossRef]
- Barwa, E.; Pascher, T.F.; Ončák, M.; van der Linde, C.; Beyer, M.K. Carbon Dioxide Activation at Metal Centers: Evolution of Charge Transfer from Mg.+ to CO2 in [MgCO2 (H2O) n].+, n= 0–8. Angew. Chem. Int. Ed. 2020, 59, 7467–7471. [Google Scholar] [CrossRef] [Green Version]
- Iskra, A.; Gentleman, A.S.; Cunningham, E.M.; Mackenzie, S.R. Carbon dioxide binding to metal oxides: Infrared spectroscopy of NbO2+ (CO2) n and TaO2+ (CO2) n complexes. Int. J. Mass Spectrom. 2019, 435, 93–100. [Google Scholar] [CrossRef]
- Yin, X.; Moss, J.R. Recent developments in the activation of carbon dioxide by metal complexes. Coord. Chem. Rev. 1999, 181, 27–59. [Google Scholar] [CrossRef]
- Iskra, A.; Gentleman, A.S.; Kartouzian, A.; Kent, M.J.; Sharp, A.P.; Mackenzie, S.R. Infrared spectroscopy of gas-phase M+ (CO2) n (M = Co, Rh, Ir) ion–molecule complexes. J. Phys. Chem. A 2017, 121, 133–140. [Google Scholar] [CrossRef]
- Thompson, M.C.; Ramsay, J.; Weber, J.M. Solvent-driven reductive activation of CO2 by bismuth: Switching from metalloformate complexes to oxalate products. Angew. Chem. Int. Ed. 2016, 55, 15171–15174. [Google Scholar] [CrossRef]
- Knurr, B.J.; Weber, J.M. Solvent-driven reductive activation of carbon dioxide by gold anions. J. Am. Chem. Soc. 2012, 134, 18804–18808. [Google Scholar] [CrossRef]
- Zheng, H.; Kong, X.; Wang, C.; Wang, T.; Yang, D.; Li, G.; Xie, H.; Zhao, Z.; Shi, R.; Han, H. Spectroscopic Identification of Transition-Metal M [η2-(O, O) C] Species for Highly-Efficient CO2 Activation. J. Phys. Chem. Lett. 2020, 12, 472–477. [Google Scholar] [CrossRef]
- Zimmermann, N.; Bernhardt, T.M.; Bakker, J.M.; Barnett, R.N.; Landman, U.; Lang, S.M. Infrared Spectroscopy of Gas-Phase Mn x O y (CO2) z+ Complexes. J. Phys. Chem. A 2020, 124, 1561–1566. [Google Scholar] [CrossRef]
- Paparo, A.; Okuda, J. Carbon dioxide complexes: Bonding modes and synthetic methods. Coord. Chem. Rev. 2017, 334, 136–149. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.; Luo, L.; Du, J.; Zeng, J. Symmetry-Breaking Sites for Activating Linear Carbon Dioxide Molecules. Acc. Chem. Res. 2021, 54, 1454–1464. [Google Scholar] [CrossRef]
- Ye, R.-P.; Ding, J.; Gong, W.; Argyle, M.D.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 2019, 10, 5698. [Google Scholar] [CrossRef] [Green Version]
- Dietz, L.; Piccinin, S.; Maestri, M. Mechanistic Insights into CO2 activation via reverse water–gas shift on metal surfaces. J. Phys. Chem. C 2015, 119, 4959–4966. [Google Scholar] [CrossRef]
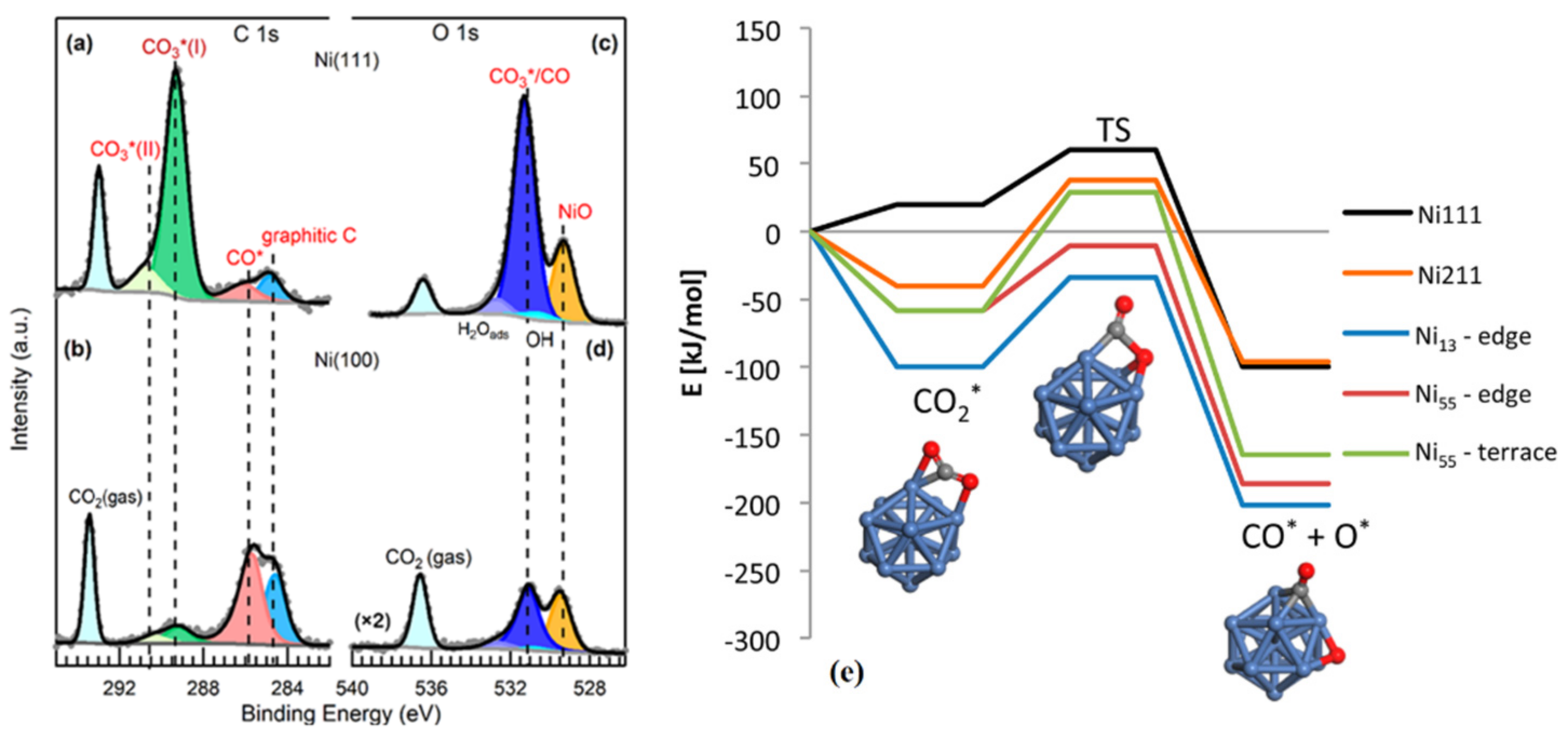
- Ko, J.; Kim, B.-K.; Han, J.W. Density functional theory study for catalytic activation and dissociation of CO2 on bimetallic alloy surfaces. J. Phys. Chem. C 2016, 120, 3438–3447. [Google Scholar] [CrossRef]
- Wang, S.-G.; Liao, X.-Y.; Cao, D.-B.; Huo, C.-F.; Li, Y.-W.; Wang, J.; Jiao, H. Factors controlling the interaction of CO2 with transition metal surfaces. J. Phys. Chem. C 2007, 111, 16934–16940. [Google Scholar] [CrossRef]
- Ding, X.; De Rogatis, L.; Vesselli, E.; Baraldi, A.; Comelli, G.; Rosei, R.; Savio, L.; Vattuone, L.; Rocca, M.; Fornasiero, P. Interaction of carbon dioxide with Ni (110): A combined experimental and theoretical study. Phys. Rev. B 2007, 76, 195425. [Google Scholar] [CrossRef]
- Eren, B.; Weatherup, R.S.; Liakakos, N.; Somorjai, G.A.; Salmeron, M. Dissociative carbon dioxide adsorption and morphological changes on Cu (100) and Cu (111) at ambient pressures. J. Am. Chem. Soc. 2016, 138, 8207–8211. [Google Scholar] [CrossRef] [PubMed]
- Hammami, R.; Dhouib, A.; Fernandez, S.; Minot, C. CO2 adsorption on (0 0 1) surfaces of metal monoxides with rock-salt structure. Catal. Today 2008, 139, 227–233. [Google Scholar] [CrossRef]
- Solymosi, F. The bonding, structure and reactions of CO2 adsorbed on clean and promoted metal surfaces. J. Mol. Catal. 1991, 65, 337–358. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.K.; Roldan, A.; de Leeuw, N.H. CuO surfaces and CO2 activation: A dispersion-corrected DFT+ U study. J. Phys. Chem. C 2016, 120, 2198–2214. [Google Scholar] [CrossRef]
- Yang, T.; Gu, T.; Han, Y.; Wang, W.; Yu, Y.; Zang, Y.; Zhang, H.; Mao, B.; Li, Y.; Yang, B. Surface orientation and pressure dependence of CO2 activation on Cu surfaces. J. Phys. Chem. C 2020, 124, 27511–27518. [Google Scholar] [CrossRef]
- Yu, H.; Cao, D.; Fisher, A.; Johnston, R.L.; Cheng, D. Size effect on the adsorption and dissociation of CO2 on Co nanoclusters. Appl. Surf. Sci. 2017, 396, 539–546. [Google Scholar] [CrossRef]
- Etim, U.J.; Song, Y.; Zhong, Z. Improving the Cu/ZnO-Based Catalysts for Carbon Dioxide Hydrogenation to Methanol, and the Use of Methanol As a Renewable Energy Storage Media. Front. Energy Res. 2020, 8, 545431. [Google Scholar] [CrossRef]
- Fu, S.S.; Somorjai, G.A. Interactions of O2, CO, CO2, and D2 with the stepped Cu (311) crystal face: Comparison to Cu (110). Surf. Sci. 1992, 262, 68–76. [Google Scholar] [CrossRef]
- Rasmussen, P.; Taylor, P.; Chorkendorff, I. The interaction of carbon dioxide with Cu (100). Surf. Sci. 1992, 269, 352–359. [Google Scholar] [CrossRef]
- Muttaqien, F.; Hamamoto, Y.; Inagaki, K.; Morikawa, Y. Dissociative adsorption of CO2 on flat, stepped, and kinked Cu surfaces. J. Chem. Phys. 2014, 141, 034702. [Google Scholar] [CrossRef]
- Pohl, M.; Otto, A. Adsorption and reaction of carbon dioxide on pure and alkali-metal promoted cold-deposited copper films. Surf. Sci. 1998, 406, 125–137. [Google Scholar] [CrossRef]
- Wang, S.-G.; Cao, D.-B.; Li, Y.-W.; Wang, J.; Jiao, H. Chemisorption of CO2 on nickel surfaces. J. Phys. Chem. B 2005, 109, 18956–18963. [Google Scholar] [CrossRef]
- Cao, D.-B.; Li, Y.-W.; Wang, J.; Jiao, H. CO2 dissociation on Ni (2 1 1). Surf. Sci. 2009, 603, 2991–2998. [Google Scholar] [CrossRef]
- Illing, G.; Heskett, D.; Plummer, E.; Freund, H.-J.; Somers, J.; Lindner, T.; Bradshaw, A.; Buskotte, U.; Neumann, M.; Starke, U. Adsorption and reaction of CO2 on Ni {110}: X-ray photoemission, near-edge X-ray absorption fine-structure and diffuse leed studies. Surf. Sci. 1988, 206, 1–19. [Google Scholar] [CrossRef]
- Heine, C.; Lechner, B.A.; Bluhm, H.; Salmeron, M. Recycling of CO2: Probing the chemical state of the Ni (111) surface during the methanation reaction with ambient-pressure X-ray photoelectron spectroscopy. J. Am. Chem. Soc. 2016, 138, 13246–13252. [Google Scholar] [CrossRef] [PubMed]
- Roiaz, M.; Monachino, E.; Dri, C.; Greiner, M.; Knop-Gericke, A.; Schlögl, R.; Comelli, G.; Vesselli, E. Reverse water–gas shift or Sabatier methanation on Ni (110)? Stable surface species at near-ambient pressure. J. Am. Chem. Soc. 2016, 138, 4146–4154. [Google Scholar] [CrossRef]
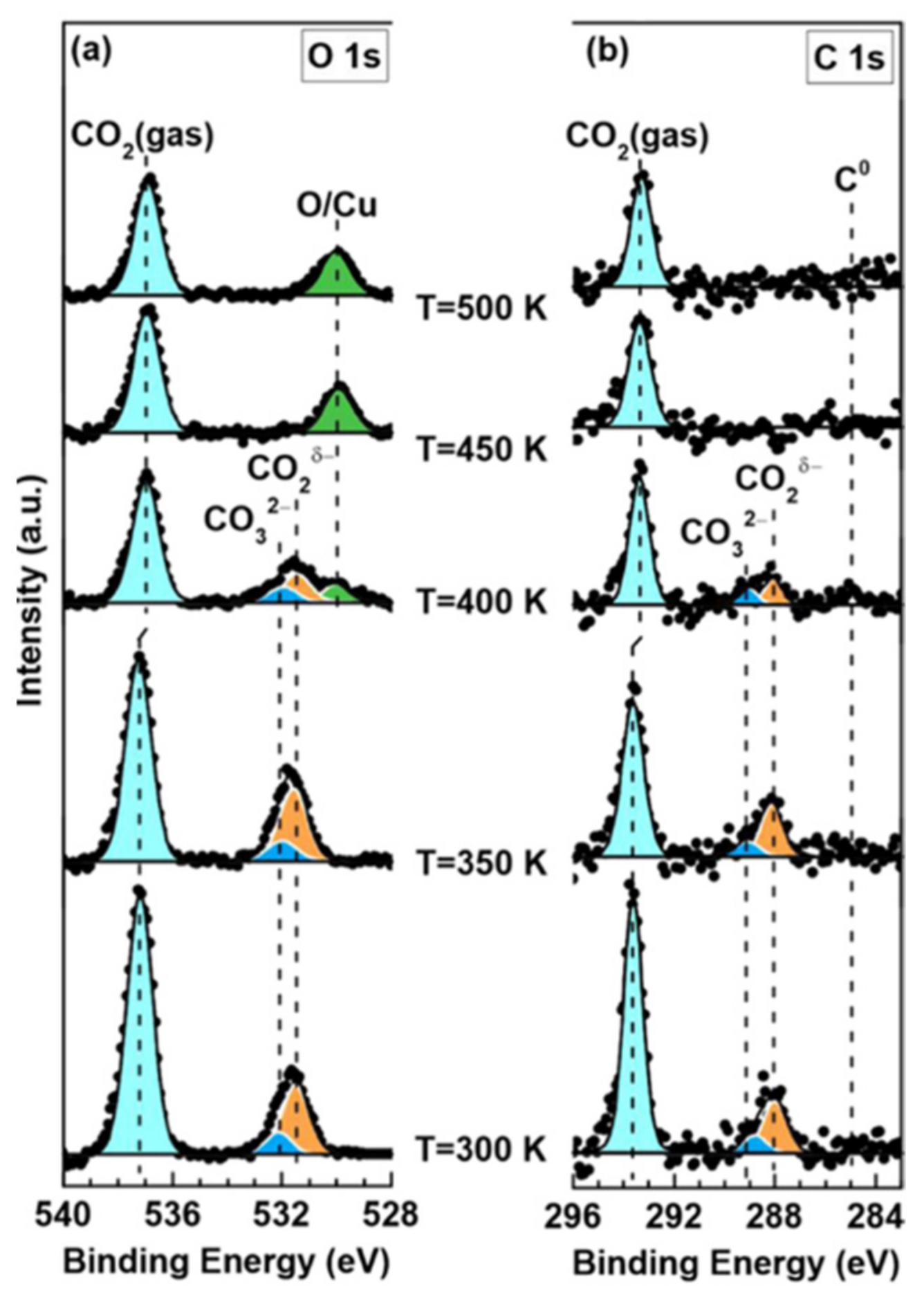
- Cai, J.; Han, Y.; Chen, S.; Crumlin, E.J.; Yang, B.; Li, Y.; Liu, Z. CO2 activation on Ni (111) and Ni (100) surfaces in the presence of H2O: An ambient-pressure X-ray photoelectron spectroscopy study. J. Phys. Chem. C 2019, 123, 12176–12182. [Google Scholar] [CrossRef]
- Silaghi, M.-C.; Comas-Vives, A.; Coperet, C. CO2 Activation on Ni/γ–Al2O3 catalysts by first-principles calculations: From ideal surfaces to supported nanoparticles. ACS Catal. 2016, 6, 4501–4505. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Y.; Solymosi, F.; White, J. Spectroscopic study of K-induced activation of CO2 on Pt (111). Surf. Sci. 1991, 245, 289–304. [Google Scholar] [CrossRef]
- Ricart, J.M.; Habas, M.P.; Clotet, A.; Curulla, D.; Illas, F. Theoretical study of CO2 activation on Pt (111) induced by coadsorbed K atoms. Surf. Sci. 2000, 460, 170–181. [Google Scholar] [CrossRef]
- Huang, M.; Adnot, A.; Suppiah, S.; Kaliaguine, S. XPS observation of surface interaction between H2 and CO2 on platinum foil. J. Mol. Catal. A: Chem. 1995, 104, L131–L137. [Google Scholar] [CrossRef]
- Su, H.; Ye, Y.; Lee, K.-J.; Zeng, J.; Mun, B.S.; Crumlin, E.J. Probing the surface chemistry for reverse water gas shift reaction on Pt (1 1 1) using ambient pressure X-ray photoelectron spectroscopy. J. Catal. 2020, 391, 123–131. [Google Scholar] [CrossRef]
- Víctor, A.; González, S.; Illas, F.; Fierro, J.L. Evidence for spontaneous CO2 activation on cobalt surfaces. Chem. Phys. Lett. 2008, 454, 262–268. [Google Scholar]
- Jia, J.; Qian, C.; Dong, Y.; Li, Y.F.; Wang, H.; Ghoussoub, M.; Butler, K.T.; Walsh, A.; Ozin, G.A. Heterogeneous catalytic hydrogenation of CO2 by metal oxides: Defect engineering–perfecting imperfection. Chem. Soc. Rev. 2017, 46, 4631–4644. [Google Scholar] [CrossRef]
- Seiferth, O.; Wolter, K.; Dillmann, B.; Klivenyi, G.; Freund, H.-J.; Scarano, D.; Zecchina, A. IR investigations of CO2 adsorption on chromia surfaces: Cr2O3 (0001)/Cr (110) versus polycrystalline α-Cr2O3. Surf. Sci. 1999, 421, 176–190. [Google Scholar] [CrossRef]
- Gao, P.; Li, S.; Bu, X.; Dang, S.; Liu, Z.; Wang, H.; Zhong, L.; Qiu, M.; Yang, C.; Cai, J. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 2017, 9, 1019–1024. [Google Scholar] [CrossRef]
- Liu, L.; Fan, W.; Zhao, X.; Sun, H.; Li, P.; Sun, L. Surface dependence of CO2 adsorption on Zn2GeO4. Langmuir 2012, 28, 10415–10424. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Liu, P.; Stacchiola, D.J.; Senanayake, S.D.; White, M.G.; Chen, J.G. Hydrogenation of CO2 to methanol: Importance of metal–oxide and metal–carbide interfaces in the activation of CO2. ACS Catal. 2015, 5, 6696–6706. [Google Scholar] [CrossRef]
- Hezam, A.; Namratha, K.; Drmosh, Q.A.; Ponnamma, D.; Wang, J.; Prasad, S.; Ahamed, M.; Cheng, C.; Byrappa, K. CeO2 nanostructures enriched with oxygen vacancies for photocatalytic CO2 reduction. ACS Appl. Nano Mater. 2019, 3, 138–148. [Google Scholar] [CrossRef] [Green Version]
- Bu, Y.; Weststrate, C.; Niemantsverdriet, J.; Fredriksson, H.O. Role of ZnO and CeO x in Cu-Based Model Catalysts in Activation of H2O and CO2 Dynamics Studied by in Situ Ultraviolet–Visible and X-ray Photoelectron Spectroscopy. ACS Catal. 2016, 6, 7994–8003. [Google Scholar] [CrossRef]
- Chen, S.; Cao, T.; Gao, Y.; Li, D.; Xiong, F.; Huang, W. Probing surface structures of CeO2, TiO2, and Cu2O nanocrystals with CO and CO2 chemisorption. J. Phys. Chem. C 2016, 120, 21472–21485. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Wang, T.; Li, Y.; Li, X.; Yin, J.; Wang, C. CO2 photoreduction with H2O vapor on highly dispersed CeO2/TiO2 catalysts: Surface species and their reactivity. J. Catal. 2016, 337, 293–302. [Google Scholar] [CrossRef]
- Di, J.; Zhu, C.; Ji, M.; Duan, M.; Long, R.; Yan, C.; Gu, K.; Xiong, J.; She, Y.; Xia, J. Defect-rich Bi12O17Cl2 nanotubes self-accelerating charge separation for boosting photocatalytic CO2 reduction. Angew. Chem. Int. Ed. 2018, 57, 14847–14851. [Google Scholar] [CrossRef]
- Yan, S.C.; Ouyang, S.X.; Gao, J.; Yang, M.; Feng, J.Y.; Fan, X.X.; Wan, L.J.; Li, Z.S.; Ye, J.H.; Zhou, Y. A room-temperature reactive-template route to mesoporous ZnGa2O4 with improved photocatalytic activity in reduction of CO2. Angew. Chem. 2010, 122, 6544–6548. [Google Scholar] [CrossRef]
- Guo, J.; Ouyang, S.; Kako, T.; Ye, J. Mesoporous In(OH)3 for photoreduction of CO2 into renewable hydrocarbon fuels. Appl. Surf. Sci. 2013, 280, 418–423. [Google Scholar] [CrossRef]
- Cao, A.; Wang, Z.; Li, H.; Nørskov, J.K. Relations between Surface Oxygen Vacancies and Activity of Methanol Formation from CO2 Hydrogenation over In2O3 Surfaces. ACS Catal. 2021, 11, 1780–1786. [Google Scholar] [CrossRef]
- Staudt, T.; Lykhach, Y.; Tsud, N.; Skála, T.S.; Prince, K.C.; Matolín, V.R.; Libuda, J.R. Electronic structure of magnesia–ceria model catalysts, CO2 adsorption, and CO2 activation: A synchrotron radiation photoelectron spectroscopy study. J. Phys. Chem. C 2011, 115, 8716–8724. [Google Scholar] [CrossRef]
- Graciani, J.; Mudiyanselage, K.; Xu, F.; Baber, A.E.; Evans, J.; Senanayake, S.D.; Stacchiola, D.J.; Liu, P.; Hrbek, J.; Sanz, J.F. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science 2014, 345, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Sherman, B.J.; Lo, C.S. Carbon dioxide activation and dissociation on ceria (110): A density functional theory study. J. Chem. Phys. 2013, 138, 014702. [Google Scholar] [CrossRef]
- Yang, C.; Bebensee, F.; Chen, J.; Yu, X.; Nefedov, A.; Wöll, C. Carbon dioxide adsorption on CeO2 (110): An XPS and NEXAFS study. ChemPhysChem 2017, 18, 1874–1880. [Google Scholar] [CrossRef]
- Hahn, K.R.; Iannuzzi, M.; Seitsonen, A.P.; Hutter, J.R. Coverage effect of the CO2 adsorption mechanisms on CeO2 (111) by first principles analysis. J. Phys. Chem. C 2013, 117, 1701–1711. [Google Scholar] [CrossRef] [Green Version]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Thompson, T.L.; Diwald, O.; Yates, J.T. CO2 as a probe for monitoring the surface defects on TiO2 (110) temperature-programmed desorption. J. Phys. Chem. B 2003, 107, 11700–11704. [Google Scholar] [CrossRef]
- Funk, S.; Burghaus, U. Adsorption of CO2 on oxidized, defected, hydrogen and oxygen covered rutile (1 × 1)-TiO2 (110). Phys. Chem. Chem. Phys. 2006, 8, 4805–4813. [Google Scholar] [CrossRef]
- Suriye, K.; Praserthdam, P.; Jongsomjit, B. Control of Ti3+ surface defect on TiO2 nanocrystal using various calcination atmospheres as the first step for surface defect creation and its application in photocatalysis. Appl. Surf. Sci. 2007, 253, 3849–3855. [Google Scholar] [CrossRef]
- Suriye, K.; Jongsomjit, B.; Satayaprasert, C.; Praserthdam, P. Surface defect (Ti3+) controlling in the first step on the anatase TiO2 nanocrystal by using sol–gel technique. Appl. Surf. Sci. 2008, 255, 2759–2766. [Google Scholar] [CrossRef]
- Sorescu, D.C.; Al-Saidi, W.A.; Jordan, K.D. CO2 adsorption on TiO2 (101) anatase: A dispersion-corrected density functional theory study. J. Chem. Phys. 2011, 135, 124701. [Google Scholar] [CrossRef]
- Liao, L.-F.; Lien, C.-F.; Shieh, D.-L.; Chen, M.-T.; Lin, J.-L. FTIR study of adsorption and photoassisted oxygen isotopic exchange of carbon monoxide, carbon dioxide, carbonate, and formate on TiO2. J. Phys. Chem. B 2002, 106, 11240–11245. [Google Scholar] [CrossRef]
- Lee, J.; Sorescu, D.C.; Deng, X. Electron-induced dissociation of CO2 on TiO2 (110). J. Am. Chem. Soc. 2011, 133, 10066–10069. [Google Scholar] [CrossRef]
- Nolan, M. Adsorption of CO2 on heterostructures of Bi2O3 nanocluster-modified TiO2 and the role of reduction in promoting CO2 activation. ACS Omega 2018, 3, 13117–13128. [Google Scholar] [CrossRef] [Green Version]
- Pavelec, J.; Hulva, J.; Halwidl, D.; Bliem, R.; Gamba, O.; Jakub, Z.; Brunbauer, F.; Schmid, M.; Diebold, U.; Parkinson, G.S. A multi-technique study of CO2 adsorption on Fe3O4 magnetite. J. Chem. Phys. 2017, 146, 014701. [Google Scholar] [CrossRef] [Green Version]
- Hakim, A.; Marliza, T.S.; Abu Tahari, N.M.; Wan Isahak, R.W.; Yusop, R.M.; Mohamed Hisham, W.M.; Yarmo, A.M. Studies on CO2 adsorption and desorption properties from various types of iron oxides (FeO, Fe2O3, and Fe3O4). Ind. Eng. Chem. Res. 2016, 55, 7888–7897. [Google Scholar] [CrossRef]
- Li, X.; Paier, J. Vibrational properties of CO2 adsorbed on the Fe3O4 (111) surface: Insights gained from DFT. J. Chem. Phy 2020, 152, 104702. [Google Scholar] [CrossRef]
- Mirabella, F.; Zaki, E.; Ivars-Barcelo, F.; Schauermann, S.; Shaikhutdinov, S.; Freund, H.-J. CO2 adsorption on magnetite Fe3O4 (111). J. Phys. Chem. C 2018, 122, 27433–27441. [Google Scholar] [CrossRef]
- Su, T.; Qin, Z.; Huang, G.; Ji, H.; Jiang, Y.; Chen, J. Density functional theory study on the interaction of CO2 with Fe3O4 (111) surface. Appl. Surf. Sci. 2016, 378, 270–276. [Google Scholar] [CrossRef]
- Tang, Q.-L.; Hong, Q.-J.; Liu, Z.-P. CO2 fixation into methanol at Cu/ZrO2 interface from first principles kinetic Monte Carlo. J. Catal. 2009, 263, 114–122. [Google Scholar] [CrossRef]
- Fisher, I.A.; Bell, A.T. In-situinfrared study of methanol synthesis from H2/CO2 over Cu/SiO2 and Cu/ZrO2/SiO2. J. Catal. 1997, 172, 222–237. [Google Scholar] [CrossRef]
- Jin, X.; Lv, C.; Zhou, X.; Ye, L.; Xie, H.; Liu, Y.; Su, H.; Zhang, B.; Chen, G. Oxygen vacancy engineering of Bi24O31Cl10 for boosted photocatalytic CO2 conversion. ChemSusChem 2019, 12, 2740–2747. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Shi, W.; Ling, P.; Sun, Y.; Jiao, X.; Gao, S.; Liang, L.; Xu, J.; Yan, W. Efficient visible-light-driven CO2 reduction mediated by defect-engineered BiOBr atomic layers. Angew. Chem. 2018, 130, 8855–8859. [Google Scholar] [CrossRef]
- Huygh, S.; Bogaerts, A.; Neyts, E.C. How oxygen vacancies activate CO2 dissociation on TiO2 anatase (001). J. Phys. Chem. C 2016, 120, 21659–21669. [Google Scholar] [CrossRef]
- Pan, Y.-x.; Liu, C.-J.; Mei, D.; Ge, Q. Effects of Hydration and Oxygen Vacancy on CO2 Adsorption and Activation on β-Ga2O3(100). Langmuir 2010, 26, 5551–5558. [Google Scholar] [CrossRef]
- Ye, J.; Liu, C.; Mei, D.; Ge, Q. Active oxygen vacancy site for methanol synthesis from CO2 hydrogenation on In2O3 (110): A DFT study. ACS Catal. 2013, 3, 1296–1306. [Google Scholar] [CrossRef]
- Sarkar, A.; Khan, G.G. The formation and detection techniques of oxygen vacancies in titanium oxide-based nanostructures. Nanoscale 2019, 11, 3414–3444. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Na, W.; Gao, W.; Wang, H. Carbon-Modified CuO/ZnO Catalyst with High Oxygen Vacancy for CO2 Hydrogenation to Methanol. Energy Technol. 2020, 8, 2000194. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, W.; Zhang, L.; Zheng, Y.; Wang, Z. Insights into the surface-defect dependence of photoreactivity over CeO2 nanocrystals with well-defined crystal facets. ACS Catal. 2015, 5, 4851–4858. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Zhang, X.; Wei, M.; Evans, D.G.; Duan, X. Catalytic behavior of supported Ru nanoparticles on the {1 0 0},{1 1 0}, and {1 1 1} facet of CeO2. J. Catal. 2015, 329, 177–186. [Google Scholar] [CrossRef]
- Bielz, T.; Lorenz, H.; Jochum, W.; Kaindl, R.; Klauser, F.; Klötzer, B.; Penner, S. Hydrogen on In2O3: Reducibility, Bonding, Defect Formation, and Reactivity. J. Phys. Chem. C 2010, 114, 9022–9029. [Google Scholar] [CrossRef]
- Rui, N.; Wang, Z.; Sun, K.; Ye, J.; Ge, Q.; Liu, C.-J. CO2 hydrogenation to methanol over Pd/In2O3: Effects of Pd and oxygen vacancy. Appl. Catal. B: Environ. 2017, 218, 488–497. [Google Scholar] [CrossRef]
- Wang, M.; Shen, M.; Jin, X.; Tian, J.; Li, M.; Zhou, Y.; Zhang, L.; Li, Y.; Shi, J. Oxygen vacancy generation and stabilization in CeO2–x by Cu introduction with improved CO2 photocatalytic reduction activity. ACS Catal. 2019, 9, 4573–4581. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Jiang, X.; Huang, J. Enhanced photocatalytic activity of Ga-N Co-doped anatase TiO2 for water decomposition to hydrogen. Int. J. Electrochem. Sci 2012, 7, 11519–11527. [Google Scholar]
- Wang, P.; Qi, C.; Wen, P.; Hao, L.; Xu, X.; Agathopoulos, S. Synthesis of Si, N co-doped nano-sized TiO2 with high thermal stability and photocatalytic activity by mechanochemical method. Nanomaterials 2018, 8, 294. [Google Scholar] [CrossRef] [Green Version]
- Qiu, M.; Tian, Y.; Chen, Z.; Yang, Z.; Li, W.; Wang, K.; Wang, L.; Wang, K.; Zhang, W. Synthesis of Ti3+ self-doped TiO2 nanocrystals based on Le Chatelier’s principle and their application in solar light photocatalysis. RSC Adv. 2016, 6, 74376–74383. [Google Scholar] [CrossRef]
- Zhu, K.; Shi, F.; Zhu, X.; Yang, W. The roles of oxygen vacancies in electrocatalytic oxygen evolution reaction. Nano Energy 2020, 73, 104761. [Google Scholar] [CrossRef]
- Guo, S.; Di, J.; Chen, C.; Zhu, C.; Duan, M.; Lian, C.; Ji, M.; Zhou, W.; Xu, M.; Song, P. Oxygen vacancy mediated bismuth stannate ultra-small nanoparticle towards photocatalytic CO2-to-CO conversion. Appl. Catal. B Environ. 2020, 276, 119156. [Google Scholar] [CrossRef]
- Santara, B.; Giri, P.; Imakita, K.; Fujii, M. Evidence of oxygen vacancy induced room temperature ferromagnetism in solvothermally synthesized undoped TiO2 nanoribbons. Nanoscale 2013, 5, 5476–5488. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Neyts, E.C.; Cao, X.; Zhang, X.; Jang, B.W.-L.; Liu, C.-J. Catalyst preparation with plasmas: How does it work? ACS Catal. 2018, 8, 2093–2110. [Google Scholar] [CrossRef]
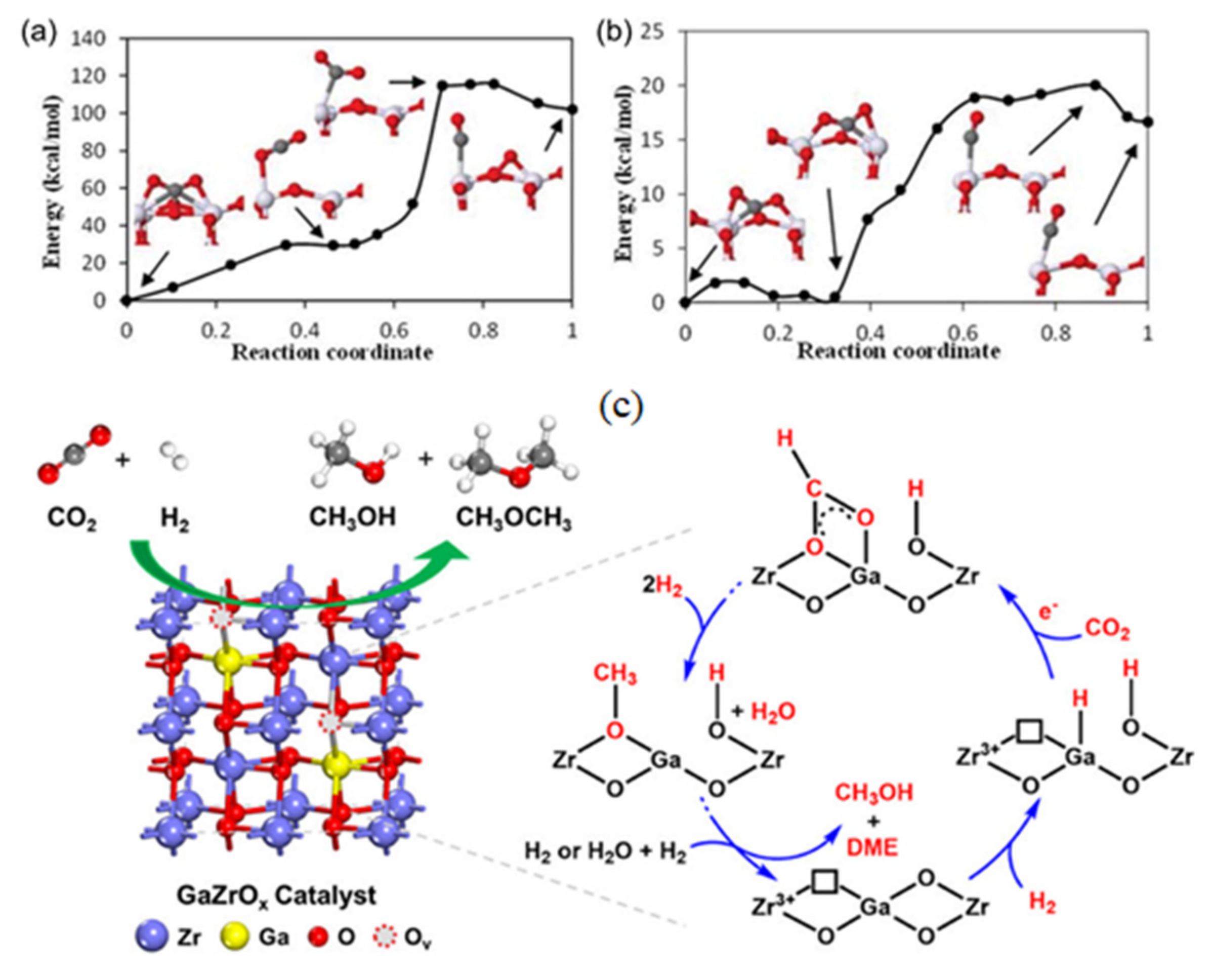
- Feng, W.-H.; Yu, M.-M.; Wang, L.-J.; Miao, Y.-T.; Shakouri, M.; Ran, J.; Hu, Y.; Li, Z.; Huang, R.; Lu, Y.-L. Insights into Bimetallic Oxide Synergy during Carbon Dioxide Hydrogenation to Methanol and Dimethyl Ether over GaZrO x Oxide Catalysts. ACS Catal. 2021, 11, 4704–4711. [Google Scholar] [CrossRef]
- Tabatabaei, J.; Sakakini, B.; Waugh, K. On the mechanism of methanol synthesis and the water-gas shift reaction on ZnO. Catal. Lett. 2006, 110, 77–84. [Google Scholar] [CrossRef]
- Singh, R.; Tripathi, K.; Pant, K.K. Investigating the role of oxygen vacancies and basic site density in tuning methanol selectivity over Cu/CeO2 catalyst during CO2 hydrogenation. Fuel 2021, 303, 121289. [Google Scholar] [CrossRef]
- Rhodes, M.D.; Bell, A.T. The effects of zirconia morphology on methanol synthesis from CO and H2 over Cu/ZrO2 catalysts: Part I. Steady-state studies. J. Catal. 2005, 233, 198–209. [Google Scholar] [CrossRef]
- Martin, O.; Mondelli, C.; Cervellino, A.; Ferri, D.; Curulla-Ferré, D.; Pérez-Ramírez, J. Operando synchrotron X-ray powder diffraction and modulated-excitation infrared spectroscopy elucidate the CO2 promotion on a commercial methanol synthesis catalyst. Angew. Chem. Int. Ed. 2016, 55, 11031–11036. [Google Scholar] [CrossRef]
- Kattel, S.; Yan, B.; Chen, J.G.; Liu, P. CO2 hydrogenation on Pt, Pt/SiO2 and Pt/TiO2: Importance of synergy between Pt and oxide support. J. Catal. 2016, 343, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; He, S.; Chen, H.; Wang, B.; Zheng, L.; Wei, M.; Evans, D.G.; Duan, X. Active site dependent reaction mechanism over Ru/CeO2 catalyst toward CO2 methanation. J. Am. Chem. Soc. 2016, 138, 6298–6305. [Google Scholar] [CrossRef]
- Srinivas, B.; Shubhamangala, B.; Lalitha, K.; Anil Kumar Reddy, P.; Durga Kumari, V.; Subrahmanyam, M.; De, B.R. Photocatalytic Reduction of CO2 over Cu-TiO2/Molecular Sieve 5A Composite. Photochem. Photobiol. 2011, 87, 995–1001. [Google Scholar] [CrossRef]
- Liu, B.-J.; Torimoto, T.; Matsumoto, H.; Yoneyama, H. Effect of solvents on photocatalytic reduction of carbon dioxide using TiO2 nanocrystal photocatalyst embedded in SiO2 matrices. J. Photochem. Photobiol. A Chem. 1997, 108, 187–192. [Google Scholar] [CrossRef]
- Baltrusaitis, J.; Schuttlefield, J.; Zeitler, E.; Grassian, V.H. Carbon dioxide adsorption on oxide nanoparticle surfaces. Chem. Eng. J. 2011, 170, 471–481. [Google Scholar] [CrossRef]
- Li, H.; Rameshan, C.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Bukhtiyarov, V.I.; Rupprechter, G. CO2 activation on ultrathin ZrO2 film by H2O co-adsorption: In situ NAP-XPS and IRAS studies. Surf. Sci. 2019, 679, 139–146. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, J.; Shen, L.; Ford, M.E.; Gao, J.; Xu, J.; Wachs, I.E.; Han, Y.-F. Probing the surface of promoted CuO-Cr2O3-Fe2O3 catalysts during CO2 activation. Appl. Catal. B: Environ. 2020, 271, 118943. [Google Scholar] [CrossRef]
- Posada-Borbón, A.; Grönbeck, H. CO2 adsorption on hydroxylated In2O3 (110). Phys. Chem. Chem. Phys. 2019, 21, 21698–21708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghuman, K.K.; Hoch, L.B.; Wood, T.E.; Mims, C.; Singh, C.V.; Ozin, G.A. Surface analogues of molecular frustrated Lewis pairs in heterogeneous CO2 hydrogenation catalysis. ACS Catal. 2016, 6, 5764–5770. [Google Scholar] [CrossRef]
- Martin, O.; Martin, A.J.; Mondelli, C.; Mitchell, S.; Segawa, T.F.; Hauert, R.; Drouilly, C.; Curulla-Ferre, D.; Perez-Ramirez, J. Indium Oxide as a Superior Catalyst for Methanol Synthesis by CO2 Hydrogenation. Angew. Chem. Int. Ed. 2016, 55, 6261–6265. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Gu, M.; Qiu, R.; Yang, Z.; Xuan, F.; Zhu, M. Tunable Carbon Dioxide Activation Pathway over Iron Oxide Catalysts: Effects of Potassium. Ind. Eng. Chem. Res. 2021, 60, 8705–8713. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Wongsakulphasatch, S.; Vo, D.-V.N. Understanding the role of surface basic sites of catalysts in CO2 activation in dry reforming of methane: A short review. Catal. Sci. Technol. 2020, 10, 35–45. [Google Scholar] [CrossRef]
- Nie, X.; Meng, L.; Wang, H.; Chen, Y.; Guo, X.; Song, C. DFT insight into the effect of potassium on the adsorption, activation and dissociation of CO2 over Fe-based catalysts. Phys. Chem. Chem. Phys. 2018, 20, 14694–14707. [Google Scholar] [CrossRef]
- Miao, L.; Wang, J.; Zhang, P. Review on manganese dioxide for catalytic oxidation of airborne formaldehyde. Appl. Surf. Sci. 2019, 466, 441–453. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, L.; Luo, Q.; Cao, Y.; Dai, Y.; Li, Z.; Li, H.; Zheng, X.; Yan, W.; Yang, J. Molecular-level insight into how hydroxyl groups boost catalytic activity in CO2 hydrogenation into methanol. Chem 2018, 4, 613–625. [Google Scholar] [CrossRef] [Green Version]
- Stacchiola, D.J.; Senanayake, S.D.; Liu, P.; Rodriguez, J.A. Fundamental studies of well-defined surfaces of mixed-metal oxides: Special properties of MOx/TiO2 (110){M= V, Ru, Ce, or W}. Chem. Rev. 2013, 113, 4373–4390. [Google Scholar] [CrossRef]
- Witoon, T.; Numpilai, T.; Phongamwong, T.; Donphai, W.; Boonyuen, C.; Warakulwit, C.; Chareonpanich, M.; Limtrakul, J. Enhanced activity, selectivity and stability of a CuO-ZnO-ZrO2 catalyst by adding graphene oxide for CO2 hydrogenation to methanol. Chem. Eng. J. 2018, 334, 1781–1791. [Google Scholar] [CrossRef]
- Ma, Q.; Geng, M.; Zhang, J.; Zhang, X.; Zhao, T.S. Enhanced Catalytic Performance for CO2 Hydrogenation to Methanol over N-doped Graphene Incorporated Cu-ZnO-Al2O3 Catalysts. ChemistrySelect 2019, 4, 78–83. [Google Scholar] [CrossRef]
- Qi, Y.; Jiang, J.; Liang, X.; Ouyang, S.; Mi, W.; Ning, S.; Zhao, L.; Ye, J. Fabrication of black In2O3 with dense oxygen vacancy through dual functional carbon doping for enhancing photothermal CO2 hydrogenation. Adv. Funct. Mater. 2021, 31, 2100908. [Google Scholar] [CrossRef]
- Tomboc, G.M.; Tesfaye Gadisa, B.; Jun, M.; Chaudhari, N.K.; Kim, H.; Lee, K. Carbon Transition-metal Oxide Electrodes: Understanding the Role of Surface Engineering for High Energy Density Supercapacitors. Chem.—Asian J. 2020, 15, 1628–1647. [Google Scholar] [CrossRef]
- Chatterjee, D.P.; Nandi, A.K. A review on the recent advances in hybrid supercapacitors. J. Mater. Chem. A 2021, 9, 15880–15918. [Google Scholar] [CrossRef]
- Yang, Y.; White, M.G.; Liu, P. Theoretical study of methanol synthesis from CO2 hydrogenation on metal-doped Cu (111) surfaces. J. Phys. Chem. C 2012, 116, 248–256. [Google Scholar] [CrossRef]
- Surnev, S.; Fortunelli, A.; Netzer, F.P. Structure–property relationship and chemical aspects of oxide–metal hybrid nanostructures. Chem. Rev. 2013, 113, 4314–4372. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L.; Lin, D.; Feng, X.; Niu, Y.; Zhang, B.; Xiao, F.-S. Strong metal–support interactions on gold nanoparticle catalysts achieved through Le Chatelier’s principle. Nat. Catal. 2021, 4, 418–424. [Google Scholar] [CrossRef]
- Meng, C.; Zhao, G.; Shi, X.-R.; Chen, P.; Liu, Y.; Lu, Y. Oxygen-deficient metal oxides supported nano-intermetallic InNi3C0. 5 toward efficient CO2 hydrogenation to methanol. Sci. Adv. 2021, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]




| Equation | Reaction | ΔH° 298 K (kJ·mol−1) | ΔG° 298 K (kJ·mol−1) |
|---|---|---|---|
| (1) | 293.0 | 257.2 | |
| (2) | 41.2 | 28.6 | |
| (3) | −49.5 | 3.5 | |
| (4) | −86.7 | −32.4 | |
| (5) | −165.0 | −113.5 | |
| (6) | −132.1 | −78.7 | |
| (7) | −125.0 | −70.9 | |
| (8) | −121.6 | −66.9 | |
| (9) | −64.0 | −28.7 | |
| (10) | −83.6 | −42.1 | |
| (11) | −90.3 | −45.2 |
| Property | Boiling Point (° C) | Density Gas (g/L) | Dipole Moment | Bond Polarity | ∆Hf (298 K) (kJ·mol−1) | Bond Energy (kJ·mol−1) | C–O Bond Distance (Å) | Bond Angle (degree) | Band Gap | Charge | a HOMO Structure | b LUMO Structure |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO2 | −78.5 (sublimes) | 1.98 | 0 | 1.0 | −393.5 | 806 | 1.16 | 180 | ca. 8 e | C: −0.360 O: +719 | 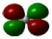 |  |
| Metal | Physisorption | Chemisorption | ||
|---|---|---|---|---|
| Binding Energy (kJ·mol−1) | OCO Angle/° | Binding Energy (kJ·mol−1) | Net Charge/e | |
| Fe (110) | −23 | 121 | −90 | −1.11 |
| Ir (111) | −33 | 128 | −34 | −0.47 |
| Pd (111) | −32 | 140 | −17 | −0.35 |
| Ru (0001) | −31 | 123 | −61 | −0.83 |
| Rh (111) | −32 | 135 | −35 | −0.46 |
| Ni (111) | −26 | 136 | −20 | −0.50 |
| Co (0001) | −25 | 139 | −30 | −0.64 |
| Pt (111) | −21 | 131 | −3 | −0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Etim, U.J.; Zhang, C.; Zhong, Z. Impacts of the Catalyst Structures on CO2 Activation on Catalyst Surfaces. Nanomaterials 2021, 11, 3265. https://doi.org/10.3390/nano11123265
Etim UJ, Zhang C, Zhong Z. Impacts of the Catalyst Structures on CO2 Activation on Catalyst Surfaces. Nanomaterials. 2021; 11(12):3265. https://doi.org/10.3390/nano11123265
Chicago/Turabian StyleEtim, Ubong J., Chenchen Zhang, and Ziyi Zhong. 2021. "Impacts of the Catalyst Structures on CO2 Activation on Catalyst Surfaces" Nanomaterials 11, no. 12: 3265. https://doi.org/10.3390/nano11123265
APA StyleEtim, U. J., Zhang, C., & Zhong, Z. (2021). Impacts of the Catalyst Structures on CO2 Activation on Catalyst Surfaces. Nanomaterials, 11(12), 3265. https://doi.org/10.3390/nano11123265





