CeO2-Promoted PtSn/SiO2 as a High-Performance Catalyst for the Oxidative Dehydrogenation of Propane with Carbon Dioxide
Abstract
1. Introduction
2. Experimental
2.1. Catalyst Preparation
2.2. Catalyst Characterizations
2.3. Catalytic Tests
3. Results
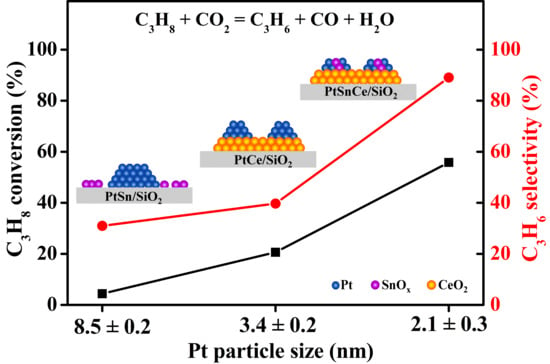
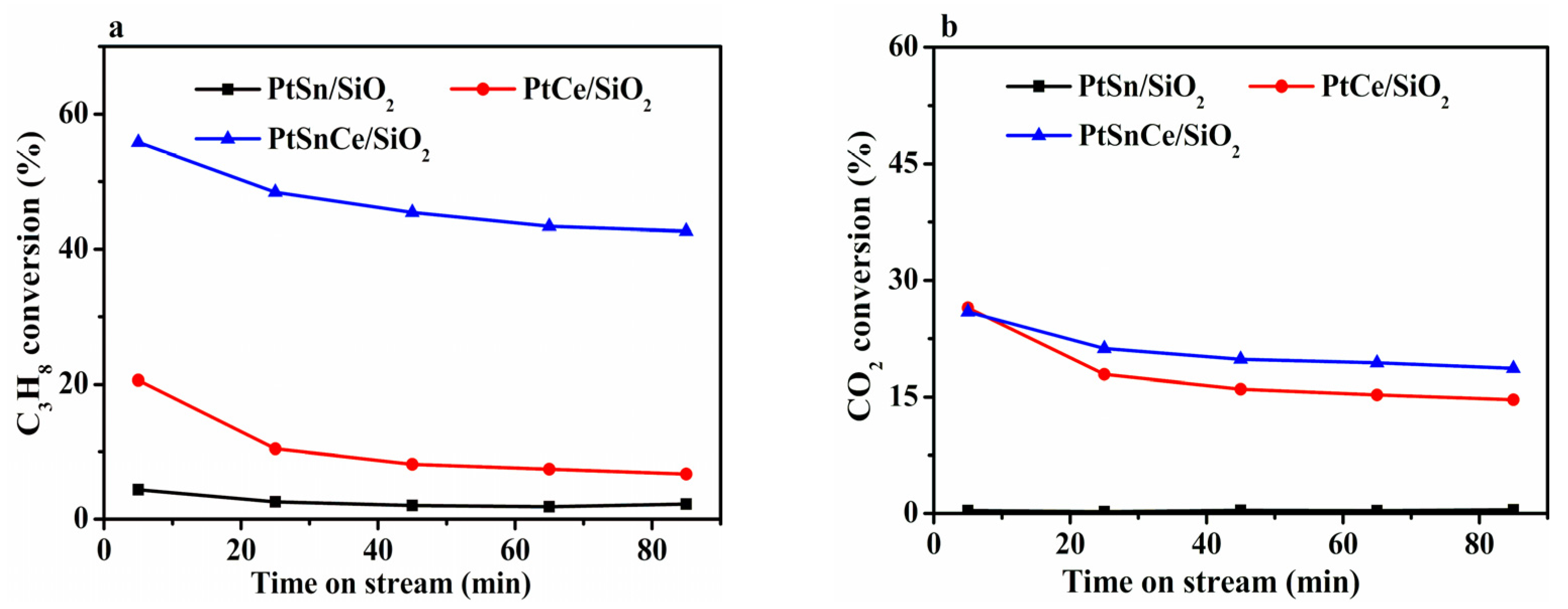
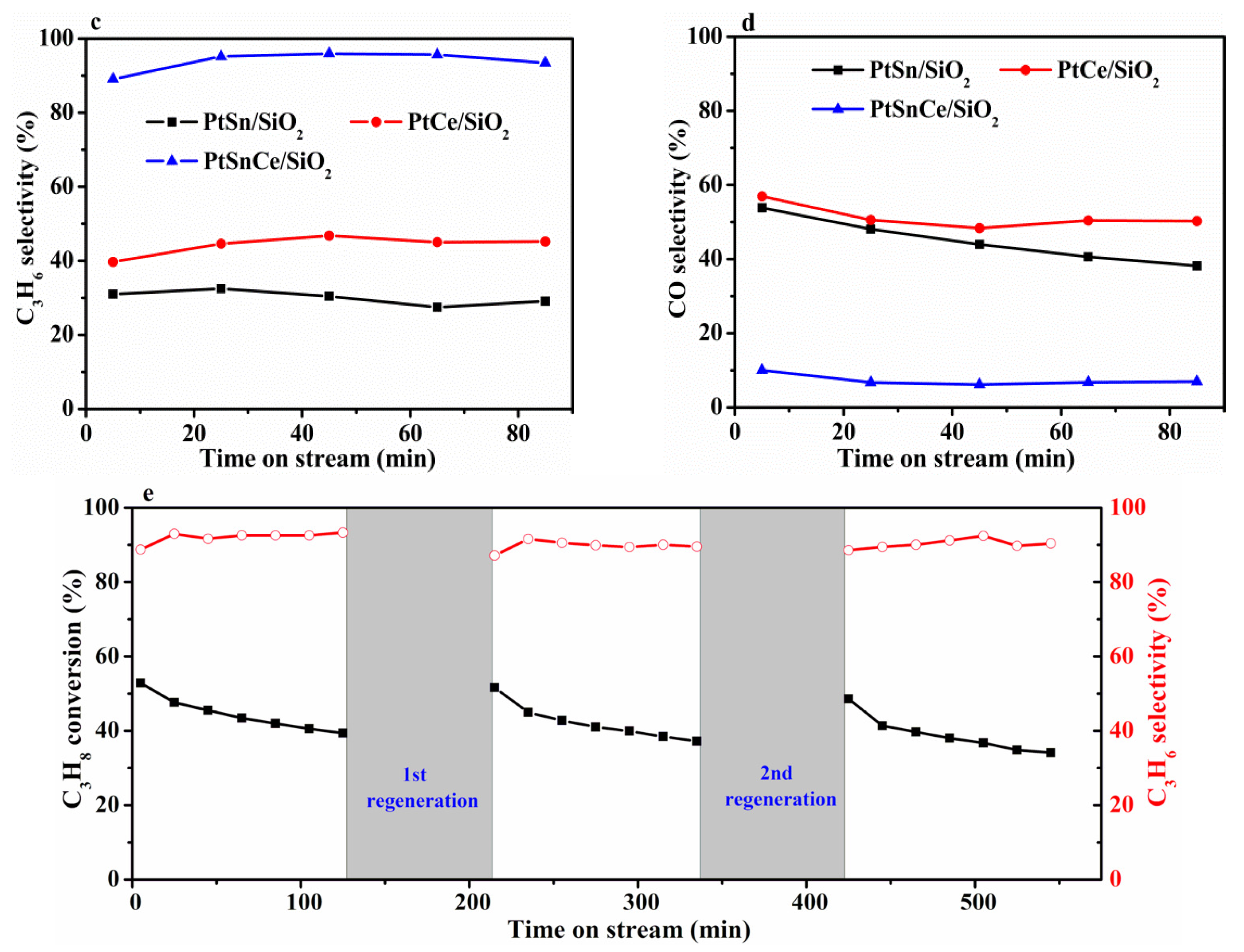
3.1. Catalytic Results of CO2-ODP
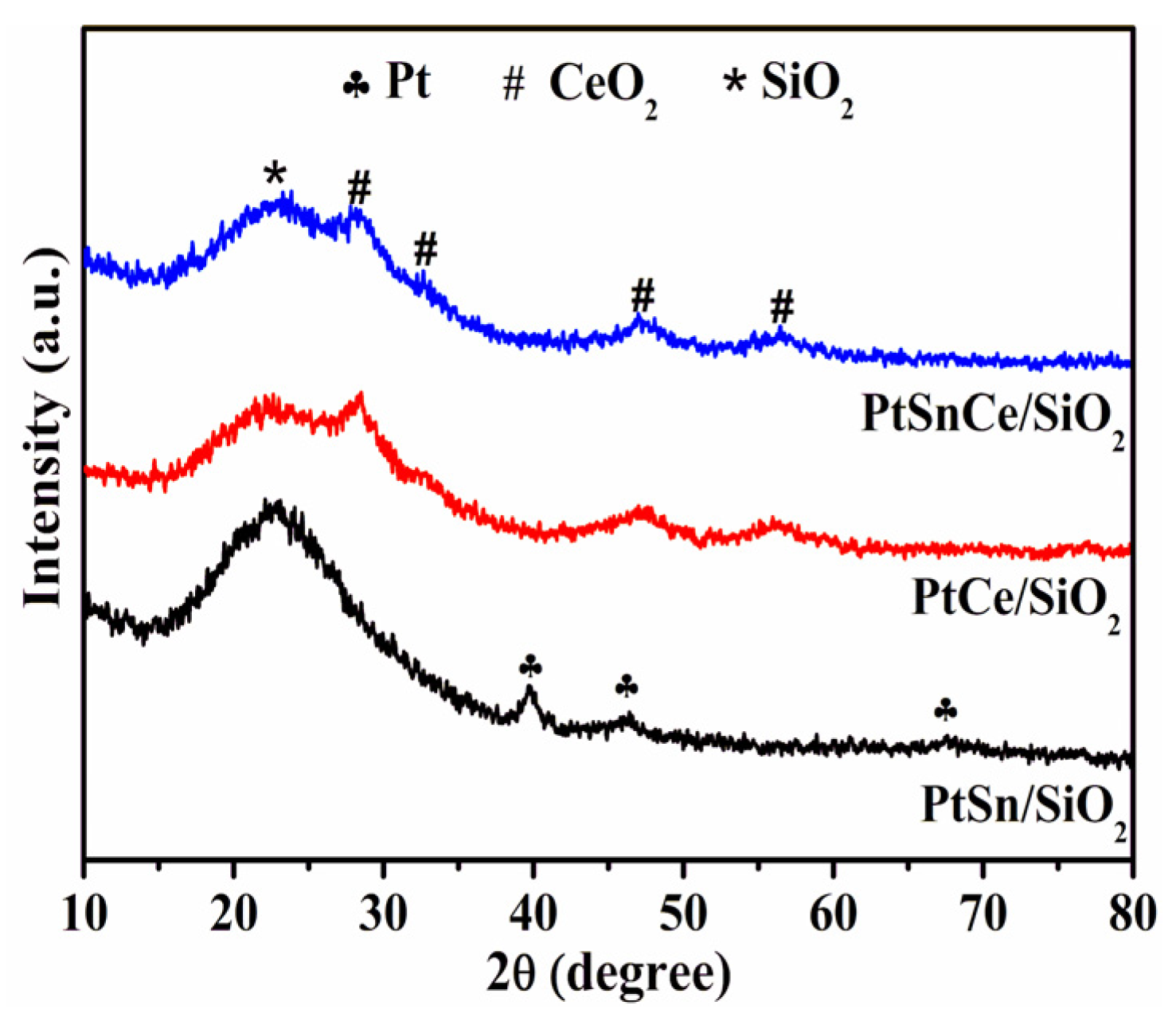
3.2. Textural and Structural Properties
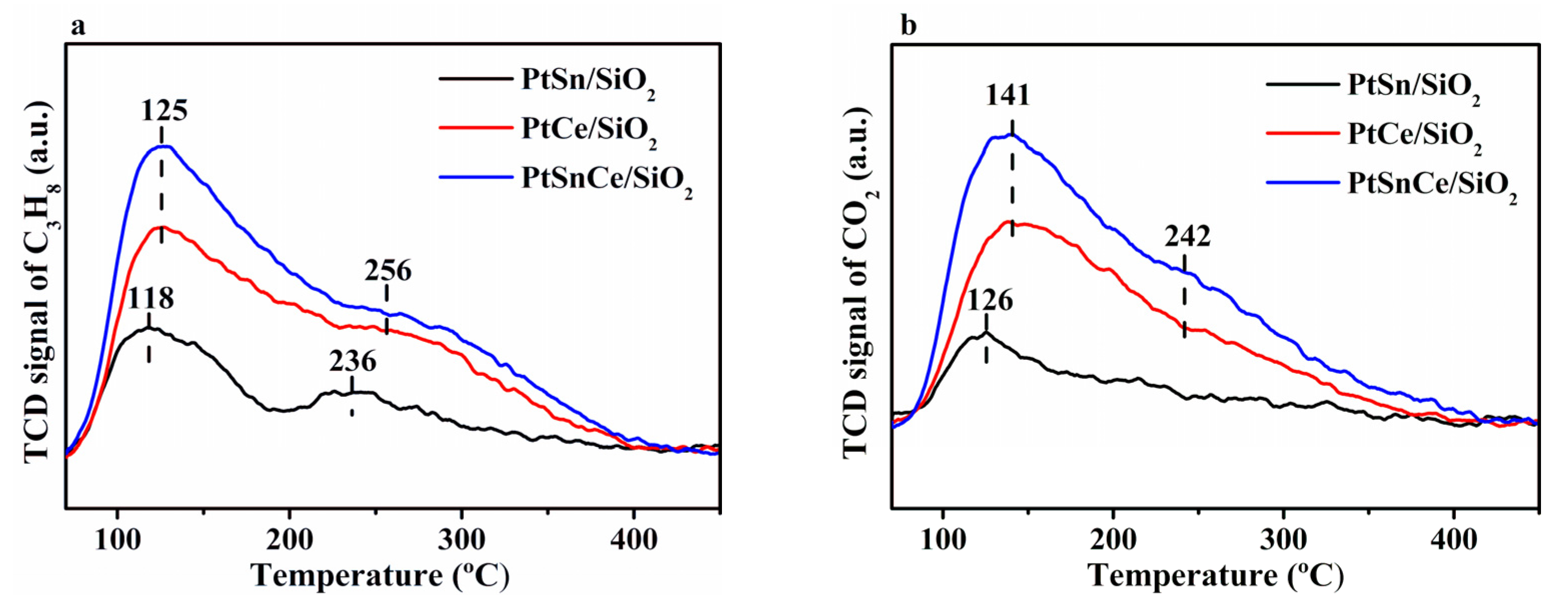
3.3. Reduction Behavior
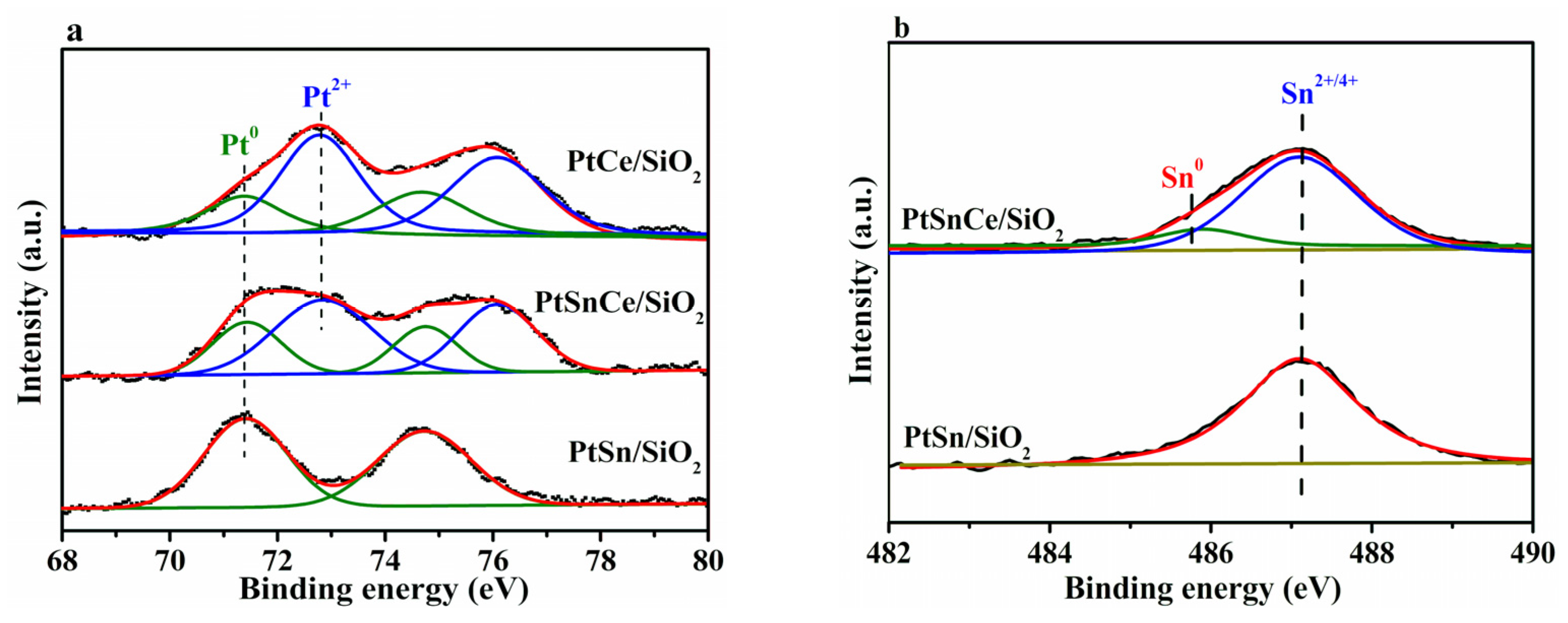
3.4. Chemical States
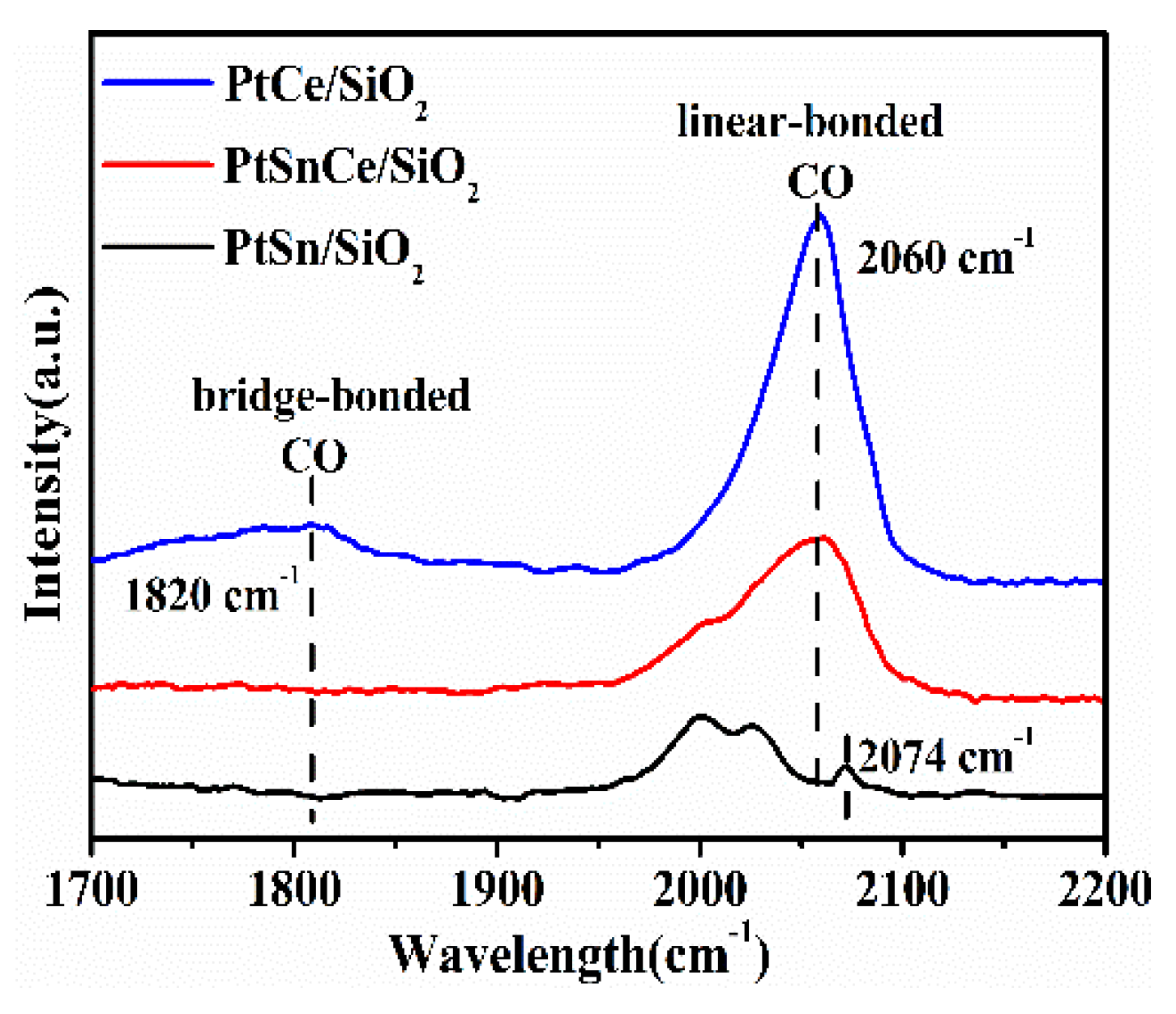
3.5. CO-DRIFTS Studies
4. Discussion
4.1. Key Factors of Catalytic Activity
4.2. Insights into Product Selectivity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amghizar, I.; Vandewalle, L.A.; Van, K.M.; Marin, G.B. New trends in olefin production. Engineering 2017, 3, 171–178. [Google Scholar] [CrossRef]
- Galvis, H.M.T.; Jong, K.P. Catalysts for production of lower olefins from synthesis gas: A review. ACS Catal. 2013, 3, 2130–2149. [Google Scholar] [CrossRef]
- Sattler, J.J.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef] [PubMed]
- Atanga, M.A.; Rezaei, F.; Jawad, A.; Fitch, M.; Rownaghi, A.A. Oxidative dehydrogenation of propane to propylene with carbon dioxide. Appl. Catal. B 2018, 220, 429–445. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.-Y.; Song, J.; Lang, Y.; Chen, J.-G.; Luo, Q.-X.; He, Z.-H.; Wang, K.; Liu, Z.-W.; Liu, Z.-T. Facile synthesis of SiO2 supported GaN as an active catalyst for CO2 enhanced dehydrogenation of propane. J. CO2 Util. 2020, 38, 306–313. [Google Scholar] [CrossRef]
- Jiang, X.; Sharma, L.; Fung, V.; Park, S.J.; Jones, C.W.; Sumpter, B.G.; Baltrusaitis, J.; Wu, Z. Oxidative dehydrogenation of propane to propylene with soft oxidants via heterogeneous catalysis. ACS Catal. 2021, 11, 2182–2234. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, L.; Mu, R.; Xiong, C.; Zhao, Z.J.; Zhao, C.; Pei, C.; Peng, L.; Luo, J.; Fan, L.S.; et al. Modulating lattice oxygen in dual-functional Mo-V-O mixed oxides for chemical looping oxidative dehydrogenation. J. Am. Chem. Soc. 2019, 141, 18653–18657. [Google Scholar] [CrossRef]
- Li, G.; Liu, C.; Cui, X.; Yang, Y.; Shi, F. Oxidative dehydrogenation of light alkanes with carbon dioxide. Green Chem. 2021, 23, 689. [Google Scholar] [CrossRef]
- Carrero, C.A.; Schloegl, R.; Wachs, I.E.; Schomaecker, R. Critical literature review of the kinetics for the oxidative dehydrogenation of propane over well-defined supported vanadium oxide catalysts. ACS Catal. 2014, 4, 3357–3380. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Z.H. Catalytic conversion of alkanes to olefins by carbon dioxide oxidative dehydrogenations—A review. Energy Fuel 2004, 18, 1126–1139. [Google Scholar] [CrossRef]
- Wegrzyniak, A.; Jarczewski, S.; Wegrzynowicz, A.; Michorczyk, B.; Kustrowski, P.; Michorczyk, P. Catalytic behavior of chromium oxide supported on nanocasting-prepared mesoporous alumina in dehydrogenation of propane. Nanomaterials 2017, 7, 249. [Google Scholar] [CrossRef] [PubMed]
- Ruthwik, N.; Kavya, D.; Shadab, A.; Lingaiah, N.; Sumana, C. Thermodynamic analysis of chemical looping combustion integrated oxidative dehydrogenation of propane to propylene with CO2. Chem. Eng. Process. 2020, 153, 107959. [Google Scholar] [CrossRef]
- Gomez, E.; Kattel, S.; Yan, B.; Yao, S.; Liu, P.; Chen, J.G. Combining CO2 reduction with propane oxidative dehydrogenation over bimetallic catalysts. Nat. Commun. 2018, 9, 1398. [Google Scholar] [CrossRef] [PubMed]
- Ascoop, I.; Galvita, V.V.; Alexopoulos, K.; Reyniers, M.-F.; Voort, P.V.D.; Bliznuk, V.; Marin, G.B. The role of CO2 in the dehydrogenation of propane over WOx-VOx/SiO2. J. Catal. 2016, 335, 1–10. [Google Scholar] [CrossRef]
- Shishido, T.; Shimamura, K.; Teramura, K.; Tanaka, T. Role of CO2 in dehydrogenation of propane over Cr-based catalysts. Catal. Today 2012, 185, 151–156. [Google Scholar] [CrossRef]
- Nowicka, E.; Reece, C.; Althahban, S.M.; Mohammed, K.M.H.; Kondrat, S.A.; Morgan, D.J.; He, Q.; Willock, D.J.; Golunski, S.; Kiely, C.J.; et al. Elucidating the role of CO2 in the soft oxidative dehydrogenation of propane over ceria-based catalysts. ACS Catal. 2018, 8, 3454–3468. [Google Scholar] [CrossRef]
- Dai, Y.; Gao, X.; Wang, Q.; Wan, X.; Zhou, C.; Yang, Y. Recent progress in heterogeneous metal and metal oxide catalysts for direct dehydrogenation of ethane and propane. Chem. Rev. 2021, 50, 5590–5630. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, Y.; Cai, X.; Chen, Y.; Peng, M.; Jia, Z.; Jiang, Z.; Ren, P.; Yao, S.; Xie, J.; et al. Tin-assisted fully exposed platinum clusters stabilized on defect-rich graphene for dehydrogenation reaction. ACS Catal. 2019, 9, 5998–6005. [Google Scholar] [CrossRef]
- Kumar, M.S.; Chen, D.; Holmen, A.; Walmsley, J.C. Dehydrogenation of propane over Pt-SBA-15 and Pt-Sn-SBA-15: Effect of Sn on the dispersion of Pt and catalytic behavior. Catal. Today 2009, 142, 17–23. [Google Scholar] [CrossRef]
- Chen, S.; Chang, X.; Sun, G.; Zhang, T.; Xu, Y.; Wang, Y.; Pei, C.; Gong, J. Propane dehydrogenation: Catalyst development, new chemistry, and emerging technologies. Chem. Rev. 2021, 50, 3315–3354. [Google Scholar] [CrossRef]
- Etim, U.J.; Zhang, C.; Zhong, Z. Impacts of the catalyst structures on CO2 activation on catalyst surfaces. Nanomaterials 2021, 11, 3265. [Google Scholar] [CrossRef]
- Wang, H.; Yang, G.-Q.; Song, Y.-H.; Liu, Z.-T.; Liu, Z.-W. Defect-rich Ce1-xZrxO2 solid solutions for oxidative dehydrogenation of ethylbenzene with CO2. Catal. Today 2019, 324, 39–48. [Google Scholar] [CrossRef]
- Xue, M.; Zhou, Y.; Zhang, Y.; Liu, X.; Duan, Y.; Sheng, X. Effect of cerium addition on catalytic performance of PtSnNa/ZSM-5 catalyst for propane dehydrogenation. J. Nat. Gas Chem. 2012, 21, 324–331. [Google Scholar] [CrossRef]
- Motagamwala, A.H.; Almallahi, R.; Wortman, J.; Igenegbai, V.O.; Linic, S. Stable and selective catalysts for propane dehydrogenation operating at thermodynamic limit. Science 2021, 373, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, N.; Fan, Q.; Zeng, L.; Mayoral, A.; Miao, S.; Yang, R.; Jiang, Z.; Zhou, W.; Zhang, J.; et al. Subnanometer bimetallic platinum-zinc clusters in zeolites for propane dehydrogenation. Angew. Chem. Int. Ed. 2020, 59, 2–11. [Google Scholar] [CrossRef]
- Akhtar, M.; Tompkins, F.C. The hydrogen-oxygen titration on platinum films: Determination of the catalytically active area. Trans. Faraday Soc. 1971, 67, 2454–2460. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, F.; Liu, G.; Zeng, L.; Zhao, Z.; Gong, J. Effects of Ga doping on Pt/CeO2-Al2O3 catalysts for propane dehydrogenation. AIChE J. 2016, 62, 4365–4376. [Google Scholar] [CrossRef]
- Lin, C.; Yang, Z.; Pan, H.; Cui, J.; Lv, Z.; Liu, X.; Tian, P.; Xiao, Z.; Li, P.; Xu, J.; et al. Ce-introduced effects on modification of acidity and Pt electronic states on Pt-Sn/γ-Al2O3 catalysts for catalytic reforming. Appl. Catal. A Gen. 2021, 617, 118116. [Google Scholar] [CrossRef]
- Baek, J.; Yun, H.J.; Yun, D.; Choi, Y.; Yi, J. Preparation of highly dispersed chromium oxide catalysts supported on mesoporous silica for the oxidative dehydrogenation of propane using CO2: Insight into the nature of catalytically active chromium sites. ACS Catal. 2012, 2, 1893–1903. [Google Scholar] [CrossRef]
- Deng, L.; Miura, H.; Ohkubo, T.; Shishido, T.; Wang, Z.; Hosokawa, S.; Teramura, K.; Tanaka, T. The importance of direct reduction in the synthesis of highly active Pt-Sn/SBA-15 for n-butane dehydrogenation. Catal. Sci. Technol. 2019, 9, 947–956. [Google Scholar] [CrossRef]
- Kaneko, S.; Izuka, M.; Takahashi, A.; Ohshima, M.; Kurokawa, H.; Miura, H. Pt dispersion control in Pt/SiO2 by calcination temperature using chloroplatinic acid as catalyst precursor. Appl. Catal. A Gen. 2012, 427–428, 85–91. [Google Scholar] [CrossRef]
- Wang, H.; Cao, F.-X.; Song, Y.-H.; Yang, G.-Q.; Ge, H.-Q.; Liu, Z.-T.; Qu, Y.-Q.; Liu, Z.-W. Two-step hydrothermally synthesized Ce1-xZrxO2 for oxidative dehydrogenation of ethylbenzene with carbon dioxide. J. CO2 Util. 2019, 34, 99–107. [Google Scholar] [CrossRef]
- Deng, L.; Miura, H.; Shishido, T.; Wang, Z.; Hosokawa, S.; Teramura, K.; Tanaka, T. Elucidating strong metal-support interactions in Pt-Sn/SiO2 catalyst and its consequences for dehydrogenation of lower alkanes. J. Catal. 2018, 365, 277–291. [Google Scholar] [CrossRef]
- Huang, L.; Xu, B.; Yang, L.; Fan, Y. Propane dehydrogenation over the PtSn catalyst supported on alumina-modified SBA-15. Catal. Commun. 2008, 9, 2593–2597. [Google Scholar] [CrossRef]
- Wang, H.; Huang, H.; Bashir, K.; Li, C. Isolated Sn on mesoporous silica as a highly stable and selective catalyst for the propane dehydrogenation. Appl. Catal. A Gen. 2020, 590, 117291. [Google Scholar] [CrossRef]
- Chen, A.; Zhou, Y.; Ta, N.; Li, Y.; Shen, W. Redox properties and catalytic performance of ceria-zirconia nanorods. Catal. Sci. Technol. 2015, 5, 4184–4192. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Zhang, Y.; Jiao, Y.; Ren, C.; Gong, M.; Chen, Y. A study on H2 -TPR of Pt/Ce0.27Zr0.73O2 and Pt/Ce0.27Zr0.70 La0.03Ox for soot oxidation. Appl. Surf. Sci. 2016, 377, 48–55. [Google Scholar] [CrossRef]
- Lee, J.; Ryou, Y.; Chan, X.; Kim, T.J.; Kim, D.H. How Pt interacts with CeO2 under the reducing and oxidizing environments at elevated temperature: The origin of improved thermal stability of Pt/CeO2 compared to CeO2. J. Phys. Chem. C 2016, 120, 25870–25879. [Google Scholar] [CrossRef]
- Xiang, X.; Zhao, H.; Yang, J.; Zhao, J.; Yan, L.; Song, H.; Chou, L. Nickel based mesoporous silica-ceria-zirconia composite for carbon dioxide reforming of methane. Appl. Catal. A Gen. 2016, 520, 140–150. [Google Scholar] [CrossRef]
- Ono, L.K.; Croy, J.R.; Heinrich, H.; Roldan Cuenya, B. Oxygen chemisorption, formation, and thermal stability of Pt oxides on Pt nanoparticles supported on SiO2/Si(001): Size effects. J. Phys. Chem. C 2011, 115, 16856–16866. [Google Scholar] [CrossRef]
- Bruix, A.; Lykhach, Y.; Matolínová, I.; Neitzel, A.; Skála, T.; Tsud, N.; Vorokhta, M.; Stetsovych, V.; Ševčíková, K.; Mysliveček, J.; et al. Maximum noble-metal efficiency in catalytic materials: Atomically dispersed surface platinum. Angew. Chem. Int. Ed. 2014, 53, 10525–10530. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Lin, S.; Goetze, J.; Pletcher, P.; Guo, H.; Kovarik, L.; Artyushkova, K.; Weckhuysen, B.M.; Datye, A.K. Thermally stable and regenerable platinum-tin clusters for propane dehydrogenation prepared by atom trapping on ceria. Angew. Chem. Int. Ed. 2017, 56, 8986–8991. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Anjum, D.H.; Wang, Q.; Abou-Hamad, E.; Emsley, L.; Dong, H.; Laveille, P.; Li, L.; Samal, A.K.; Basset, J.-M. Sn surface-enriched Pt-Sn bimetallic nanoparticles as a selective and stable catalyst for propane dehydrogenation. J. Catal. 2014, 320, 52–62. [Google Scholar] [CrossRef]
- Xu, H.; Wen, C.; Liu, H.; Li, Z.P.; Shen, W.Z. Relationship of microstructure properties to oxygen impurities in nanocrystalline silicon photovoltaic materials. J. Appl. Phys. 2013, 113, 093501. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Sachdev, A.; Schwank, J. Chemisorption and FTIR study of bimetallic Pt-Au/SiO2 catalysts. J. Catal. 1990, 121, 441–455. [Google Scholar] [CrossRef][Green Version]
- Podda, N.; Corva, M.; Mohamed, F.; Feng, Z.; Dri, C.; Dvorák, F.; Matolin, V.; Comelli, G.; Peressi, M.; Vesselli, E. Experimental and theoretical investigation of the restructuring process induced by CO at near ambient pressure: Pt nanoclusters on graphene/Ir(111). ACS Nano 2016, 11, 1041–1053. [Google Scholar] [CrossRef]
- Arteaga, G.J.; Anderson, J.A.; Rochester, C.H. FTIR study of CO adsorption on coked Pt-Sn/Al2O3 catalysts. Catal. Lett. 1999, 58, 189–194. [Google Scholar] [CrossRef]
- Wang, H.-Z.; Zhang, W.; Jiang, J.-W.; Sui, Z.-J.; Zhu, Y.-A.; Ye, G.-H.; Chen, D.; Zhou, X.-G.; Yuan, W.-K. The role of H2S addition on Pt/Al2O3 catalyzed propane dehydrogenation: A mechanistic study. Catal. Sci. Technol. 2019, 9, 867–876. [Google Scholar] [CrossRef]
- Boccuzzi, F.; Chiorino, A.; Guglielminotti, E. Effects of structural defects and alloying on the FTIR spectra of CO adsorbed on PtZnO. Surf. Sci. 1996, 268, 264–269. [Google Scholar] [CrossRef]
- Wang, Q.; Tichit, D.; Meunier, F.; Guesmi, H. Combined DRIFTS and DFT study of CO adsorption and segregation modes in Pt-Sn nanoalloys. J. Phys. Chem. C 2020, 124, 9979–9989. [Google Scholar] [CrossRef]
- Moscu, A.; Schuurman, Y.; Veyre, L.; Thieuleux, C.; Meunier, F. Direct evidence by in situ IR CO monitoring of the formation and the surface segregation of a Pt-Sn alloy. Chem. Commun. 2014, 50, 8590. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Jiang, J.; Sui, Z.; Zhu, Y.; Chen, D.; Zhou, X. Size Dependence of Pt catalysts for propane dehydrogenation: From atomically dispersed to nanoparticles. ACS Catal. 2020, 10, 12932–12942. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, W.; Yang, G.-Q.; Zhang, Y.-W.; Song, Y.-H.; Jiang, N.; Liu, Z.-T.; Liu, Z.-W. Insights into the oxidative dehydrogenation of ethylbenzene with CO2 catalyzed by the ordered mesoporous V2O5-Ce0.5Zr0.5O2-Al2O3. Ind. Eng. Chem. Res. 2019, 58, 21372–21381. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.-H.; Liu, Z.-T.; Liu, Z.-W. Active and selective nature of supported CrOx for the oxidative dehydrogenation of propane with carbon dioxide. Appl. Catal. B 2021, 297, 120400. [Google Scholar] [CrossRef]
- Hu, Z.-P.; Wang, Y.; Yang, D.; Yuan, Z.-Y. CrOx supported on high-silica HZSM-5 for propane dehydrogenation. J. Energy Chem. 2020, 47, 225–233. [Google Scholar] [CrossRef]
- Burri, D.R.; Choi, K.M.; Han, D.S.; Jiang, N.; Burri, A.; Park, S.E. Oxidative dehydrogenation of ethylbenzene to styrene with CO2 over SnO2-ZrO2 mixed oxide nanocomposite catalysts. Catal. Today 2008, 131, 173–178. [Google Scholar] [CrossRef]
- Choi, H.; Oh, S.; Park, J.Y. High methane selective Pt cluster catalyst supported on Ga2O3 for CO2 hydrogenation. Catal. Today 2020, 352, 212–219. [Google Scholar] [CrossRef]
- Yu, C.; Ge, Q.; Xu, H.; Li, W. Effects of Ce addition on the Pt-Sn/γ-Al2O3 catalyst for propane dehydrogenation to propylene. Appl. Catal. A Gen. 2006, 315, 58–67. [Google Scholar] [CrossRef]
- Shi, L.; Deng, G.M.; Li, W.C.; Miao, S.; Wang, Q.N.; Zhang, W.P.; Lu, A.H. Al2O3 nanosheets rich in pentacoordinate Al3+ ions stabilize Pt-Sn clusters for propane dehydrogenation. Angew. Chem. Int. Ed. 2015, 54, 13994–18998. [Google Scholar] [CrossRef]
- Yang, G.-Q.; He, Y.-J.; Song, Y.-H.; Wang, J.; Liu, Z.-T.; Liu, Z.-W. Oxidative dehydrogenation of propane with carbon dioxide catalyzed by ZnxZr1-xO2-x solid solutions. Ind. Eng. Chem. Res. 2021, 60, 17850–17861. [Google Scholar] [CrossRef]
- Wang, H.-M.; Chen, Y.; Yan, X.; Lang, W.-Z.; Guo, Y.-J. Cr doped mesoporous silica spheres for propane dehydrogenation in the presence of CO2: Effect of Cr adding time in sol-gel process. Microporous and Mesoporous Mater. 2019, 284, 69–77. [Google Scholar] [CrossRef]
- Michorczyk, P.; Ogonowski, J.; Zeńczak, K. Activity of chromium oxide deposited on different silica supports in the dehydrogenation of propane with CO2—A comparative study. J. Mol. Catal. A Chem. 2011, 349, 1–12. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, R.; Yue, Y.; Yang, W.; Gu, S.; Miao, C.; Hua, W.; Gao, Z. Chromium oxide supported on ZSM-5 as a novel efficient catalyst for dehydrogenation of propane with CO2. Microporous and Mesoporous Mater. 2011, 145, 194–199. [Google Scholar] [CrossRef]
- Xue, X.-L.; Lang, W.-Z.; Yan, X.; Guo, Y.J. Dispersed vanadium in three-dimensional dendritic mesoporous silica nanospheres: Active and stable catalysts for the oxidative dehydrogenation of propane in the presence of CO2. ACS Appl. Mater. Interfaces 2017, 9, 15408–15423. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Y.; He, Z.-H.; Xia, Y.; Zhang, L.; Wang, K.; Wang, W.; Yang, Y.; Chen, J.-G.; Liu, Z.-T. Oxidative dehydrogenation of propane to propylene in the presence of CO2 over gallium nitride supported on NaZSM-5. Ind. Eng. Chem. Res. 2021, 60, 2807–2817. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, J.; Wang, P.; Wang, X.; Pang, F.; Zhang, Z.; Tan, Y. Dehydrogenation of propane over a hydrothermal-synthesized Ga2O3-Al2O3 catalyst in the presence of carbon dioxide. Catal. Sci. Technol. 2016, 6, 5183–5195. [Google Scholar] [CrossRef]
- Chen, M.; Xu, J.; Cao, Y.; He, H.-Y.; Fan, K.-N.; Zhuang, J.-H. Dehydrogenation of propane over In2O3-Al2O3 mixed oxide in the presence of carbon dioxide. J. Catal. 2010, 272, 101–108. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, F.; Hua, W.; Yue, Y.; Gao, Z. ZnO supported on high silica HZSM-5 as new catalysts for dehydrogenation of propane to propene in the presence of CO2. Catal. Today 2009, 148, 316–322. [Google Scholar] [CrossRef]
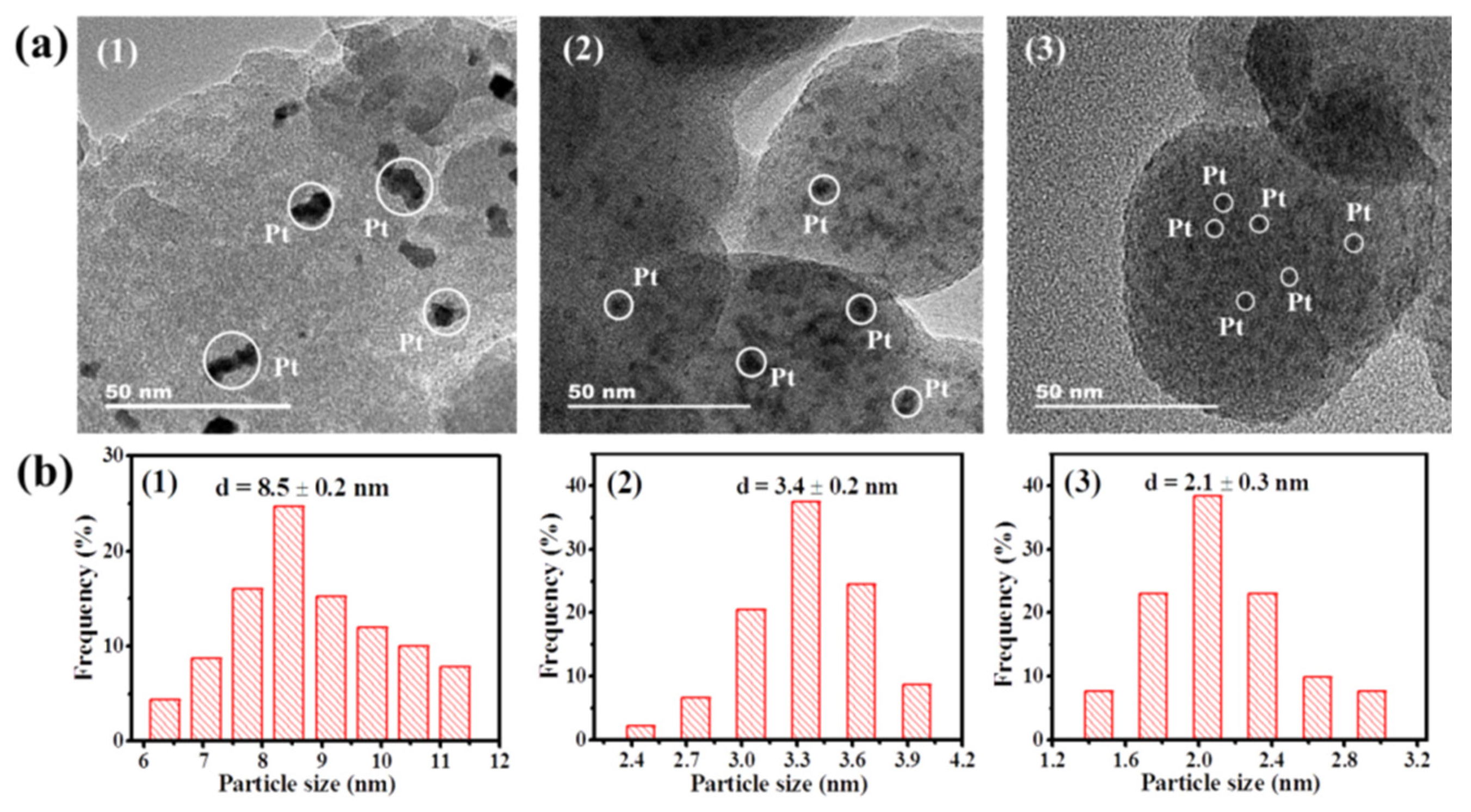

| Catalyst | Specific Surface Area (m2/g) | Mean Pore Size (nm) | Total Pore Volume (cm3/g) | Crystallite Size (nm) | Metal Dispersion *** (%) | |
|---|---|---|---|---|---|---|
| Pt * | CeO2 ** | |||||
| PtSn/SiO2 | 568.9 | 3.19 | 0.42 | 8.5 ± 0.2 | - | 13.4 |
| PtCe/SiO2 | 536.4 | 3.25 | 0.43 | 3.4 ± 0.2 | 5.9 | 20.9 |
| PtSnCe/SiO2 | 527.3 | 3.16 | 0.41 | 2.1 ± 0.3 | 5.6 | 41.3 |
| Catalyst | H2 Consumption (mol/g) | Pt0 (%) | Pt2+ (%) | Sn0 (%) | Ce3+ (%) |
|---|---|---|---|---|---|
| PtSn/SiO2 | 23.5 | 100.0 | 0 | 0 | - |
| PtCe/SiO2 | 78.5 | 30.5 | 69.5 | - | 31.9 |
| PtSnCe/SiO2 | 140.6 | 33.1 | 66.9 | 12.0 | 43.2 |
| Catalyst | C3H8 | CO2 | C3H6 | Coke Content (wt%) | ID/IG | Coking Rate (g/mol) |
|---|---|---|---|---|---|---|
| (mmol/g) * | ||||||
| PtSn/SiO2 | 0.09 | 0.04 | 0.10 | - | - | - |
| PtCe/SiO2 | 0.22 | 0.11 | 0. 23 | 1.71 | 0.73 | 2.09 |
| PtSnCe/SiO2 | 0.30 | 0.14 | 0.26 | 2.53 | 0.81 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Yang, G.-Q.; Ren, X.; Liu, Z.-W. CeO2-Promoted PtSn/SiO2 as a High-Performance Catalyst for the Oxidative Dehydrogenation of Propane with Carbon Dioxide. Nanomaterials 2022, 12, 417. https://doi.org/10.3390/nano12030417
Wang L, Yang G-Q, Ren X, Liu Z-W. CeO2-Promoted PtSn/SiO2 as a High-Performance Catalyst for the Oxidative Dehydrogenation of Propane with Carbon Dioxide. Nanomaterials. 2022; 12(3):417. https://doi.org/10.3390/nano12030417
Chicago/Turabian StyleWang, Li, Guo-Qing Yang, Xing Ren, and Zhong-Wen Liu. 2022. "CeO2-Promoted PtSn/SiO2 as a High-Performance Catalyst for the Oxidative Dehydrogenation of Propane with Carbon Dioxide" Nanomaterials 12, no. 3: 417. https://doi.org/10.3390/nano12030417
APA StyleWang, L., Yang, G.-Q., Ren, X., & Liu, Z.-W. (2022). CeO2-Promoted PtSn/SiO2 as a High-Performance Catalyst for the Oxidative Dehydrogenation of Propane with Carbon Dioxide. Nanomaterials, 12(3), 417. https://doi.org/10.3390/nano12030417