Abstract
We studied the magnetic properties of WSe/MoSe powder. The coercivity field reaches 2600 Oe at 5 K, 4233 Oe at 100 K and 1300 Oe at 300 K. These are the highest values reported for two-dimensional transition metal dichalcogenides. This study is different from the widely reported vacancy and zigzag structure-induced ferromagnetism studies. Importantly, a Raman peak red shift was observed, and that supports the chemical bonding at the interface between WSe and MoSe. The large coercivity field originates from the chemical bonding-induced structural distortion at the interface between WSe and MoSe.
1. Introduction
Spintronics is an approach to the manipulation of spin polarization and to realizing spin-base functionalities [1]. The dilute magnetic semiconductor (DMS) is one of the promising materials for spintronics applications. The original idea is doping a magnetic element into a semiconductor host, thereby making a material possessing both semiconductor and magnetic behaviors. The DMS has been widely studied in III–V and II–VI group semiconductor based systems, and has intrinsic ferromagnetism. However, the low Curie temperature and the intrinsic/extrinsic mechanism disputation limit its application potential. The combination of strong spin-orbit coupling and surface bonding has been shown to be very effective at generating magnetism in nanoparticles of metals [2] and semiconductors [3].
Two-dimensional transition-metal dichalcogenides (2D TMDs) have strong spin-orbit coupling and exhibit semiconductor behavior with an appropriate tunable bandgap [1,4,5,6]. Theoretical and experimental works have demonstrated that magnetism can be induced through doping magnetic elements, structure defects, or edge manipulation [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. Reports show that the low coercivity field and oxidation in the MoS and WS with various element dopings or physical treatments would be too abrupt for applications. Differently from the broadly studied MoS and WS, WSe and MoSe exhibit resistance to oxidation and humid ambiance [27,28]. WSe and MoSe also have a stronger spin-orbit interaction than MoS and WS, and that might enhance the spin manipulation efficiency. However, there are rare reports about ferromagnetism of WSe and MoSe. Both WSe and MoSe have the same crystallographic structure where 2D sheets are bounded in 3D stacks by van der Waals interactions. Experimental studies have reported room temperature ferromagnetism in Co, Ni and V-doped WSe [29,30,31,32,33,34]. However, the coercivity fields and ferromagnetism were still weak. A recent report revealed that the Co and Nb co-doped WSe has a strong coercivity field and magnetization, and it reached 1.2 kOe and 60.62 emu/g in the 1% Nb–4% Co co-doped WSe at 10 K [35]. It is understood that the vacancy and/or defect-induced pinning effect leads to the high coercivity and magnetization. Similarly to the magnetic element dopant, a theoretical calculation suggests that the ferromagnetism can be induced via structural defects (W or Se vacancies), and such structural defects could be achieved experimentally.
The structural defects and/or edge band bonding could lead to structural distortion that would induce ferromagnetism [36,37]. Recent studies have shown that element replacement might induce intrinsic ferromagnetism. It is interesting to know how it would be in mixed TMD materials. In this work, we demonstrate the thermally annealed WSe/MoSe mixed powder. The WSe and MoSe blocks were chemically bonded. Our experimental results show high coercivity, 1324 Oe (2695 Oe) at room temperature (5 K). This is the highest coercivity ever reported in a TMD system.
2. Experimental Methods
The mixed WSe/MoSe powder is a commercial product and was purchased from SixCarbon Technology. Co. (ShenZhen, China) The purchased WSe/MoSe powder was vacuum-sealed in a glass tube with a pressure of 10 torr, and then thermally annealed. The WSe/MoSe powder was heated up to 1000 C by a rate of 2.7 C/min, and maintained at 1000 C for 1 h. After thermal annealing, it was naturally cooled down to room temperature. The X-ray diffraction (XRD) was performed in D2 phaser using the Cu K radiation with a scan step of 0.1. Raman spectroscopy was performed in the HORIBA, HR 800 (HORIBA Taiwan, Inc., Zhubei City, HsinChiu county, Taiwan) with wavelength 633 nm, power 16 mW and step 0.3 cm. X-ray photoelectron spectroscopy (XPS) was performed in ULVAC-PHI, PHI 5000 Versa Probe (ULVAC-PHI, Inc., Kanagawa ken, Japan), and used to detect the sample’s phase composition. The electron probe micro-analyzer (EPMA, JEOL Ltd., Musashino, Akishima, Tokyo, Japan) was performed in JEOL, JXA-8530F (JEOL, akishima Japan) and used to determine the material composition ratio. Magnetism measurements were performed using the standard technique in a SQUID MPMS-3 magnetometer (Quantum Design North America, Pacific Center Court, San Diego, CA, USA) in the temperature range of 5 to 300 K under an applied magnetic field of up to 5 T. The magnetic field step was 1000 Oe for sample 1 and 50 Oe for sample 2.
3. Results and Discussion
Figure 1 shows the XRD spectrum of the WSe/MoSe powder. It shows sharp peaks and these peaks are consistent with the data on WSe/MoSe powder [38]. The hexagonal structure is consistent with the structure of WSe and MoSe. The XRD peak intensity over background noise reached 440 for the (002) peak, and the full-width at half-maximum (FWHM) was 0.2. These results support that the WSe/MoSe powders are highly crystallized. The crystallographic structure of WSe and MoSe domains might orient the same way in the whole grain. Figure 1 inset shows the SEM image of the WSe/MoSe powder in backscattered emission imaging (BEI) mode. It reveals zones with different black and white intensity. The energy dispersive spectroscopy (EDS) supports that the light zone is WSe and the dark zone is MoSe. It shows that there are no obvious cracks or geometric gaps between light zones and dark zones. Figure 1 inset exhibits that the WSe and MoSe are mainly individual blocks and do not appear in the WSeTe form, which would lead to a wide range of gray intensity in the BEI mode. The EPMA supports that W:Se = 1:2 in the light zone and Mo:Se = 1:2 in the dark zone, and : 1:1.

Figure 1.
The XRD spectrum of the WSe/MoSe powder. The peak position is consistent with the database. The sharp peaks imply that the sample is highly crystallized. The top-right inset shows that SEM image in the backscattering emission image mode. The light area is the WSe, and the dark area is the MoSe. The phases of WSe and MoSe are separated.
Figure 2a shows the WSe/MoSe magnetization as a function of magnetic fields and it reveals the diamagnetization at high magnetic fields. The M–H loop shows a clear hysteresis loop, and that is a ferromagnetism feature. The diamagnetic background is superimposed onto the ferromagnetic loop. The ferromagnetism is known to be saturated at critical magnetic fields, and the diamagnetism is negatively linearly correlated with magnetic fields. After subtracting out the diamagnetic contribution that is determined from the magnetism at high magnetic fields, one could extract the ferromagnetism signal. Figure 2b shows the extracted ferromagnetic loops. The coercivity field was 1300 Oe at 300 K and 2600 Oe at 5 K, and these coercivity fields are larger than all values reported for 2D TMDs. To further confirm this large coercivity field, the other sample was prepared from the same raw material and under the same treatment conditions. Figure 2c shows that the M–H loop shows ferromagnetic loops for the second sample. The M–H curve is similar to the curve of the first sample. Figure 2d exhibits ferromagnetic loops after subtracting out the diamagnetic background. It exhibits the ferromagnetic features with a coercivity field of 1100 Oe at 300 K and 2299 Oe at 5 K. This is consistent with the results of the first sample, and confirms that this large coercivity is an intrinsic feature.
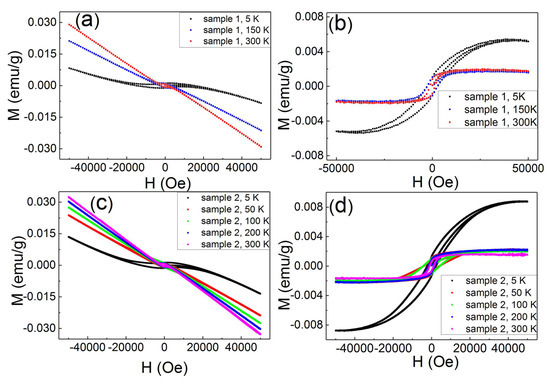
Figure 2.
(a,c) The M–H curves at temperatures for sample 1 and sample 2. They exhibit hysteresis loops at low magnetic fields and diamagnetism at high magnetic fields. (b,d) The M–H curves at temperature for sample 1 and sample 2; the diamagnetic contribution was subtracted.
Figure 3a shows the saturation magnetization as a function of temperature, and it reveals consistent values in the two samples. The saturation magnetization is roughly consistent with the values reported for 2D TMDs. Figure 3b shows the temperature-dependent coercivity fields. It shows a smooth curve and a maximum value of 4233 Oe at 100 K. Table 1 lists the reported saturation magnetization and coercivity fields in 2D materials. These hysteresis loop coercivity fields have a wide range of values. Our observations of 2600 Oe at 5 K, 4233 Oe at 100 K and 1300 Oe at 300 K are the largest values reported for 2D TMDs at those temperatures.
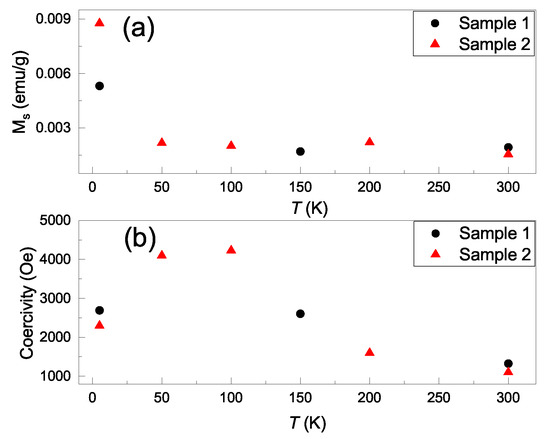
Figure 3.
(a) The saturation magnetization as a function of temperature for two WSe/MoSe powders. (b) The temperature dependent coercivity fields of two WSe/MoSe powders.

Table 1.
List of the reported coercivity and saturation magnetization values of two-dimensional transition metal dichalcogenides.
A slight magnetic or transition element dopant might lead to strong ferromagnetism in 2D TMDs [29,30,31,32,33,34]. Our EDS analysis supports that there were no un-avoided magnetic or transition elements in our system. The saturation magnetization was 0.001 emu/g. If this magnetism originated from Ni, Co or/and Fe, the magnetic elements would have reached a 0.01% atomic ratio, which is within the detectable range of the EMPA, but our EMPA experiment showed no detectable magnetic elements in our samples. This supports that the external element dopants are not the main mechanism of the observed ferromagnetism in WSe/MoSe powder.
Apart from the magnetic element dopants, theoretical calculations and experimental work support that the structural defects can induce ferromagnetism. The coercivity field is sensitive to the host material, number of defects and defect structure [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. The vacancies and defects were expected to be uniformly distributed in the entirety of WSe-MoSe blocks, and not only in specific one material (WSe or MoSe). Table 1 shows that the defect-induced coercivity field in WSe was roughly one order of magnitude higher than that in MoSe. In a case where the observed hysteresis loop originates from the vacancy or defect in the WSe and MoSe, one would expect to observe two hysteresis loop steps in our samples. Only one hysteresis loop was observed though; see Figure 2. On the other hand, we report that the thermal annealing induced S vacancies in WS and MoS. The XPS shows no obvious Mo, W and Se vacancies in the WSe/MoSe powder. The structure of vacancies might impact the XRD peak intensity suppression and XRD peak shift. As mentioned in Figure 1, the XRD peaks are extremely sharp and show no XRD peak shift. These results indicate extremely little vacancy in our system, and we do not think that this slight, unavoidable structural defect could have led to the large coercivity fields observed. This implies that the structural defects are not the dominant mechanism.
Ferromagnetism was studied in the MoSSe nanosheet, and the results revealed that the ferromagnetism is sensitive to the ratio. MoSSe exhibited the most ferromagnetism in the Mo(SSe) nanosheet, in which the ratio was the largest [36]. The magnetism decreased as more Se or S were substituted into the Mo(SSe) nanosheet. This supports the idea that the element replacement might lead to the ferromagnetism. The chemical bonding at the WSe and MoSe interface would be in the WSeTe form. This would lead to structural distortion, and the observed hysteresis loop might originate from the interface. On the other hand, it is reported that ferromagnetism and magnetoresistance hysteresis can be observed in a molecular-beam epitaxy grown non-magnetic group IV GeSn thin film. A GeSn alloy forms at the interface between Ge and Sn thin films. The observed ferromagnetism is understood as the inversion symmetry breaking from atomic disordering in the alloy [37].
The Raman spectrometer, a sensitive tool for detecting lattice bonding, was used to identify the chemical bonding at the interface between WSe and MoSe. Figure 4 shows the Raman spectra of different zones. The WSe/MoSe powder size was two orders of magnitude larger than the Raman laser spot dot size of about 1 m. The Raman spectra might have detected the signal of only WSe, MoSe or chemically bonded WSe/MoSe. That means the Raman spectra exhibit slight different peak positions and peak intensity at different zones. The spectra are consistent with the database of WSe and MoSe. The peaks of MoSe A (242 cm), WSe A (250 cm) and WSe 2LA(M) (256 cm) were labeled with dotted lines. It is noticeable that there are different peaks positions of WSe in zone 5. The peak positions are 253 and 249 cm in zone 5. The measured step was 0.3 cm, which is much smaller than the peak difference in the A and 2LA(M), and the red shifting of these peaks might have originated from the intrinsic lattice vibration mode in the WSe/MoSe powder. It also shows a larger red shift in the WMoSe with more Mo replacement [39]. As shown in the Figure 1 inset, there is a merge combination. The peaks at 253 and 249 cm were expected to have red shifts: peak 2LA(M) to 256 cm for A, and the 250 cm peak for WSe. This is evidence of the chemical bonding in the WSe/MoSe powder. Focusing on the MoSSe nanosheet, our WSe/MoSe powder is a three-dimensional chemical bonding system. Compared to the nanosheet, a three-dimensional system would possess more interface chemical bonding between WSe and MoSe, and that would greatly enhance the total amount of structural distortion. This could have led to large coercivity fields in our WSe/MoSe powder.
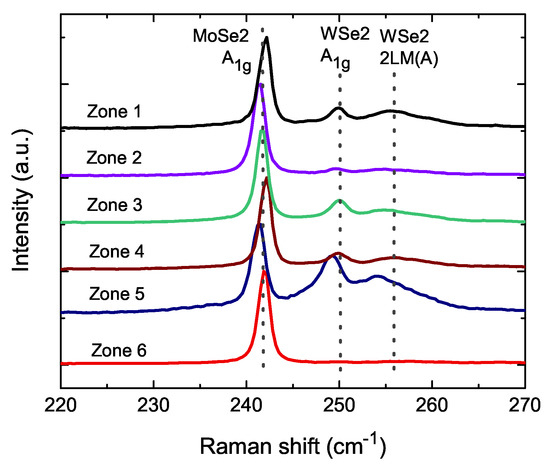
Figure 4.
The Raman shifts of two WSe/MoSe powders in different zones. We exhibit the standard peaks of WSe and MoSe. Red shifts of WSe peaks are shown in zone 5.
To further identify the source of the observed ferromagnetism, another mixed WSe and MoSe powder from the same raw materials was prepared. The sample was a mixture of WSe and MoSe flakes containing only one material within each flake. Figure 5a shows these MoSe and MoSe were individually distributed with no geometric connection between two materials. Figure 5b shows a diamagnetic feature with the backward and forward magnetic field strength from −3000–0 Oe and 0–3000 Oe, respectively. The backward and forward sweeping completely overlap and no hysteresis loops were detected—indicating the absence of ferromagnetism in the sample. Figure 5c shows that only individual peaks of 242 cm for MoSe, and 250 cm and 256 cm for WSe were observed in the Raman spectra. No mixed peaks or red-shifted peaks were observed. This shows that the individual MoSe or WSe in our source material would not lead to the ferromagnetism. This supports the sample having no ferromagnetism due to the lack of chemical bonding between WSe and MoSe blocks, so the ferromagnetism might originate from the structural distortion at the interface between WSe and MoSe.
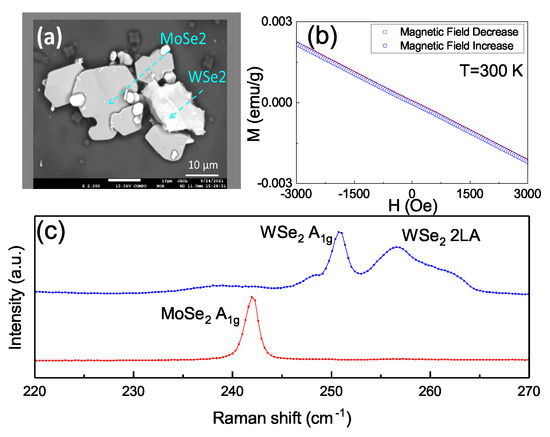
Figure 5.
(a) The SEM image in the backscattering emission imaging mode. The light area is the WSe and the dark area is the MoSe. The sample was a mixture of WSe and MoSe flakes containing only one material within each flake. (b) The M–H curve shows the diamagnetism feature, and no hysteresis loops were detected. (c) The 242 cm for MoSe, and 250 cm and 256 cm for WSe, are shown in the Raman spectra. No mixed peaks or red-shifted peaks were observed.
4. Conclusions
An investigation of the magnetism of WSe/MoSe powder was performed. The coercivity field reaches 2600 Oe at 5 K, 4233 Oe at 100 K and 1300 Oe at 300 K. These are the largest values reported for two-dimensional transition metal dichalcogenides, distinguishing them from the widely reported vacancy and zigzag structure-induced ferromagnetism values. A Raman peak red shift was observed, which supports the chemical bonding at the interface of WSe and MoSe. The large coercivity field originates from the chemical bonding-induced structural distortion at the interface between WSe and MoSe.
Author Contributions
Conceptualization, methodology, formal data analysis and manuscript preparation, S.-M.H.; experiment operation, P.-C.C. and P.-C.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the Ministry of Science and Technology, Taiwan through grant numbers 109-2112-M-110-018 and 110-2112-M-110-021; and Center of Crystal Research at National Sun Yat-sen University. Service plan of core-facility center at NSYSU through MOST110-2731-M-110-001, MOST108-2731-M-110-001 and MOST107-2731-M-110-001- and MOST106-2731-M-110-001-.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sethulakshmi, N.; Mishra, A.; Ajayan, P.M.; Kawazoe, Y.; Roy, A.K.; Singh, A.K.; Tiwary, C.S. Magnetism in two-dimensional materials beyond graphene. Mater. Today 2019, 27, 107–122. [Google Scholar] [CrossRef]
- Hernando, A.; Crespo, P.; Garcia, M.A. Origin of Orbital Ferromagnetism and Giant Magnetic Anisotropy at the Nanoscale. Phys. Rev. Lett. 2006, 96, 057206. [Google Scholar] [CrossRef] [Green Version]
- Garcia, M.A.; Merino, J.M.; Fernandez Pinel, E.; Quesada, A.; de la Venta, J.; Ruiz Gonzalez, M.L.; Castro, G.R.; Crespo, P.; Llopis, J.; Gonzalez-Calbet, J.M. Magnetic Properties of ZnO Nanoparticles. Nano. Lett. 2007, 7, 1489–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef]
- Ahmed, S.; Ding, X.; Murmu, P.P.; Bao, N.N.; Liu, R.; Kennedy, J.; Ding, J.; Yia, J.B. Magnetic properties of Co doped WSe2 by implantation. J. Alloys Compd. 2018, 731, 25. [Google Scholar] [CrossRef]
- Li, H.; Lu, G.; Wang, Y.; Yin, Z.; Cong, C.; He, Q.; Wang, L.; Ding, F.; Yu, T.; Zhang, H. Mechanical Exfoliation and Characterization of Single- and Few-Layer Nanosheets of WSe2, TaS2, and TaSe2. Small 2013, 9, 1974. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Meng, F.; Zhao, S.; Song, Y.; Yu, J.; Wang, X.; Liu, Z.; Wang, Y.; Li, B.; Wang, Y.; et al. Experimental and theoretical evidence for the ferromagnetic edge in WSe2 nanosheets. Nanoscale 2017, 9, 4898–4906. [Google Scholar] [CrossRef] [PubMed]
- Matte, H.S.S.R.; Maitra, U.; Kumar, P.; Govinda Rao, B.; Pramoda, K.; Rao, C.N.R. Synthesis, Characterization, and Properties of Few-layer Metal Dichalcogenides and their Nanocomposites with Noble Metal Particles, Polyaniline, and Reduced Graphene Oxide. Z. Anorg. Allg. Chem. 2012, 638, 2617–2624. [Google Scholar] [CrossRef]
- Huo, N.; Li, Y.; Kang, J.; Li, R.; Xia, Q.; Li, J. Edge-states ferromagnetism of WS2 nanosheets. Appl. Phys. Lett. 2014, 104, 202406. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, D.; Zhang, J.; Xu, Q.; Shi, S.; Tao, K.; Xue, D. Realization of high Curie temperature ferromagnetism in atomically thin MoS2 and WS2 nanosheets with uniform and flower-like morphology. Nanoscale 2015, 7, 650–658. [Google Scholar] [CrossRef]
- Mao, X.; Xu, Y.; Xue, Q.; Wang, W.; Gao, D. Ferromagnetism in exfoliated tungsten disulfide nanosheets. Nanoscale Res. Lett. 2013, 8, 430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, X.; Liu, T.; Ahmed, S.; Bao, N.; Ding, J.; Yi, J.B. Enhanced ferromagnetism in WS2 via defect engineering. J. Alloys Compd. 2019, 772, 740–744. [Google Scholar] [CrossRef]
- Joseph, A.; Kumar Tadi, K.; Anju, K.S.; Aneesh, P.M. Structural, optical, magnetic and electrochemical properties of hydrothermally synthesized WS2 nanoflakes. J. Mater. Res. 2021, 36, 884–895. [Google Scholar] [CrossRef]
- Xia, B.; Gao, D.; Liu, P.; Liu, Y.; Shia, S.; Taoa, K. Zigzag-edge related ferromagnetism in MoSe2 nanoflakes. Phys. Chem. Chem. Phys. 2015, 17, 32505–32510. [Google Scholar] [CrossRef] [Green Version]
- Xing, X.; Wang, X.; Wu, C.; Lu, Y.; Yan, M. Room temperature ferromagnetism and its origin for amorphous MoSe2 nanoflowers. Appl. Phys. Lett. 2018, 112, 122407. [Google Scholar] [CrossRef]
- Cai, L.; He, J.; Liu, Q.; Yao, T.; Chen, L.; Yan, W.; Hu, F.; Jiang, Y.; Zhao, Y.; Hu, T.; et al. Vacancy-Induced Ferromagnetism of MoS2 Nanosheets. J. Am. Chem. Soc. 2015, 137, 2622–2627. [Google Scholar] [CrossRef]
- Sanikop, R.; Sudakar, C. Tailoring Magnetically Active Defect Sites in MoS2 Nanosheets for Spintronics Applications. ACS Appl. Nano Mater. 2020, 3, 576–587. [Google Scholar] [CrossRef]
- Mathew, S.; Gopinadhan, K.; Chan, T.K.; Yu, X.J.; Zhan, D.; Cao, L.; Rusydi, A.; Breese, M.B.H.; Dhar, S.; Shen, Z.X.; et al. Magnetism in MoS2 induced by proton irradiation. Appl. Phys. Lett. 2012, 101, 102103. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Zhang, L.; Xiang, G. Web buckle-mediated room-temperature ferromagnetism in strained MoS2 thin films. Appl. Phys. Lett. 2020, 116, 012401. [Google Scholar] [CrossRef]
- Ahmed, S.; Viboon, P.; Ding, X.; Bao, N.N.; Du, Y.H.; Herng, T.S.; Ding, J.; Yi, J.B. Annealing effect on the ferromagnetism of MoS2 nanoparticles. J. Alloys Compd. 2018, 746, 399–404. [Google Scholar] [CrossRef]
- Tongay, S.; Varnoosfaderani, S.S.; Appleton, B.R.; Wu, J.; Hebard, A.F. Magnetic properties of MoS2: Existence of ferromagnetism. Appl. Phys. Lett. 2012, 101, 123105. [Google Scholar] [CrossRef]
- Zhou, Q.; Su, S.; Cheng, P.; Hu, X.; Zeng, M.; Gao, X.; Zhang, Z.; Liu, J.M. Robust ferromagnetism in zigzag-edge rich MoS2 pyramids. Nanoscale 2018, 10, 11578–11584. [Google Scholar] [CrossRef]
- Hu, W.; Tan, H.; Duan, H.; Li, G.; Li, N.; Ji, Q.; Lu, Y.; Wang, Y.; Sun, Z.; Hu, F.; et al. Synergetic Effect of Substitutional Dopants and Sulfur Vacancy in Modulating the Ferromagnetism of MoS2 Nanosheets. ACS Appl. Mater. Interfaces 2019, 11, 31155–31161. [Google Scholar] [CrossRef]
- Han, S.W.; Park, Y.; Hwang, Y.H.; Jekal, S.; Kang, M.; Lee, W.G.; Yang, W.; Lee, G.-D.; Hong, S.C. Electron beam-formed ferromagnetic defects on MoS2 surface along 1T phase transition. Sci. Rep. 2016, 6, 38730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Li, Y.; Qi, J.; Gao, D. Ferromagnetism in ultrathin MoS2 nanosheets: From amorphous to crystalline. Nanoscale Res. Lett. 2014, 9, 586. [Google Scholar] [CrossRef] [Green Version]
- Qi, R.; Wang, S.; Wang, M.; Liu, W.; Yan, Z.; Bi, X.; Huang, Q. Towards well-defined MoS2 nanoribbons on a large scale. Chem. Commun. 2017, 53, 9757–9760. [Google Scholar] [CrossRef]
- Kubart, T.; Polcar, T.; Kopecký, L.; Novak, R.; Novakova, D. Temperature dependence of tribological properties of and MoSe2 coatings. Surf. Coat. Technol. 2005, 193, 230–233. [Google Scholar] [CrossRef]
- Dominguez-Meister, S.; Cristina Rojas, T.; Brizuela, M.; Carlos Sanchez-Lopez, J. Solid lubricant behavior of MoS2 and WSe2-based nanocomposite coatings. Sci. Technol. Adv. Mater. 2017, 18, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Habib, M.; Muhammad, Z.; Khan, R.; Wu, C.; Rehman, Z.; Zhou, Y.; Liu, H.; Song, L. Ferromagnetism in CVT Grown Tungsten Diselenide Single Crystals with Nickel Doping. Nanotechnology 2018, 29, 115701. [Google Scholar] [CrossRef]
- Martinez, L.M.; Delgado, J.A.; Saiz, C.L.; Cosio, A.; Wu, Y.; Villagran, D.; Gandha, K.; Karthik, C.; Nlebedim, I.C.; Singamaneni, S.R. Magnetic and electrocatalytic properties of transition metal doped MoS2 nanocrystals. J. Appl. Phys. 2018, 124, 153903. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Sun, F.; Yang, S.; Li, Y.; Zhao, C.; Xu, M.; Zhang, Y.; Zeng, H. Robust ferromagnetism in Mn-doped MoS2 nanostructures. Appl. Phys. Lett. 2016, 109, 092401. [Google Scholar] [CrossRef]
- Ahmed, S.; Ding, X.; Bao, N.; Bian, P.; Zheng, R.; Wang, Y.; Murmu, P.; Kennedy, J.; Liu, R.; Fan, H.; et al. Inducing High Coercivity in MoS2 Nanosheets by Transition Element Doping. Chem. Mater. 2017, 29, 9066–9074. [Google Scholar] [CrossRef]
- Zhang, Q.; Ren, Z.; Wu, N.; Wang, W.; Gao, Y.; Zhang, Q.; Shi, J.; Zhuang, L.; Sun, X.; Fu, L. Nitrogen-doping induces tunable magnetism in ReS2. NPJ 2D Mater. Appl. 2018, 2, 22. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Xing, T.; Zhong, M.; Huang, L.; Lei, N.; Zhang, J.; Li, J.; Wei, Z. A two-dimensional Fe-doped SnS2 magnetic semiconductor. Nat. Commun. 2017, 8, 1985. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ding, X.; Murmu, P.P.; Bao, N.; Liu, R.; Kennedy, J.; Wang, L.; Ding, J.; Wu, T.; Vinu, A.; et al. High Coercivity and Magnetization in WSe2 by Codoping Co and Nb. Small 2019, 16, 1903173. [Google Scholar] [CrossRef]
- Xia, B.; An, L.; Gao, D.; Shi, S.; Xib, P.; Xue, D. Hierarchical ultrathin Mo(SxSe1-x)2 nanosheets with tunable ferromagnetism and efficient hydrogen evolution reaction activity: Towards defect site effect. CrystEngComm 2015, 17, 6240–6425. [Google Scholar] [CrossRef]
- Lin, B.-C.; Ye, X.G.; Wang, N.; Zhang, C.X.; Deng, H.X.; Fang, J.Z.; Cui, H.N.; Wang, S.; Liu, J.; Wei, Z.; et al. Spontaneous ferromagnetism and magnetoresistance hysteresis in Ge1-xSnx alloys. Sci. Bull. 2021, 66, 1375. [Google Scholar] [CrossRef]
- JCPDS cards No. 87-2419 for MoSe2, and JCPDS cards No. 87-2418 for WSe2.
- Sun, Y.; Fujisawa, K.; Lin, Z.; Lei, Y.; Mondschein, J.S.; Terrones, M.; Schaak, R.E. Low-Temperature Solution Synthesis of Transition Metal Dichalcogenide Alloys with Tunable Optical Properties. J. Am. Chem. Soc. 2017, 139, 11096–11105. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).