Abstract
Nowadays, Mn-doping is considered as a promising dissolution for the heavy usage of toxic lead in CsPbX3 perovskite material. Interestingly, Mn-doping also introduces an additional photoluminescence band, which is favorable to enrich the emission gamut of this cesium lead halide. Here, a solution spraying strategy was employed for the direct preparation of CsPbxMn1−x(Br,Cl)3 film through MnCl2 doping in host CsPbBr3 material. The possible fabrication mechanism of the provided approach and the dependences of material properties on Mn-doping were investigated in detail. As the results shown, Pb was partially substituted by Mn as expected. With the ratio of PbBr2:MnCl2 increasing from 3:0 to 1:1, the obtained film separately featured green, cyan, orange-red and pink-red emission, which was caused by the energy transferring process. Moreover, the combining energy of Cs, Pb, and Mn gradually red-shifted resulted from the formation of Cs-Cl, Pb-Cl and Mn-Br coordination bonding as MnCl2 doping increased. In addition, the weight of short decay lifetime of prepared samples increased with the doping rising, which indicated a better exciton emission and less defect-related transition. The aiming of current work is to provide a new possibility for the facile preparation of Mn-doping CsPbX3 film material.
1. Introduction
All-inorganic CsPbX3 (X=Cl, Br, or I) perovskite material has become a promising candidate for light emitting diodes (LEDs) [1], solar cells [2], lasers [3,4] and so forth [5,6] due to their superior optical properties [7], such as high color purity [8], high photo-luminescence quantum yield (PLQY) [5], excellent carrier characteristics [9] and tunable band gap [10]. Unfortunately, the toxicity of lead is a great challenge for the development of lead halide perovskite [11,12]. How to reduce or replace lead by less-toxic or non-toxic metals is one of the key issues for its commercial application. Currently, great efforts have been made to partially or completely replace Pb by doping divalent cations (such as Sn2+, Ge2+, Zn2+) as the core elements of CsPbX3 material [13]. However, the susceptible oxidation of Sn and Ge from bivalent state to tetravalent state exposed under ambient environment is risky for the material stability. Regarding Zn2+, the doping concentration in the host material is limited, which is unfavorable to further eliminate the toxic Pb use [14].
Interestingly, Mn-doped CsPbX3 material has new pleasant chemical and physical properties. It has become a promising candidate for lead-less perovskite material [15]. As suggested by previous reports [16], Mn2+ doping can improve the material stability, which derives from Mn-X bond having higher binding energy than Pb-X bond. The most interesting result after partial substitutions of Mn for Pb element is that there is an additional photoluminescence (PL) band in the orange region compared with the pure CsPbX3 material [17,18]. Commonly, Mn-doped CsPbX3 can be fabricated by halide exchange driven cation exchange strategy [19,20]. Sheldon and co-workers reported the first attempt at Mn-doping based on host material of CsPbCl3 by hot injection method [17]. Later, Zhu et al. found that Mn also can doped into CsPbBr3 structure via MnCl2 to form mixed halide perovskites in 2017 [21]. These mixed halide perovskites can support a much higher Mn doping ratio while retaining the original perovskite emission [15,16,22]. Furthermore, Cl-to-Br anion exchange was adopted to modify the compositions of Mn-doped perovskite material, leading to multi-color luminescence [22]. It is known that the methods of hot injection [23], thermal solvent [24], and anti-solvent [20] are prevalent for the fabrication of Mn-doping CsPbX3 material. However, these approaches are limited by the shortcomings of low yields, complex procedures, and toxic chemicals. In addition, the obtained product prepared by the above methods is in a powder-state rather than in film-state. It is well known that the solution spraying method is widely used for the multi-element material fabrication, such as ZnS [25], Cu2SnS3 [26], Cu2ZnSnS4 [27], etc., due to its prominent merits of low cost, simple operation, low time-consuming and scalable production. Yang et al. reported their work about the fabrication of CsPbBr3 thin film through a spraying method combined with raw CsPbBr3 nanocrystals (NCs) [28]. However, the direct deposition for this perovskite film by spraying strategy is rare to date, especially in CsPbxMn1−x(Br,Cl)3 film material.
Herein, the solution spraying strategy was employed for the direct preparation of CsPbxMn1−x(Br,Cl)3 film through MnCl2 doping in host CsPbBr3 material for the first time. The soft polyethylene terephthalate (PET) material was used as the substrate. It is well known that the flexible substrate features distinguished plasticity and tailorability, which is favorable to widen the application of prepared CsPbxMn1−x(Br,Cl)3 material such as curved displaying or wearable devices [29]. In the current work, the influences of MnCl2 doping on the material properties including photoluminescence, structure, morphology, chemical composition, carrier decay lifetime, etc., were investigated in detail. Moreover, the fabrication mechanism of the proposed spraying approach was also analyzed. This work aims to provide a new possibility for the facile preparation of Mn-doping CsPbX3 film material.
2. Experimental Details
2.1. Preparation of CsPbBr3 and CsPbxMn1−x(Br,Cl)3 Thin Films
Typically, the precursors were prepared by 0.2 mmol CsBr (cesium bromide, 99%, AR), 0.2 mmol PbBr2 (lead bromide, 99%, AR), 40 ul EAC (ethyl acetate, 90%, AR), 40 ul OLA (oleylamine, 70%, AR) and 10 mL DMSO (Dimethyl sulfoxide, 90%) were successively added into the beaker with stirring until reactants were dissolved sufficiently. Then, the transparent solution was poured into spraying devices, and sprayed onto flexible PET substrate at 110 ℃ within 60 s to obtain the expected sample. Concerning to the fabrication of CsPbxMn1−x(Br,Cl)3 films, MnCl2 was introduced to partially substitute PbBr2. For MnCl2 (manganese chloride, 99%, AR) doping, the ratio of PbBr2 and MnCl2 was set as 3:1, 3:2 and 1:1, and other experimental conditions were the same as the preparation of CsPbBr3 film. All operations are carried out in the atmosphere. In addition, CsBr and DMSO were provided by Shanghai Titan Scientific Co., Ltd. (Shanghai, China). PbBr2, EAC, OLA and MnCl2 were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China).
2.2. Characterizations for the As-Obtained Samples
The film’s surface morphology was measured by scanning electron microscopy (SEM, Quanta 200, FEI, Eindhoven, The Netherlands). The crystalline structure was characterized by X-ray diffraction (XRD, Ultima IV, Rigaku, Tokyo, Japan). An Ultraviolet-Visible-Infrared spectrophotometer (UV-vis-IR, UH 4150, Hitachi, Tokyo, Japan) was used to record the absorption spectra of the prepared films. The PL properties were measured by fluorescence spectrophotometer (FS5, Edinburgh Instruments, Edinburgh, UK). Energy dispersive X-ray spectroscopy (EDS, Genesis Apollo X, Pittsburgh, PA, USA) and X-ray photoelectron spectroscopy (XPS, Axis Ultra DLD, Kratos, Manchester, UK) were employed for the study of chemical constitution.
3. Results and Discussion
In this work, the preparation diagram was described in Figure 1a. For CsPbBr3 fabrication, all reactants were sufficiently dissolved in DMSO solvent to prepare the precursor solution. In this solution, the mental ions Cs+, Pb2+ dispersed homogeneously and freely. Halide ions Br− were released from mental halides of CsBr and PbBr2, then acted with OLA to form Br-OLA complexes. During the spraying process, the DMSO solvent was evaporated by the heating treatment. Br− anions were separated from Br-OLA complexes and combined with Cs+, Pb2+ cations to form the expected CsPbBr3 products. Meanwhile, these CsPbBr3 products may be encapsulated and passivated by OLA molecules as suggested by previous works [30]. For CsPbxMn1−xX3 film fabrication, Mn2+ replaced part of Pb2+ (as shown in Figure 1b) and Cl− exchanged with part Br− after MnCl2 adding in the precursor solution. Here, the partial substitution of Pb2+ by Mn2+ was driven by the halide exchange process of Br-to-Cl. During the Br-to-Cl exchange, the rigid octahedron structure of PbBr64− would be opened up, and then the Mn2+ would be incorporated into this structure to replace Pb2+. The above halide exchange driven cation exchange process has also been demonstrated by early studies [31].
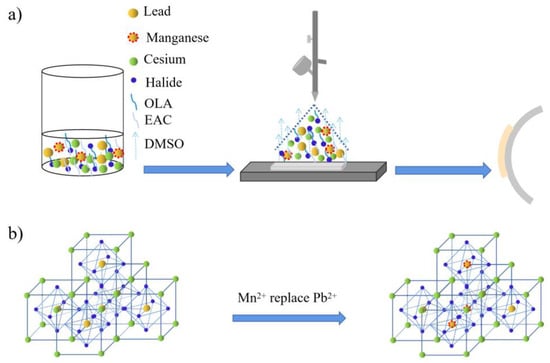
Figure 1.
(a) The schematic diagram of solution spraying process; (b) Mn2+ replaced part of Pb2+.
The PL spectra of CsPbBr3 and CsPbxMn1−x(Br,Cl)3 films were shown in Figure 2a. For CsPbBr3 film, an emission band centered at 515 nm is observed, while the peaks at 450/470 nm could be attributed to EAC. When doped with MnCl2, two peaks at around 509/616 nm were observed. It was found that the main peak of 515 nm in pure CsPbBr3 film gradually blue-shifted to 505 nm with the ratio of PbBr2:MnCl2 increasing from 3:1 to 1:1. This may be caused by the band gap varying, which resulted from the increasing Br-to-Cl exchanging after MnCl2 incorporation [22,32]. The peak locating at 616 nm was derived from the energy transferring of 4T1→6A1 caused by Mn substitution for Pb in CsPbxMn1−x(Br,Cl)3 material. In CsPbxMn1−x(Br,Cl)3, after band to band optical excitation, the energy is transferred from the main material to the Mn2+ excited state [33]. In addition, the Mn2+ emission slightly red-shifted from 616 nm to 619 nm as the Mn ratio increased. This may be caused by the lattice contraction induced modification of Mn2+ ligand-field and the Mn2+ reabsorption/emission effect as suggested [22]. As observed, the PLQY values of the samples, prepared with different PbBr2: MnCl2 ratios of 3:0, 3:1, 3:2, 1:1, were 15.68%, 1.26%, 3.85% and 3.09%, respectively. It was found that the PLQY of MnCl2 doped samples displayed a downward trend when the PbBr2: MnCl2 ratio was over 3:2. This may be caused by a quenching effect due to excessive Mn. Figure 2b depicted the Commission Internationale de I’Eclairage (CIE) color space coordinates of CsPbBr3 and CsPbxMn1−x(Br,Cl)3 films. For the CsPbBr3 sample, the CIE was (0.2329, 0.5913) and showed green light under 365 nm UV excitation. For the CsPbxMn1−x(Br,Cl)3 sample, the corresponding CIE of different Pb/Mn ratios (3:1, 3:2, 1:1) were (0.2891, 0.3657), (0.3853, 0.3625), and (0.4294, 0.3757), respectively, and the as-prepared samples featured cyan, orange-red, pink-red separately. The cyan color for the sample fabricated under 3:1 Pb/Mn ratio may occur because the emission of main material was dominant while the energy transferring was in a low degree. With the increasing Mn content, the energy transferring was accelerated. This was well suggested by the color changing and the variation of normalized Mn2+ emission intensity. Additionally, the color purity was 53.8%, 20.1%, 24.4% and 41.6%, while the color temperature was 7344, 7839, 3749 and 2889 K, for 3:0, 3:1, 3:2 and 1:1 Pb/Mn ratio, respectively.
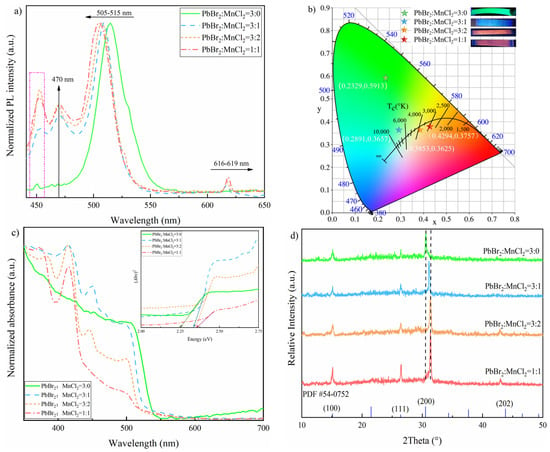
Figure 2.
Optical properties of as-obtained samples prepared by different PbBr2/MnCl2 ratios: (a) PL spectra; (b) CIE diagram; (c) absorption spectra (inset plots of (Ahv)2 vs. (hv)); and (d) XRD pattern.
Furthermore, the absorbance spectra of as-obtained samples were determined by UV-vis spectrophotometer and displayed in Figure 2c. As observed, all samples had absorption edges around 510 nm, which was attributed to main materials CsPb(Br/Cl)3. Additionally, this edge bule-shifted gradually, with the increasing Cl−. The bandgap energy Eg of these samples were calculated by extrapolating the straight line of (Ahv)2 vs. (hv) plots displayed in the inset of Figure 2c. Here, A is absorbance, h is Planck’s constant, v is frequency. As observed, the Eg values for Mn-doped CsPbBr3 films (with the ratio of PbBr2:MnCl2 3:0, 3:1, 3:2, 1:1) were 2.25, 2.33, 2.34 and 2.35 eV, separately. The crystal structure of prepared films was determined by XRD treatment as shown in Figure 2d. Regarding to the undoped sample, there were three peaks, locating around at 15.02°, 26.39°, and 30.56°, could be attributed to the (100), (111), and (200) planes of cubic CsPbBr3 (JCPDS 54-0752). This indicated the undoped sample was in pure perovskite phase. It was found that the diffraction peak of (202) plane gradually appeared at around 42.80° with MnCl2 doping increasing. Interestingly, the diffraction peak of (200) plane gradually shifted toward to higher angles as MnCl2 doping increased. As suggested by early studies [22], this was caused by the lattice contraction after the smaller Mn2+ (0.97 Å) and Cl− incorporation into the lattice to separately replace Pb2+ (1.33 Å) and Br−. This is also confirmed by the changing of crystal size of prepared films. As calculated based on (200) plane by Scherrer formula, the crystal sizes corresponding to the different ratios of PbBr2:MnCl2 (3:0, 3:1, 3:2, 1:1) were 22.03, 21.31, 20.86, and 19.96 nm, respectively, which presented a downward trend.
The surface morphologies of the samples prepared in 3:0, 3:1, 3:2 and 1:1 PbBr2:MnCl2 ratios were shown in Figure 3a–d, respectively. It was found that the undoped sample was in bulk-shape, and was dense and compact. This was benefitted from the Br-rich state as suggested by previous studies [34]. A few particles also appeared on the surface. As suggested by previous studies [30], the bulk-shaped or particle-shaped products were composed of agglomerated NCs by soft agglomeration of electrostatic force or Van der Waals force, especially in the presence of OLA. Interestingly, the dense and compact state of as-fabricated samples deteriorated as the MnCl2 ratio increased. More and more particles appeared as well as the pins. This was because Br was partially replaced by Cl to change the Br-rich state after MnCl2 doping. Thus, the morphology featured a deterioration trend with more Cl incorporating into the prepared film samples. Furthermore, the EDS mappings were also described in Figure 3a–d to determine the chemical composition of as-obtained samples prepared with different PbBr2:MnCl2 ratios. As observed, all elements including Cs, Pb/Mn, Br/Cl dispersed homogeneously on the sample surfaces. Concerning the Mn atom percent in prepared samples, the value increased from 0 to 10.73% with the Pb/Mn ratio varying from 3:0 to 1:1. The actual Pb/Mn ratios were 3:0, 2.8:1, 3.1:2 and 0.93:1, which were close to the expected values of 3:0, 3:1, 3:2 and 1:1. This indicated Pb was successfully substituted by Mn in host material as expected. In addition, the Cs:(Pb+Mn):(Cl+Br) ratio of fabricated samples was close to the stoichiometric value 1:1:3.
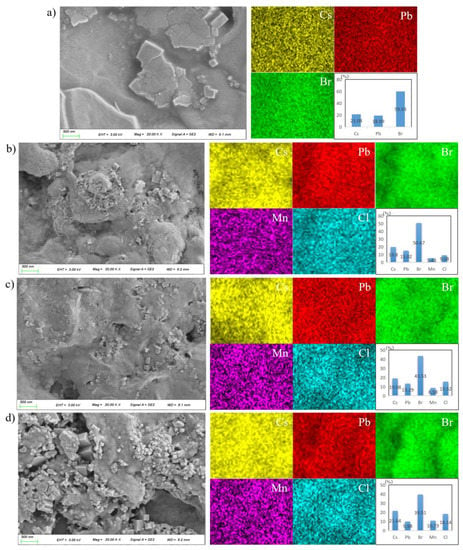
Figure 3.
Morphologies and EDS mappings of the samples prepared by different PbBr2/MnCl2 ratios: (a) 3:0; (b) 3:1; (c) 3:2; (d) 1:1.
XPS survey was employed to further investigate the composition of as-prepared samples. As observed in Figure 4a, the orbital peaks of Mn 2p and Cl 2p appeared as expected in MnCl2 dopped sample comparing to the pure CsPbBr3 film. Cs, Pb and Br all had two split peaks locating at around 737.78 and 723.81 eV, 142.34 and 137.45 eV, and 68.45 and 67.56 eV, which were assigned to Cs 3d3/2 and Cs 3d5/2, Pb 4f5/2 and Pb 4f7/2, and Br 3d3/2 and Br 3d5/2 electronic levels, respectively, as displayed in Figure 4b–d [35]. Note that these peaks all slightly shifted to high binding energy as MnCl2 increased, especially for Pb 4f. This may be caused by the coordination bonding of Cs-Cl, Pb-Cl and Mn-Br on the surface of host material. This was favorable for the stability improvement of Cs 3d, Pb 4f and Br 3d. In addition, the obvious red-shifting of Pb 4f may also be a result from the changing of chemical environment and electron density after Mn doping as suggested by previous work [32,36]. Figure 4e showed two characteristic peaks located at 641.63 eV and 650.31 eV corresponding to Mn 2p3/2 and Mn 2p1/2, respectively. This indicated Mn was in divalent state, and also suggested Pb was successfully substituted by Mn as expected [35]. Similar to Cs and Pb, the binding energy of Mn 2p1/2 also slightly varied to a higher value as MnCl2 doping increased. For Cl, there were two peaks that appeared at 197.25 and 198.95 eV, as observed in Figure 4f.
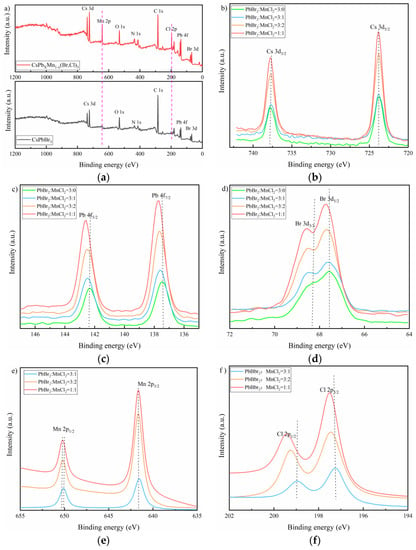
Figure 4.
(a) XPS survey spectra of Mn-doped and undoped samples; the combining energy of different elements: (b) Cs 3d, (c) Pb 4f, (d) Br 3d, (e) Mn 2p, and (f) Cl 2p.
Figure 5 displayed the normalized time-resolved PL decay curves of as-prepared films. The curves were fitted by a bi-exponential function as shown in Equation (1):
where, τ1, τ2 are short-lived and long-lived lifetime originating from exciton radiative recombination and the trap-assisted nonradiative recombination, respectively. A1 and A2 are the corresponding occupied percent of τ1 and τ2, respectively. A0 is a constant, the average lifetime τave is calculated by Equation (2). As observed, the values of τ1, τ2 and τave all featured a downward trend as MnCl2 doping increased. It is worth mentioning that the value of τ1 decreased from 1.94 ns to 1.14 ns, which may be derived from the bandgap increasing caused by Br-to-Cl anion exchange [22]. Moreover, the occupied percent of τ1 was increased from 35.70% to 49.00% which meant a higher ratio of radiative to nonradiative recombination. Above indicated a better exciton emission and less defect-related transition, as suggested by previous studies [30].
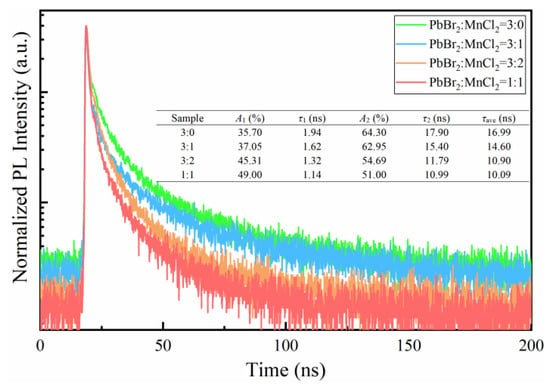
Figure 5.
Time-resolved PL decay curves of the samples prepared by different PbBr2/MnCl2 ratios.
4. Conclusions
In conclusion, a CsPbxMn1−x(Br,Cl)3 film was successfully prepared by facile solution spraying approach with flexible PET substrate through MnCl2 doping in host CsPbBr3 material. As the results showed, the green emission in CsPbBr3 film gradually transferred to be cyan, orange-red, and pink-red as MnCl2 increased, which was derived from the energy transferring between host material and Mn2+ excited state. It was found that the dense and compact film was varied to be a coexisting state of bulk and nanoparticles products with the MnCl2 increasing in doped material. The EDS mapping displayed that all chemical dispersed uniformly on the film surface, and Pb was successfully substituted by Mn as expected, which was further confirmed by XPS investigation. Moreover, with MnCl2 doping rising, the combining energy of Cs, Pb, and Mn featured a red-shifting variation which may be caused by the formation of Cs-Cl, Pb-Cl and Mn-Br coordination bonding. Time-resolved PL decay study indicated the weight of short-lifetime presented an upward trend as doping increased, which suggested a better exciton emission and less defect-related transition.
Author Contributions
Conceptualization, Y.S. and J.C.; methodology, Y.S., J.C. and C.Z.; formal analysis, J.C., Y.S. and J.K.; investigation, Y.S., Y.Y., X.P. and R.H.; writing—original draft preparation, Y.S.; writing—review and editing, Y.S., J.C. and F.W.; supervision, F.W.; project administration, J.C. and J.W.; funding acquisition, J.C., Y.J. and J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
The project was sponsored by Shanghai Sailing Program, China (No.18YF1422500, No. 20YF1447600), and Collaborative innovation project of Shanghai Institute of Technology (No. XTCX2020-12).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gualdron-Reyes, A.F.; Yoon, S.J.; Barea, E.M.; Agouram, S.; Munoz-Sanjose, V.; Melendez, A.M.; Nino-Gomez, M.E.; Mora-Sero, I. Controlling the phase segregation in mixed halide perovskites through nanocrystal size. ACS Energy Lett. 2019, 4, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Selopal, G.S.; Zhao, H.G.; Wang, Z.M.; Rosei, F. Core/Shell Quantum Dots Solar Cells. Adv. Funct. Mater. 2020, 30, 1908762. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Lim, S.S.; Yantara, N.; Liu, X.; Sabba, D.; Grtzel, M.; Mhaisalkar, S.; Sum, T.C. Low-temperature solution-processed wavelength-tunable perovskites for lasing. Nat. Mater. 2014, 13, 476–480. [Google Scholar] [CrossRef]
- Park, Y.S.; Bae, W.K.; Baker, T.; Lim, J.; Klimov, V.I. Effect of auger recombination on lasing in heterostructured quantum dots with engineered core/shell interfaces. Nano Lett. 2015, 15, 7319–7328. [Google Scholar] [CrossRef]
- Andrés Fabian, G.R.; Jhonatan, R.P.; Eliseo, A.G.; Jorge, R.P.; Rogelio, O.; Sofia, M.; Joon, Y.; Juan, T.; Franklin, J.; Said, A.; et al. Unravelling the photocatalytic behavior of all-inorganic mixed halide perovskites: The role of surface chemical states. ACS Appl. Mater. Interfaces 2019, 12, 914–924. [Google Scholar]
- Suarez, I.; Juarez-Perez, E.J.; Bisquert, J.; Mora-Sero, I.; Martinez-Pastor, J.P. Polymer/perovskite amplifying waveguides for aactive hybrid silicon photonics. Adv. Mater. 2015, 27, 6157–6162. [Google Scholar] [CrossRef] [PubMed]
- Manav, R.K.; Smaranika, R.; Saikat, B. State of the art and prospects of metal halide perovskite core@shell nanocrystals and nanocomposites. Mater. Today Chem. 2021, 20, 100424. [Google Scholar]
- Abhishek, S.; Ramya, C.; Vikash, K.R.; Mir, I.; Arindam, C.; Angshuman, N. Colloidal CsPbBr3 perovskite nanocrystals: Luminescence beyond traditional quantum dots. Angew. Chem. 2015, 127, 15644–15648. [Google Scholar]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX(3), X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Wang, Z.; Xue, Y.; Ou, Q.; Polavarapu, L.; Zheng, J.; Qi, X.; Bao, Q. Synthesis, properties, and optical applications of low-dimensional perovskites. Chem. Commun. 2016, 52, 13637–13655. [Google Scholar] [CrossRef]
- Babayigit, A.; Ethirajan, A.; Muller, M.; Conings, B. Toxicity of organometal halide perovskite solar cells. Nat. Mater. 2016, 15, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Stam, W.V.D.; Geuchies, J.J.; Altantzis, T.; Bos, K.H.V.D.; Meeldijk, J.D.; Aert, S.V.; Bals, S.; Vanmaekelbergh, D. Highly emissive divalent-ion-doped colloidal CsPb1−xMxBr3 perovskite nanocrystals through cation exchange. J. Am. Chem. Soc. 2017, 139, 4087–4097. [Google Scholar] [CrossRef]
- Guo, Y.; Su, J.; Wang, L.; Lin, Z.; Hao, Y.; Chang, J. Improved doping and optoelectronic properties of Zn-doped CsPbBr3 perovskite through Mn codoping approach. J. Phys. Chem. Lett. 2021, 12, 3393–3400. [Google Scholar] [CrossRef]
- Liu, W.; Lin, Q.; Li, H.; Wu, K.; Klimov, V.I. Mn2+-doped lead halide perovskite nanocrystals with dual-color emission controlled by halide content. J. Am. Chem. Soc. 2016, 138, 14954–14961. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Liu, Y.; Li, J.; Liu, C.; Feng, R.; Jiang, F.; Li, Y.; Song, J.; Zeng, H.; Hong, M.; et al. Stabilizing cesium lead halide perovskite lattice through Mn (II)-substitution for air-stable light-emitting diodes. J. Am. Chem. Soc. 2017, 139, 11443–11450. [Google Scholar] [CrossRef] [PubMed]
- Parobek, D.; Roman, B.J.; Dong, Y.; Jin, H.; Lee, E.; Sheldon, M.; Son, D.H. Exciton-to-dopant energy transfer in Mn-doped cesium lead halide perovskite nanocrystals. Nano Lett. 2016, 16, 7376–7380. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, Q.A.; Meggiolaro, D.; Dang, Z.; Angelis, F.D.; Manna, L. Fluorescent alloy CsPbxMn1–xI3 perovskite nanocrystals with high structural and optical stability. ACS Energy Lett. 2017, 2, 2183–2186. [Google Scholar] [CrossRef]
- Szeremeta, J.; Antoniak, M.A.; Wawrzynczyk, D.; Nyk, M.; Samoc, M. The two-photon absorption cross-section studies of CsPbX3 (X = I, Br, Cl) nanocrystals. Nanomaterials 2020, 10, 1054. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, C.; Rao, L.; Lu, H.; Yan, C.; Cao, K.; Ding, X.; Yu, B.; Tang, Y. Synthesis of highly photoluminescent all-inorganic CsPbX3 nanocrystals via interfacial anion exchange reactions. Nanomaterials 2019, 9, 1296. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yang, X.; Zhu, Y.; Wang, Y.; Cai, J.; Shen, J.; Sun, L.; Li, C. Room temperature synthesis of Mn-doped cesium lead halide quantum dots with high Mn substitution ratio. J. Phys. Chem. Lett. 2017, 8, 4167–4171. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Fang, G.; Chen, X.; Lei, L.; Zhong, J.; Mao, Q.; Zhou, S.; Li, J. Mn-doped CsPbCl3 perovskite nanocrystals: Solvothermal synthesis, dual-color luminescence and improved stability. J. Mater. Chem. C 2018, 6, 8990–8998. [Google Scholar] [CrossRef]
- Mei, S.; Yang, B.; Wei, X.; Dai, H.; Chen, Z.; Cui, Z.; Zhang, G.; Xie, F.; Zhang, W.; Guo, R. Facile synthesis and optical properties of CsPbX3/ZIF-8 composites for wide-color-gamut display. Nanomaterials 2019, 9, 832. [Google Scholar] [CrossRef] [PubMed]
- Skorupska, E.; Jeziorna, A.; Potrzebowski, M.J. Thermal solvent-free method of loading of pharmaceutical cocrystals into the pores of silica particles: A case of naproxen/picolinamide cocrystal. J. Mater. Chem. C 2016, 120, 13169–13180. [Google Scholar] [CrossRef]
- Wang, Y.; Han, S.; Hao, L.; He, L.; Wei, G.; Wu, M.; Wang, H.; Liu, Y.; Chen, D. New type of neutron image scintillator based on H310BO3/ZnS(Ag). Phys. Procedia 2013, 43, 216–222. [Google Scholar] [CrossRef][Green Version]
- Hadi, Z.L.; Essa, M.S.; Chiad, B.T. Ternary Cu2SnS3 thin films deposited by fully controlled system of spray pyrolysis. J. Phys. Conf. Ser. 2019, 1234, 012041. [Google Scholar] [CrossRef]
- Wang, F.; Peng, X.; Sun, Y.; Chen, J.; Ju, J.; Jin, Y.; Zhang, C.; Kong, J.; Yang, J.; Chen, Q.; et al. Influences of rapid thermal annealing on Cu2ZnSnS4 thin film fabricated by spraying approach. J. Mater. Sci. Mater. Electron. 2021, 32, 7153–7161. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, M.; Li, J.; Dou, J.; Qiu, H.; Shao, J. Spray-coated CsPbBr3 quantum dot Films for perovskite photodiodes. ACS Appl. Mater. Interfaces 2018, 10, 26387–26395. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Zhu, L.; Zhai, W.; Berbille, A.; Li, L.; Wang, Z. Flexible piezoelectric nanogenerators based on P(VDF-TrFE)/CsPbBr3 quantum dot composite films. ACS Appl. Electron. Mater. 2021, 3, 2136–2144. [Google Scholar] [CrossRef]
- Wang, F.; Peng, X.; Wang, C.; Dong, H.; Sun, Y.; Kong, J.; Zhang, C.; Chen, J.; Li, L.; Xu, J. Synthesis of CsPbBr3 and CsPbBrXI(3−X) films by spray-coating technique. ECS J. Solid State SC 2020, 9, 126007. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, S.; Fang, G.; Chen, X.; Zhong, J. Fast room-temperature cation exchange synthesis of Mn-doped CsPbCl3 nanocrystals driven by dynamic halogen exchange. ACS Appl. Mater. Interfaces 2018, 10, 39872–39878. [Google Scholar] [CrossRef]
- Xu, K.; Vickers, E.T.; Luo, B.; Allen, A.C.; Chen, E.; Roseman, G.; Wang, Q.; Kliger, D.S.; Millhauser, G.L.; Yang, W.; et al. First synthesis of Mn-doped cesium lead bromide perovskite magic sized clusters at room temperature. J. Phys. Chem. Lett. 2020, 11, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, N.; Peng, X. Efficient and color-tunable Mn-doped ZnSe nanocrystal emitters: Control of optical performance via greener synthetic chemistry. J. Am. Chem. Soc. 2007, 129, 3339–3347. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, A.; Zhao, C.; Guo, Q.; Wang, Y.; Wang, F.; Bian, X.; Alsaedi, A.; Hayat, T.; Tan, Z. Efficient and stable pure green all-inorganic perovskite CsPbBr3 light-emitting diodes with a solution-processed NiOx interlayer. J. Phys. Chem. C 2017, 121, 28132–28138. [Google Scholar] [CrossRef]
- He, M.; Cheng, Y.; Shen, L.; Zhang, H.; Shen, C.; Xiang, W.; Liang, X. Doping manganese into CsPb(Cl/Br)3 quantum dots glasses: Dual-color emission and super thermal stability. J. Am. Ceram. Soc. 2019, 102, 1090–1100. [Google Scholar] [CrossRef]
- Liu, M.; Zhong, G.; Yin, Y.; Miao, J.; Li, K.; Wang, C.; Xu, X.; Shen, C.; Meng, H. Aluminum-doped cesium lead bromide perovskite nanocrystals with stable blue photoluminescence used for display backlight. Adv. Sci. 2017, 4, 1700335. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).