Nanomaterials: Synthesis and Applications in Theranostics
Abstract
1. Introduction
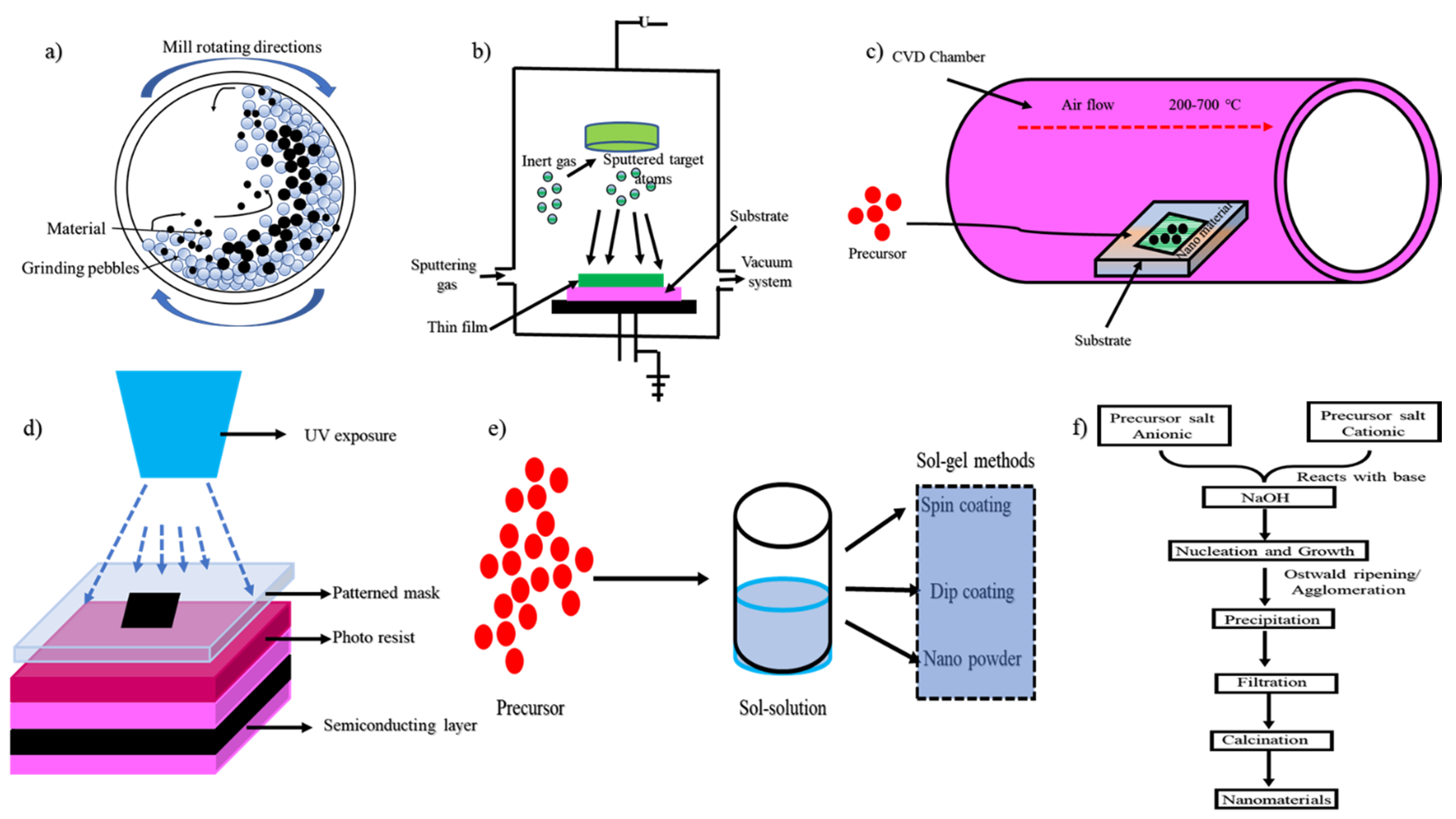
2. Synthesis of Nanomaterials
2.1. Ball Milling through the Mechanical Method
2.2. Physical Vapor Deposition (PVD) Method
2.3. Lithography
2.4. Sol-Gel Method
2.5. Chemical Vapor Deposition Method (CVD)
2.6. Chemical Co-Precipitation Method
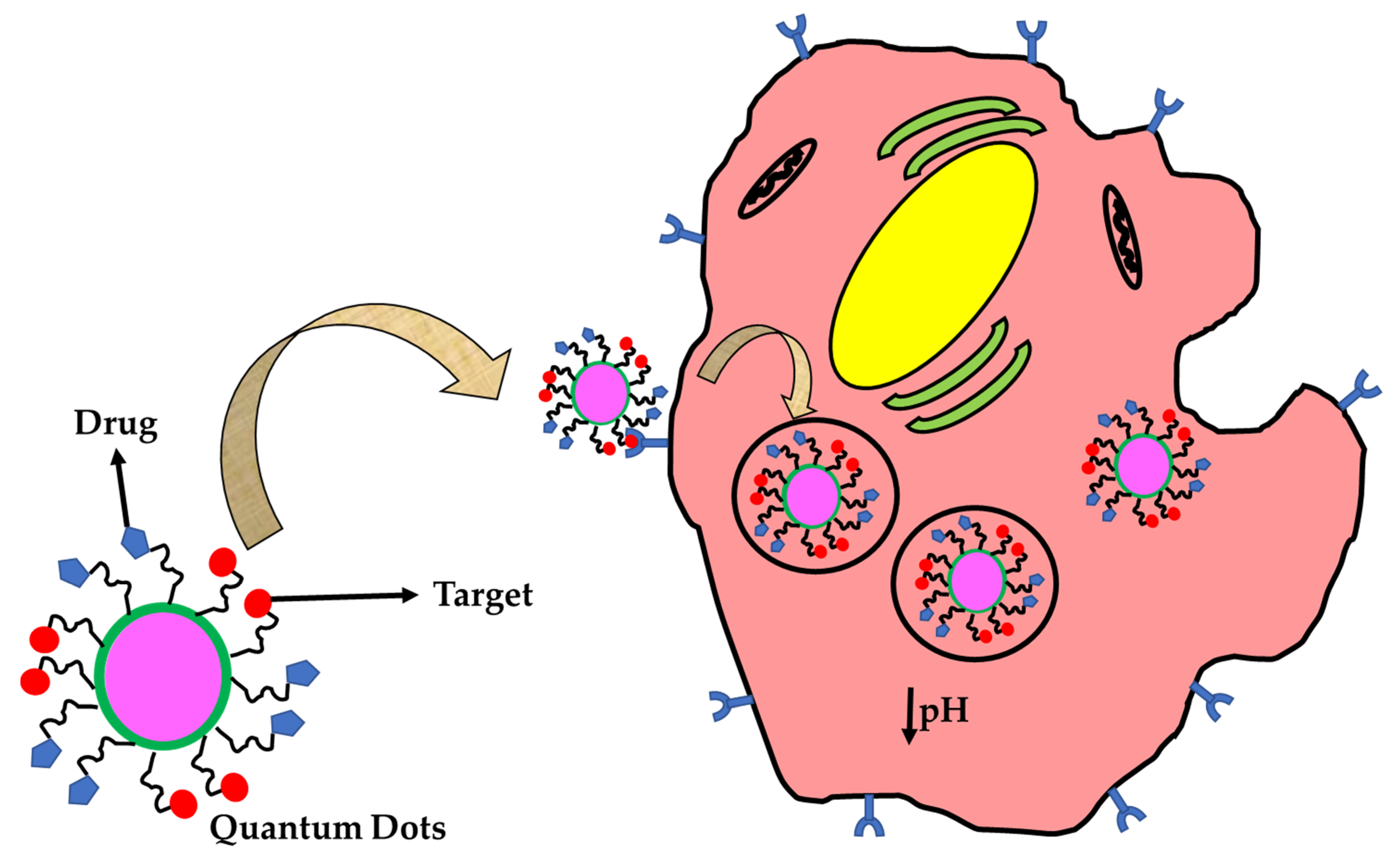
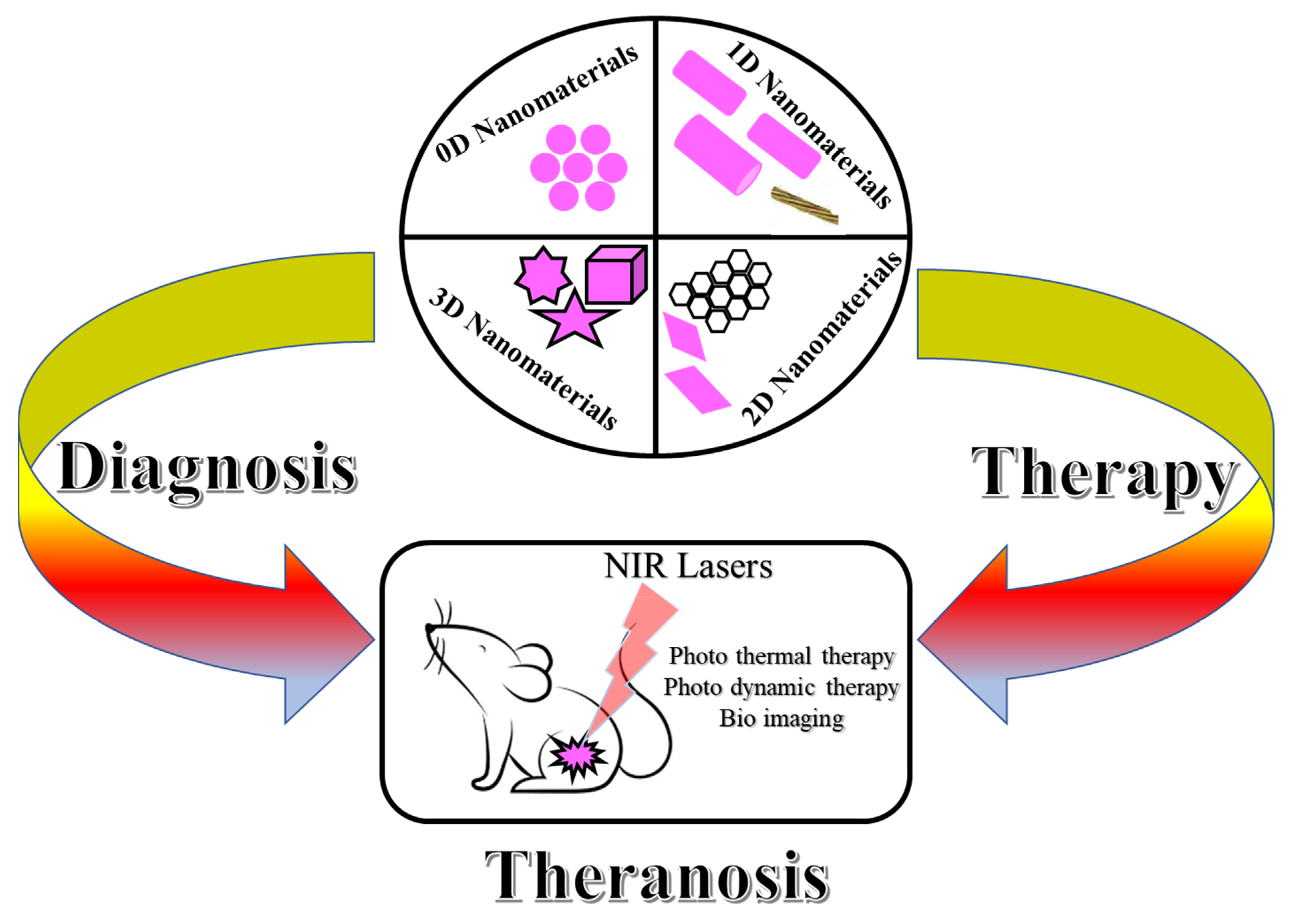
3. Zero Dimensional (0D) Nanomaterials in Theranostics
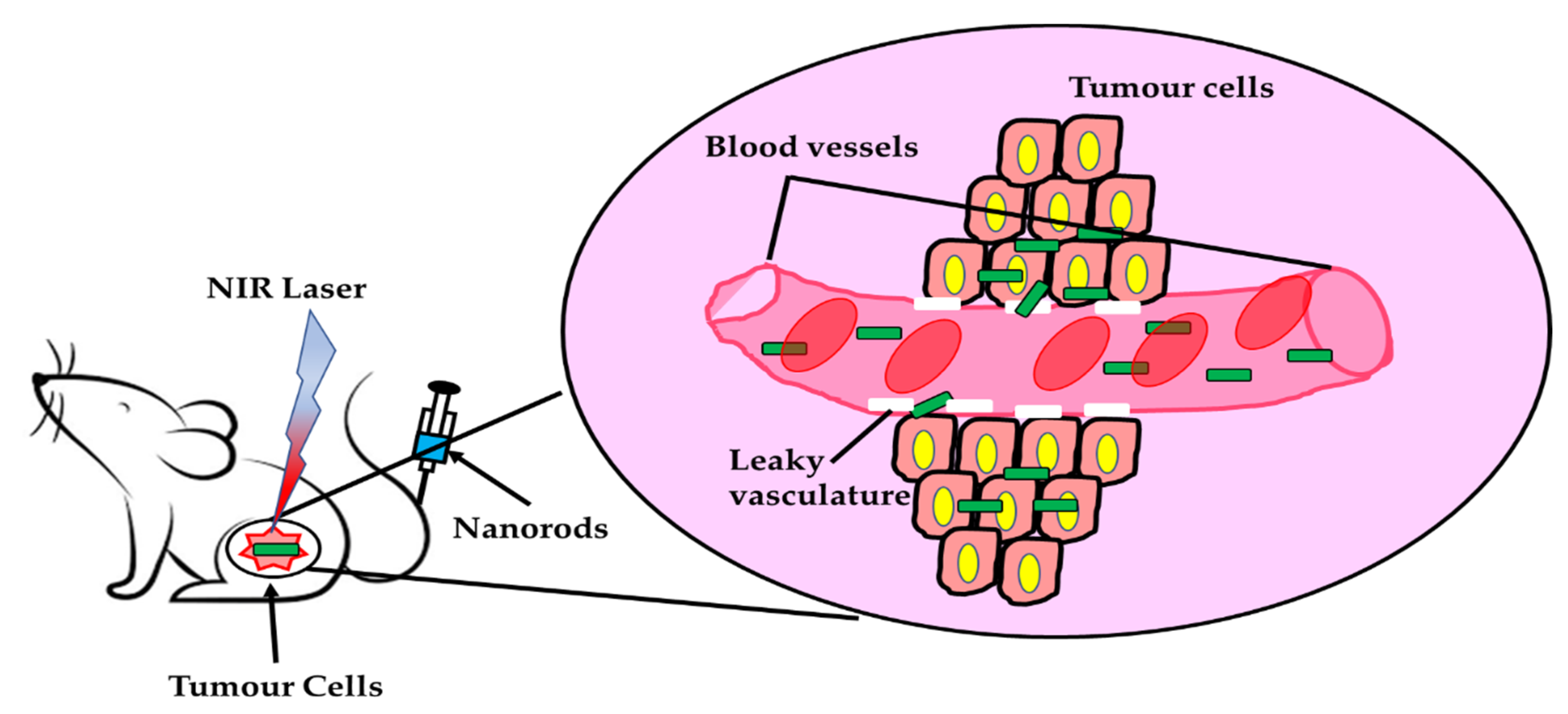
4. One Dimensional (1D) Nanomaterial in Theranosis
5. Two Dimensional (2D) Nanomaterials in Theranostics
6. Three Dimensional (3D) Nanomaterials in Theranostics
Characteristics of Theranostic Nanomaterials
7. The Recent Process on the Nanomaterials in Theranosis
8. Theranosis: Future Direction and Challenges
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jadvar, H.; Chen, X.; Cai, W.; Mahmood, U. Radiotheranostics in cancer diagnosis and management. Radiology 2018, 286, 388–400. [Google Scholar] [CrossRef]
- Funkhouser, J. Reinventing pharma: The theranostic revolution. Curr. Drug Discov. 2002, 2, 17–19. [Google Scholar]
- DeNardo, G.L.; DeNardo, S.J. Concepts, consequences, and implications of theranosis. In Seminars in Nuclear Medicine; Elsevier: Amsterdam, The Netherlands, 2012; pp. 147–150. [Google Scholar]
- Radiology, E.S.O. Medical imaging in personalised medicine: A white paper of the research committee of the European Society of Radiology (ESR). Insights Imaging 2015, 6, 141–155. [Google Scholar]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical–physical applications to nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef]
- Baptista, A.; Silva, F.; Porteiro, J.; Míguez, J.; Pinto, G. Sputtering physical vapour deposition (PVD) coatings: A critical review on process improvement and market trend demands. Coatings 2018, 8, 402. [Google Scholar] [CrossRef]
- Wani, I.A. Nanomaterials, novel preparation routes, and characterizations. In Nanotechnology Applications for Improvements in Energy Efficiency and Environmental Management; IGI Global: Hershey, PA, USA, 2015; pp. 1–40. [Google Scholar]
- Saxena, J.; Jyoti, A. Nanomaterials: Novel Preparation Routes, Characterizations, and Applications. In Nanobiotechnology; Apple Academic Press: Cambridge, MA, USA, 2020; pp. 23–33. [Google Scholar]
- Shaban, M.; Hasanzadeh, M. Biomedical applications of dendritic fibrous nanosilica (DFNS): Recent progress and challenges. RSC Adv. 2020, 10, 37116–37133. [Google Scholar] [CrossRef]
- Raliya, R.; Singh Chadha, T.; Haddad, K.; Biswas, P. Perspective on nanoparticle technology for biomedical use. Curr. Pharm. Des. 2016, 22, 2481–2490. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Huo, D.; Kim, M.J.; Lyu, Z.; Shi, Y.; Wiley, B.J.; Xia, Y. One-dimensional metal nanostructures: From colloidal syntheses to applications. Chem. Rev. 2019, 119, 8972–9073. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yang, Y.; Liang, H.W.; Liu, J.W.; Yu, S.H. Nanowire genome: A magic toolbox for 1D nanostructures. Adv. Mater. 2019, 31, 1902807. [Google Scholar] [CrossRef] [PubMed]
- Mauro, N.; Utzeri, M.A.; Varvarà, P.; Cavallaro, G. Functionalization of Metal and Carbon Nanoparticles with Potential in Cancer Theranostics. Molecules 2021, 26, 3085. [Google Scholar] [CrossRef] [PubMed]
- Pascui, O.F.; Lohwasser, R.; Sommer, M.; Thelakkat, M.; Thurn-Albrecht, T.; Saalwachter, K. High crystallinity and nature of crystal− crystal phase transformations in regioregular poly (3-hexylthiophene). Macromolecules 2010, 43, 9401–9410. [Google Scholar] [CrossRef]
- Salata, O.V. Applications of nanoparticles in biology and medicine. J. Nanobiotechnology 2004, 2, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Daraio, C.; Jin, S. Synthesis and patterning methods for nanostructures useful for biological applications. In Nanotechnology for Biology and Medicine; Springer: Berlin/Heidelberg, Germany, 2012; pp. 27–44. [Google Scholar]
- Delogu, F.; Gorrasi, G.; Sorrentino, A. Fabrication of polymer nanocomposites via ball milling: Present status and future perspectives. Prog. Mater. Sci. 2017, 86, 75–126. [Google Scholar] [CrossRef]
- Sun, J.; Wang, M.; Zhao, Y.; Li, X.; Liang, B. Synthesis of titanium nitride powders by reactive ball milling of titanium and urea. J. Alloy. Compd. 2009, 482, L29–L31. [Google Scholar] [CrossRef]
- Pentimalli, M.; Imperi, E.; Zaccagnini, A.; Padella, F. Nanostructured metal hydride–Polymer composite as fixed bed for sorption technologies. Advantages of an innovative combined approach by high-energy ball milling and extrusion techniques. Renew. Energy 2017, 110, 69–78. [Google Scholar] [CrossRef]
- Bi, S.; Xiao, B.; Ji, Z.; Liu, B.; Liu, Z.; Ma, Z. Dispersion and damage of carbon nanotubes in carbon nanotube/7055Al composites during high-energy ball milling process. Acta Metall. Sin. (Engl. Lett.) 2021, 34, 196–204. [Google Scholar] [CrossRef]
- Ma, P.C.; Wang, S.Q.; Kim, J.-K.; Tang, B.Z. In-situ amino functionalization of carbon nanotubes using ball milling. J. Nanosci. Nanotechnol. 2009, 9, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Gou, J.; Zhuge, J.; Liang, F. Processing of polymer nanocomposites. In Manufacturing Techniques for Polymer Matrix Composites (PMCs); Elsevier: Amsterdam, The Netherlands, 2012; pp. 95–119. [Google Scholar]
- Hecht, D.S.; Hu, L.; Irvin, G. Emerging transparent electrodes based on thin films of carbon nanotubes, graphene, and metallic nanostructures. Adv. Mater. 2011, 23, 1482–1513. [Google Scholar] [CrossRef] [PubMed]
- Piras, C.C.; Fernández-Prieto, S.; De Borggraeve, W.M. Ball milling: A green technology for the preparation and functionalisation of nanocellulose derivatives. Nanoscale Adv. 2019, 1, 937–947. [Google Scholar] [CrossRef]
- Karousis, N.; Tagmatarchis, N.; Tasis, D. Current progress on the chemical modification of carbon nanotubes. Chem. Rev. 2010, 110, 5366–5397. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.; Cavin, O.; McKamey, C.; Scarbrough, J. Preparation of ‘‘amorphous’’Ni60Nb40 by mechanical alloying. Appl. Phys. Lett. 1983, 43, 1017–1019. [Google Scholar] [CrossRef]
- Fox-Rabinovich, G.; Gershman, I.; Veldhuis, S. Thin-Film PVD Coating Metamaterials Exhibiting Similarities to Natural Processes under Extreme Tribological Conditions. Nanomaterials 2020, 10, 1720. [Google Scholar] [CrossRef] [PubMed]
- El-Eskandarany, M.S.; Al-Salem, S.M.; Ali, N. Top-down reactive approach for the synthesis of disordered zrn nanocrystalline bulk material from solid waste. Nanomaterials 2020, 10, 1826. [Google Scholar] [CrossRef]
- Venables, J.; Spiller, G. Nucleation and growth of thin films. Surf. Mobilities Solid Mater. 1983, 86, 341–404. [Google Scholar]
- Abegunde, O.O.; Akinlabi, E.T.; Oladijo, O.P.; Akinlabi, S.; Ude, A.U. Overview of thin film deposition techniques. AIMS Mater. Sci. 2019, 6, 174–199. [Google Scholar] [CrossRef]
- Knotek, O.; Löffler, F.; Krämer, G. Process and advantage of multicomponent and multilayer PVD coatings. Surf. Coat. Technol. 1993, 59, 14–20. [Google Scholar] [CrossRef]
- Mattox, D.M. Physical vapor deposition (PVD) processes. Met. Finish. 1999, 97, 417–430. [Google Scholar] [CrossRef]
- Kim, S.; Sojoudi, H.; Zhao, H.; Mariappan, D.; McKinley, G.H.; Gleason, K.K.; Hart, A.J. Ultrathin high-resolution flexographic printing using nanoporous stamps. Sci. Adv. 2016, 2, e1601660. [Google Scholar] [CrossRef]
- Lee, K.-H.; Kim, S.-M.; Jeong, H.; Jung, G.-Y. Spontaneous nanoscale polymer solution patterning using solvent evaporation driven double-dewetting edge lithography. Soft Matter 2012, 8, 465–471. [Google Scholar] [CrossRef]
- Jang, J.H.; Ullal, C.K.; Maldovan, M.; Gorishnyy, T.; Kooi, S.; Koh, C.; Thomas, E.L. 3D micro-and nanostructures via interference lithography. Adv. Funct. Mater. 2007, 17, 3027–3041. [Google Scholar] [CrossRef]
- Pimpin, A.; Srituravanich, W. Review on micro-and nanolithography techniques and their applications. Eng. J. 2012, 16, 37–56. [Google Scholar] [CrossRef]
- Paik, S.; Kim, G.; Chang, S.; Lee, S.; Jin, D.; Jeong, K.-Y.; Lee, I.S.; Lee, J.; Moon, H.; Lee, J. Near-field sub-diffraction photolithography with an elastomeric photomask. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Del Barrio, J.; Sánchez-Somolinos, C. Light to shape the future: From photolithography to 4D printing. Adv. Opt. Mater. 2019, 7, 1900598. [Google Scholar] [CrossRef]
- Vollenbroek, F.A.; Spiertz, E.J. Photoresist systems for microlithography. In Electronic Applications; Springer: Berlin/Heidelberg, Germany, 1988; pp. 85–111. [Google Scholar]
- Sha, D.; Hsieh, L.; Chen, K. Wafer rework strategies at the photolithography stage. Int. J. Ind. Eng. Theory Appl. Pract. 2001, 8, 122–130. [Google Scholar]
- Altissimo, M. E-beam lithography for micro-/nanofabrication. Biomicrofluidics 2010, 4, 026503. [Google Scholar] [CrossRef]
- Ferrera, J.F.U. Nanometer-Scale Placement in Electron-Beam Lithography. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2000. [Google Scholar]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.; Ingber, D.E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 2001, 3, 335–373. [Google Scholar] [CrossRef]
- Sahin, O.; Ashokkumar, M.; Ajayan, P.M. Micro-and nanopatterning of biomaterial surfaces. In Fundamental Biomaterials: Metals; Elsevier: Amsterdam, The Netherlands, 2018; pp. 67–78. [Google Scholar]
- Quate, C.F. Scanning probes as a lithography tool for nanostructures. Surf. Sci. 1997, 386, 259–264. [Google Scholar] [CrossRef]
- Zhang, K.; Fu, Q.; Pan, N.; Yu, X.; Liu, J.; Luo, Y.; Wang, X.; Yang, J.; Hou, J. Direct writing of electronic devices on graphene oxide by catalytic scanning probe lithography. Nat. Commun. 2012, 3, 1–6. [Google Scholar] [CrossRef]
- Kühnel, M.; Fröhlich, T.; Füßl, R.; Hoffmann, M.; Manske, E.; Rangelow, I.W.; Reger, J.; Schäffel, C.; Sinzinger, S.; Zöllner, J.-P. Towards alternative 3D nanofabrication in macroscopic working volumes. Meas. Sci. Technol. 2018, 29, 114002. [Google Scholar] [CrossRef]
- Lyles, V.D. Surface studies of Organic Thin Films Using Scanning Probe Microscopy and Nanofabrication. Ph.D. Thesis, Louisiana State University and Agricultural and Mechanical College, Baton Rouge, LA, USA, 2013. [Google Scholar]
- Zhao, C.; Liu, Q.; Cheung, K.M.; Liu, W.; Yang, Q.; Xu, X.; Man, T.; Weiss, P.S.; Zhou, C.; Andrews, A.M. Narrower Nanoribbon Biosensors Fabricated by Chemical Lift-off Lithography Show Higher Sensitivity. ACS Nano 2020, 15, 904–915. [Google Scholar] [CrossRef]
- Rane, A.V.; Kanny, K.; Abitha, V.; Thomas, S. Methods for synthesis of nanoparticles and fabrication of nanocomposites. In Synthesis of Inorganic Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 121–139. [Google Scholar]
- Kickelbick, G. Introduction to hybrid materials. Hybrid Mater. 2007, 1, 2. [Google Scholar]
- Hakim, S.H.; Shanks, B.H. A comparative study of macroporous metal oxides synthesized via a unified approach. Chem. Mater. 2009, 21, 2027–2038. [Google Scholar] [CrossRef][Green Version]
- Rahman, I.A.; Padavettan, V. Synthesis of silica nanoparticles by sol-gel: Size-dependent properties, surface modification, and applications in silica-polymer nanocomposites—A review. J. Nanomater. 2012, 2012, 8. [Google Scholar] [CrossRef]
- Kwiatkowski, K.C.; Lukehart, C.M. Nanocomposites prepared by sol-gel methods: Synthesis and characterization. In Nanostructured Materials and Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2002; pp. 57–91. [Google Scholar]
- Tillotson, T.; Gash, A.; Simpson, R.; Hrubesh, L.; Satcher, J., Jr.; Poco, J. Nanostructured energetic materials using sol–gel methodologies. J. Non Cryst. Solids 2001, 285, 338–345. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, G.; Gao, L.; Yang, J.; Chhowalla, M.; Gharahcheshmeh, M.H.; Gleason, K.K.; Choi, Y.S.; Hong, B.H.; Liu, Z. Chemical vapour deposition. Nat. Rev. Methods Primers 2021, 1, 1–20. [Google Scholar] [CrossRef]
- Koponen, S.E.; Gordon, P.G.; Barry, S.T. Principles of precursor design for vapour deposition methods. Polyhedron 2016, 108, 59–66. [Google Scholar] [CrossRef]
- Kara, F.; Öztürk, B. Comparison and optimization of PVD and CVD method on surface roughness and flank wear in hard-machining of DIN 1.2738 mold steel. Sens. Rev. 2019, 29, 24–33. [Google Scholar] [CrossRef]
- Muüller, R.; Gelme, O.; Scholz, J.-P.; Huber, F.; Mundszinger, M.; Li, Y.; Madel, M.; Minkow, A.; Kaiser, U.; Herr, U. Epitaxial ZnO layer growth on Si (111) substrates with an intermediate aln nucleation layer by methane-based chemical vapor deposition. Cryst. Growth Des. 2020, 20, 6170–6185. [Google Scholar] [CrossRef]
- Mochalov, L.; Logunov, A.; Kitnis, A.; Vorotyntsev, V. Plasma-chemistry of arsenic selenide films: Relationship between film properties and plasma power. Plasma Chem. Plasma Process. 2020, 40, 407–421. [Google Scholar] [CrossRef]
- Lee, J.-I.; Hwang, N.-M. Generation of negative-charge carriers in the gas phase and their contribution to the growth of carbon nanotubes during hot-filament chemical vapor deposition. Carbon 2008, 46, 1588–1592. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Kim, D.-S.; Hwang, N.-M. Comparison of diamond nanoparticles captured on the floating and grounded membranes in the hot filament chemical vapor deposition process. RSC Adv. 2021, 11, 5651–5657. [Google Scholar] [CrossRef]
- Prawer, S.; Nugent, K.; Jamieson, D.; Orwa, J.; Bursill, L.A.; Peng, J. The Raman spectrum of nanocrystalline diamond. Chem. Phys. Lett. 2000, 332, 93–97. [Google Scholar] [CrossRef]
- Andhare, D.; Jadhav, S.; Khedkar, M.; Somvanshi, S.B.; More, S.; Jadhav, K. Structural and chemical properties of ZnFe2O4 nanoparticles synthesised by chemical co-precipitation technique. In Proceedings of the Journal of Physics: Conference Series, International Web Conference on Advanced Material Science and Nanotechnology (NANOMAT -2020), Nandgaon Khandeshwar, India, 20–21 June 2020; IOP Publishing: Bristol, UK, 2020; p. 012014. [Google Scholar]
- Anbarasu, M.; Anandan, M.; Chinnasamy, E.; Gopinath, V.; Balamurugan, K. Synthesis and characterization of polyethylene glycol (PEG) coated Fe3O4 nanoparticles by chemical co-precipitation method for biomedical applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Gnanaprakash, G.; Mahadevan, S.; Jayakumar, T.; Kalyanasundaram, P.; Philip, J.; Raj, B. Effect of initial pH and temperature of iron salt solutions on formation of magnetite nanoparticles. Mater. Chem. Phys. 2007, 103, 168–175. [Google Scholar] [CrossRef]
- Bloemen, M.; Brullot, W.; Luong, T.T.; Geukens, N.; Gils, A.; Verbiest, T. Improved functionalization of oleic acid-coated iron oxide nanoparticles for biomedical applications. J. Nanoparticle Res. 2012, 14, 1–10. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, J.; Zhou, J.; Zeng, Y.; Zhang, W.; Shi, B. Highly efficient removal of Cr (III)-poly (acrylic acid) complex by coprecipitation with polyvalent metal ions: Performance, mechanism, and validation. Water Res. 2020, 178, 115807. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.; Helwani, Z.; Fernando, W. Synthetic hydrotalcites from different routes and their application as catalysts and gas adsorbents: A review. Appl. Organomet. Chem. 2009, 23, 335–346. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Q.; Lee, B.; Jun, Y.-S. Aluminum affects heterogeneous Fe (III)(Hydr) oxide nucleation, growth, and ostwald ripening. Environ. Sci. Technol. 2014, 48, 299–306. [Google Scholar] [CrossRef]
- Li, J.; Sun, X.; Liu, S.; Li, X.; Li, J.-G.; Huo, D. A homogeneous co-precipitation method to synthesize highly sinterability YAG powders for transparent ceramics. Ceram. Int. 2015, 41, 3283–3287. [Google Scholar] [CrossRef]
- Maleki, H.; Haselpour, M.; Fathi, R. The effect of calcination conditions on structural and magnetic behavior of bismuth ferrite synthesized by co-precipitation method. J. Mater. Sci. Mater. Electron. 2018, 29, 4320–4326. [Google Scholar] [CrossRef]
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum dots in imaging, drug delivery and sensor applications. Int. J. Nanomed. 2017, 12, 5421. [Google Scholar] [CrossRef] [PubMed]
- Bohn, B.J.; Simon, T.; Gramlich, M.; Richter, A.F.; Polavarapu, L.; Urban, A.S.; Feldmann, J. Dephasing and quantum beating of excitons in methylammonium lead iodide perovskite nanoplatelets. ACS Photonics 2018, 5, 648–654. [Google Scholar] [CrossRef]
- Xie, R.; Chen, K.; Chen, X.; Peng, X. InAs/InP/ZnSe core/shell/shell quantum dots as near-infrared emitters: Bright, narrow-band, non-cadmium containing, and biocompatible. Nano Res. 2008, 1, 457–464. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.J.; Jing, L.; Behrens, A.M.; Jayawardena, S.; Tang, W.; Gao, M.; Langer, R.; Jaklenec, A. Biocompatible semiconductor quantum dots as cancer imaging agents. Adv. Mater. 2018, 30, 1706356. [Google Scholar] [CrossRef]
- Subramaniyan, S.B.; Veerappan, A. Water soluble cadmium selenide quantum dots for ultrasensitive detection of organic, inorganic and elemental mercury in biological fluids and live cells. RSC Adv. 2019, 9, 22274–22281. [Google Scholar] [CrossRef]
- Gogoi, S.; Kalita, S.; Hazarika, R.; Puzari, P. Preparation of an integrated porous substrate of 11-mercaptoundecanoic acid and chitosan on gold for electrochemical study of pores and pore forming interactions in lipid bilayers. Electrochim. Acta 2020, 329, 135174. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, B.; Zheng, Z.; Liu, T. Semiconductor quantum dots in tumor research. J. Lumin. 2019, 209, 61–68. [Google Scholar] [CrossRef]
- Tang, S.-Y.; Yang, C.-C.; Su, T.-Y.; Yang, T.-Y.; Wu, S.-C.; Hsu, Y.-C.; Chen, Y.-Z.; Lin, T.-N.; Shen, J.-L.; Lin, H.-N. Design of Core–Shell Quantum Dots–3D WS2 Nanowall Hybrid Nanostructures with High-Performance Bifunctional Sensing Applications. ACS Nano 2020, 14, 12668–12678. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, Q.; Wang, Q.; Yu, M.; Gong, X.; Li, S.; Xiao, J.; Guo, Y.; Chen, G.; Lai, X. Flexible translucent chitosan–glycerin/QD nanocomposite glue for anti-counterfeiting films with strong adhesion and stability. RSC Adv. 2020, 10, 23410–23416. [Google Scholar] [CrossRef]
- Rahman, M.F.; Hossain, J.; Kuddus, A.; Tabassum, S.; Rubel, M.H.; Shirai, H.; Ismail, A.B.M. A novel synthesis and characterization of transparent CdS thin films for CdTe/CdS solar cells. Appl. Phys. A 2020, 126, 1–11. [Google Scholar] [CrossRef]
- Zayed, D.G.; AbdElhamid, A.S.; Freag, M.S.; Elzoghby, A.O. Hybrid quantum dot-based theranostic nanomedicines for tumor-targeted drug delivery and cancer imaging. Future Med. 2019, 14. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Jafari, S.; Khosroushahi, A.Y. A sight on the current nanoparticle-based gene delivery vectors. Nanoscale Res. Lett. 2014, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.-S.; Meng, X.-Y.; Zhang, Y.-Y.; Yang, L.; Zhang, J.-H.; Wang, X.-R.; Lu, S.-Y.; Ren, H.-L.; Liu, Z.-S. Development of an immunochromatographic strip and its application in the simultaneous determination of Hg (II), Cd (II) and Pb (II). Sens. Actuators B Chem. 2013, 183, 303–309. [Google Scholar] [CrossRef]
- Hong, G.; Antaris, A.L.; Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 1–22. [Google Scholar] [CrossRef]
- Farshbaf, M.; Davaran, S.; Rahimi, F.; Annabi, N.; Salehi, R.; Akbarzadeh, A. Carbon quantum dots: Recent progresses on synthesis, surface modification and applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1331–1348. [Google Scholar] [CrossRef]
- Du, J.; Xu, N.; Fan, J.; Sun, W.; Peng, X. Carbon dots for in vivo bioimaging and theranostics. Small 2019, 15, 1805087. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Gillies, J.M. Synthesis, characterisation and bioconjugation of [109Cd] CdSe/ZnS core/shell quantum dots as “proof of principle” for the potential development of an anti-cancer theranostic. Inorg. Chim. Acta 2019, 495, 119001. [Google Scholar] [CrossRef]
- Wu, H.; Su, W.; Xu, H.; Zhang, Y.; Li, Y.; Li, X.; Fan, L. Applications of carbon dots on tumour theranostics. View 2021, 2, 20200061. [Google Scholar] [CrossRef]
- Bwatanglang, I.B.; Mohammad, F.; Yusof, N.A.; Abdullah, J.; Alitheen, N.B.; Hussein, M.Z.; Abu, N.; Mohammed, N.E.; Nordin, N.; Zamberi, N.R. In vivo tumor targeting and anti-tumor effects of 5-fluororacil loaded, folic acid targeted quantum dot system. J. Colloid Interface Sci. 2016, 480, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.-T.; Ding, H.; Roy, I.; Law, W.-C.; Bergey, E.J.; Maitra, A.; Prasad, P.N. Imaging pancreatic cancer using bioconjugated InP quantum dots. ACS Nano 2009, 3, 502–510. [Google Scholar] [CrossRef]
- Unnikrishnan, B.; Wu, R.-S.; Wei, S.-C.; Huang, C.-C.; Chang, H.-T. Fluorescent carbon dots for selective labeling of subcellular organelles. ACS Omega 2020, 5, 11248–11261. [Google Scholar] [CrossRef] [PubMed]
- Atmaja, B.; Lui, B.H.; Hu, Y.; Beck, S.E.; Frank, C.W.; Cochran, J.R. Targeting of cancer cells using quantum dot–polypeptide hybrid assemblies that function as molecular imaging agents and carrier systems. Adv. Funct. Mater. 2010, 20, 4091–4097. [Google Scholar] [CrossRef]
- Li, N.; Zhao, P.; Astruc, D. Anisotropic gold nanoparticles: Synthesis, properties, applications, and toxicity. Angew. Chem. Int. Ed. 2014, 53, 1756–1789. [Google Scholar] [CrossRef]
- Zhou, G.; Lü, M.; Yang, Z.; Zhang, H.; Zhou, Y.; Wang, S.; Wang, S.; Zhang, A. Surfactant-assisted synthesis and characterization of silver nanorods and nanowires by an aqueous solution approach. J. Cryst. Growth 2006, 289, 255–259. [Google Scholar] [CrossRef]
- Lee, G.-J.; Shin, S.-I.; Kim, Y.-C.; Oh, S.-G. Preparation of silver nanorods through the control of temperature and pH of reaction medium. Mater. Chem. Phys. 2004, 84, 197–204. [Google Scholar] [CrossRef]
- Roach, L.; Booth, M.E.; Ingram, N.; Paterson, D.A.; Batchelor, D.V.; Moorcroft, S.C.; Bushby, R.J.; Critchley, K.; Coletta, P.L.; Evans, S.D. Evaluating Phospholipid-Functionalized Gold Nanorods for In Vivo Applications. Small 2021, 17, 2006797. [Google Scholar] [CrossRef]
- Matthews, J.R.; Payne, C.M.; Hafner, J.H. Analysis of phospholipid bilayers on gold nanorods by plasmon resonance sensing and surface-enhanced raman scattering. Langmuir 2015, 31, 9893–9900. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Wang, H.; Fu, Q.; Peng, J.; Wang, Y.; Du, J.; Zhou, Y.; Zhan, L. Gold nanorod-based localized surface plasmon resonance biosensor for sensitive detection of hepatitis B virus in buffer, blood serum and plasma. Biosens. Bioelectron. 2010, 26, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Banik, B.; Barman, J.; Das, M.K. Recent advancement in photo nanotheranostics for cancer treatment. In Multifunctional Theranostic Nanomedicines in Cancer; Elsevier: Amsterdam, The Netherlands, 2021; pp. 163–176. [Google Scholar]
- Li, H.; Jin, H.; Wan, W.; Wu, C.; Wei, L. Cancer nanomedicine: Mechanisms, obstacles and strategies. Nanomedicine 2018, 13, 1639–1656. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Bian, Q.; Lei, S.; Deng, Y.; Zhao, K.; Sun, S.; Fu, Q.; Xiao, Y.; Cheng, B. Bi 19 S 27 I 3 nanorods: A new candidate for photothermal therapy in the first and second biological near-infrared windows. Nanoscale 2021, 13, 5369–5382. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Gill, G.S.; Jeet, K. Applications of carbon nanotubes in drug delivery: A comprehensive review. In Characterization and Biology of Nanomaterials for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–135. [Google Scholar]
- Rao, R.; Pint, C.L.; Islam, A.E.; Weatherup, R.S.; Hofmann, S.; Meshot, E.R.; Wu, F.; Zhou, C.; Dee, N.; Amama, P.B. Carbon nanotubes and related nanomaterials: Critical advances and challenges for synthesis toward mainstream commercial applications. ACS Nano 2018, 12, 11756–11784. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Malaiya, A.; Kesharwani, P.; Soni, D.; Jain, A. Biomedical applications and toxicities of carbon nanotubes. Drug Chem. Toxicol. 2020, 43, 1–16. [Google Scholar] [CrossRef]
- Jeyamohan, P.; Hasumura, T.; Nagaoka, Y.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Accelerated killing of cancer cells using a multifunctional single-walled carbon nanotube-based system for targeted drug delivery in combination with photothermal therapy. Int. J. Nanomed. 2013, 8, 2653. [Google Scholar]
- Hayashi, K.; Nakamura, M.; Miki, H.; Ozaki, S.; Abe, M.; Matsumoto, T.; Ishimura, K. Near-Infrared Fluorescent Silica/Porphyrin Hybrid Nanorings for In Vivo Cancer Imaging. Adv. Funct. Mater. 2012, 22, 3539–3546. [Google Scholar] [CrossRef]
- Son, S.J.; Reichel, J.; He, B.; Schuchman, M.; Lee, S.B. Magnetic nanotubes for magnetic-field-assisted bioseparation, biointeraction, and drug delivery. J. Am. Chem. Soc. 2005, 127, 7316–7317. [Google Scholar] [CrossRef]
- Hajba, L.; Guttman, A. The use of magnetic nanoparticles in cancer theranostics: Toward handheld diagnostic devices. Biotechnol. Adv. 2016, 34, 354–361. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, X.; Gong, F.; Liu, T.; Liu, Z. 2D nanomaterials for cancer theranostic applications. Adv. Mater. 2020, 32, 1902333. [Google Scholar] [CrossRef]
- Kühne, M.; Börrnert, F.; Fecher, S.; Ghorbani-Asl, M.; Biskupek, J.; Samuelis, D.; Krasheninnikov, A.V.; Kaiser, U.; Smet, J.H. Reversible superdense ordering of lithium between two graphene sheets. Nature 2018, 564, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.Y.; Laurent, S.; Chen, W.; Akhavan, O.; Imani, M.; Ashkarran, A.A.; Mahmoudi, M. Graphene: Promises, facts, opportunities, and challenges in nanomedicine. Chem. Rev. 2013, 113, 3407–3424. [Google Scholar] [CrossRef]
- Anju, S.; Ashtami, J.; Mohanan, P. Black phosphorus, a prospective graphene substitute for biomedical applications. Mater. Sci. Eng. C 2019, 97, 978–993. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-E.; Koo, H.; Sun, I.-C.; Ryu, J.H.; Kim, K.; Kwon, I.C. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev. 2012, 41, 2656–2672. [Google Scholar] [CrossRef] [PubMed]
- Jonoush, Z.A.; Farahani, M.; Bohlouli, M.; Niknam, Z.; Golchin, A.; Hatamie, S.; Rezaei-Tavirani, M.; Omidi, M.; Zali, H. Surface Modification of Graphene and its Derivatives for Drug Delivery Systems. Mini Rev. Org. Chem. 2021, 18, 78–92. [Google Scholar] [CrossRef]
- Lee, X.J.; Lim, H.N.; Gowthaman, N.; Rahman, M.B.A.; Abdullah, C.A.C.; Muthoosamy, K. In-situ surface functionalization of superparamagnetic reduced graphene oxide–Fe3O4 nanocomposite via Ganoderma lucidum extract for targeted cancer therapy application. Appl. Surf. Sci. 2020, 512, 145738. [Google Scholar] [CrossRef]
- Tas, A.; Keklikcioglu Cakmak, N. Synthesis of PEGylated nanographene oxide as a nanocarrier for docetaxel drugs and anticancer activity on prostate cancer cell lines. Hum. Exp. Toxicol. 2021, 40, 172–182. [Google Scholar] [CrossRef]
- Darrigues, E.; Nima, Z.A.; Griffin, R.J.; Anderson, J.M.; Biris, A.S.; Rodriguez, A. 3D cultures for modeling nanomaterial-based photothermal therapy. Nanoscale Horiz. 2020, 5, 400–430. [Google Scholar] [CrossRef]
- Fu, Q.; Zhu, R.; Song, J.; Yang, H.; Chen, X. Photoacoustic imaging: Contrast agents and their biomedical applications. Adv. Mater. 2019, 31, 1805875. [Google Scholar] [CrossRef] [PubMed]
- Knights, O.B.; McLaughlan, J.R. Gold nanorods for light-based lung cancer theranostics. Int. J. Mol. Sci. 2018, 19, 3318. [Google Scholar] [CrossRef]
- Lee, H.; Choi, M.; Lim, J.; Jo, M.; Han, J.-Y.; Kim, T.M.; Cho, Y. Magnetic nanowire networks for dual-isolation and detection of tumor-associated circulating biomarkers. Theranostics 2018, 8, 505. [Google Scholar] [CrossRef]
- Su, Y.; Wei, X.; Peng, F.; Zhong, Y.; Lu, Y.; Su, S.; Xu, T.; Lee, S.-T.; He, Y. Gold nanoparticles-decorated silicon nanowires as highly efficient near-infrared hyperthermia agents for cancer cells destruction. Nano Lett. 2012, 12, 1845–1850. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Shao, D.; Zhang, L.; Zhang, X.-L.; Li, J.; Feng, J.; Xia, H.; Huo, Q.-S.; Dong, W.-F.; Sun, H.-B. Gold nanorods-silica Janus nanoparticles for theranostics. Appl. Phys. Lett. 2015, 106, 173705. [Google Scholar] [CrossRef]
- Khan, M.; Boumati, S.; Arib, C.; Diallo, A.T.; Djaker, N.; Doan, B.-T.; Spadavecchia, J. Doxorubicin (DOX) Gadolinium–Gold-Complex: A New Way to Tune Hybrid Nanorods as Theranostic Agent. Int. J. Nanomed. 2021, 16, 2219. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Shi, L.; Wang, P. MXene Ti3C2: An effective 2D light-to-heat conversion material. ACS Nano 2017, 11, 3752–3759. [Google Scholar] [CrossRef]
- Shim, G.; Ko, S.; Park, J.Y.; Suh, J.H.; Le, Q.-V.; Kim, D.; Kim, Y.B.; Im, G.H.; Kim, H.N.; Choe, Y.S. Tannic acid-functionalized boron nitride nanosheets for theranostics. J. Control. Release 2020, 327, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, Y.; Liu, H.; Wang, Y.; Liu, L.; Lv, F.; Li, Y.; Wang, S. Graphdiyne materials as nanotransducer for in vivo photoacoustic imaging and photothermal therapy of tumor. Chem. Mater. 2017, 29, 6087–6094. [Google Scholar] [CrossRef]
- Gulzar, A.; Xu, J.; Xu, L.; Yang, P.; He, F.; Yang, D.; An, G.; Ansari, M.B. Redox-responsive UCNPs-DPA conjugated NGO-PEG-BPEI-DOX for imaging-guided PTT and chemotherapy for cancer treatment. Dalton Trans. 2018, 47, 3921–3930. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhu, X.; Chen, X.; Zhao, Y.; Liu, J. Gd 3+-Doped MoSe 2 nanosheets used as a theranostic agent for bimodal imaging and highly efficient photothermal cancer therapy. Biomater. Sci. 2018, 6, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Escudero, A.; Becerro, A.I.; Carrillo-Carrión, C.; Nunez, N.O.; Zyuzin, M.V.; Laguna, M.; González-Mancebo, D.; Ocaña, M.; Parak, W.J. Rare earth based nanostructured materials: Synthesis, functionalization, properties and bioimaging and biosensing applications. Nanophotonics 2017, 6, 881–921. [Google Scholar] [CrossRef]
- Mahmoud, M.; El-Sayed, M. Metallic double shell hollow nanocages: The challenges of their synthetic techniques. Langmuir 2012, 28, 4051–4059. [Google Scholar] [CrossRef]
- Xu, Y.H.; Cai, Q.Q.; Ma, H.X.; He, Y.; Zhang, H.; Ma, C.A. Optimisation of electrocatalytic dechlorination of 2, 4-dichlorophenoxyacetic acid on a roughened silver–palladium cathode. Electrochim. Acta 2013, 96, 90–96. [Google Scholar] [CrossRef]
- De Sio, L.; Placido, T.; Comparelli, R.; Curri, M.L.; Striccoli, M.; Tabiryan, N.; Bunning, T.J. Next-generation thermo-plasmonic technologies and plasmonic nanoparticles in optoelectronics. Prog. Quantum Electron. 2015, 41, 23–70. [Google Scholar] [CrossRef]
- Yang, W.; Yu, Y.; Wang, L.; Yang, C.; Li, H. Controlled synthesis and assembly into anisotropic arrays of magnetic cobalt-substituted magnetite nanocubes. Nanoscale 2015, 7, 2877–2882. [Google Scholar] [CrossRef]
- Evans, R.J.; Lavin, B.; Phinikaridou, A.; Chooi, K.Y.; Mohri, Z.; Wong, E.; Boyle, J.J.; Krams, R.; Botnar, R.; Long, N.J. Targeted molecular iron oxide contrast agents for imaging atherosclerotic plaque. Nanotheranostics 2020, 4, 184. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.H.; Park, M.; Do, M.J.; Lee, N.; Ryu, J.H.; Kim, G.W.; Kim, C.; Park, T.G.; Hyeon, T. Chitosan oligosaccharide-stabilized ferrimagnetic iron oxide nanocubes for magnetically modulated cancer hyperthermia. ACS Nano 2012, 6, 5266–5273. [Google Scholar] [CrossRef] [PubMed]
- Kuthala, N.; Vankayala, R.; Chiang, C.S.; Hwang, K.C. Unprecedented Theranostic LaB6 Nanocubes-Mediated NIR-IIb Photodynamic Therapy to Conquer Hypoxia-Induced Chemoresistance. Adv. Funct. Mater. 2020, 30, 2002940. [Google Scholar] [CrossRef]
- Wolfram, J.; Ferrari, M. Clinical cancer nanomedicine. Nano Today 2019, 25, 85–98. [Google Scholar] [CrossRef]
| S. No. | Nanomaterial | Elements | Size (nm) | Absorption (nm) | Functionalization | Application | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Quantum dots | CdSe, ZnS | <10 | 579 nm | Core/Shell-ZnS | Cd109-SPECT imaging agent incorporated for imaging and diagnostics to identify the tumour | [93] |
| 2 | Quantum dots | CdTe, CdS, BSA | ~550 | λex = 400 nm λem = 528 to 650 nm | Protein (BSA) | QD-BSA was used for long term fluorescence observation because emission decreased by 4.06% after being irradiated at 365 nm for 1 h | [84] |
| 3 | Carbon quantum dots | large amino acid-mimicking (LAAM), tetramino-anthraquinone (TAAQ), and citric acid | ~3 | 230 nm, 280 nm, 650 nm | α-carboxyl, amino groups | LAT-1-mediated targeting tumour theranostics | [94] |
| 4 | Quantum dot | Mn, ZnS | 218 | 287 | Chitosan biopolymer and conjugated with folic acid | Diagnosis and treatment of anticancer activity of 5-fluorouracil for breast cancer therapy | [95] |
| 5 | Quantum dot | In, P, Zn | 15–20 | 645 | Mercapto-succinic acid | This is a non-cadmium based QD used in diagnostic imaging in the early detection of cancer | [96] |
| S. No. | Nanomaterial | Elements | Size (nm) | Absorption (nm) | Functionalization | Application | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Nanorods | Au | 2.9–4.2 (aspect ratio) | 770–811 nm | Citrate, CTAB | Petawatts (PW) lasers are used to treat lung cancer cells instead of continuous wave (CW) lasers in theranostics. The laser energy used was 19 mJ/cm2. | [113] |
| 2. | Nanowire | Magnetic polypyrrole, PEI, Antibody | 10 | --- | Biotin, Antibody | Magnetic nano wires are used to recover rare circulating biomarkers which improves cancer diagnostics and prognostics | [114] |
| 3 | Nanowire | Au, Si | Au~500 nm, Si nanowire~17 aspect ratio | 530 nm | Gold nanoparticles decorated on silicon nanowire | AuNP@SiNW acts as a NIR hyperthermia agent which destroys the cancer cells within 3 min upon NIR radiations | [115] |
| 4. | Nanorods | Au, Si | 3–4 aspect ratio | AuNRs~600 nm Au@SiNRs~725 nm Au-Janus~650 nm | AuNRs functionalized with silica nanoparticles | Au@Si-Janus nanoparticles act as a carrier to deliver imaging agents and drugs. It also useful for combined photo-thermo or chemo cancer therapy | [116] |
| 5 | Nanorods | Gd, Au | 20–40 | >750 nm | PEG functionalized Gd@AuNRs | Gd@AuNRs acts as a strong theranostics agent to image and treat MIAPaCa-2 cells. For NIR 808 lasers used and for MRI T1 features at 7T | [117] |
| S. No. | Nanomaterial | Elements | Size (nm) | Absorption (nm) | Functionalization | Application | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | MXene | Ti3C2 | 500 | 800 | --- | MXene acts as light to heat convert material with 100% efficiency in PTT | [128] |
| 2 | Nanosheet | Boron nitride | 100 | 650 | Tannic acid (TA) | The TA-Fe coordinated complex on boron nitride nanosheet configuring T1 weighted MRI- Theranostics. It is also useful for MRI guided photo-therapy | [129] |
| 3 | Nanosheet | Graphdiyne (GDY) | 360 | 700 | PEG | GDY-PEG acts as a photothermal-acoustic wave transducer in PAI and PTT for treating cancer | [130] |
| 4 | Nanosheet | Graphene | 50 | --- | PEG, BPEI, DOX | The graphene oxide nanocomposites act as theranostics agents for UCL image-driven combinatorial PTT and chemotherapy to treat cancer. The NIR laser used at 980 nm with 13.5 photothermal conversion efficiency | [131] |
| 5 | Nanosheet | (Gd3+)MoSe2 | 100–150 | 700–850 | PEG | Gd provides T1 weighted MR-imaging. (Gd3+)MoSe2 acts as photothermal agents in cancer therapy | [132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paramasivam, G.; Palem, V.V.; Sundaram, T.; Sundaram, V.; Kishore, S.C.; Bellucci, S. Nanomaterials: Synthesis and Applications in Theranostics. Nanomaterials 2021, 11, 3228. https://doi.org/10.3390/nano11123228
Paramasivam G, Palem VV, Sundaram T, Sundaram V, Kishore SC, Bellucci S. Nanomaterials: Synthesis and Applications in Theranostics. Nanomaterials. 2021; 11(12):3228. https://doi.org/10.3390/nano11123228
Chicago/Turabian StyleParamasivam, Gokul, Vishnu Vardhan Palem, Thanigaivel Sundaram, Vickram Sundaram, Somasundaram Chandra Kishore, and Stefano Bellucci. 2021. "Nanomaterials: Synthesis and Applications in Theranostics" Nanomaterials 11, no. 12: 3228. https://doi.org/10.3390/nano11123228
APA StyleParamasivam, G., Palem, V. V., Sundaram, T., Sundaram, V., Kishore, S. C., & Bellucci, S. (2021). Nanomaterials: Synthesis and Applications in Theranostics. Nanomaterials, 11(12), 3228. https://doi.org/10.3390/nano11123228







