New Phenotype and Mineralization of Biogenic Iron Oxide in Magnetotactic Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Collection of MTB
2.2. Electron Microscopy
3. Results and Discussion
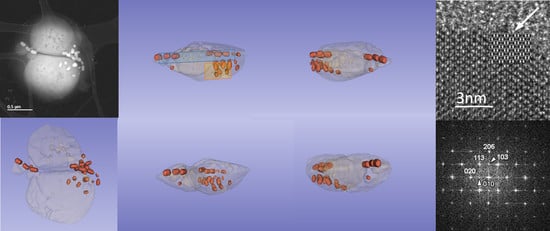
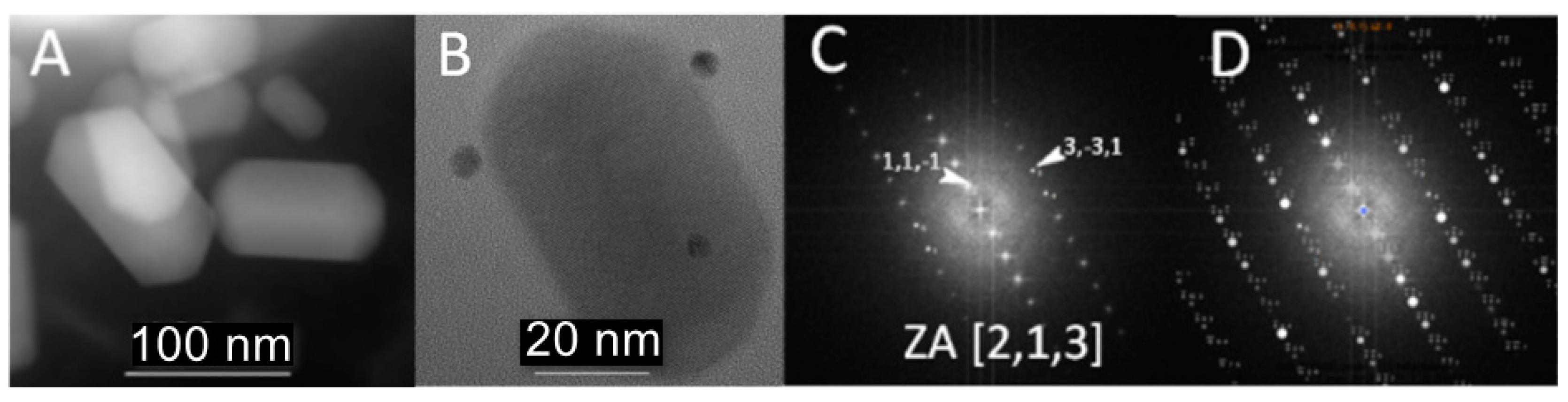
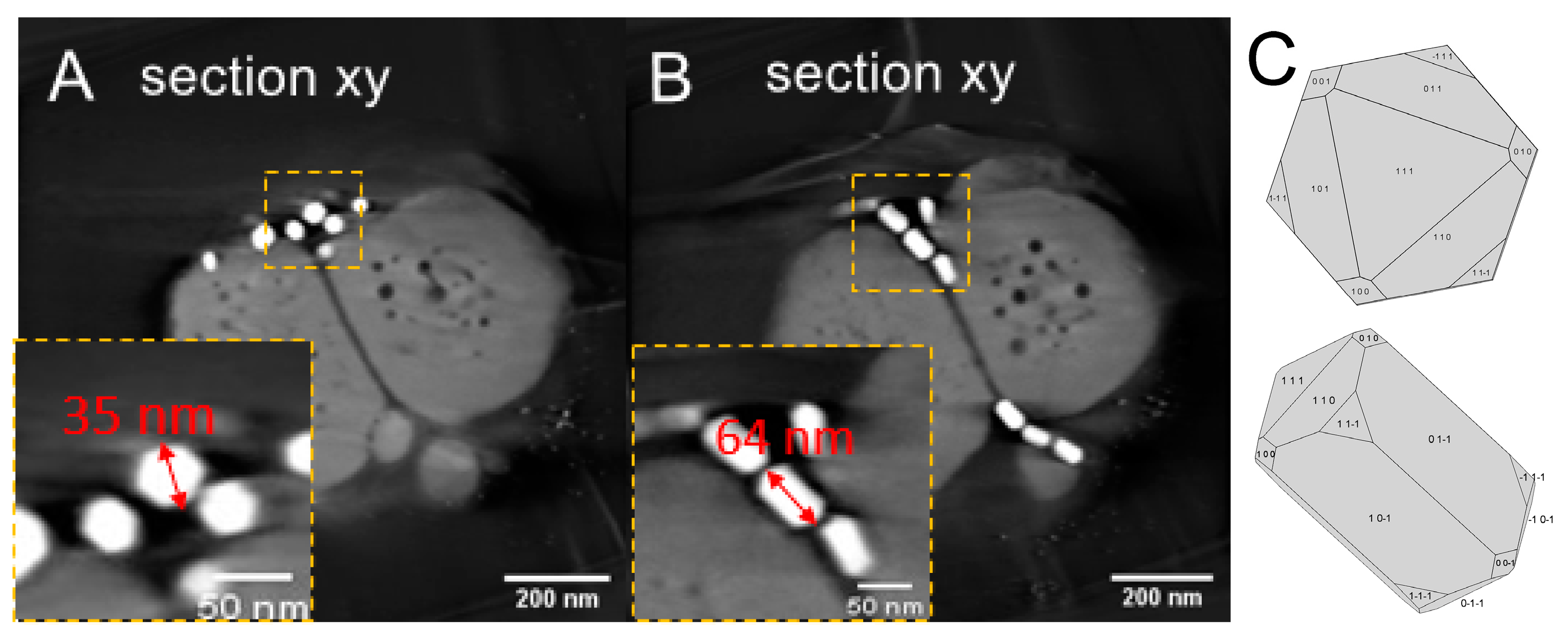
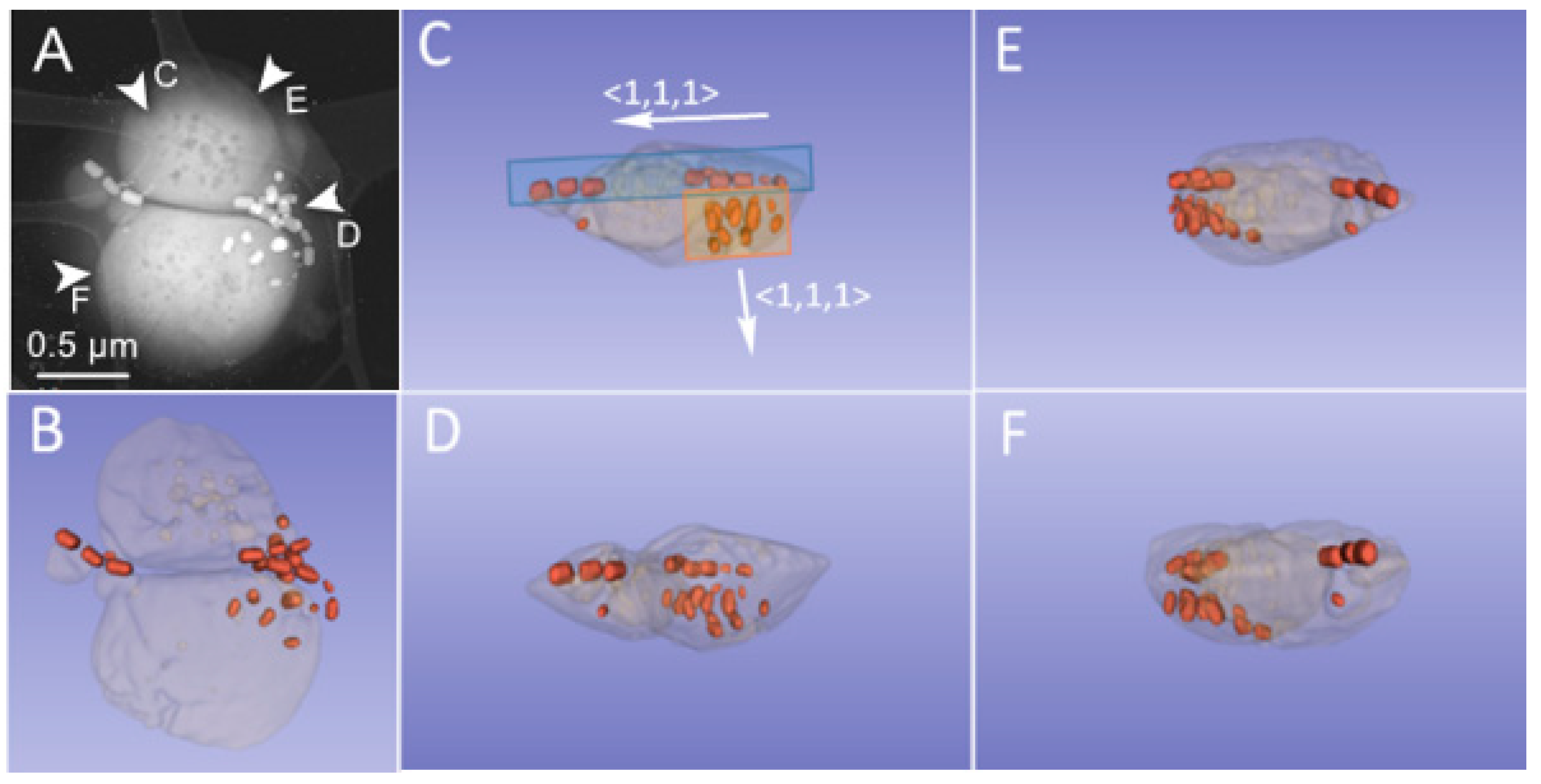
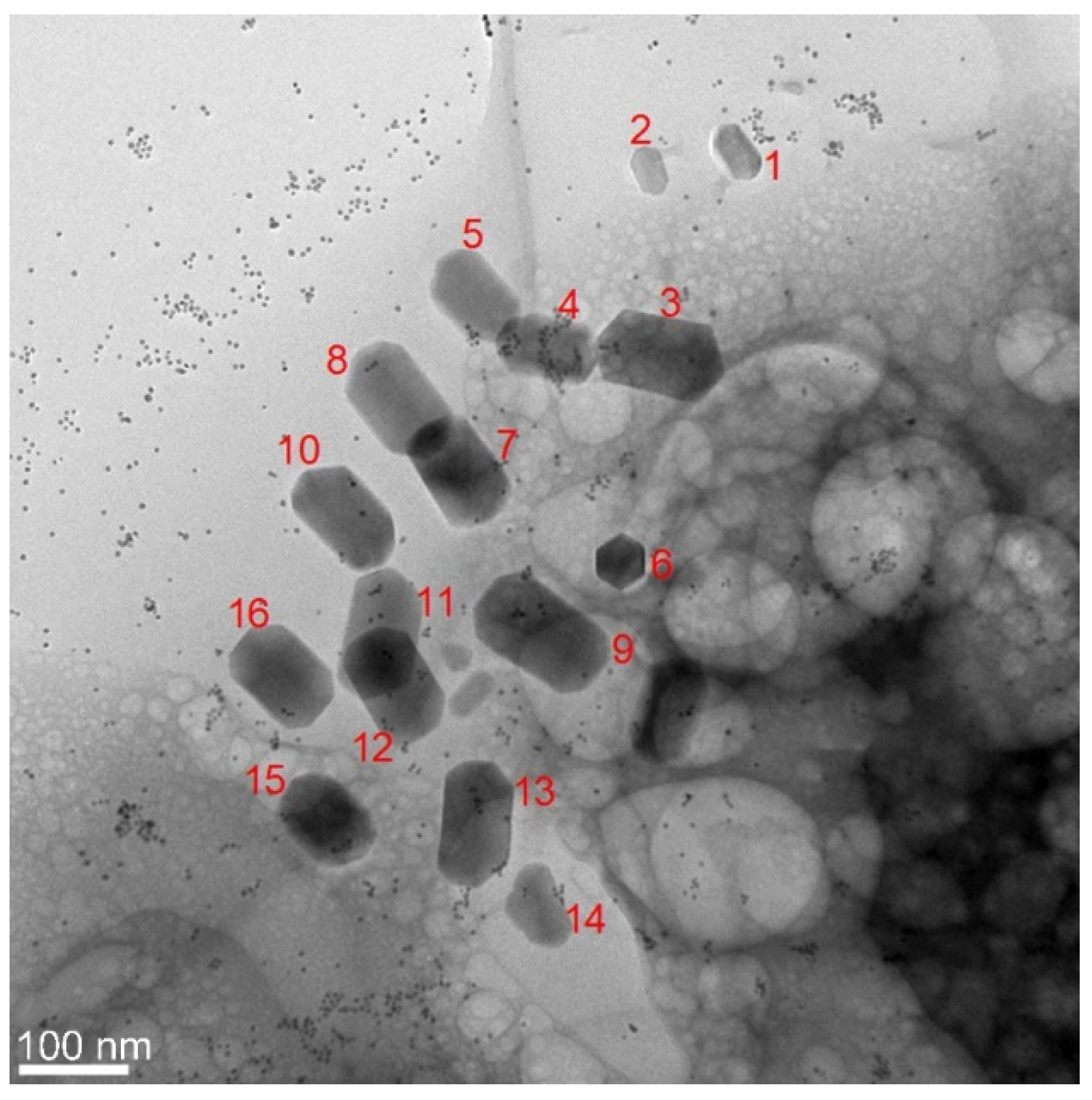
3.1. Phenotype and Crystal Morphology
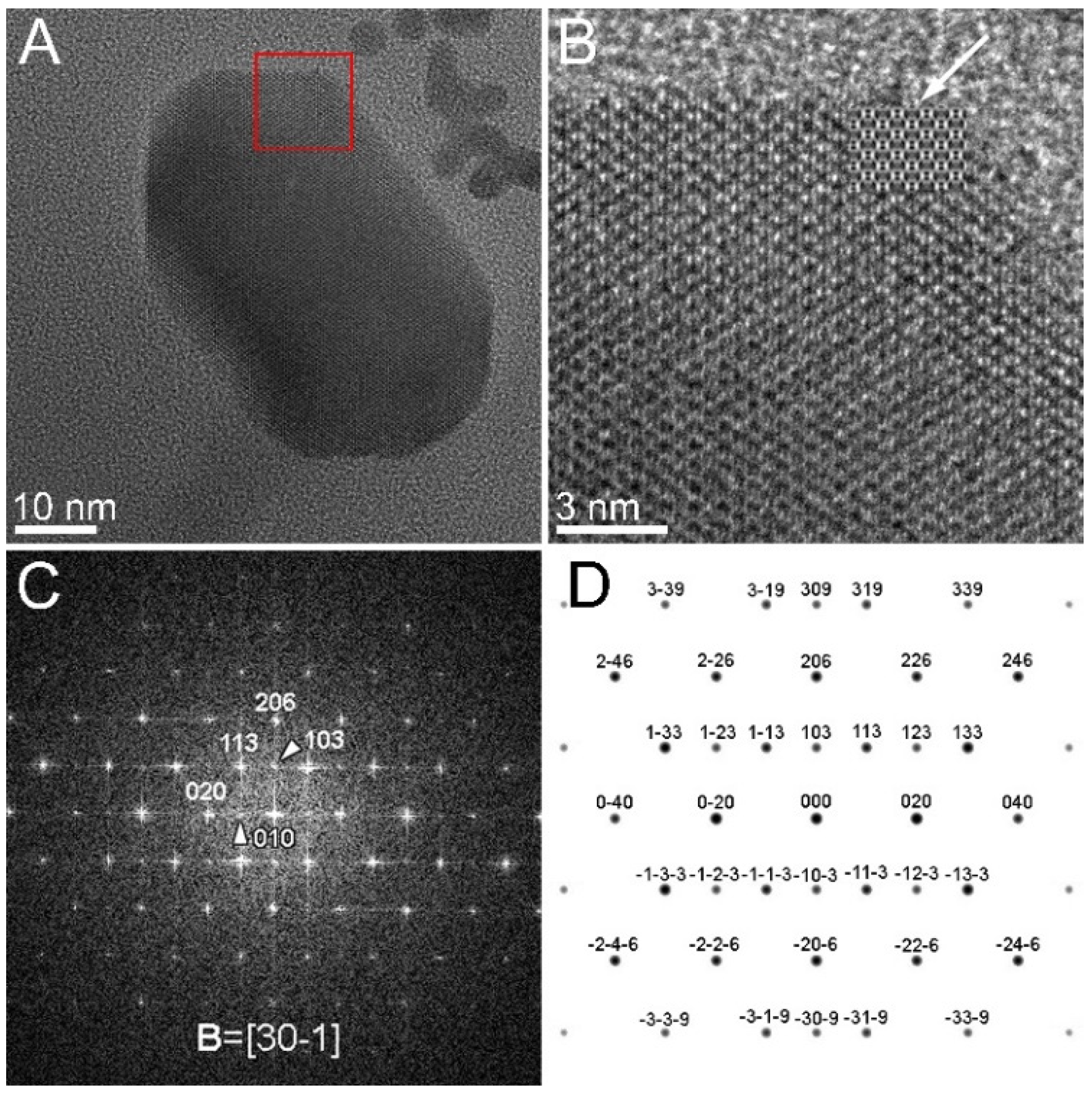
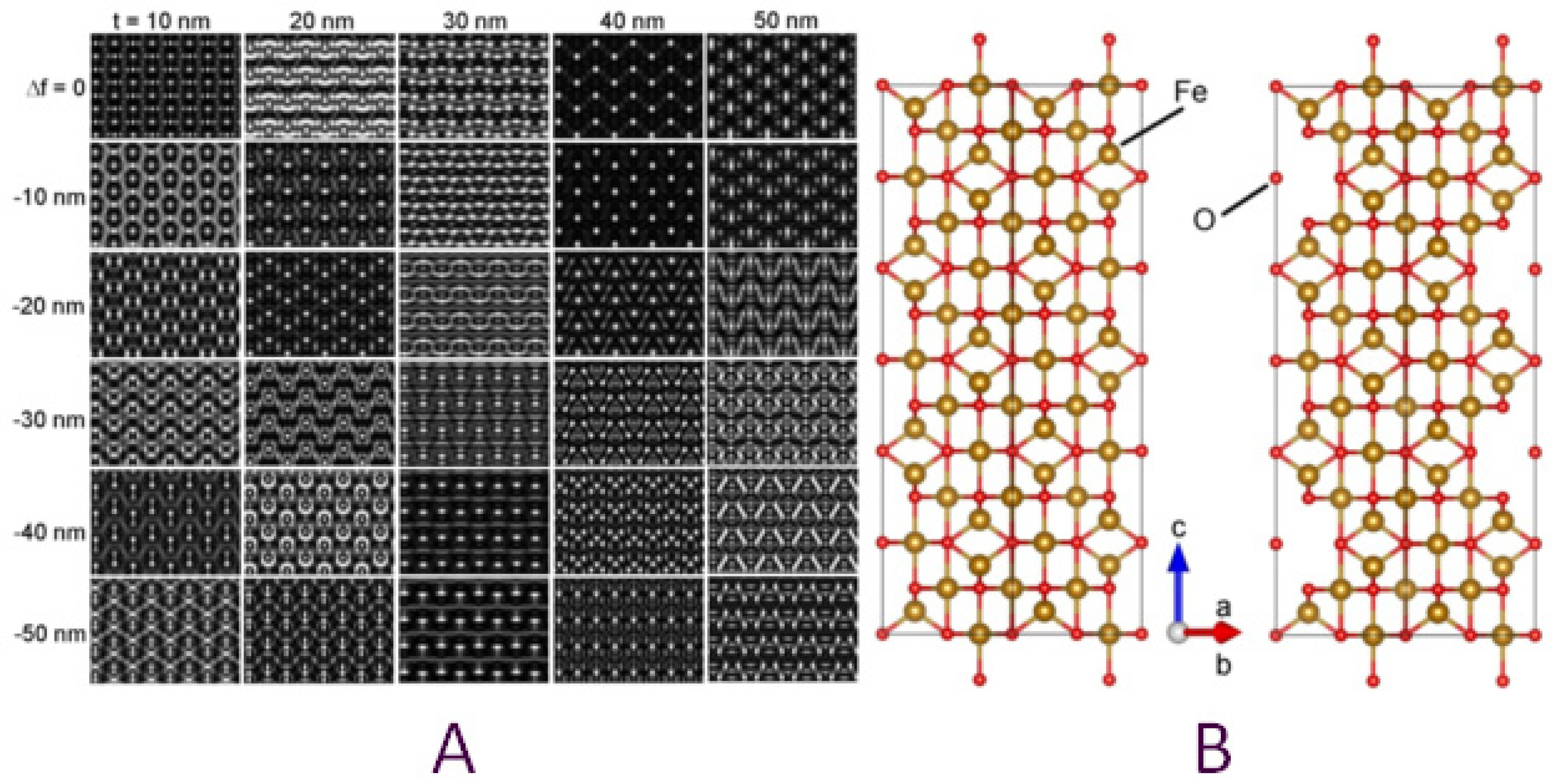
3.2. Crystallographic Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blakemore, R. Magnetotactic bacteria. Science 1975, 190, 377−379. [Google Scholar] [CrossRef] [PubMed]
- Rahn-Lee, L.; Byrne, M.; Zhang, M.; Le Sage, D.; Glenn, D.R.; Milbourne, T.; Walsworth, R.L.; Vali, H.; Komeili, A. A Genetic Strategy for Probing the Functional Diversity of Magnetosome Formation. PLoS Genet. 2015, 11, e1004811. [Google Scholar] [CrossRef] [PubMed]
- Cypriano, J.; Werckmann, J.; Vargas, G.; dos Santos, A.L.; Silva, K.T.; Leão, P.; Almeida, F.P.; Bazylinski, D.A.; Farina, M.; Lins, U.; et al. Uptake and persistence of bacterial magnetite magnetosomes in a mammalian cell line: Implications for medical and biotechnological applications. PLoS ONE 2019, 14, e0215657. [Google Scholar] [CrossRef] [PubMed]
- Cypriano, J.; Bahri, M.; Dembelé, K.; Baaziz, W.; Leão, P.; Bazylinski, D.A.; Abreu, F.; Ersen, O.; Farina, M.; Werckmann, J. Insight on thermal stability of magnetite magnetosomes: Implications for the fossil record and biotechnology. Sci. Rep. 2020, 10, 6706. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.B.R.; Kirschvink, J.L. Magnetofossils, the Magnetization of Sediments, and the Evolution of Magnetite Biomineralization. Annu. Rev. Earth Planet. Sci. 1989, 17, 169–195. [Google Scholar] [CrossRef]
- Lefèvre, C.T.; Bazylinski, D.A. Ecology, Diversity, and Evolution of Magnetotactic Bacteria. Microbiol. Mol. Biol. Rev. 2013, 77, 497–526. [Google Scholar] [CrossRef]
- Alkwill, D.L.; Maratea, D.; Blakemore, R.P. Ultrastructure of a magnetotactic spirillum. J. Bacteriol. 1980, 141, 1399−1408. [Google Scholar]
- DeLong, E.F.; Frankel, R.B.; Bazylinski, D.A. Multiple Evolutionary Origins of Magnetotaxis in Bacteria. Science 1993, 259, 803–806. [Google Scholar] [CrossRef]
- Abreu, F.; Cantão, M.E.; Nicolás, M.F.; Barcellos, F.G.; Morillo, V.; Almeida, L.G.; Nascimento, F.F.D.; Lefevre, C.; Bazylinski, D.A.; De Vasconcelos, A.T.R.; et al. Common ancestry of iron oxide- and iron-sulfide-based biomineralization in magnetotactic bacteria. ISME J. 2011, 5, 1634–1640. [Google Scholar] [CrossRef]
- Faivre, D.; Schüler, D. Magnetotactic Bacteria an Magnetosomes. Chem. Rev. 2008, 108, 4875–4898. [Google Scholar] [CrossRef]
- Bazylinski, D.A.; Frankel, R.B. Magnetosome formation in prokaryotes. Nat. Rev. Genet. 2004, 2, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, A.; Tanaka, M.; Lenders, J.J.M.; Thiesbrummel, J.; Sommerdijk, N.A.J.M.; Matsunaga, T.; Arakaki, A. Control of magnetite nanocrystal morphology in magnetotactic bacteria by regulation of mms7 gene expression. Sci. Rep. 2016, 6, 29785. [Google Scholar] [CrossRef] [PubMed]
- Raschdorf, O.; Forstner, Y.; Kolinko, I.; Uebe, R.; Plitzko, J.M.; Schüler, D. Genetic and Ultrastructural Analysis Reveals the Key Players and Initial Steps of Bacterial Magnetosome Membrane Biogenesis. PLoS Genet. 2016, 12, e1006101. [Google Scholar] [CrossRef] [PubMed]
- Grünberg, K.; Wawer, C.; Tebo, B.M.; Schüler, D. A Large Gene Cluster Encoding Several Magnetosome Proteins Is Conserved in Different Species of Magnetotactic Bacteria. Appl. Environ. Microbiol. 2001, 67, 4573–4582. [Google Scholar] [CrossRef] [PubMed]
- Nudelman, H.; Zarivach, R. Structure prediction of magnetosome-associated proteins. Front. Microbiol. 2014, 5, 9. [Google Scholar] [CrossRef]
- Komeili, A. Molecular Mechanisms of Magnetosome Formation. Annu. Rev. Biochem. 2007, 76, 351–366. [Google Scholar] [CrossRef][Green Version]
- Jinhua, L.; Pan, Y. Environmental Factors Affect Magnetite Magnetosome Synthesis in Magnetospirillum magneticum AMB-1: Implications for Biologically Controlled Mineralization. Geomicrobiol. J. 2012, 29, 362–373. [Google Scholar]
- Scheffel, A.; Gruska, M.; Faivre, D.; Linaroudis, A.; Plitzko, J.; Schüler, D. An acidic protein aligns magnetosomes along a filamentous structure in magnetotactic bacteria. Nature 2005, 440, 110–114. [Google Scholar] [CrossRef]
- Rivas-Lamelo, S.; Benzerara, K.; Lefèvre, C.; Monteil, C.; Jézéquel, D.; Menguy, N.; Viollier, E.; Guyot, F.; Férard, C.; Poinsot, M.; et al. Magnetotactic bacteria as a new model for P sequestration in the ferruginous Lake Pavin. Geochem. Persp. Let. 2017, 5, 35–41. [Google Scholar] [CrossRef]
- Devouard, B.; Posfai, M.; Hua, X.; Bazylinski, D.A.; Frankel, R.B.; Buseck, P.R. Magnetite from magnetotactic bacteria: Size distributions and twinning. Am. Mineral. 1998, 83, 1387–1398. [Google Scholar] [CrossRef]
- Pósfai, M.; Lefèvre, C.T.; Trubitsyn, D.; Bazylinski, D.A.; Richard, B.; Frankel, R.B. Phylogenetic significance of composition and crystal morphology of magnetosome minerals. Front. Microbiol. 2013, 4, 344. [Google Scholar] [CrossRef]
- Baumgartner, J.; Morin, G.; Menguy, N.; Gonzalez, T.P.; Widdrat, M.; Cosmidis, J.; Faivre, D. Magnetotactic bacteria form magnetite from a phosphate-rich ferric hydroxide via nanometric ferric (oxyhydr)oxide intermediates. Proc. Natl. Acad. Sci. USA 2013, 110, 14883–14888. [Google Scholar] [CrossRef]
- Leão, P.; Le Nagard, L.; Yuan, H.; Cypriano, J.; Da Silva-Neto, I.; Bazylinski, D.A.; Acosta-Avalos, D.; de Barros, H.L.; Hitchcock, A.P.; Lins, U.; et al. Magnetosome magnetite biomineralization in a flagellated protist: Evidence for an early evolutionary origin for magnetoreception in eukaryotes. Environ. Microbiol. 2019, 22, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Toro-Nahuelpan, M.; Müller, F.D.; Klumpp, S.; Plitzko, J.M.; Bramkamp, M.; Schüler, D. Segregation of prokaryotic magnetosomes organelles is driven by treadmilling of a dynamic actin-like MamK filament. BMC Biol. 2016, 14, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Jinhua, L.; Menguy, N.; Arrio, M.-A.; Sainctavit, P.; Juhin, A.; Wang, Y.; Chen, H.; Bunau, O.; Otero, E.; Ohresser, P.; et al. Controlled cobalt doping in the spinel structure of magnetosome magnetite: New evidences from element- and site-specific X-ray magnetic circular dichroism analyses. J. R. Soc. Interface 2016, 13, 20160355. [Google Scholar] [CrossRef]
- Pecharromán, C.; Gonzalez-Carreno, T.; Iglesias, J.E. The infrared dielectric properties of maghemite, γ-Fe2O3, from reflectance measurement on pressed powders. Phys. Chem. Miner. 1995, 22, 21–29. [Google Scholar] [CrossRef]
- Lins, U.; Freitas, F.; Keim, C.N.; Farina, M. Electron Spectroscopic Imaging of Magnetotactic Bacteria: Magnetosome Morphology and Diversity. Microsc. Microanal. 2000, 6, 463–470. [Google Scholar] [CrossRef]
- Guo, F.F.; Yang, W.; Jiang, W.; Geng, S.; Peng, T.; Li, J.L. Magnetosomes eliminate intracellular reactive oxygen species in Magnetospirillum gryphiswaldense MSR. Environ. Microbiol. 2012, 14, 1722–1729. [Google Scholar] [CrossRef]
- Li, K.; Wang, P.; Chen, C.; Chen, C.; Li, L.; Song, T. Light irradiation helps magnetotactic bacteria eliminate intracellular reactive oxygen species. Environ. Microbiol. 2017, 19, 3638–3648. [Google Scholar] [CrossRef]
- Muñoz, D.; Marcano, L.; Martín-Rodríguez, R.; Simonelli, L.; Serrano, A.; García-Prieto, A.; Fdez-Gubieda, M.L.; Muela, A. Magnetosomes could be protective shields against metal stress in magnetotactic bacteria. Sci. Rep. 2020, 10, 11430. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baaziz, W.; Ghica, C.; Cypriano, J.; Abreu, F.; Anselme, K.; Ersen, O.; Farina, M.; Werckmann, J. New Phenotype and Mineralization of Biogenic Iron Oxide in Magnetotactic Bacteria. Nanomaterials 2021, 11, 3189. https://doi.org/10.3390/nano11123189
Baaziz W, Ghica C, Cypriano J, Abreu F, Anselme K, Ersen O, Farina M, Werckmann J. New Phenotype and Mineralization of Biogenic Iron Oxide in Magnetotactic Bacteria. Nanomaterials. 2021; 11(12):3189. https://doi.org/10.3390/nano11123189
Chicago/Turabian StyleBaaziz, Walid, Corneliu Ghica, Jefferson Cypriano, Fernanda Abreu, Karine Anselme, Ovidiu Ersen, Marcos Farina, and Jacques Werckmann. 2021. "New Phenotype and Mineralization of Biogenic Iron Oxide in Magnetotactic Bacteria" Nanomaterials 11, no. 12: 3189. https://doi.org/10.3390/nano11123189
APA StyleBaaziz, W., Ghica, C., Cypriano, J., Abreu, F., Anselme, K., Ersen, O., Farina, M., & Werckmann, J. (2021). New Phenotype and Mineralization of Biogenic Iron Oxide in Magnetotactic Bacteria. Nanomaterials, 11(12), 3189. https://doi.org/10.3390/nano11123189