Sunlight-Driven AO7 Degradation with Perovskites (La,Ba)(Fe,Ti)O3 as Heterogeneous Photocatalysts
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Catalysts Characterization
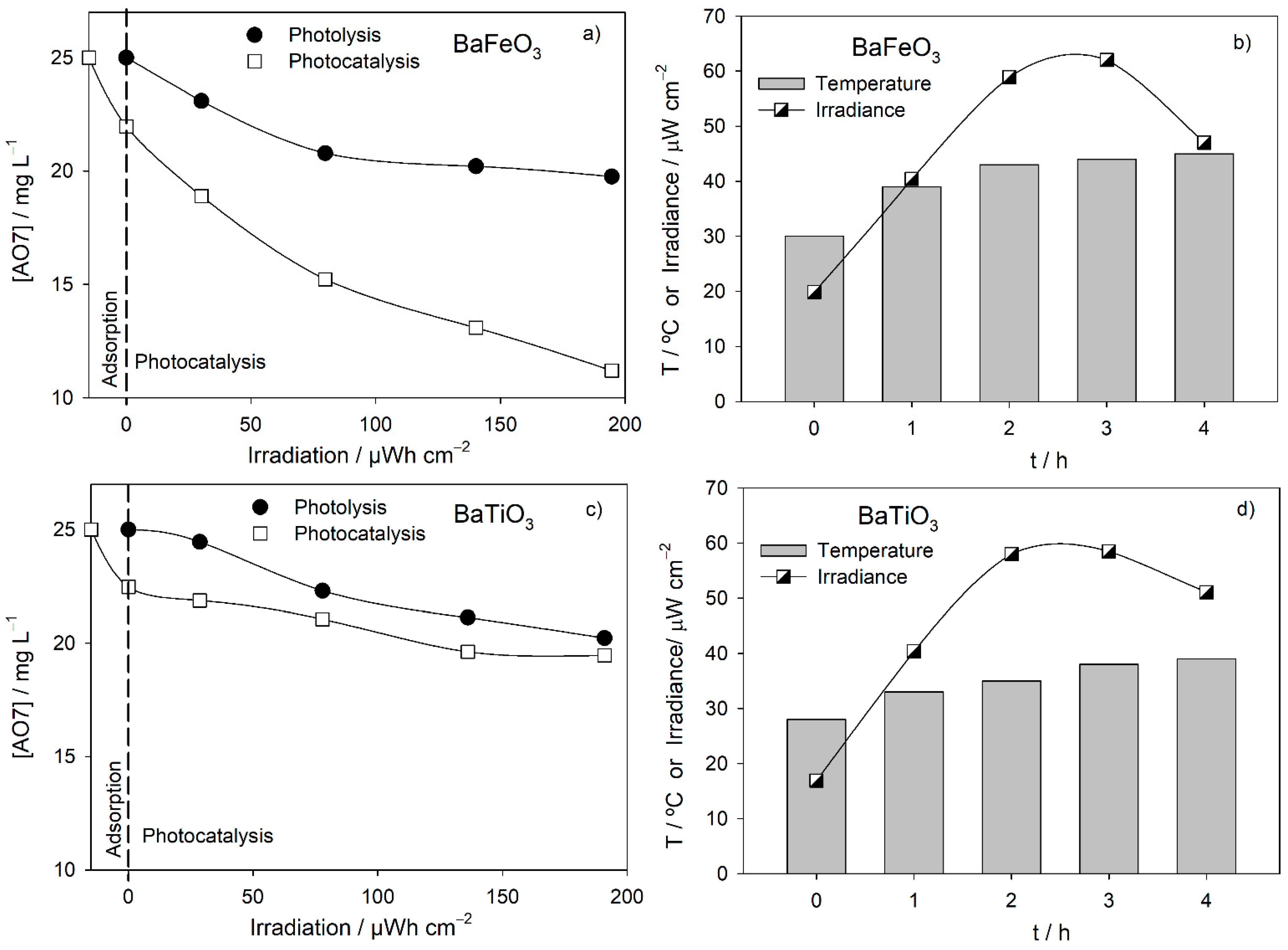
3.2. Degradation Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, S.; Cui, L.; Zhao, Y.; Wu, Y.; Xia, L.; Su, X.; Zhang, C.; Wang, D.; Guo, W.; Sun, J. Crystal structure and photocatalytic properties of perovskite MSn(OH)6(M=Cu and Zn) composites with d10-d10 configuration. Appl. Surf. Sci. 2019, 463, 659–667. [Google Scholar] [CrossRef]
- Grabowska, E. Selected perovskite oxides: Characterization, preparation and photocatalytic properties—A review. Appl. Catal. B-Environ. 2016, 186, 97–126. [Google Scholar] [CrossRef]
- Zhang, M.; Qi, Y.; Zhang, Z. AgBr/BiOBr nano-heterostructure-decorated polyacrylonitrile nanofibers: A recyclable high-performance photocatalyst for dye degradation under visible-light irradiation. Polymers 2019, 11, 1718. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Schuerings, C.; Kumar, S.; Kumar, A.; Krishnan, V. Perovskite-structured CaTiO3 coupled with g-C3N4 as a heterojunction photocatalyst for organic pollutant degradation. Beilstein J. Nanotechnol. 2018, 9, 671–685. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Zhou, X.; Zhang, Y.; Yao, Q.; Qian, Y.; Chu, H.; Chen, J. Carbamazepine degradation by heterogeneous activation of peroxymonosulfate with lanthanum cobaltite perovskite: Performance, mechanism and toxicity. J. Environ. Sci. 2020, 91, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Ye, X.; Chen, Q.; Long, H.; Rao, Y. The oxidative degradation of diclofenac using the activation of peroxymonosulfate by BiFeO3 microspheres—kinetics, role of visible light and decay pathways. Sep. Purif. Technol. 2020, 232, 115967. [Google Scholar] [CrossRef]
- Rao, Y.; Han, F.; Chen, Q.; Wang, D.; Xue, D.; Wang, H.; Pu, S. Efficient degradation of diclofenac by LaFeO3-Catalyzed peroxymonosulfate oxidation-kinetics and toxicity assessment. Chemosphere 2019, 218, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.Y.A.; Chen, Y.C.; Lin, Y.F. LaMO3 perovskites (M=Co, Cu, Fe and Ni) as heterogeneous catalysts for activating peroxymonosulfate in water. Chem. Eng. Sci. 2017, 160, 96–105. [Google Scholar] [CrossRef]
- Kanhere, P.; Chen, Z. A review on visible light active perovskite-based photocatalysts. Molecules 2014, 19, 19995–20022. [Google Scholar] [CrossRef] [Green Version]
- Rojas-Cervantes, M.L.; Castillejos, E. Perovskites as catalysts in advanced oxidation processes for wastewater treatment. Catalysts 2019, 9, 230. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Lv, S.; Luo, Z. A study on the photocatalytic degradation performance of a [KNbO3]0.9-[BaNi0.5Nb0.5O3− δ]0.1 perovskite. RSC Adv. 2020, 10, 1275–1280. [Google Scholar] [CrossRef] [Green Version]
- Pang, X.; Guo, Y.; Zhang, Y.; Xu, B.; Qi, F. LaCoO3 perovskite oxide activation of peroxymonosulfate for aqueous 2-phenyl-5-sulfobenzimidazole degradation: Effect of synthetic method and the reaction mechanism. Chem. Eng. J. 2016, 304, 897–907. [Google Scholar] [CrossRef]
- Zhu, J.; Li, H.; Zhong, L.; Xiao, P.; Xu, X.; Yang, X.; Zhao, Z.; Li, J. Perovskite oxides: Preparation, characterizations, and applications in heterogeneous catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- Chen, H.; Motuzas, J.; Martens, W.; da Costa, J.C.D. Degradation of azo dye Orange II under dark ambient conditions by calcium strontium copper perovskite. Appl. Catal. B Environ. 2018, 221, 691–700. [Google Scholar] [CrossRef] [Green Version]
- Pena, M.A.; Fierro, J.L.G. Chemical structures and performance of perovskite oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Meng, J.; Wu, S.; Pei, J.; Lin, Q.; Wei, X.; Li, J.; Zhang, Z. Room temperature synthesized BaTiO3 for photocatalytic hydrogen evolution. J. Alloy. Compd. 2018, 754, 184–189. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, Y.; Sun, Y.; Wang, J.; Bie, L.; Duan, Y. Sunlight-activated AlFeO3/TiO2 photocatalyst. Sci. China Ser. B-Chem. 2006, 49, 67–74. [Google Scholar] [CrossRef]
- Majumdar, A.; Pal, A. Optimized synthesis of Bi4NbO8Cl perovskite nanosheets for enhanced visible light assisted photocatalytic degradation of tetracycline antibiotics. J. Environ. Chem. Eng. 2020, 8, 103645. [Google Scholar] [CrossRef]
- Bresolin, B.M. Synthesis and Performance of Metal-Halide Perovskites as New Visible Light Photocatalysts. Ph.D. Thesis, Acta Universitatis Lappeenrantaensis 947, Lappeenranta-Lahti University of Technology LUT, Lappeenranta, Finland, 2021. Available online: https://lutpub.lut.fi/bitstream/handle/10024/162090/Bianca%20Maria%20Bresolin%20A4.pdf?sequence=1&isAllowed=y (accessed on 16 September 2021).
- Yakout, S.M. Influence of Na and Na/Fe doping on the dielectric constant, ferromagnetic and sunlight photocatalytic properties of BaTiO3 perovskite. J. Solid State Chem. 2020, 290, 121517. [Google Scholar] [CrossRef]
- Bharati, B.; Sonkar, A.K.; Singh, N.; Dash, D.; Rath, C. Enhanced photocatalytic degradation of dyes under sunlight using biocompatible TiO2 nanoparticles. Mater. Res. Express 2017, 4, 085503. [Google Scholar] [CrossRef]
- Wei, Z.X.; Wang, Y.; Liu, J.P.; Xiao, C.M.; Zeng, W.W.; Ye, S.B. Synthesis, magnetization, and photocatalytic activity of LaFeO3 and LaFe0.9Mn0.1O3−δ. J. Mater. Sci. 2013, 48, 1117–1126. [Google Scholar] [CrossRef]
- Tummino, M.L.; Laurenti, E.; Deganello, F.; Prevot, A.B.; Magnacca, G. Revisiting the catalytic activity of a doped SrFeO3 for water pollutants removal: Effect of light and temperature. Appl. Catal. B Environ. 2017, 207, 174–181. [Google Scholar] [CrossRef]
- Shirazi, P.; Rahbar, M.; Behpour, M.; Ashrafi, M. La2MnTiO6 double perovskite nanostructures as highly efficient visible light photocatalysts. New J. Chem. 2020, 44, 231–238. [Google Scholar] [CrossRef]
- Thirumalairajan, S.; Girija, K.; Hebalkar, N.Y.; Mangalaraj, D.; Viswanathan, C.; Ponpandian, N. Shape evolution of perovskite LaFeO3 nanostructures: A systematic investigation of growth mechanism, properties and morphology dependent photocatalytic activities. RSC Adv. 2013, 3, 7549–7561. [Google Scholar] [CrossRef]
- Ghiasi, M.; Malekzadeh, A. Solar photocatalytic degradation of methyl orange over La0.7Sr0.3MnO3 nano-perovskite. Sep. Purif. Technol. 2014, 134, 12–19. [Google Scholar] [CrossRef]
- Feng, Y.N.; Wang, H.C.; Luo, Y.D.; Shen, Y.; Lin, Y. Ferromagnetic and photocatalytic behaviors observed in Ca-doped BiFeO3 nanofibres. J. App. Phys. 2013, 113, 146101. [Google Scholar] [CrossRef]
- Wei, K.; Yousef, F.; Yao, G.; Xie, R.; Lai, B. Strategies for improving perovskite photocatalysts reactivity for organic pollutants degradation: A review on recent progress. Chem. Eng. J. 2021, 414, 128783. [Google Scholar] [CrossRef]
- Nunes, M.J.; Lopes, A.; Pacheco, M.J.; Ciríaco, L.; Fiadeiro, P.T. Photocatalytic degradation of the AO7 under visible light with Bi-doped SrTiO3. In Textiles, Identity and Innovation: In Touch; Montagna, G., Carvalho, C., Eds.; Taylor & Francis Group: London, UK, 2020; pp. 349–355. [Google Scholar] [CrossRef]
- Guan, S.; Yang, H.; Sun, X.; Xian, T. Preparation and promising application of novel LaFeO3/BiOBr heterojunction photocatalysts for photocatalytic and photo-Fenton removal of dyes. Opt. Mater. 2020, 100, 109644. [Google Scholar] [CrossRef]
- Carvalho, C.; Fernandes, A.; Lopes, A.; Pinheiro, H.; Gonçalves, I. Electrochemical degradation applied to the metabolites of Acid Orange 7 anaerobic biotreatment. Chemosphere 2007, 67, 1316–1324. [Google Scholar] [CrossRef]
- Shao, S.; Zhang, J.; Zhang, Z.; Zheng, P.; Zhao, M.; Li, J.; Wang, C. High piezoelectric properties and domain configuration in BaTiO3 ceramics obtained through the solid-state reaction route. J. Phys. D-Appl. Phys. 2009, 42, 189801. [Google Scholar] [CrossRef]
- Vinothini, V.; Singh, P.; Balasubramanian, M. Synthesis of barium titanate nanopowder using polymeric precursor method. Ceram. Int. 2006, 32, 99–103. [Google Scholar] [CrossRef]
- Kakihana, M.; Okubo, T.; Arima, M.; Uchiyama, O.; Yashima, M.; Yoshimura, M.; Nakamura, Y. Polymerized complex synthesis of perovskite lead titanate at reduced temperatures: Possible formation of heterometallic (Pb,Ti)-citric acid complex. Chem. Mater. 1997, 9, 451–456. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Nunes, M.J.; Lopes, A.; Silva, J.N.; Ciríaco, L.; Pacheco, M.J. Electrodegradation of naphthalenic amines: Influence of the relative position of the substituent groups, anode material and electrolyte on the degradation products and kinetics. Chemosphere 2018, 205, 433–442. [Google Scholar] [CrossRef]
- Holland, T.J.B.; Redfern, S.A.T. Unit cell refinement from powder diffraction data: The use of regression diagnostics. Mineral. Mag. 1997, 61, 65–77. [Google Scholar] [CrossRef]
- Pacheco, M.J.; Santos, V.; Ciríaco, L.; Lopes, A. Electrochemical degradation of aromatic amines on BDD electrodes. J. Hazard. Mat. 2011, 186, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.S.; Jorge, M.E.M.; Ciríaco, L.; Pacheco, M.J.; Lopes, A. Perovskites (La,Ba)(Fe,Ti)O3: AO7 photocatalysis under visible light. Rev. Adv. Mater. Sci. 2020, 59, 151–159. [Google Scholar] [CrossRef]
- Bresolin, B.M.; Hammouda, S.B.; Sillanpää, M. Methylammonium iodo bismuthate perovskite (CH3NH3)3Bi2I9 as new effective visible light-responsive photocatalyst for degradation of environment pollutants. J. Photochem. Photobiol. A Chem. 2019, 376, 116–126. [Google Scholar] [CrossRef]
- Kappadan, S.; Gebreab, T.W.; Thomas, S.; Kalarikkal, N. Tetragonal BaTiO3 nanoparticles: An efficient photocatalyst for the degradation of organic pollutants. Mater. Sci. Semicond. Process. 2016, 51, 42–47. [Google Scholar] [CrossRef]
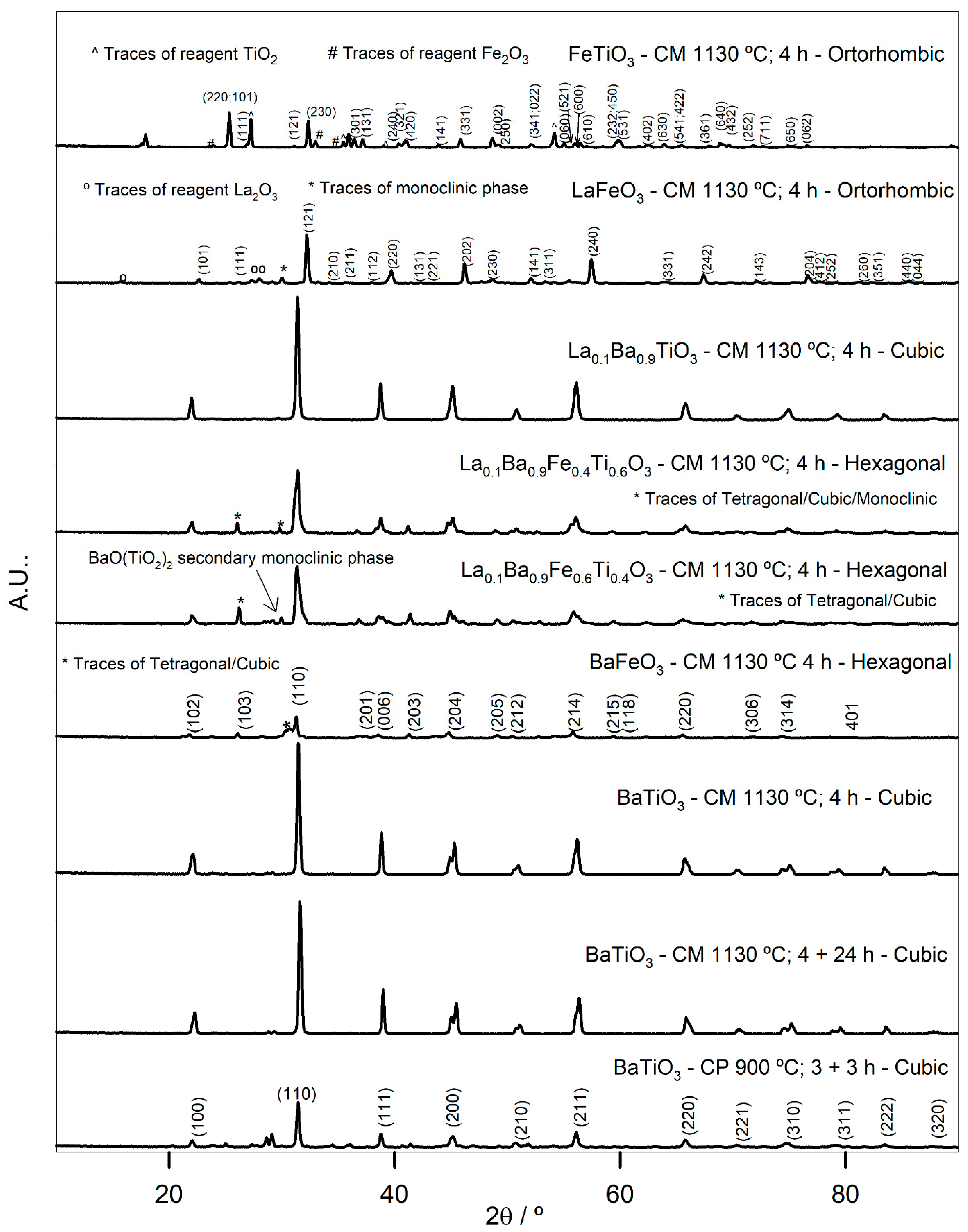
| Perovskite/Preparation Method 1 | Crystallite Size/nm | Unit Cell 2 | Cell Parameters/nm | Eg/eV | EDS | SEM |
|---|---|---|---|---|---|---|
| FeTiO3 CM | 70.74 | Orthorhombic | a = 0.9783 b = 1.0010 c = 0.3745 | 2.20 | Fe/Ti = 0.7 |  |
| LaFeO3 CM | 32.15 | Orthorhombic | a = 0.5571 b = 0.7842 c = 0.5560 | 2.30 | La/Fe = 1.3 |  |
| La0.1Ba0.9TiO3 CM | 55.12 | Cubic | a = 0.4009 | 3.29 | Ba/La = 10.5 Ti/La = 13.5 |  |
| La0.1Ba0.9Fe0.4Ti0.6O3 CM | 36.36 | Hexagonal | a = 0.5670 c = 1.4030 | 3.23 | Ba/La = 7.6 Ti/La = 13.5 Ti/Fe = 3.7 |  |
| La0.1Ba0.9Fe0.6Ti0.4O3 CM | 38.66 | Hexagonal | a = 0.5698 c = 1.4004 | 2.89 | Ba/La = 6.5 Ti/La = 4.9 Ti/Fe = 1.6 |  |
| BaFeO3 CM | 36.37 | Hexagonal | a = 0.5673 c = 1.4039 | 2.96 | Ba/Fe = 1.06 |  |
| BaTiO3 CM | 55.16 | Cubic | a = 0.4007 | 3.24 | Ba/Ti = 0.85 |  |
| BaTiO3 CP | 55.76 | Cubic | a = 0.4014 | 3.24 | Ba/Ti = 0.96 |  |
| Catalyst | AN, SA and Carboxylic Acids Final Concentration 1 | AO7 Removal/% | ||||
|---|---|---|---|---|---|---|
| AN | SA | Maleic Acid | Oxamic Acid | Acetic Acid | ||
| -- (Photolysis) | − | ++ | − | − | − | 46 |
| FeTiO3, CM_1130 °C, 4 h | ++ | + | − | − | − | 17 |
| LaFeO3, CM_1130 °C, 4 h | ++ | + | − | − | − | 36 |
| La0.1Ba0.9TiO3, CM_1130 °C, 4 h | ++ | + | − | − | − | 28 |
| La0.1Ba0.9Fe0.4Ti0.6O3, CM_1130 °C, 4 h | ++ | + | ++ | +++ | − | 20 |
| La0.1Ba0.9Fe0.6Ti0.4O3, CM_1130 °C, 4 h | ++ | + | − | ++ | − | 21 |
| BaFeO3, CM_1130 °C, 4 h | ++ | + | − | − | + | 74 |
| BaTiO3, CM_1130 °C, 4 h | ++ | + | − | − | − | 28 |
| BaTiO3, CM_1130 °C, 4 + 24 h | ++ | + | − | − | − | 24 |
| BaTiO3, CP_900 °C, 3 + 3 h | ++ | + | ++ | − | − | 53 |
| Type of Assay | [AO7]0/ mg L−1 | AN, SA and Carboxylic Acids Final Concentration 1 | AO7 Absolute Removal/mg L−1 (AO7 Removal/%) | ||||
|---|---|---|---|---|---|---|---|
| AN | SA | Maleic Acid | Oxamic Acid | Acetic Acid | |||
| Photolysis | 5 | − | − | − | − | − | 2.3 (46) |
| 10 | − | ++ | − | ++ | − | 4.0 (40) | |
| 20 | ++ | ++ | − | − | − | 7.2 (36) | |
| Adsorption | 5 | n.d. 2 | n.d. | n.d. | n.d. | n.d. | 0.6 (11) |
| 10 | n.d. | n.d. | n.d. | n.d. | n.d. | 1.8 (18) | |
| 20 | n.d. | n.d. | n.d. | n.d. | n.d. | 3.6 (18) | |
| Photocatalysis | 5 | ++ | ++ | − | − | + | 3.7 (74) |
| 10 | ++ | ++ | ++ | +++ | + | 8.4 (84) | |
| 20 | ++ | ++ | − | +++ | + | 17.4 (87) | |
| Catalyst | [Catalyst]/ mg L−1 | AN, SA and Carboxylic Acids Final Concentration 1 | AO7 Absolute Removal/mg L−1 (AO7 Removal/%) | ||||
|---|---|---|---|---|---|---|---|
| AN | SA | Maleic Acid | Oxamic Acid | Acetic Acid | |||
| BaFeO3_CM_1130 °C_4 h | 250 | ++ | ++ | − | ++ | + | 0.3 (6) |
| 500 | ++ | ++ | − | − | + | 3.7 (74) | |
| 750 | ++ | + | ++ | ++ | + | 4.6 (92) | |
| BaTiO3_CP_ 900 °C_3 + 3 h | 250 | − | + | ++ | ++ | − | 0.8 (15) |
| 500 | ++ | + | ++ | − | − | 2.7 (53) | |
| 750 | − | + | − | − | − | 1.6 (32) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, A.S.; Ciríaco, L.; Pacheco, M.J.; Fernandes, A.; Mogo, S.; Lopes, A. Sunlight-Driven AO7 Degradation with Perovskites (La,Ba)(Fe,Ti)O3 as Heterogeneous Photocatalysts. Nanomaterials 2021, 11, 3142. https://doi.org/10.3390/nano11113142
Rodrigues AS, Ciríaco L, Pacheco MJ, Fernandes A, Mogo S, Lopes A. Sunlight-Driven AO7 Degradation with Perovskites (La,Ba)(Fe,Ti)O3 as Heterogeneous Photocatalysts. Nanomaterials. 2021; 11(11):3142. https://doi.org/10.3390/nano11113142
Chicago/Turabian StyleRodrigues, Ana Sofia, Lurdes Ciríaco, Maria José Pacheco, Annabel Fernandes, Sandra Mogo, and Ana Lopes. 2021. "Sunlight-Driven AO7 Degradation with Perovskites (La,Ba)(Fe,Ti)O3 as Heterogeneous Photocatalysts" Nanomaterials 11, no. 11: 3142. https://doi.org/10.3390/nano11113142
APA StyleRodrigues, A. S., Ciríaco, L., Pacheco, M. J., Fernandes, A., Mogo, S., & Lopes, A. (2021). Sunlight-Driven AO7 Degradation with Perovskites (La,Ba)(Fe,Ti)O3 as Heterogeneous Photocatalysts. Nanomaterials, 11(11), 3142. https://doi.org/10.3390/nano11113142








