Challenges and Recent Advances in Enzyme-Mediated Wastewater Remediation—A Review
Abstract
:1. Introduction
2. Removal of Emerging Pollutants by Various Methods
2.1. Physiochemical Methods
2.2. Biological Methods
2.2.1. Microbial Bioremediation
2.2.2. Enzymatic Bioremediation (The Use of Peroxidase and Laccase Enzymes)
3. Enzyme Immobilization
4. Major Challenges and Recent Progress with Enzyme-Based Approaches
4.1. Stability
4.1.1. Stability of Immobilized Enzymes
4.1.2. Enzyme Engineering and Evolution
4.2. Recyclability and Reusability
4.2.1. Immobilization on Membranes
4.2.2. Immobilization on Solid Supports
4.3. Cost
4.4. Scaling-Up and Bioreactors
4.4.1. Fixed Bed Reactors
4.4.2. Fluidized-Bed Reactors
4.4.3. Stirred Tank Bioreactors
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Petrovic, M. Analysis and removal of emerging contaminants in wastewater and drinking water. TrAC Trends Anal. Chem. 2003, 22, 685–696. [Google Scholar] [CrossRef] [Green Version]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Tovar-Sánchez, A.; Sánchez-Quiles, D.; Basterretxea, G.; Benedé, J.L.; Chisvert, A.; Salvador, A.; Moreno-Garrido, I.; Blasco, J. Sunscreen Products as Emerging Pollutants to Coastal Waters. PLoS ONE 2013, 8, e65451. [Google Scholar] [CrossRef] [Green Version]
- Farre, M.; Pérez, S.; Kantiani, L.; Barceló, D. Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment. TrAC Trends Anal. Chem. 2008, 27, 991–1007. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef]
- Bonefeld-Jorgensen, E.C.; Long, M.; Bossi, R.; Ayotte, P.; Asmund, G.; Krüger, T.; Ghisari, M.; Mulvad, G.; Kern, P.; Nzulumiki, P.; et al. Perfluorinated compounds are related to breast cancer risk in greenlandic inuit: A case control study. Environ. Health 2011, 10, 88. [Google Scholar] [CrossRef] [Green Version]
- Corbel, V.; Stankiewicz, M.; Pennetier, C.; Fournier, D.; Stojan, J.; Girard, E.; Dimitrov, M.; Molgó, J.; Hougard, J.-M.; Lapied, B. Evidence for inhibition of cholinesterases in insect and mammalian nervous systems by the insect repellent deet. BMC Biol. 2009, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Barnes, K.K.; Kolpin, D.W.; Furlong, E.; Zaugg, S.D.; Meyer, M.; Barber, L.B. A national reconnaissance of pharmaceuticals and other organic wastewater contaminants in the United States—I) Groundwater. Sci. Total Environ. 2008, 402, 192–200. [Google Scholar] [CrossRef]
- Sorensen, J.; Lapworth, D.; Nkhuwa, D.; Stuart, M.; Gooddy, D.; Bell, R.; Chirwa, M.; Kabika, J.; Liemisa, M.; Chibesa, M.; et al. Emerging contaminants in urban groundwater sources in Africa. Water Res. 2014, 72, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Rodil, R.; Quintana, J.B.; Concha-Graña, E.; López-Mahía, P.; Muniategui, S.; Prada-Rodríguez, D. Emerging pollutants in sewage, surface and drinking water in Galicia (NW Spain). Chemosphere 2012, 86, 1040–1049. [Google Scholar] [CrossRef]
- Peña-Guzmán, C.; Ulloa-Sánchez, S.; Mora, K.; Helena-Bustos, R.; López, E.; Alvarez, J.; Rodriguez-Pinzón, M. Emerging pollutants in the urban water cycle in Latin America: A review of the current literature. J. Environ. Manag. 2019, 237, 408–423. [Google Scholar] [CrossRef]
- Papadakis, E.-N.; Tsaboula, A.; Kotopoulou, A.; Kintzikoglou, K.; Vryzas, Z.; Papadopoulou-Mourkidou, E. Pesticides in the surface waters of Lake Vistonis Basin, Greece: Occurrence and environmental risk assessment. Sci. Total Environ. 2015, 536, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; He, Y.; Jekel, M.; Reinhard, M.; Gin, K.Y.-H. Emerging contaminants of public health significance as water quality indicator compounds in the urban water cycle. Environ. Int. 2014, 71, 46–62. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sanchez-Polo, M.; García, M.F.; Prados-Joya, G.; Perez, R.O. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Drugs in the environment: Emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources—A review. Chemosphere 2001, 45, 957–969. [Google Scholar] [CrossRef]
- Montes-Grajales, D.; Fennix-Agudelo, M.A.; Miranda-Castro, W. Occurrence of personal care products as emerging chemicals of concern in water resources: A review. Sci. Total Environ. 2017, 595, 601–614. [Google Scholar] [CrossRef]
- Postigo, C.; Barceló, D. Synthetic organic compounds and their transformation products in groundwater: Occurrence, fate and mitigation. Sci. Total Environ. 2015, 503–504, 32–47. [Google Scholar] [CrossRef]
- Azzouz, A.; Ballesteros, E. Influence of seasonal climate differences on the pharmaceutical, hormone and personal care product removal efficiency of a drinking water treatment plant. Chemosphere 2013, 93, 2046–2054. [Google Scholar] [CrossRef]
- Teodosiu, C.; Gilca, A.-F.; Barjoveanu, G.; Fiore, S. Emerging pollutants removal through advanced drinking water treatment: A review on processes and environmental performances assessment. J. Clean. Prod. 2018, 197, 1210–1221. [Google Scholar] [CrossRef]
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Al-Maqdi, K.A.; Hisaindee, S.; Rauf, M.A.; Ashraf, S.S. Detoxification and degradation of sulfamethoxazole by soybean peroxidase and UV + H2O2 remediation approaches. Chem. Eng. J. 2018, 352, 450–458. [Google Scholar] [CrossRef]
- Gupta, V. Suhas Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, Y.; Zhou, A.; Wang, A.; Wu, Z.; Lv, L.; Li, X.; Zhang, K.; Zhu, T. Removal of azo dye in an up-flow membrane-less bioelectrochemical system integrated with bio-contact oxidation reactor. Chem. Eng. J. 2017, 326, 454–461. [Google Scholar] [CrossRef]
- Sophia, A.C.; Lima, E.C. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S. Application of adsorption process for effective removal of emerging contaminants from water and wastewater. Environ. Pollut. 2021, 280, 116995. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Vikrant, K.; Giri, B.S.; Raza, N.; Roy, K.; Kim, K.-H.; Rai, B.N.; Singh, R.S. Recent advancements in bioremediation of dye: Current status and challenges. Bioresour. Technol. 2018, 253, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Rondon, H.; El-Cheikh, W.; Boluarte, I.A.R.; Chang, C.-Y.; Bagshaw, S.; Farago, L.; Jegatheesan, V.; Shu, L. Application of enhanced membrane bioreactor (eMBR) to treat dye wastewater. Bioresour. Technol. 2015, 183, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Dastidar, M.G.; Sreekrishnan, T. Bioremediation of chromium complex dyes and treatment of sludge generated during the process. Int. Biodeterior. Biodegrad. 2017, 119, 448–460. [Google Scholar] [CrossRef]
- Ito, T.; Adachi, Y.; Yamanashi, Y.; Shimada, Y. Long–term natural remediation process in textile dye–polluted river sediment driven by bacterial community changes. Water Res. 2016, 100, 458–465. [Google Scholar] [CrossRef]
- Rauf, M.A.; Ashraf, S.S. Survey of recent trends in biochemically assisted degradation of dyes. Chem. Eng. J. 2012, 209, 520–530. [Google Scholar] [CrossRef]
- Bharagava, R.N.; Purchase, D.; Saxena, G.; Mulla, S.I. Chapter 26—Applications of Metagenomics in Microbial Bioremediation of Pollutants: From Genomics to Environmental Cleanup. In Microbial Diversity in the Genomic Era; Das, S., Dash, H.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 459–477. [Google Scholar] [CrossRef]
- Timmis, K.N.; Pieper, D.H. Bacteria designed for bioremediation. Trends Biotechnol. 1999, 17, 201–204. [Google Scholar] [CrossRef]
- Alhefeiti, M.A.; Athamneh, K.; Vijayan, R.; Ashraf, S.S. Bioremediation of various aromatic and emerging pollutants by Bacillus cereus sp. isolated from petroleum sludge. Water Sci. Technol. 2021, 83, 1535–1547. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Malik, A. Degradation of Reactive Black 5 dye by a newly isolated bacterium Pseudomonas entomophila BS1. Can. J. Microbiol. 2016, 62, 220–232. [Google Scholar] [CrossRef] [Green Version]
- Lade, H.; Kadam, A.; Paul, D.; Govindwar, S. Biodegradation and detoxification of textile azo dyes by bacterial consortium under sequential microaerophilic/aerobic processes. EXCLI J. 2015, 14, 158–174. [Google Scholar] [CrossRef]
- Nnenna, F.-P.; Lekiah, P.; Obemeata, O. Degradation of antibiotics by bacteria and fungi from the aquatic environment. JTEHS 2011, 3, 275–285. [Google Scholar] [CrossRef]
- Yoo, E.S.; Libra, J.; Adrian, L. Mechanism of Decolorization of Azo Dyes in Anaerobic Mixed Culture. J. Environ. Eng. 2001, 127, 844–849. [Google Scholar] [CrossRef]
- Chen, K.-C.; Wu, J.-Y.; Liou, D.-J.; Hwang, S.-C.J. Decolorization of the textile dyes by newly isolated bacterial strains. J. Biotechnol. 2003, 101, 57–68. [Google Scholar] [CrossRef]
- Srivastava, J.; Naraian, R.; Kalra, S.J.S.; Chandra, H. Advances in microbial bioremediation and the factors influencing the process. Int. J. Environ. Sci. Technol. 2013, 11, 1787–1800. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, M.; da Fonseca, M.M.; De Carvalho, C.C.C.R. Bioaugmentation and biostimulation strategies to improve the effectiveness of bioremediation processes. Biodegradation 2010, 22, 231–241. [Google Scholar] [CrossRef]
- Mishra, M.; Singh, S.K.; Kumar, A. Chapter 32—Role of omics approaches in microbial bioremediation. In Microbe Mediated Remediation of Environmental Contaminants; Kumar, A., Singh, V.K., Singh, P., Mishra, V.K., Eds.; Woodhead Publishing: Southston, UK, 2021; pp. 435–445. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Sharma, K. Microbial Biodiversity and Bioremediation Assessment Through Omics Approaches. Front. Environ. Chem. 2020, 1, 9. [Google Scholar] [CrossRef]
- Imran, M.; Asad, M.J.; Hadri, S.H.; Mehmood, S. Production and industrial applications of laccase enzyme. J. Cell Mol. Biol. 2012, 10, 1–11. [Google Scholar]
- Mayer, A. Laccase: New functions for an old enzyme. Phytochemistry 2002, 60, 551–565. [Google Scholar] [CrossRef]
- Asadgol, Z.; Forootanfar, H.; Rezaei, S.; Mahvi, A.H.; Faramarzi, M.A. Removal of phenol and bisphenol-A catalyzed by laccase in aqueous solution. J. Environ. Health Sci. Eng. 2014, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Hongyan, L.; Zexiong, Z.; Shiwei, X.; He, X.; Yinian, Z.; Haiyun, L.; Zhongsheng, Y. Study on transformation and degradation of bisphenol A by Trametes versicolor laccase and simulation of molecular docking. Chemosphere 2019, 224, 743–750. [Google Scholar] [CrossRef]
- Auriol, M.; Filali-Meknassi, Y.; Tyagi, R.D.; Adams, C.D. Laccase-catalyzed conversion of natural and synthetic hormones from a municipal wastewater. Water Res. 2007, 41, 3281–3288. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, K.; Chang, Y.-S.; Kim, Y.-M.; Jeon, J.-R.; Kim, E.-J. Enhanced transformation of triclosan by laccase in the presence of redox mediators. Water Res. 2010, 44, 298–308. [Google Scholar] [CrossRef]
- Singh, R.L.; Singh, P.K.; Singh, R.P. Enzymatic decolorization and degradation of azo dyes—A review. Int. Biodeterior. Biodegrad. 2015, 104, 21–31. [Google Scholar] [CrossRef]
- Teerapatsakul, C.; Parra, R.; Keshavarz, T.; Chitradon, L. Repeated batch for dye degradation in an airlift bioreactor by laccase entrapped in copper alginate. Int. Biodeterior. Biodegrad. 2017, 120, 52–57. [Google Scholar] [CrossRef]
- Alneyadi, A.; Rauf, M.A.; Ashraf, S.S. Oxidoreductases for the remediation of organic pollutants in water—A critical review. Crit. Rev. Biotechnol. 2018, 38, 971–988. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Parra, R.; Hu, H.; Wang, W.; Zhang, X.; Iqbal, H.M. Immobilized ligninolytic enzymes: An innovative and environmental responsive technology to tackle dye-based industrial pollutants—A review. Sci. Total Environ. 2016, 576, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Rasheed, T.; Iqbal, H.; Hu, H.; Wang, W.; Zhang, X. Horseradish peroxidase immobilization by copolymerization into cross-linked polyacrylamide gel and its dye degradation and detoxification potential. Int. J. Biol. Macromol. 2018, 113, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Almaqdi, K.A.; Morsi, R.; Alhayuti, B.; Alharthi, F.; Ashraf, S.S. LC-MSMS based screening of emerging pollutant degradation by different peroxidases. BMC Biotechnol. 2019, 19, 83. [Google Scholar] [CrossRef] [PubMed]
- Morsi, R.; Al-Maqdi, K.; Bilal, M.; Iqbal, H.; Khaleel, A.; Shah, I.; Ashraf, S. Immobilized Soybean Peroxidase Hybrid Biocatalysts for Efficient Degradation of Various Emerging Pollutants. Biomolecules 2021, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- l-Maqdi, K.A.; Hisaindee, S.M.; Rauf, M.A.; Ashraf, S.S. Comparative Degradation of a Thiazole Pollutant by an Advanced Oxidation Process and an Enzymatic Approach. Biomolecules 2017, 7, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, S.; Christena, R.; Rajaram, Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, R.A. Enzyme Immobilization: The Quest for Optimum Performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Gholami-Borujeni, F.; Mahvi, A.H.; Naseri, S.; Faramarzi, M.A.; Nabizadeh, R.; Alimohammadi, M. Application of immobilized horseradish peroxidase for removal and detoxification of azo dye from aqueous solution. Res. J. Chem. Environ. 2011, 15, 217–222. [Google Scholar]
- Kim, J.; Grate, J.W.; Wang, P. Nanobiocatalysis and its potential applications. Trends Biotechnol. 2008, 26, 639–646. [Google Scholar] [CrossRef]
- dos Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Rodrigues, R.C.; Fernandez-Lafuente, R. Importance of the Support Properties for Immobilization or Purification of Enzymes. ChemCatChem 2015, 7, 2413–2432. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; A Cargill, A.; Medintz, I.L.; Claussen, J.C. Increasing the activity of immobilized enzymes with nanoparticle conjugation. Curr. Opin. Biotechnol. 2015, 34, 242–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, K.; Fernandez-Lafuente, R. Control of protein immobilization: Coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzym. Microb. Technol. 2011, 48, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.K.H.; Tan, H.K.; Lau, S.Y.; Yap, P.-S.; Danquah, M.K. Potential and challenges of enzyme incorporated nanotechnology in dye wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103261. [Google Scholar] [CrossRef]
- Lee, S.H.; Doan, T.T.N.; Won, K.; Ha, S.H.; Koo, Y.-M. Immobilization of lipase within carbon nanotube–silica composites for non-aqueous reaction systems. J. Mol. Catal. B Enzym. 2010, 62, 169–172. [Google Scholar] [CrossRef]
- Salis, A.; Casula, M.F.; Bhattacharyya, M.S.; Pinna, M.; Solinas, V.; Monduzzi, M. Physical and Chemical Lipase Adsorption on SBA-15: Effect of Different Interactions on Enzyme Loading and Catalytic Performance. ChemCatChem 2010, 2, 322–329. [Google Scholar] [CrossRef]
- Hartmann, M.; Kostrov, X. Immobilization of enzymes on porous silicas—Benefits and challenges. Chem. Soc. Rev. 2013, 42, 6277–6289. [Google Scholar] [CrossRef]
- Bhattacharyya, M.; Singh, A.; Banerjee, U. Immobilization of intracellular carbonyl reductase from Geotrichum candidum for the stereoselective reduction of 1-naphthyl ketone. Bioresour. Technol. 2010, 101, 1581–1586. [Google Scholar] [CrossRef]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilizations: A review. Enzym. Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Pesic, M.; López, C.; Alvaro, G.; Lopez-Santin, J. A novel immobilized chloroperoxidase biocatalyst with improved stability for the oxidation of amino alcohols to amino aldehydes. J. Mol. Catal. B Enzym. 2012, 84, 144–151. [Google Scholar] [CrossRef]
- Cho, E.J.; Jung, S.; Kim, H.J.; Lee, Y.G.; Nam, K.C.; Lee, H.-J.; Bae, H.-J. Co-immobilization of three cellulases on Au-doped magnetic silicananoparticles for the degradation of cellulose. Chem. Commun. 2011, 48, 886–888. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.A.; Freitas, L.; de Carvalho, A.K.F.; de Oliveira, P.C.; de Castro, H.F. Immobilization of a Commercial Lipase from Penicillium camembertii (Lipase G) by Different Strategies. Enzym. Res. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lathouder, K.; van Benthem, D.; Wallin, S.; Mateo, C.; Lafuente, R.F.; Guisan, J.; Kapteijn, F.; Moulijn, J. Polyethyleneimine (PEI) functionalized ceramic monoliths as enzyme carriers: Preparation and performance. J. Mol. Catal. B Enzym. 2008, 50, 20–27. [Google Scholar] [CrossRef]
- Cui, R.; Bai, C.; Jiang, Y.; Hu, M.; Li, S.; Zhai, Q. Well-defined bioarchitecture for immobilization of chloroperoxidase on magnetic nanoparticles and its application in dye decolorization. Chem. Eng. J. 2015, 259, 640–646. [Google Scholar] [CrossRef]
- Jun, L.Y.; Yon, L.S.; Mubarak, N.; Bing, C.H.; Pan, S.; Danquah, M.K.; Abdullah, E.; Khalid, M. An overview of immobilized enzyme technologies for dye and phenolic removal from wastewater. J. Environ. Chem. Eng. 2019, 7, 102961. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.; Shah, S.Z.H.; Hu, H.; Wang, W.; Zhang, X. Horseradish peroxidase-assisted approach to decolorize and detoxify dye pollutants in a packed bed bioreactor. J. Environ. Manag. 2016, 183, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jin, X.; Long, N.; Zhang, R. Improved biodegradation of synthetic azo dye by horseradish peroxidase cross-linked on nano-composite support. Int. J. Biol. Macromol. 2017, 95, 1049–1055. [Google Scholar] [CrossRef]
- Alessa, A.H.A.; Tee, K.L.; Gonzalez-Perez, D.; Ali, H.E.M.O.; Evans, C.A.; Trevaskis, A.; Xu, J.-H.; Wong, T.S. Accelerated directed evolution of dye-decolorizing peroxidase using a bacterial extracellular protein secretion system (BENNY). Bioresour. Bioprocess. 2019, 6, 20. [Google Scholar] [CrossRef]
- Sivapragasam, M.; Moniruzzaman, M.; Goto, M. Recent advances in exploiting ionic liquids for biomolecules: Solubility, stability and applications. Biotechnol. J. 2016, 11, 1000–1013. [Google Scholar] [CrossRef]
- Ali, M.; Ishqi, H.M.; Husain, Q. Enzyme engineering: Reshaping the biocatalytic functions. Biotechnol. Bioeng. 2020, 117, 1877–1894. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. Developments in support materials for immobilization of oxidoreductases: A comprehensive review. Adv. Colloid Interface Sci. 2018, 258, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gupta, G.; Ahmad, T.; Mansoor, S.; Kaur, B. Enzyme Engineering: Current Trends and Future Perspectives. Food Rev. Int. 2019, 37, 121–154. [Google Scholar] [CrossRef]
- Rong, J.; Wang, Y.; Ni, L.; Zhang, W.; Li, C.; Han, J.; Zhou, Z. Immobilization of Horseradish Peroxidase on Multi-Armed Magnetic Graphene Oxide Composite. Food Technol. Biotechnol. 2019, 57, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Mazlan, S.Z.; Abu Hanifah, S. Effects of Temperature and pH on Immobilized Laccase Activity in Conjugated Methacrylate-Acrylate Microspheres. Int. J. Polym. Sci. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Samui, A.; Happy; Sahu, S.K. Integration of α-amylase into covalent organic framework for highly efficient biocatalyst. Microporous Mesoporous Mater. 2020, 291, 109700. [Google Scholar] [CrossRef]
- Wu, E.; Li, Y.; Huang, Q.; Yang, Z.; Wei, A.; Hu, Q. Laccase immobilization on amino-functionalized magnetic metal organic framework for phenolic compound removal. Chemosphere 2019, 233, 327–335. [Google Scholar] [CrossRef]
- Xu, P.; Liang, S.; Zong, M.-H.; Lou, W.-Y. Ionic liquids for regulating biocatalytic process: Achievements and perspectives. Biotechnol. Adv. 2021, 51, 107702. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2019, 297, 112038. [Google Scholar] [CrossRef]
- Gao, W.-W.; Zhang, F.-X.; Zhang, G.-X.; Zhou, C.-H. Key factors affecting the activity and stability of enzymes in ionic liquids and novel applications in biocatalysis. Biochem. Eng. J. 2015, 99, 67–84. [Google Scholar] [CrossRef]
- Naushad, M.; Alothman, Z.; Khan, A.B.; Ali, M. Effect of ionic liquid on activity, stability, and structure of enzymes: A review. Int. J. Biol. Macromol. 2012, 51, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Nao, Y.; Bhattacharjee, S.; Ono, T. Laccase Incorporated into PEG-PLA Polymer as Active and Stable Biocatalyst for Ionic Liquids Media. Appl. Mech. Mater. 2014, 625, 333–336. [Google Scholar] [CrossRef]
- Kumar, V.; Baweja, M.; Liu, H.; Shukla, P. Microbial Enzyme Engineering: Applications and Perspectives. In Recent Advances in Applied Microbiology; Shukla, P., Ed.; Springer: Singapore, 2017; pp. 259–273. [Google Scholar] [CrossRef]
- Steiner, K.; Schwab, H. RECENT ADVANCES IN RATIONAL APPROACHES FOR ENZYME ENGINEERING. Comput. Struct. Biotechnol. J. 2012, 2, e201209010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Zhang, S.; Gao, H.; Hu, N. Characterization of a cold-active esterase from Serratia sp. and improvement of thermostability by directed evolution. BMC Biotechnol. 2016, 16, 7. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, O.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef] [Green Version]
- Alneyadi, A.; Ashraf, S.S. Differential enzymatic degradation of thiazole pollutants by two different peroxidases—A comparative study. Chem. Eng. J. 2016, 303, 529–538. [Google Scholar] [CrossRef]
- Xing, H.; Zou, G.; Liu, C.; Chai, S.; Yan, X.; Li, X.; Liu, R.; Yang, Y.; Zhou, Z. Improving the thermostability of a GH11 xylanase by directed evolution and rational design guided by B-factor analysis. Enzym. Microb. Technol. 2020, 143, 109720. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Yang, G.-Y.; Zhang, Y.; Xie, Y.; Withers, S.G.; Feng, Y. A general and efficient strategy for generating the stable enzymes. Sci. Rep. 2016, 6, 33797. [Google Scholar] [CrossRef]
- ADotsenko, A.S.; Rozhkova, A.M.; Zorov, I.N.; Sinitsyn, A.P. Protein surface engineering of endoglucanase Penicillium verruculosum for improvement in thermostability and stability in the presence of 1-butyl-3-methylimidazolium chloride ionic liquid. Bioresour. Technol. 2019, 296, 122370. [Google Scholar] [CrossRef]
- Orrego, C.; Salgado, N.; Valencia, J.; Giraldo, G.; Giraldo, O.; Cardona, C. Novel chitosan membranes as support for lipases immobilization: Characterization aspects. Carbohydr. Polym. 2010, 79, 9–16. [Google Scholar] [CrossRef]
- Handayani, N.; Loos, K.; Wahyuningrum, D.; Buchari; Zulfikar, M.A. Immobilization of Mucor miehei Lipase onto Macroporous Aminated Polyethersulfone Membrane for Enzymatic Reactions. Membranes 2012, 2, 198–213. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Bhattacharya, A.; Murthy, C. Tune to immobilize lipases on polymer membranes: Techniques, factors and prospects. Biocatal. Agric. Biotechnol. 2013, 2, 171–190. [Google Scholar] [CrossRef]
- Liu, D.-M.; Chen, J.; Shi, Y.-P. Advances on methods and easy separated support materials for enzymes immobilization. TrAC Trends Anal. Chem. 2018, 102, 332–342. [Google Scholar] [CrossRef]
- Zucca, P.; Sanjust, E. Inorganic Materials as Supports for Covalent Enzyme Immobilization: Methods and Mechanisms. Molecules 2014, 19, 14139–14194. [Google Scholar] [CrossRef] [PubMed]
- Jesionowski, T.; Krysztafkiewicz, A. Preparation of the hydrophilic/hydrophobic silica particles. Colloids Surf. A: Physicochem. Eng. Asp. 2002, 207, 49–58. [Google Scholar] [CrossRef]
- Jesionowski, T. Preparation of colloidal silica from sodium metasilicate solution and sulphuric acid in emulsion medium. Colloids Surf. A Physicochem. Eng. Asp. 2001, 190, 153–165. [Google Scholar] [CrossRef]
- Kramer, M.; Cruz, J.C.; Pfromm, P.; Rezac, M.E.; Czermak, P. Enantioselective transesterification by Candida antarctica Lipase B immobilized on fumed silica. J. Biotechnol. 2010, 150, 80–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falahati, M.; Ma′Mani, L.; Saboury, A.A.; Shafiee, A.; Foroumadi, A.; Badiei, A.R. Aminopropyl-functionalized cubic Ia3d mesoporous silica nanoparticle as an efficient support for immobilization of superoxide dismutase. Biochim. Et Biophys. Acta (BBA) -Proteins Proteom. 2011, 1814, 1195–1202. [Google Scholar] [CrossRef]
- Foresti, M.; Valle, G.; Bonetto, R.; Ferreira, M.; Briand, L. FTIR, SEM and fractal dimension characterization of lipase B from Candida antarctica immobilized onto titania at selected conditions. Appl. Surf. Sci. 2009, 256, 1624–1635. [Google Scholar] [CrossRef]
- Yang, Z.; Si, S.; Zhang, C. Study on the activity and stability of urease immobilized onto nanoporous alumina membranes. Microporous Mesoporous Mater. 2008, 111, 359–366. [Google Scholar] [CrossRef]
- Siddeeg, S.M.; Tahoon, M.A.; Mnif, W.; Ben Rebah, F. Iron Oxide/Chitosan Magnetic Nanocomposite Immobilized Manganese Peroxidase for Decolorization of Textile Wastewater. Processes 2019, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, T.; Rizwan, K.; Bilal, M.; Iqbal, H.M.N. Metal-Organic Framework-Based Engineered Materials—Fundamentals and Applications. Molecules 2020, 25, 1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czaja, A.U.; Trukhan, N.; Müller, U. Industrial applications of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Rojas, S.; Horcajada, P. Metal–Organic Frameworks for the Removal of Emerging Organic Contaminants in Water. Chem. Rev. 2020, 120, 8378–8415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, Z.; Chen, B.; Liang, X. Phase-competition-driven formation of hierarchical FeNiZn-MIL-88B-on-MOF-5 octapods displaying high selectivity for the RWGS reaction. Chem. Commun. 2019, 55, 8450–8453. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Li, W.; Xu, P.; Xia, X.; Yu, H.; Zhang, S.; Li, X. In situ construction of metal–organic framework (MOF) UiO-66 film on Parylene-patterned resonant microcantilever for trace organophosphorus molecules detection. Analyst 2019, 144, 3729–3735. [Google Scholar] [CrossRef]
- Carrillo-Carrion, C.; Martínez, R.; Poupard, M.F.N.; Pelaz, B.; Polo, E.; Arenas-Vivo, A.; Olgiati, A.; Taboada, P.; Soliman, M.G.; Catalán, Ú.; et al. Aqueous Stable Gold Nanostar/ZIF-8 Nanocomposites for Light-Triggered Release of Active Cargo Inside Living Cells. Angew. Chem. Int. Ed. 2019, 58, 7078–7082. [Google Scholar] [CrossRef]
- Worrall, S.D.; Bissett, M.A.; Attfield, M.P.; Dryfe, R.A.W. Anodic dissolution growth of metal–organic framework HKUST-1 monitored via in situ electrochemical atomic force microscopy. CrystEngComm 2018, 20, 4421–4427. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Meng, L.; Shi, J.; Li, J.; Zhang, X.; Feng, M. Metal-organic frameworks/carbon-based materials for environmental remediation: A state-of-the-art mini-review. J. Environ. Manag. 2018, 232, 964–977. [Google Scholar] [CrossRef]
- Sarker, M.; Song, J.Y.; Jhung, S.H. Adsorptive removal of anti-inflammatory drugs from water using graphene oxide/metal-organic framework composites. Chem. Eng. J. 2018, 335, 74–81. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, J.; Zhang, W.; Liu, F.; Yue, X.; Liu, Y.; Yang, M.; Li, Z.; Wang, J. Interface engineering of metal organic framework on graphene oxide with enhanced adsorption capacity for organophosphorus pesticide. Chem. Eng. J. 2016, 313, 19–26. [Google Scholar] [CrossRef]
- Bayazit, S.; Yildiz, M.; Asci, Y.S.; Şahin, M.; Bener, M.; Eğlence, S.; Salam, M.A. Rapid adsorptive removal of naphthalene from water using graphene nanoplatelet/MIL-101 (Cr) nanocomposite. J. Alloy. Compd. 2017, 701, 740–749. [Google Scholar] [CrossRef]
- Xiong, W.; Zeng, G.; Yang, Z.; Zhou, Y.; Zhang, C.; Cheng, M.; Liu, Y.; Hu, L.; Wan, J.; Zhou, C.; et al. Adsorption of tetracycline antibiotics from aqueous solutions on nanocomposite multi-walled carbon nanotube functionalized MIL-53(Fe) as new adsorbent. Sci. Total Environ. 2018, 627, 235–244. [Google Scholar] [CrossRef]
- Wu, X.; Hou, M.; Ge, J. Metal–organic frameworks and inorganic nanoflowers: A type of emerging inorganic crystal nanocarrier for enzyme immobilization. Catal. Sci. Technol. 2015, 5, 5077–5085. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.-L.; Xiong, J.; Zong, M.-H.; Lou, W.-Y. Metal-organic frameworks as novel matrices for efficient enzyme immobilization: An update review. Co-Ord. Chem. Rev. 2019, 406, 213149. [Google Scholar] [CrossRef]
- Gao, X.; Zhai, Q.; Hu, M.; Li, S.; Song, J.; Jiang, Y. Design and preparation of stable CPO/HRP@H-MOF(Zr) composites for efficient bio-catalytic degradation of organic toxicants in wastewater. J. Chem. Technol. Biotechnol. 2018, 94, 1249–1258. [Google Scholar] [CrossRef]
- Gkaniatsou, E.; Sicard, C.; Ricoux, R.; Benahmed, L.; Bourdreux, F.; Zhang, Q.; Serre, C.; Mahy, J.; Steunou, N. Enzyme Encapsulation in Mesoporous Metal–Organic Frameworks for Selective Biodegradation of Harmful Dye Molecules. Angew. Chem. Int. Ed. 2018, 57, 16141–16146. [Google Scholar] [CrossRef]
- Li, S.-F.; Zhai, X.-J.; Zhang, C.; Mo, H.-L.; Zang, S.-Q. Enzyme immobilization in highly ordered macro–microporous metal–organic frameworks for rapid biodegradation of hazardous dyes. Inorg. Chem. Front. 2020, 7, 3146–3153. [Google Scholar] [CrossRef]
- Lyle, S.J.; Waller, P.J.; Yaghi, O.M. Covalent Organic Frameworks: Organic Chemistry Extended into Two and Three Dimensions. Trends Chem. 2019, 1, 172–184. [Google Scholar] [CrossRef]
- Oliveira, F.L.; França, A.D.S.; De Castro, A.M.; Souza, R.O.M.A.; Esteves, P.M.; Goncalves, R.S.B. Enzyme Immobilization in Covalent Organic Frameworks: Strategies and Applications in Biocatalysis. ChemPlusChem 2020, 85, 2051–2066. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Feng, B.; Xu, P.; Zeng, Q.; Shan, B.; Song, Y. Covalent organic frameworks and electron mediator-based open circuit potential biosensor for in vivo electrochemical measurements. Anal. Methods 2018, 10, 4320–4328. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Covalent organic frameworks (COFs) for environmental applications. Co-Ord. Chem. Rev. 2019, 400, 213046. [Google Scholar] [CrossRef]
- Gan, J.; Bagheri, A.R.; Aramesh, N.; Gul, I.; Franco, M.; Almulaiky, Y.Q.; Bilal, M. Covalent organic frameworks as emerging host platforms for enzyme immobilization and robust biocatalysis—A review. Int. J. Biol. Macromol. 2020, 167, 502–515. [Google Scholar] [CrossRef]
- Li, M.; Qiao, S.; Zheng, Y.; Andaloussi, Y.H.; Li, X.; Zhang, Z.; Li, A.; Cheng, P.; Ma, S.; Chen, Y. Fabricating Covalent Organic Framework Capsules with Commodious Microenvironment for Enzymes. J. Am. Chem. Soc. 2020, 142, 6675–6681. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Lei, J.; Zare, R.N. Protein–inorganic hybrid nanoflowers. Nat. Nanotechnol. 2012, 7, 428–432. [Google Scholar] [CrossRef]
- Al-Maqdi, K.; Bilal, M.; Alzamly, A.; Iqbal, H.; Shah, I.; Ashraf, S. Enzyme-Loaded Flower-Shaped Nanomaterials: A Versatile Platform with Biosensing, Biocatalytic, and Environmental Promise. Nanomaterials 2021, 11, 1460. [Google Scholar] [PubMed]
- Fu, M.; Xing, J.; Ge, Z. Preparation of laccase-loaded magnetic nanoflowers and their recycling for efficient degradation of bisphenol A. Sci. Total Environ. 2018, 651, 2857–2865. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Choi, H.; Lee, J.-K. Multimetal-Based Inorganic–Protein Hybrid System for Enzyme Immobilization. ACS Sustain. Chem. Eng. 2019, 7, 13633–13638. [Google Scholar] [CrossRef]
- Wang, S.; Ding, Y.; Chen, R.; Hu, M.; Li, S.; Zhai, Q.; Jiang, Y. Multilayer petal-like enzymatic-inorganic hybrid micro-spheres [CPO-(Cu/Co/Cd)3(PO4)2] with high bio-catalytic activity. Chem. Eng. Res. Des. 2018, 134, 52–61. [Google Scholar] [CrossRef]
- Rong, J.; Zhang, T.; Qiu, F.; Zhu, Y. Preparation of Efficient, Stable, and Reusable Laccase–Cu3(PO4)2 Hybrid Microspheres Based on Copper Foil for Decoloration of Congo Red. ACS Sustain. Chem. Eng. 2017, 5, 4468–4477. [Google Scholar] [CrossRef]
- Patel, S.K.; Otari, S.; Li, J.; Kim, D.R.; Kim, S.C.; Cho, B.-K.; Kalia, V.C.; Kang, Y.C.; Lee, J.-K. Synthesis of cross-linked protein-metal hybrid nanoflowers and its application in repeated batch decolorization of synthetic dyes. J. Hazard. Mater. 2018, 347, 442–450. [Google Scholar] [CrossRef]
- Altinkaynak, C.; Tavlasoglu, S.; Kalin, R.; Sadeghian, N.; Ozdemir, H.; Ocsoy, I.; Özdemir, N. A hierarchical assembly of flower-like hybrid Turkish black radish peroxidase-Cu 2+ nanobiocatalyst and its effective use in dye decolorization. Chemosphere 2017, 182, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Emli, S.; Ayadi-Zouari, D.; Ben Hlima, H.; Bejar, S. Biocatalysts: Application and engineering for industrial purposes. Crit. Rev. Biotechnol. 2014, 36, 246–258. [Google Scholar] [CrossRef]
- Global Markets for Enzymes in Industrial Applications, (n.d.). Available online: https://www.bccresearch.com/market-research/biotechnology/global-markets-for-enzymes-in-industrial-applications.html (accessed on 3 June 2021).
- Liu, Z.; Smith, S.R. Enzyme Recovery from Biological Wastewater Treatment. Waste Biomass Valorization 2020, 12, 4185–4211. [Google Scholar] [CrossRef]
- Raman, J.K.; Ting, V.F.W.; Pogaku, R. Life cycle assessment of biodiesel production using alkali, soluble and immobilized enzyme catalyst processes. Biomass Bioenergy 2011, 35, 4221–4229. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M. Sustainable bioconversion of food waste into high-value products by immobilized enzymes to meet bio-economy challenges and opportunities—A review. Food Res. Int. 2019, 123, 226–240. [Google Scholar] [CrossRef]
- Ferreira, R.; Azzoni, A.R.; Freitas, S. Techno-economic analysis of the industrial production of a low-cost enzyme using E. coli: The case of recombinant β-glucosidase. Biotechnol. Biofuels 2018, 11, 81. [Google Scholar] [CrossRef]
- Coconi-Linares, N.; Ortiz-Vázquez, E.; Fernández, F.; Loske, A.; Gómez-Lim, M.A. Recombinant expression of four oxidoreductases in Phanerochaete chrysosporium improves degradation of phenolic and non-phenolic substrates. J. Biotechnol. 2015, 209, 76–84. [Google Scholar] [CrossRef]
- Xu, H.; Guo, M.-Y.; Gao, Y.-H.; Bai, X.-H.; Zhou, X.-W. Expression and characteristics of manganese peroxidase from Ganoderma lucidum in Pichia pastoris and its application in the degradation of four dyes and phenol. BMC Biotechnol. 2017, 17, 19. [Google Scholar] [CrossRef] [Green Version]
- Recombinant Protein Expression Optimization in Escherichia coli: A Review. EBSCOhost, (n.d.). Available online: http://web.b.ebscohost.com.uaeu.idm.oclc.org/ehost/pdfviewer/pdfviewer?vid=1&sid=99a82b22-71a0-4402-a5b2-6ff56c3c93dc%40sessionmgr103 (accessed on 4 June 2021).
- Sóti, V.; Lenaerts, S.; Cornet, I. Of enzyme use in cost-effective high solid simultaneous saccharification and fermentation processes. J. Biotechnol. 2018, 270, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.-J. Recent advances in bioreactor engineering. Korean J. Chem. Eng. 2010, 27, 1035–1041. [Google Scholar] [CrossRef]
- Basso, A.; Serban, S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Illanes, A.; Altamirano, C. Enzyme Reactors. In Enzyme Biocatalysis: Principles and Applications; Illanes, A., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 205–251. [Google Scholar] [CrossRef]
- Hafeez, S.; Pallari, E.; Manos, G.; Constantinou, A. Constantinou, 6—Catalytic Conversion and Chemical Recovery. In Plastics to Energy; Al-Salem, S.M., Ed.; Plastics Design Library; William Andrew Publishing: New York, NY, USA, 2019; pp. 147–172. ISBN 978-0-12-813140-4. [Google Scholar]
- Worstell, J. Chapter 5—Scaling Fixed-Bed Reactors. In Adiabatic Fixed-Bed Reactors; Worstell, J., Ed.; Butterworth-Heinemann: Oxford, UK, 2014; pp. 81–108. [Google Scholar] [CrossRef]
- Mears, D. Diagnostic criteria for heat transport limitations in fixed bed reactors. J. Catal. 1971, 20, 127–131. [Google Scholar] [CrossRef]
- Palmieri, G.; Giardina, P.; Sannia, G. Laccase-Mediated Remazol Brilliant Blue R Decolorization in a Fixed-Bed Bioreactor. Biotechnol. Prog. 2005, 21, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Asgher, M.; Iqbal, H.M.; Hu, H.; Wang, W.; Zhang, X. Bio-catalytic performance and dye-based industrial pollutants degradation potential of agarose-immobilized MnP using a Packed Bed Reactor System. Int. J. Biol. Macromol. 2017, 102, 582–590. [Google Scholar] [CrossRef]
- Rajmohan, K.; Yadav, H.; Vaishnavi, S.; Gopinath, M.; Varjani, S. Chapter 23—Perspectives on Bio-oil Recovery from Plastic Waste. In Current Developments in Biotechnology and Bioengineering; Varjani, S., Pandey, A., Gnansounou, E., Khanal, S.K., Raveendran, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 459–480. [Google Scholar] [CrossRef]
- Zhong, J.-J. 2.14—Bioreactor Engineering. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 165–177. [Google Scholar] [CrossRef]
- Piao, M.; Zou, D.; Ren, X.; Gao, S.; Qin, C.; Piao, Y. High efficiency biotransformation of bisphenol A in a fluidized bed reactor using stabilized laccase in porous silica. Enzym. Microb. Technol. 2019, 126, 1–8. [Google Scholar] [CrossRef]
- Lloret, L.; Eibes, G.; Feijoo, G.; Moreira, M.T.; Lema, J. Continuous operation of a fluidized bed reactor for the removal of estrogens by immobilized laccase on Eupergit supports. J. Biotechnol. 2012, 162, 404–406. [Google Scholar] [CrossRef]
- Yang, C.; Mao, Z.-S. Chapter 3—Multiphase stirred reactors. In Numerical Simulation of Multiphase Reactors with Continuous Liquid Phase; Yang, C., Mao, Z.-S., Eds.; Academic Press: Oxford, UK, 2014; pp. 75–151. [Google Scholar] [CrossRef]
- López, C.; Mielgo, I.; Moreira, M.; Feijoo, G.; Lema, J. Enzymatic membrane reactors for biodegradation of recalcitrant compounds. Application to dye decolourisation. J. Biotechnol. 2002, 99, 249–257. [Google Scholar] [CrossRef]
- Flock, C.; Bassi, A.; Gijzen, M. Removal of aqueous phenol and 2-chlorophenol with purified soybean peroxidase and raw soybean hulls. J. Chem. Technol. Biotechnol. 1999, 74, 303–309. [Google Scholar] [CrossRef]
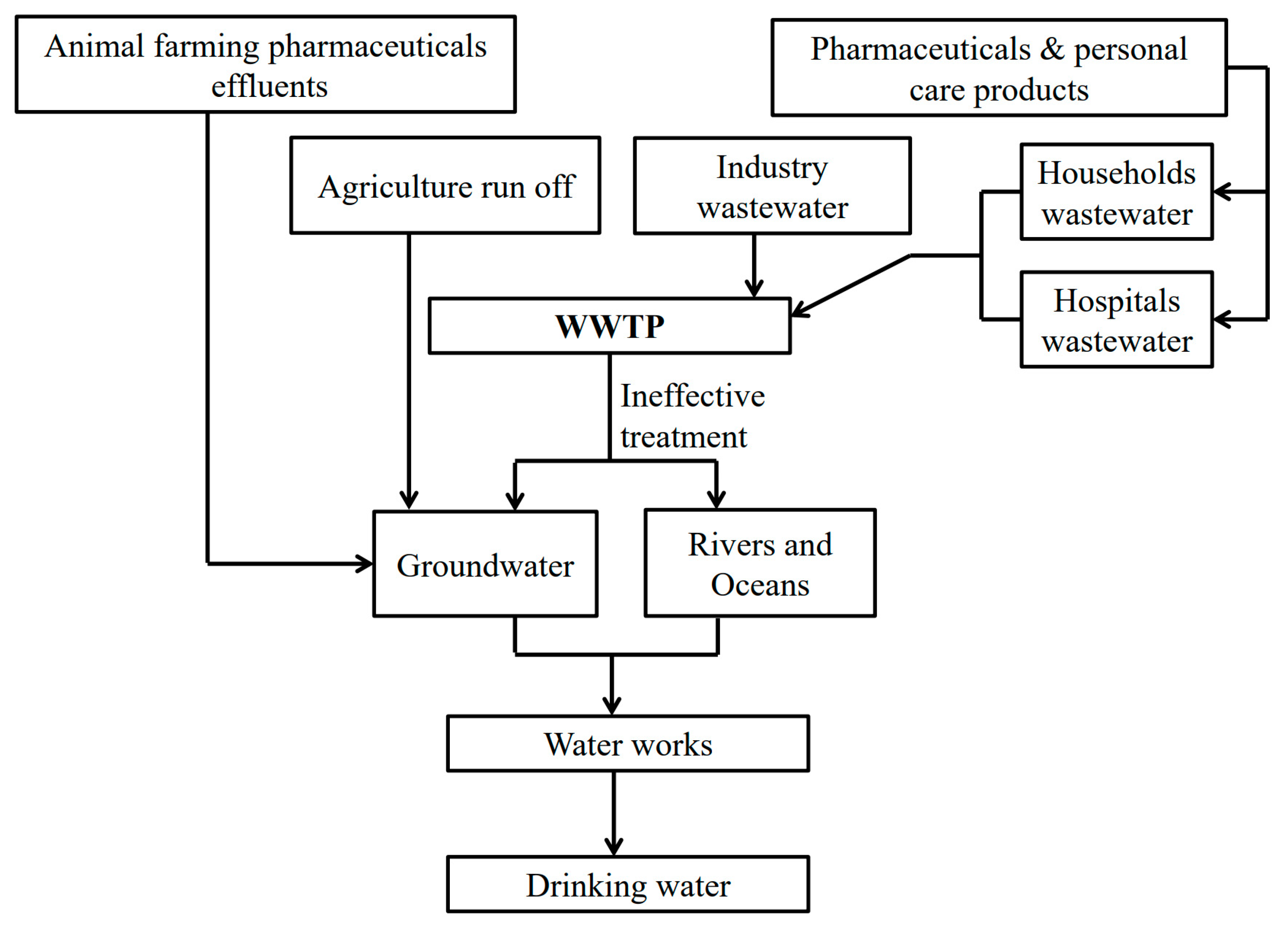
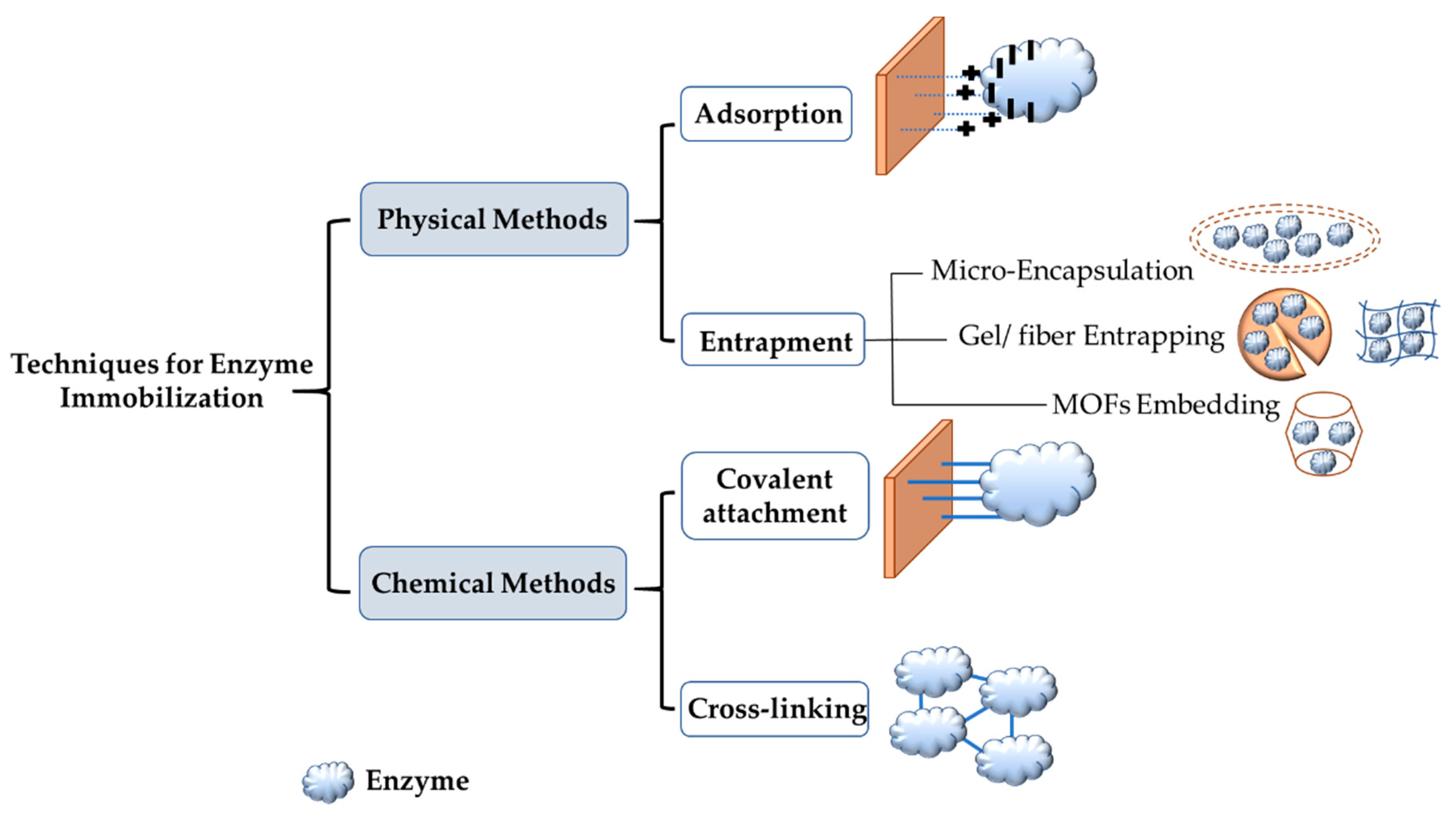
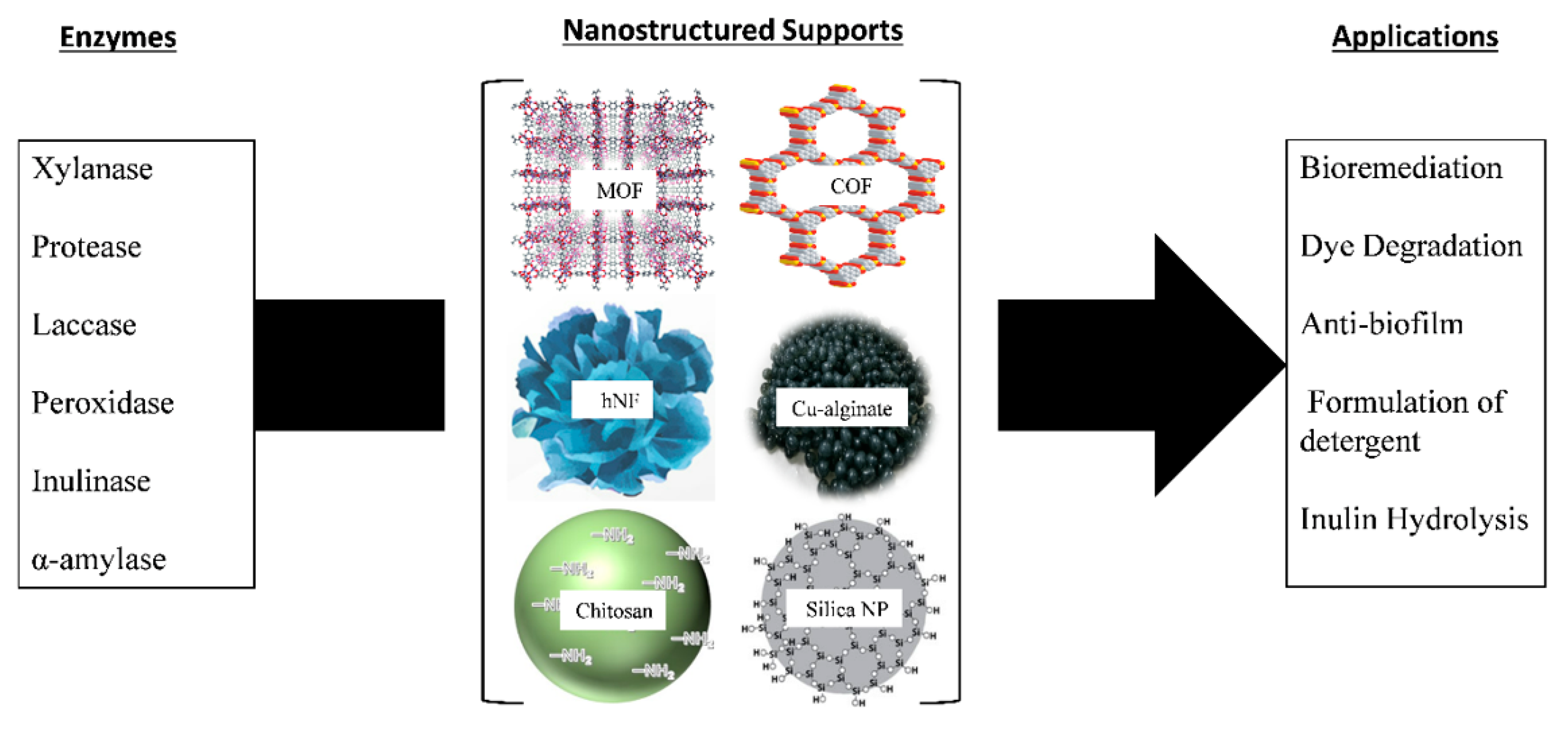
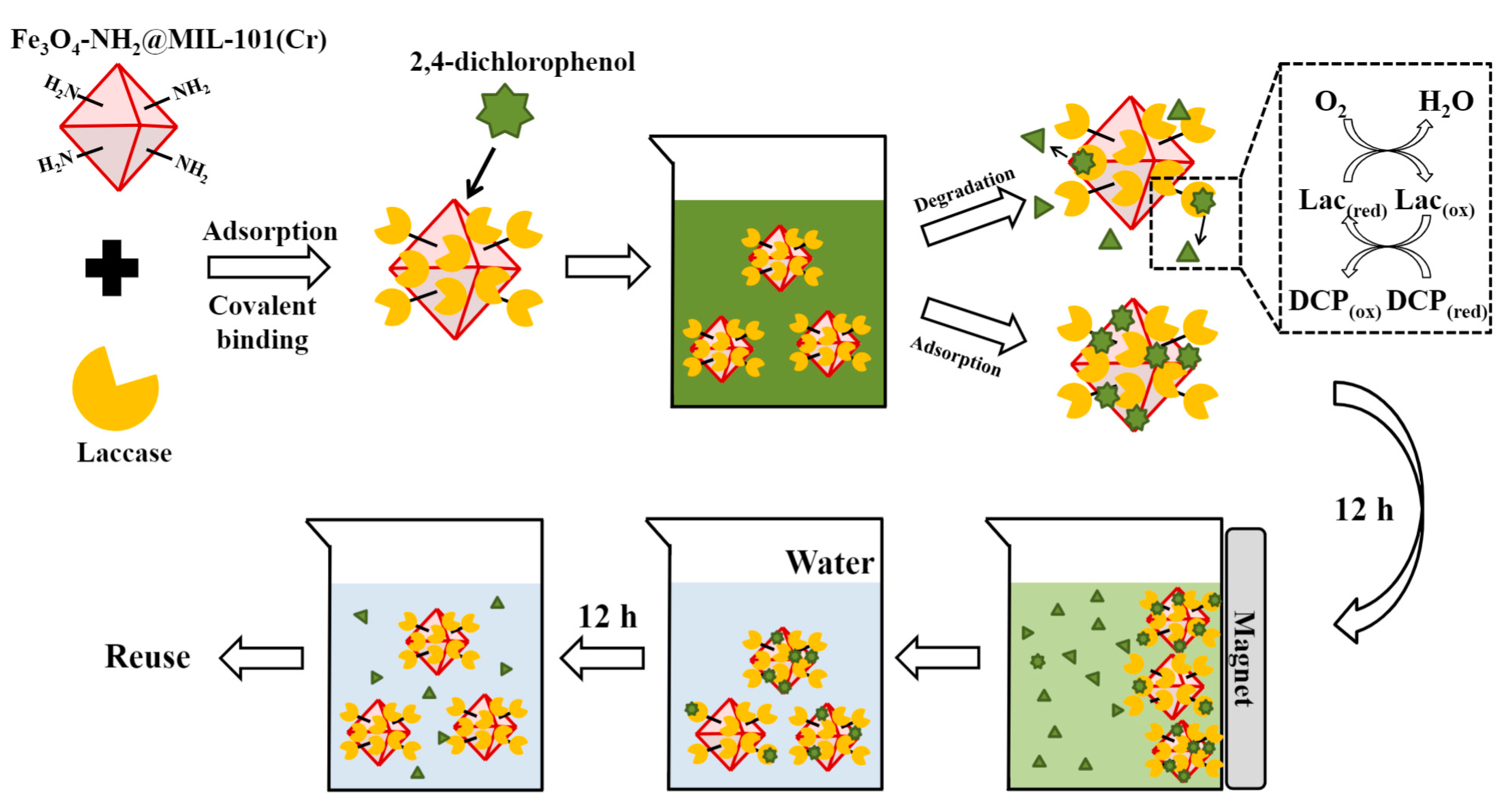
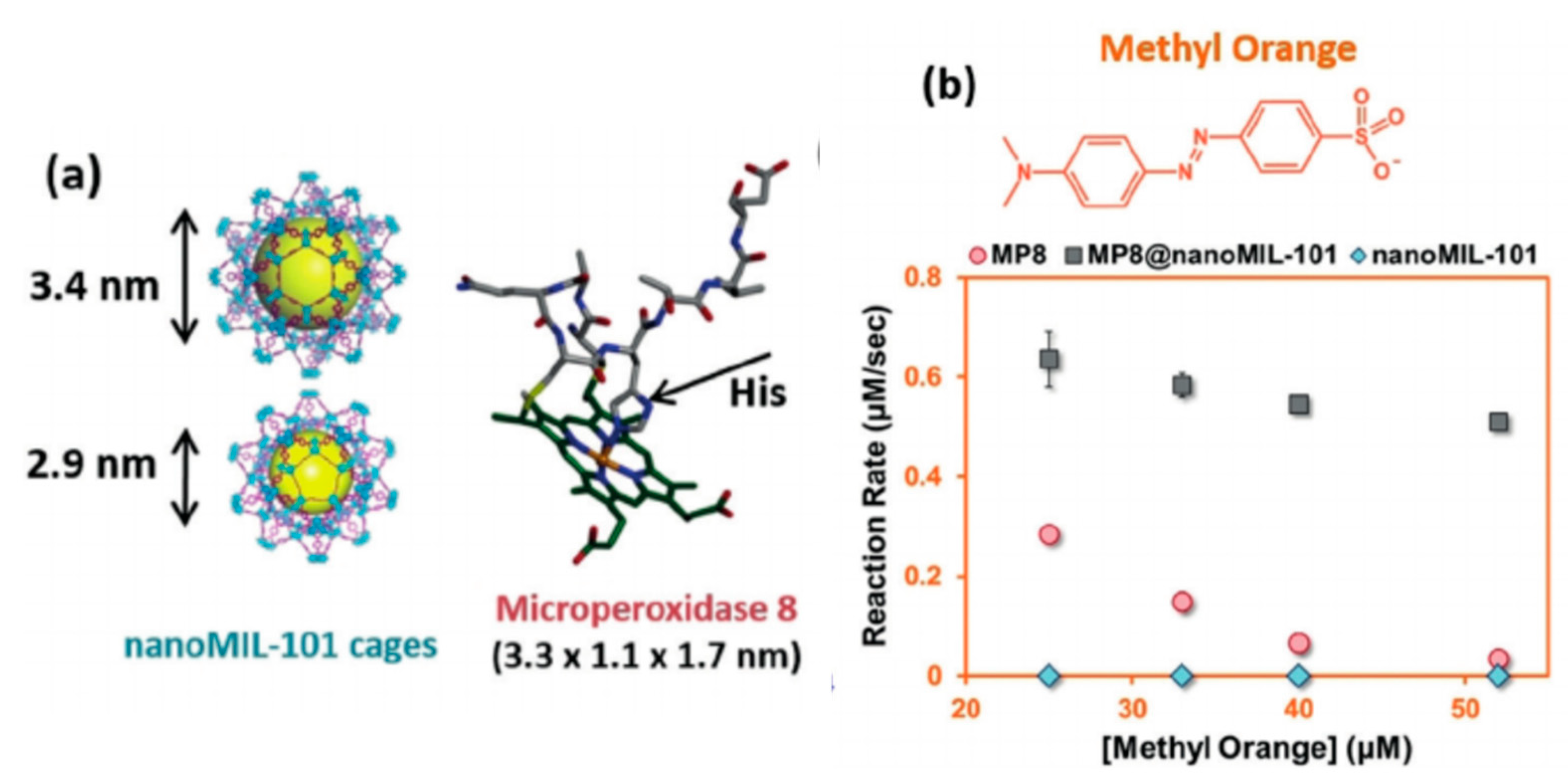
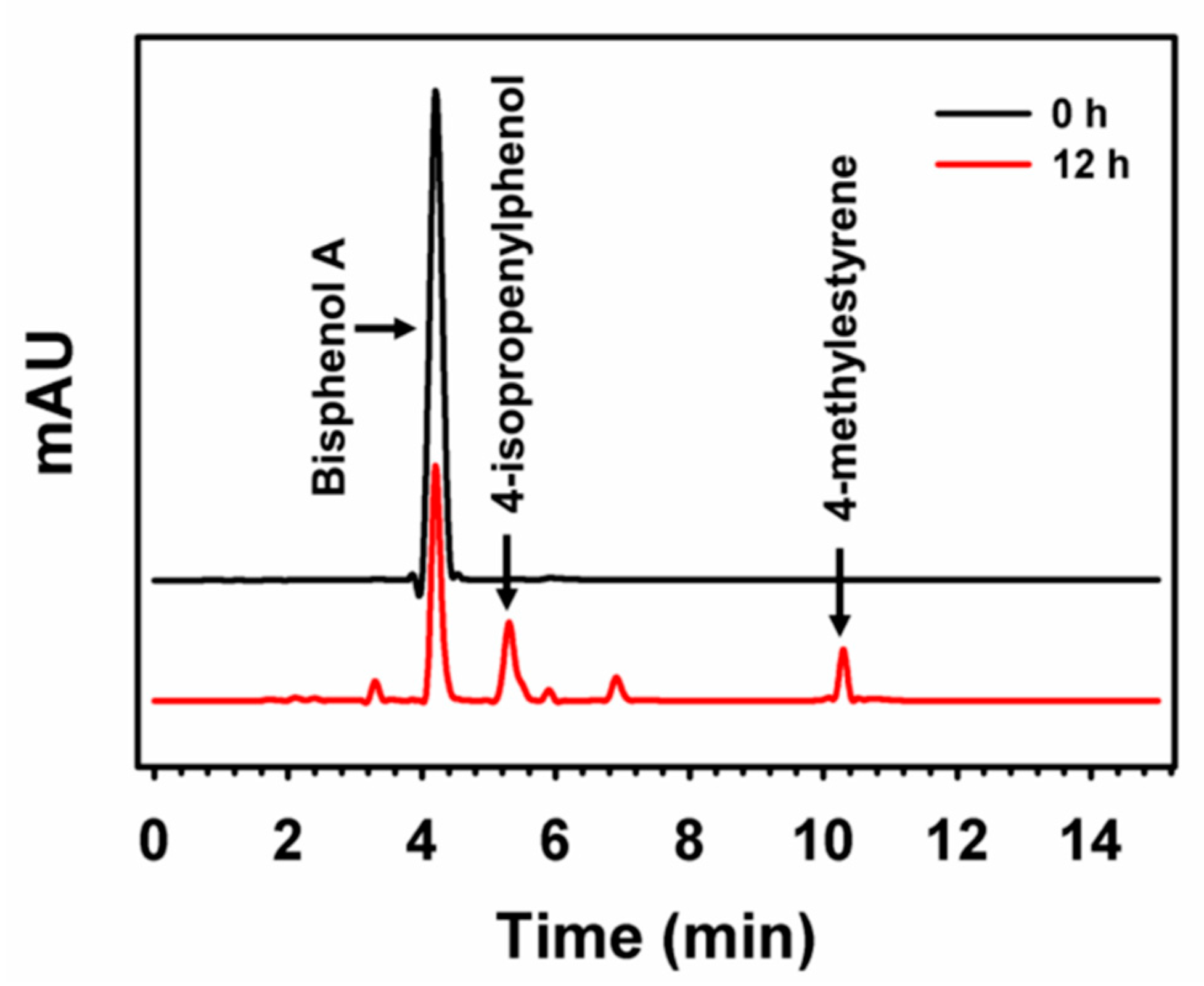

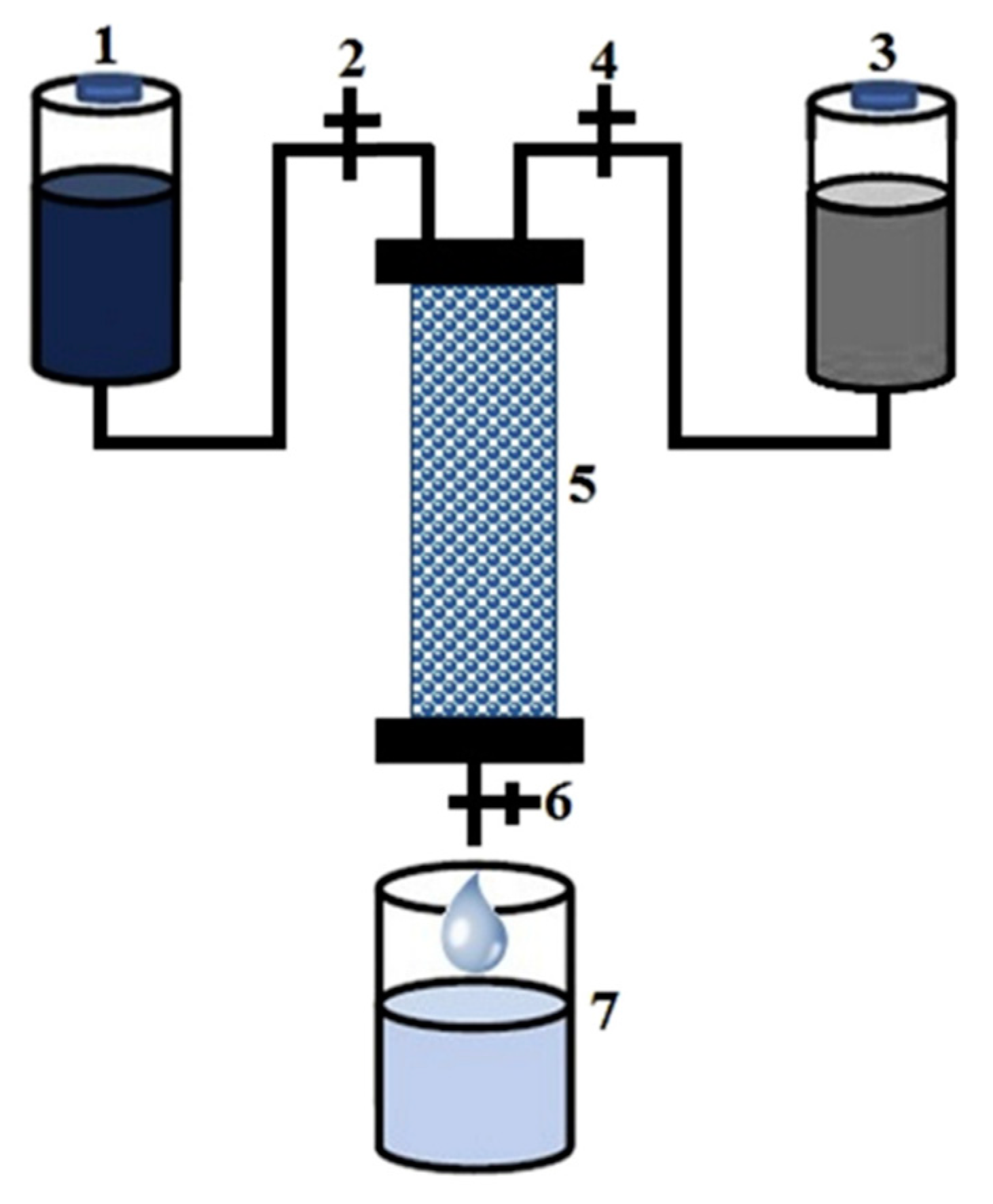
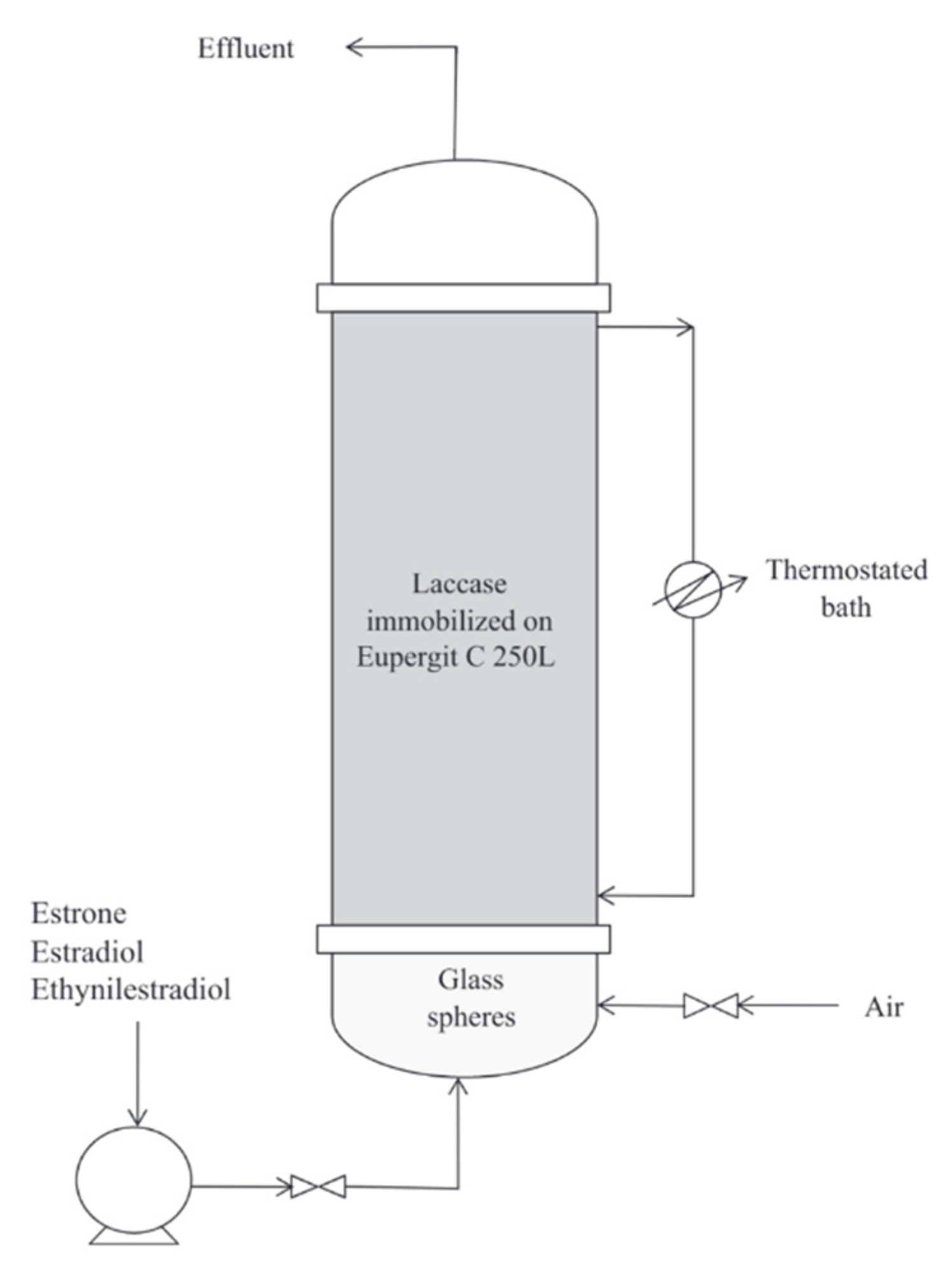
| Emerging Pollutant | Description | Examples | Reference |
|---|---|---|---|
| Pesticides | Chemical compounds that are used to manage and control pests, diseases and weeds spread. They include herbicide, insecticides, etc. They can reach the ground and surface water by irrigation. | Herbicides:
| [13] |
| Pharmaceuticals | Chemical compound that are used for the treatment and/or the prevention of disease. They include anti-inflammatory drugs, analgesics and Antibiotics. Studies show not complete removal with water treatment. |
| [14,15] |
| Personal care products | Substance that have a widespread use and being consumed daily. They include beauty products, heath products and cleaning supplies such as shampoos, mouthwash, perfume, makeup, detergents, etc. These pollutants enter water bodies though the effluents of the sewage treatment. |
| [16] |
| Food Additives | Synthesised substances such as antioxidants, thickeners, Sweeteners, Preservatives, etc. They can be found in both ground water and wastewater. |
| [17] |
| Class | Pollutant | Max Concentration (ng/L) | Reference |
|---|---|---|---|
| Pharmaceuticals | Acetylsalicylic acid | 54 | [18] |
| Carbamazepine | 245 | [18] | |
| Clofibric acid | 68 | [18] | |
| Diclofenac | 316 | [18] | |
| Florfenicol | 111 | [18] | |
| Flunixin | 145 | [18] | |
| Ibuprofen | 376 | [18] | |
| Ketoprofen | 250 | [18] | |
| Mefenamic acid | 78 | [18] | |
| Naproxen | 321 | [18] | |
| Metoprolol | 60 | [11] | |
| Bezafibrate | 160 | [11] | |
| Sulfasalazine | 780 | [11] | |
| Hormones | Estrone | 120 | [18] |
| 17β-Estradiol | 101 | [18] | |
| 17α-Ethinylestradiol | 97 | [18] | |
| Personal care product | Triclosan | 102 | [18] |
| Tonalide | 66,000 | [11] | |
| Nonylphenol | 200 | [11] | |
| Pesticides | Alphamethrin | 161 | [12] |
| Fluometuron | 88 | [12] | |
| Lambda–cyhalothrin | 41 | [12] | |
| Lindane | 30 | [12] |
| Enzyme | Class of the Enzyme | Metal Organic Framework (MOF) | Applications | Reference |
|---|---|---|---|---|
| Horseradish peroxidases (HRP) | Peroxidases | H-MOF(Zr) | Degradation of 2,4-dichlorophenol pollutant | [129] |
| Chloroperoxidase (CPO) | Peroxidases | H-MOF(Zr) | Degradation of isoproturon pollutant | [129] |
| Laccase | Laccase | Fe3O4-NH2@MIL-101(Cr) | 2,4-dichlorophenol pollutant removal | [89] |
| Microperoxidase-8 | Peroxidases | MIL-101(Cr) | Degradation of methyl orange dye | [130] |
| Horseradish peroxidases (HRP) | Peroxidases | SOM-ZIF-8 | Degradation of the hazardous dyes methyl orange (MO), Congo red (CR), rhodamine B (RB), and rhodamine 6G (R6G) | [131] |
| Enzyme | Class of Enzyme | Metal Ion | Application | Reference |
|---|---|---|---|---|
| Turkish black radish | peroxidases | Copper (II) ions | Dye decolorization | [146] |
| Chloroperoxidase (CPO) | peroxidases | Copper (II) ions | Dye decolorization | [143] |
| Chloroperoxidase (CPO) | peroxidases | Cobalt (II) ions | Dye decolorization | [143] |
| Laccase | Laccase | Copper (II) ions | Degradation of bisphenol A (BPA) pollutant | [141] |
| Laccase | Laccase | Copper (II) ions | Decolorization of Congo Red dye | [144] |
| Laccase | Laccase | Copper (II) ions | Dye decolorization | [145] |
| Laccase | Laccase | Multi-metal Copper (II) ions + Zinc (II) ions | Degradation of bisphenol A (BPA) pollutant | [142] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Maqdi, K.A.; Elmerhi, N.; Athamneh, K.; Bilal, M.; Alzamly, A.; Ashraf, S.S.; Shah, I. Challenges and Recent Advances in Enzyme-Mediated Wastewater Remediation—A Review. Nanomaterials 2021, 11, 3124. https://doi.org/10.3390/nano11113124
Al-Maqdi KA, Elmerhi N, Athamneh K, Bilal M, Alzamly A, Ashraf SS, Shah I. Challenges and Recent Advances in Enzyme-Mediated Wastewater Remediation—A Review. Nanomaterials. 2021; 11(11):3124. https://doi.org/10.3390/nano11113124
Chicago/Turabian StyleAl-Maqdi, Khadega A., Nada Elmerhi, Khawlah Athamneh, Muhammad Bilal, Ahmed Alzamly, Syed Salman Ashraf, and Iltaf Shah. 2021. "Challenges and Recent Advances in Enzyme-Mediated Wastewater Remediation—A Review" Nanomaterials 11, no. 11: 3124. https://doi.org/10.3390/nano11113124








