Dye Sensitization for Ultraviolet Upconversion Enhancement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Method
2.3.1. Synthesis of NaGdF4:49%Yb,1%Tm Core Nanocrystals
2.3.2. Synthesis of NaGdF4:49%Yb,1%Tm@NaYF4:20%Yb Core–Shell Nanocrystals
2.3.3. Synthesis of NaGdF4:49%Yb,1%Tm@NaYF4:20%Yb@NaGdF4:50%Nd,10%Yb and NaGdF4:49%Yb,1%Tm@NaYF4:20%Yb@NaGdF4:50%Nd,10%Yb@NaGdF4 Core–Multishell Nanocrystals
2.3.4. Preparation of Dye-Sensitized Upconversion Nanoparticles
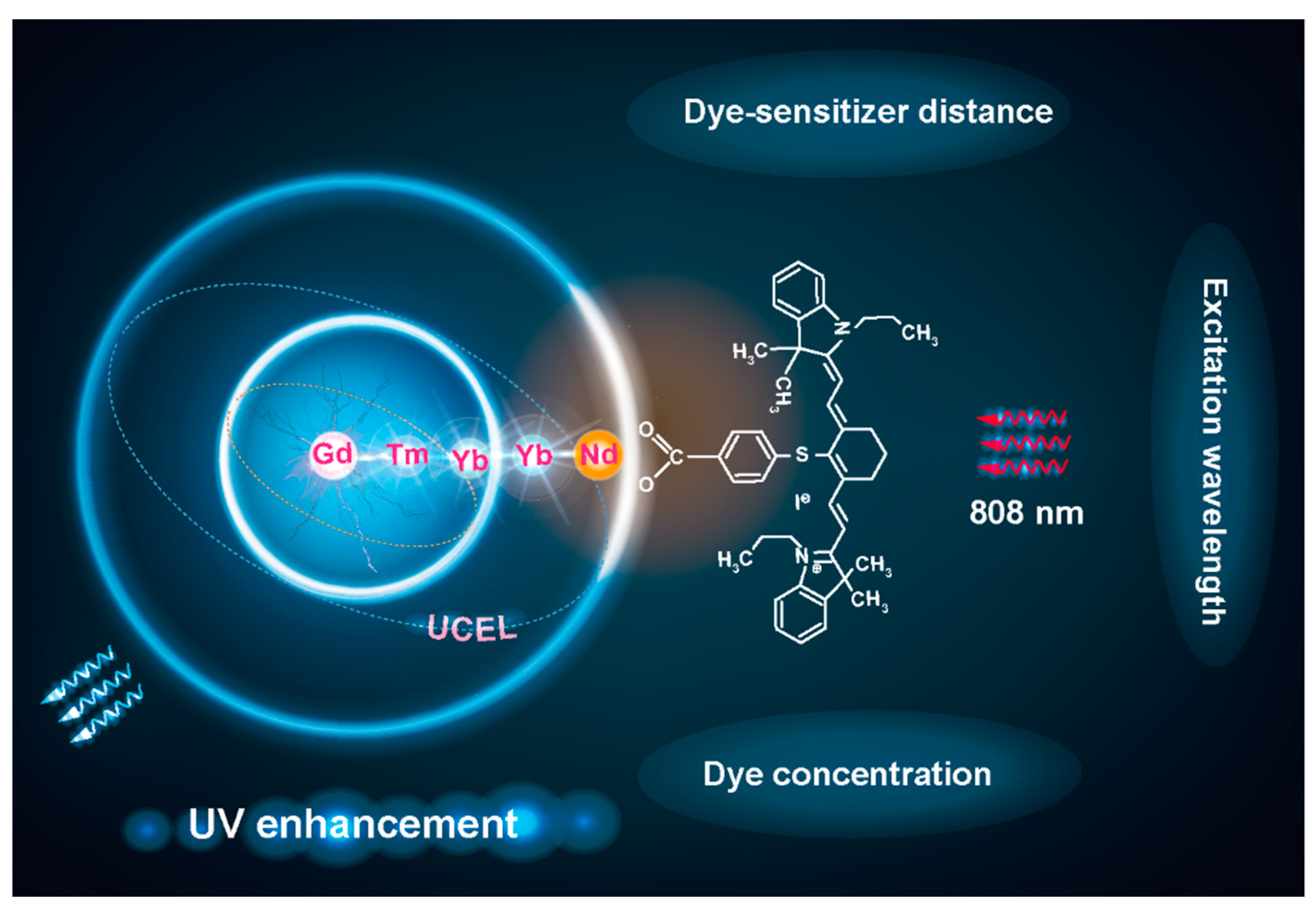
3. Results
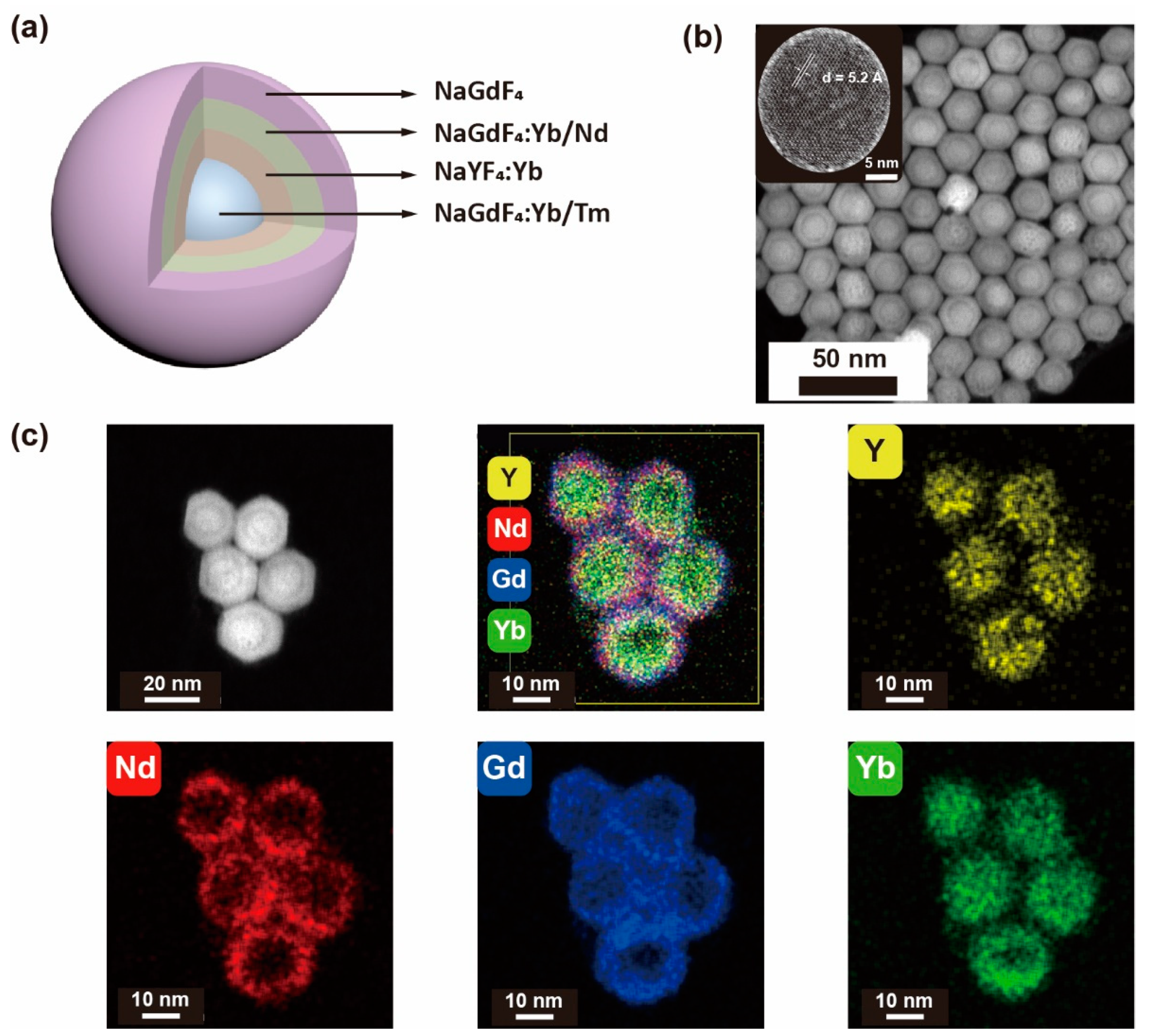
3.1. Synthesis of Core–Multishell Upconversion Nanoparticles
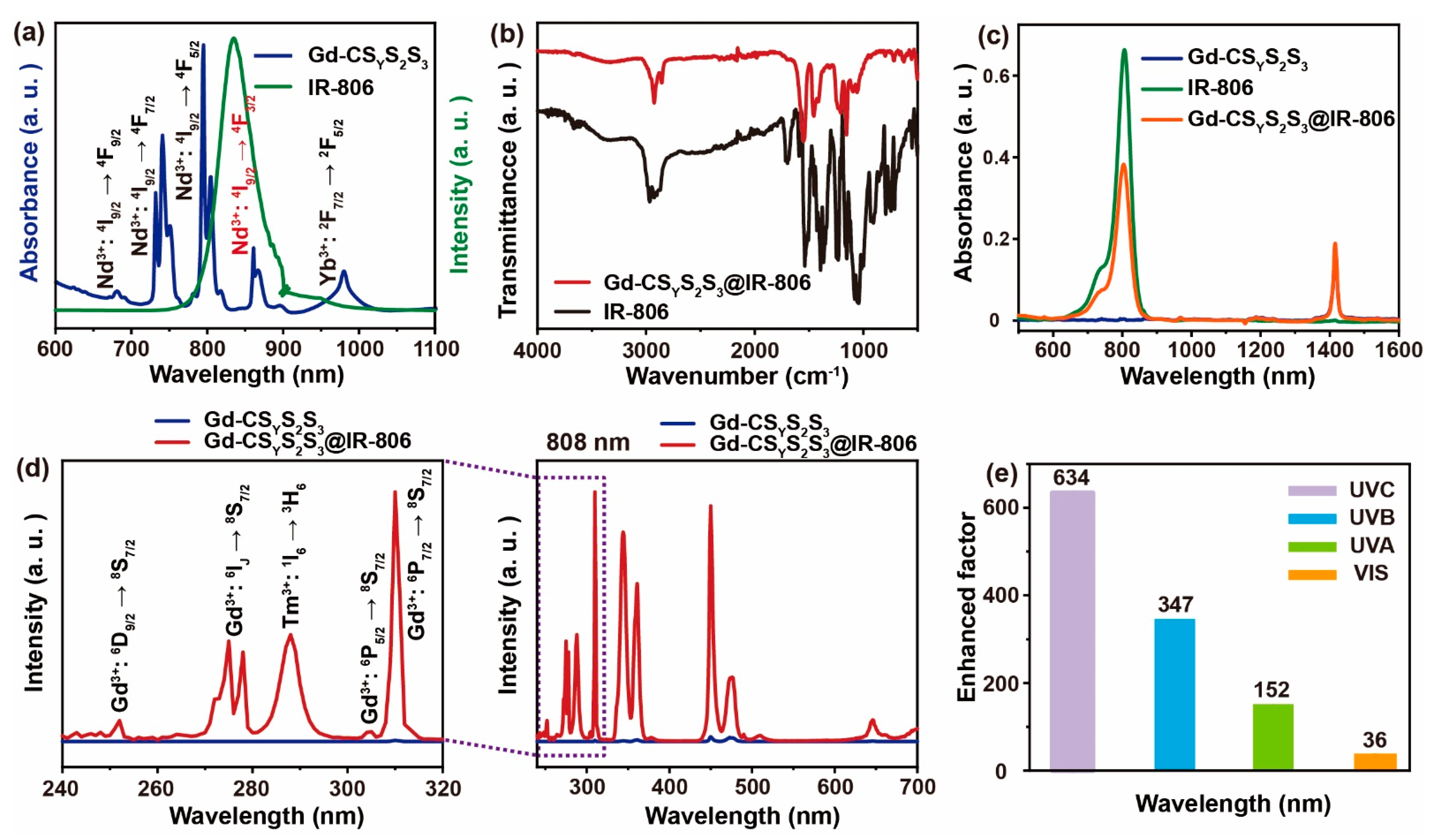
3.2. Remarkable UV Enhancement
3.3. Optimum Weight Ratio between IR-806 and Nanoparticles
3.4. The Effect of Excitation Wavelength on UV Upconversion Emission
3.5. The Effect of IR-806 Sensitizer Distance on UV Upconversion
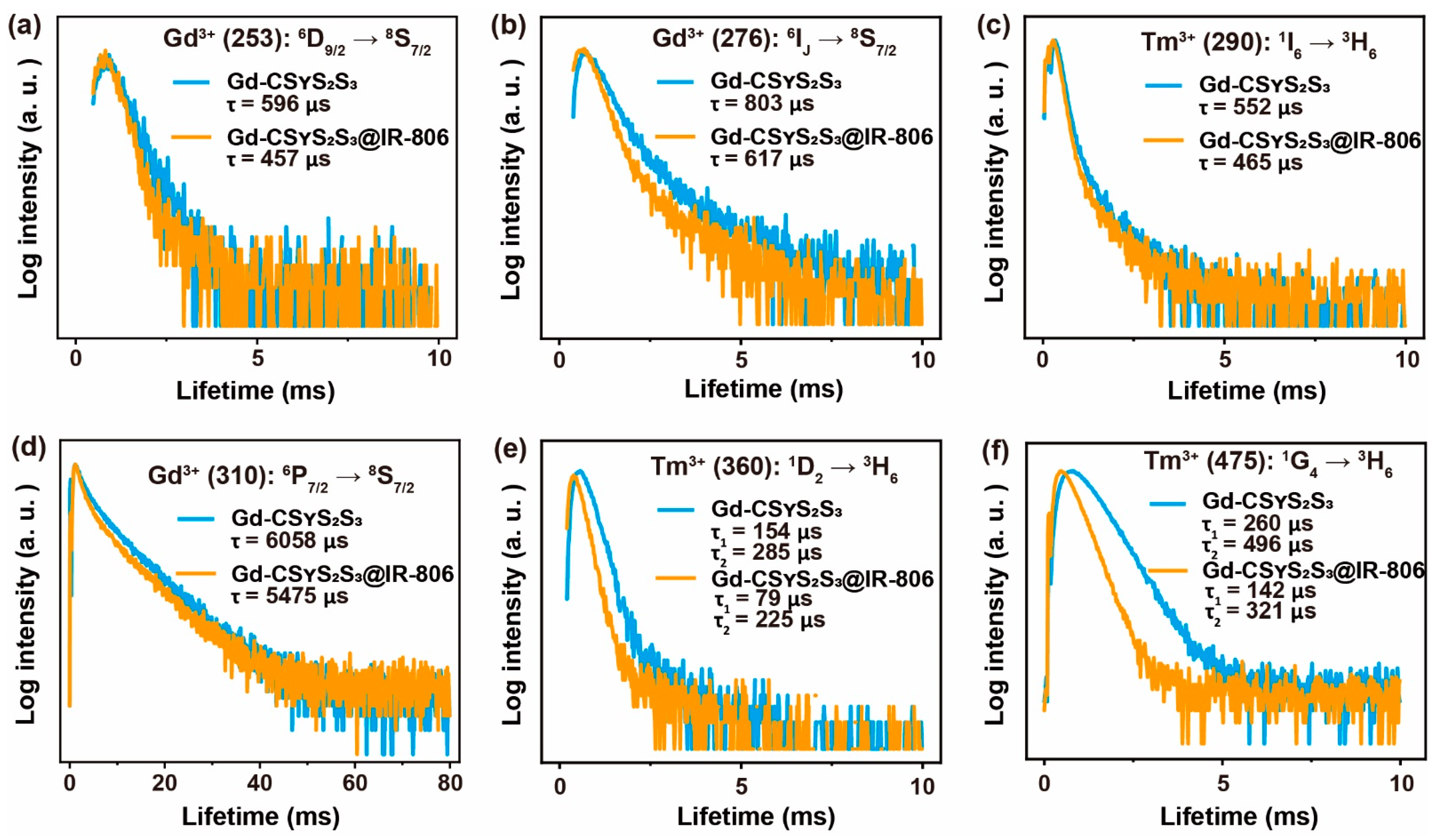
3.6. Energy Transfer Mechanism
3.7. Back Energy Transfer from Nanoparticles to IR-806
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, Y.; Guo, Z.; Yuan, W.; Kong, M.; Liu, Y.; Liu, Y.; Gao, Y.; Feng, W.; Wang, F.; Zhou, J.; et al. High-sensitivity imaging of time-domain near-infrared light transducer. Nat. Photon. 2019, 13, 525–531. [Google Scholar] [CrossRef]
- Zhu, X.; Su, Q.; Feng, W.; Li, F. Anti-stokes shift luminescent materials for bio-applications. Chem. Soc. Rev. 2017, 46, 1025–1039. [Google Scholar] [CrossRef]
- Zhao, J.; Chu, H.; Zhao, Y.; Lu, Y.; Li, L. A NIR light gated DNA nanodevice for spatiotemporally controlled imaging of MicroRNA in cells and animals. J. Am. Chem. Soc. 2019, 141, 7056–7062. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Liu, G.-Y.; Sun, L.-D.; Xiao, J.-W.; Zhou, J.-C.; Yan, C.-H. Nd3+-sensitized upconversion nanophosphors: Efficient In Vivo bioimaging probes with minimized heating effect. ACS Nano 2013, 7, 7200–7206. [Google Scholar] [CrossRef]
- Xu, J.; Yang, P.; Sun, M.; Bi, H.; Liu, B.; Yang, D.; Gai, S.; He, F.; Lin, J. Highly emissive dye-sensitized upconversion nanostructure for dual-photosensitizer photodynamic therapy and bioimaging. ACS Nano 2017, 11, 4133–4144. [Google Scholar] [CrossRef]
- Yang, D.; Ma, P.A.; Hou, Z.; Cheng, Z.; Li, C.; Lin, J. Current advances in lanthanide ion (Ln3+)-based upconversion nanomaterials for drug delivery. Chem. Soc. Rev. 2015, 44, 1416–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idris, N.M.; Gnanasammandhan, M.K.; Zhang, J.; Ho, P.C.; Mahendran, R.; Zhang, Y. In Vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nat. Med. 2012, 18, 1580–1585. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion nanoparticles: Design, nanochemistry, and applications in theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Bu, W.; Shi, J. On the latest three-stage development of nanomedicines based on upconversion nanoparticles. Adv. Mater. 2016, 28, 3987–4011. [Google Scholar] [CrossRef]
- Dai, Y.; Xiao, H.; Liu, J.; Yuan, Q.; Ma, P.A.; Yang, D.; Li, C.; Cheng, Z.; Hou, Z.; Yang, P.; et al. In Vivo multimodality imaging and cancer therapy by near-infrared light-triggered trans-platinum pro-drug-conjugated upconverison nanoparticles. J. Am. Chem. Soc. 2013, 135, 18920–18929. [Google Scholar] [CrossRef]
- Bansal, A.; Zhang, Y. Photocontrolled nanoparticle delivery systems for biomedical applications. Acc. Chem. Res. 2014, 47, 3052–3060. [Google Scholar] [CrossRef]
- Zheng, W.; Zhou, S.; Xu, J.; Liu, Y.; Huang, P.; Liu, Y.; Chen, X. Ultrasensitive luminescent in vitro detection for tumor markers based on inorganic lanthanide nano-bioprobes. Adv. Sci. 2016, 3, 1600197. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Weitemier Adam, Z.; Zeng, X.; He, L.; Wang, X.; Tao, Y.; Huang Arthur, J.Y.; Hashimotodani, Y.; Kano, M.; Iwasaki, H.; et al. Near-infrared deep brain stimulation via upconversion nanoparticle–mediated optogenetics. Science 2018, 359, 679–684. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Zhang, Y.; Takle, K.; Bilsel, O.; Li, Z.; Lee, H.; Zhang, Z.; Li, D.; Fan, W.; Duan, C.; et al. Dye-sensitized core/active shell upconversion nanoparticles for optogenetics and bioimaging applications. ACS Nano 2016, 10, 1060–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Zheng, X.; Yan, L.; Zhou, L.; Tian, G.; Yin, W.; Wang, L.; Liu, Y.; Hu, Z.; Gu, Z.; et al. Bismuth sulfide nanorods as a precision nanomedicine for in vivo multimodal imaging-guided photothermal therapy of tumor. ACS Nano 2015, 9, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Feng, W.; Chang, J.; Tan, Y.-W.; Li, J.; Chen, M.; Sun, Y.; Li, F. Temperature-feedback upconversion nanocomposite for accurate photothermal therapy at facile temperature. Nat. Commun. 2016, 7, 10437. [Google Scholar] [CrossRef]
- Zuo, J.; Tu, L.; Li, Q.; Feng, Y.; Que, I.; Zhang, Y.; Liu, X.; Xue, B.; Cruz, L.J.; Chang, Y.; et al. Near infrared light sensitive ultraviolet–blue nanophotoswitch for imaging-guided “Off–On” therapy. ACS Nano 2018, 12, 3217–3225. [Google Scholar] [CrossRef]
- Chan, M.-H.; Pan, Y.-T.; Chan, Y.-C.; Hsiao, M.; Chen, C.-H.; Sun, L.; Liu, R.-S. Nanobubble-embedded Inorganic 808 nm excited upconversion nanocomposites for tumor multiple imaging and treatment. Chem. Sci. 2018, 9, 3141–3151. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Liu, B.; Zhao, J.; Di, Z.; Chen, D.; Gu, Z.; Li, L.; Zhao, Y. Nd3+-sensitized upconversion Metal–Organic frameworks for mitochondria-targeted amplified photodynamic therapy. Angew. Chem. Int. Ed. 2020, 59, 2634–2638. [Google Scholar] [CrossRef]
- Zheng, K.; Qin, W.; Cao, C.; Zhao, D.; Wang, L. NIR to VUV: Seven-photon upconversion emissions from Gd3+ ions in fluoride nanocrystals. J. Phys. Chem. Lett. 2015, 6, 556–560. [Google Scholar] [CrossRef]
- Chen, X.; Jin, L.; Kong, W.; Sun, T.; Zhang, W.; Liu, X.; Fan, J.; Yu, S.F.; Wang, F. Confining energy migration in upconversion nanoparticles towards deep ultraviolet lasing. Nat. Commun. 2016, 7, 10304. [Google Scholar] [CrossRef]
- Zhao, C.; Kong, X.; Liu, X.; Tu, L.; Wu, F.; Zhang, Y.; Liu, K.; Zeng, Q.; Zhang, H. Li+ Ion doping: An approach for improving the crystallinity and upconversion emissions of NaYF4:Yb3+, Tm3+ Nanoparticles. Nanoscale 2013, 5, 8084–8089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, F.; Wang, J.; Zhang, D.; Qin, G.; Qin, W. Greatly enhanced size-tunable ultraviolet upconversion luminescence of monodisperse β-NaYF4:Yb,Tm nanocrystals. J. Mater. Chem. 2011, 21, 13413–13421. [Google Scholar] [CrossRef]
- Dawson, P.; Romanowski, M. Excitation modulation of upconversion nanoparticles for switch-like control of ultraviolet luminescence. J. Am. Chem. Soc. 2018, 140, 5714–5718. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, J.; Liu, X. Direct evidence of a surface quenching effect on size-dependent luminescence of upconversion nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 7456–7460. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Deng, R.; Wang, J.; Wang, Q.; Han, Y.; Zhu, H.; Chen, X.; Liu, X. Tuning upconversion through energy migration in core-shell nanoparticles. Nat. Mater. 2011, 10, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Li, Y.; Ho, W.L.; Zhu, Q.; Chen, X.; Jin, L.; Zhu, H.; Huang, B.; Lin, J.; Little, B.E.; et al. Integrating temporal and spatial control of electronic transitions for bright multiphoton upconversion. Nat. Commun. 2019, 10, 1811. [Google Scholar] [CrossRef]
- Su, Q.; Han, S.; Xie, X.; Zhu, H.; Chen, H.; Chen, C.-K.; Liu, R.-S.; Chen, X.; Wang, F.; Liu, X. The effect of surface coating on energy migration-mediated upconversion. J. Am. Chem. Soc. 2012, 134, 20849–20857. [Google Scholar] [CrossRef]
- Xie, X.; Gao, N.; Deng, R.; Sun, Q.; Xu, Q.-H.; Liu, X. Mechanistic investigation of photon upconversion in Nd3+-sensitized core–shell nanoparticles. J. Am. Chem. Soc. 2013, 135, 12608–12611. [Google Scholar] [CrossRef]
- Su, Q.; Wei, H.-L.; Liu, Y.; Chen, C.; Guan, M.; Wang, S.; Su, Y.; Wang, H.; Chen, Z.; Jin, D. Six-photon Upconverted Excitation Energy Lock-in for Ultraviolet-C Enhancement. Nat. Commun. 2021, 12, 4367. [Google Scholar] [CrossRef]
- Wen, S.; Zhou, J.; Schuck, P.J.; Suh, Y.D.; Schmidt, T.W.; Jin, D. Future and challenges for hybrid upconversion nanosystems. Nat. Photon. 2019, 13, 828–838. [Google Scholar] [CrossRef]
- Zou, W.; Visser, C.; Maduro, J.A.; Pshenichnikov, M.S.; Hummelen, J.C. Broadband dye-sensitized upconversion of near-infrared light. Nat. Photon. 2012, 6, 560–564. [Google Scholar] [CrossRef] [Green Version]
- Shao, Q.; Li, X.; Hua, P.; Zhang, G.; Dong, Y.; Jiang, J. Enhancing the upconversion luminescence and photothermal conversion properties of ~800 nm excitable core/shell nanoparticles by dye molecule sensitization. J. Colloid Interface Sci. 2017, 486, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Wang, D.; Zhang, Y.; Zuo, J.; Chang, Y.; Tu, L.; Liu, X.; Yuan, Z.; Zhao, H.; Song, J.; et al. Regulating the color output and simultaneously enhancing the intensity of upconversion nanoparticles via a dye sensitization strategy. J. Mater. Chem. C. 2019, 7, 8607–8615. [Google Scholar] [CrossRef]
- Wang, F.; Deng, R.; Liu, X. Preparation of core-shell NaGdF4 nanoparticles doped with luminescent lanthanide ions to be used as upconversion-based probes. Nat. Protoc. 2014, 9, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shen, B.; Wei, H.-L.; Liu, Z.; Chen, Z.; Zhang, Y.; Su, Y.; Zhang, J.-Z.; Wang, H.; Su, Q. Comparative investigation of the optical spectroscopic and thermal effect in Nd3+-doped nanoparticles. Nanoscale 2019, 11, 10220–10228. [Google Scholar] [CrossRef]
- Xu, H.; Han, S.; Deng, R.; Su, Q.; Wei, Y.; Tang, Y.; Qin, X.; Liu, X. Anomalous upconversion amplification induced by surface reconstruction in lanthanide sublattices. Nat. Photon. 2021, 15, 732–737. [Google Scholar] [CrossRef]
- Muhr, V.; Würth, C.; Kraft, M.; Buchner, M.; Baeumner, A.J.; Resch-Genger, U.; Hirsch, T. Particle-size-dependent förster resonance energy transfer from upconversion nanoparticles to organic dyes. Anal. Chem. 2017, 89, 4868–4874. [Google Scholar] [CrossRef]
- Ray, P.C.; Fan, Z.; Crouch, R.A.; Sinha, S.S.; Pramanik, A. Nanoscopic optical rulers beyond the fret distance limit: Fundamentals and applications. Chem. Soc. Rev. 2014, 43, 6370–6404. [Google Scholar] [CrossRef]
- Deng, R.; Wang, J.; Chen, R.; Huang, W.; Liu, X. Enabling förster resonance energy transfer from large nanocrystals through energy migration. J. Am. Chem. Soc. 2016, 138, 15972–15979. [Google Scholar] [CrossRef]
- Tu, D.; Liu, L.; Ju, Q.; Liu, Y.; Zhu, H.; Li, R.; Chen, X. Time-Resolved FRET biosensor based on amine-functionalized lanthanide-doped NaYF4 nanocrystals. Angew. Chem. Int. Ed. 2011, 50, 6306–6310. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Gu, Y.; Liu, Y.; Shi, Y.; Wu, N.; Feng, W.; Li, F. Luminescence lifetime–Based In Vivo detection with responsive rare earth–dye nanocomposite. Small 2019, 15, 1904487. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Wei, H.; Wang, S.; Hu, C.; Su, Q. Dye Sensitization for Ultraviolet Upconversion Enhancement. Nanomaterials 2021, 11, 3114. https://doi.org/10.3390/nano11113114
Wang M, Wei H, Wang S, Hu C, Su Q. Dye Sensitization for Ultraviolet Upconversion Enhancement. Nanomaterials. 2021; 11(11):3114. https://doi.org/10.3390/nano11113114
Chicago/Turabian StyleWang, Mingkai, Hanlin Wei, Shuai Wang, Chuanyu Hu, and Qianqian Su. 2021. "Dye Sensitization for Ultraviolet Upconversion Enhancement" Nanomaterials 11, no. 11: 3114. https://doi.org/10.3390/nano11113114
APA StyleWang, M., Wei, H., Wang, S., Hu, C., & Su, Q. (2021). Dye Sensitization for Ultraviolet Upconversion Enhancement. Nanomaterials, 11(11), 3114. https://doi.org/10.3390/nano11113114







