Inkjet Printing Infiltration of the Doped Ceria Interlayer in Commercial Anode-Supported SOFCs
Abstract
:1. Introduction
2. Materials and Methods
3. Experimental Section
3.1. Anode-Supported SOFC Preparation
3.2. Infiltrate Ink Synthesis
3.3. Jetting Optimisation
3.4. Inkjet Printing Infiltration
3.5. Characterisation
4. Results and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atkinson, A.; Barnett, S.; Gorte, R.J.; Irvine, J.T.S.; McEvoy, A.J.; Mogensen, M.; Singhal, S.C.; Vohs, J. Advanced anodes for high-temperature fuel cells. Nat. Mater. 2004, 3, 17–27. [Google Scholar] [CrossRef] [PubMed]
- O’Hayre, R.; Cha, S.W.; Colella, W.; Prinz, F.B. Fuel Cell Fundamentals; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Huang, K.; Goodenough, J.B. Solid Oxide Fuel Cell Technology: Principles, Performance and Operations; Woodhead Publishing Limited: Cambridge, UK, 2009. [Google Scholar]
- Irvine, J.T.S.; Neagu, D.; Verbraeken, M.C.; Chatzichristodoulou, C.; Graves, C.; Mogensen, M.B. Evolution of the electrochemical interface in high-temperature fuel cells and electrolysers. Nat. Energy 2016, 1, 15014. [Google Scholar] [CrossRef] [Green Version]
- Wachsman, E.D.; Lee, K.T. Lowering the temperature of solid oxide fuel cells. Science 2011, 334, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Ormerod, R.M. Solid oxide fuel cells. Chem. Soc. Rev. 2003, 32, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Minh, N.Q. Solid oxide fuel cell technology-features and applications. J. Am. Ceram. Soc. 2000, 76, 563–588. [Google Scholar] [CrossRef]
- Yamamoto, O. Solid oxide fuel cells: Fundamental aspects and prospects. Electrochim. Acta 2000, 45, 2423–2435. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Behling, N. Fuel Cells Current Technology Challenges and Future Research Needs, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Jiang, S.P. A comparison of O2 reduction reactions on porous (La,Sr)MnO3 and (La,Sr)(Co,Fe)O3 electrodes. Solid State Ion. 2002, 146, 1–22. [Google Scholar] [CrossRef]
- McEvoy, A.J. Thin SOFC electrolytes and their interfaces: A near-term research strategy. Solid State Ion. 2000, 132, 159–165. [Google Scholar] [CrossRef]
- Fleig, J. On the width of the electrochemically active region in mixed conducting solid oxide fuel cell cathodes. J. Power Sources 2002, 105, 228–238. [Google Scholar] [CrossRef]
- Mineshige, A.; Kobune, M.; Fujii, S.; Ogumi, Z.; Inaba, M.; Yao, T.; Kikuchi, K. Metal-insulator transition and crystal structure of La1-xSrxCoO3 as functions of Sr-content, temperature, and oxygen partial pressure. J. Solid State Chem. 1999, 142, 374–381. [Google Scholar] [CrossRef]
- Simner, S.P.; Bonnett, J.F.; Canfield, N.L.; Meinhardt, K.D.; Sprenkle, V.L.; Stevenson, J.W. Optimized lanthanum ferrite-based cathodes for anode-supported SOFCs. Electrochem. Solid-State Lett. 2002, 5, A173–A175. [Google Scholar] [CrossRef]
- Esquirol, A.; Brandon, N.P.; Kilner, J.A.; Mogensen, M. Electrochemical characterization of La0.6Sr0.4Co0.2Fe0.8O3 cathodes for intermediate-temperature SOFCs. J. Electrochem. Soc. 2004, 151, A1847–A1855. [Google Scholar] [CrossRef]
- Kostogloudis, G.C.; Tsiniarakis, G.; Ftikos, C. Chemical reactivity of perovskite oxide SOFC cathodes and yttria stabilized zirconia. Solid State Ion. 2000, 135, 529–535. [Google Scholar] [CrossRef]
- Ullmann, H.; Trofimenko, N.; Tietz, F.; Stöver, D.; Ahmad-Khanlou, A. Correlation between thermal expansion and oxide ion transport in mixed conducting perovskite-type oxides for SOFC cathodes. Solid State Ion. 2000, 138, 79–90. [Google Scholar] [CrossRef]
- Knibbe, R.; Hjelm, J.; Menon, M.; Pryds, N.; Søgaard, M.; Wang, H.J.; Neufeld, K. Cathode-electrolyte interfaces with CGO barrier layers in SOFC. J. Am. Ceram. Soc. 2010, 93, 2877–2883. [Google Scholar] [CrossRef]
- Morales, M.; Miguel-Pérez, V.; Tarancón, A.; Slodczyk, A.; Torrell, M.; Ballesteros, B.; Ouweltjes, J.P.; Bassat, J.M.; Montinaro, D.; Morata, A. Multi-scale analysis of the diffusion barrier layer of gadolinia-doped ceria in a solid oxide fuel cell operated in a stack for 3000 h. J. Power Sources 2017, 344, 141–151. [Google Scholar] [CrossRef]
- Chen, D.; Yang, G.; Shao, Z.; Ciucci, F. Nanoscaled Sm-doped CeO2 buffer layers for intermediate-temperature solid oxide fuel cells. Electrochem. Commun. 2013, 35, 131–134. [Google Scholar] [CrossRef]
- Sønderby, S.; Klemensø, T.; Christensen, B.H.; Almtoft, K.P.; Lu, J.; Nielsen, L.P.; Eklund, P. Magnetron sputtered gadolinia-doped ceria diffusion barriers for metal-supported solid oxide fuel cells. J. Power Sources 2014, 267, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Tsoga, A.; Naoumidis, A.; Stöver, D. Total electrical conductivity and defect structure of ZrO2-CeO2-Y2O3-Gd2O3 solid solutions. Solid State Ion. 2000, 135, 403–409. [Google Scholar] [CrossRef]
- Wang, F.; Nishi, M.; Brito, M.E.; Kishimoto, H.; Yamaji, K.; Yokokawa, H.; Horita, T. Sr and Zr diffusion in LSCF/10GDC/8YSZ triplets for solid oxide fuel cells (SOFCs). J. Power Sources 2014, 258, 281–289. [Google Scholar] [CrossRef]
- Plonczak, P.; Joost, M.; Hjelm, J.; Søgaard, M.; Lundberg, M.; Hendriksen, P.V. A high performance ceria based interdiffusion barrier layer prepared by spin-coating. J. Power Sources 2011, 196, 1156–1162. [Google Scholar] [CrossRef]
- Hierso, J.; Boy, P.; Vallé, K.; Vulliet, J.; Blein, F.; Laberty-Robert, C.; Sanchez, C. Nanostructured ceria based thin films (≤1 μm) as cathode/electrolyte interfaces. J. Solid State Chem. 2013, 197, 113–119. [Google Scholar] [CrossRef]
- Szymczewska, D.; Chrzan, A.; Karczewski, J.; Molin, S.; Jasinski, P. Spray pyrolysis of doped-ceria barrier layers for solid oxide fuel cells. Surf. Coat. Technol. 2017, 313, 168–176. [Google Scholar] [CrossRef]
- Constantin, G.; Rossignol, C.; Barnes, J.-P.; Djurado, E. Interface stability of thin, dense CGO film coating on YSZ for solid oxide fuel cells. Solid State Ion. 2013, 235, 36–41. [Google Scholar] [CrossRef]
- Yashiro, N.; Usui, T.; Kikuta, K. Application of a thin intermediate cathode layer prepared by inkjet printing for SOFCs. J. Eur. Ceram. Soc. 2010, 30, 2093–2098. [Google Scholar] [CrossRef]
- Sukeshini, A.M.; Cummins, R.; Reitz, T.; Miller, R.M. Inkjet printing of anode supported SOFC: Comparison of slurry pasted cathode and printed cathode. Electrochem. Solid-State Lett. 2009, 12, B176–B179. [Google Scholar] [CrossRef]
- Ding, H.; Virkar, A.V.; Liu, M.; Liu, F. Suppression of Sr surface segregation in La1-xSrxCo1-yFeyO3-d: A first principles study. Phys. Chem. Chem. Phys. 2013, 15, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Bucher, E.; Sitte, W. Long-term stability of the oxygen exchange properties of (La,Sr)1−z(Co,Fe)O3−δ in dry and wet atmospheres. Solid State Ion. 2011, 192, 480–482. [Google Scholar] [CrossRef]
- Wang, H.; Yakal-Kremski, K.J.; Yeh, T.; Rupp, G.M.; Limbeck, A.; Fleig, J.; Barnett, S.A. Mechanisms of performance degradation of (La,Sr)(Co,Fe)O3-δ solid oxide fuel cell cathodes. J. Electrochem. Soc. 2016, 163, F581–F585. [Google Scholar] [CrossRef] [Green Version]
- Oh, D.; Gostovic, D.; Wachsman, E.D. Mechanism of La0.6Sr0.4Co0.2Fe0.8O3 cathode degradation. J. Mater. Res. 2012, 27, 1992–1999. [Google Scholar] [CrossRef]
- Heide, P.A.W.V. Systematic x-ray photoelectron spectroscopic study of La1−xSrx-based perovskite-type oxides. Surf. Interface Anal. 2002, 33, 414–425. [Google Scholar] [CrossRef]
- Rupp, G.M.; Opitz, A.K.; Nenning, A.; Limbeck, A.; Fleig, J. Real-time impedance monitoring of oxygen reduction during surface modification of thin film cathodes. Nat. Mater. 2017, 16, 640–646. [Google Scholar] [CrossRef]
- Wang, Zh.; Peng, R.; Zhang, W.; Wu, X.; Xia, Ch.; Lua, Y. Oxygen reduction and transport on the La1−xSrxCo1−yFeyO3−δ cathode in solid oxide fuel cells: A first-principles study. J. Mater. Chem. A 2013, 1, 12932–12940. [Google Scholar] [CrossRef]
- Chen, J.; Liang, F.; Chi, B.; Pu, J.; Jiang, S.P.; Jian, L. Palladium and ceria infiltrated La0.8Sr0.2Co0.5Fe0.5O3−δ cathodes of solid oxide fuel cells. J. Power Sources 2009, 194, 275–280. [Google Scholar] [CrossRef]
- Nie, L.F.; Liu, M.F.; Zhang, Y.J.; Liu, M.L. La0.6Sr0.4Co0.2Fe0.8O3-δ cathodes infiltrated with samarium-doped cerium oxide for solid oxide fuel cells. J. Power Sources 2010, 195, 4704–4708. [Google Scholar] [CrossRef]
- Tomov, R.I.; Mitchel-Williams, T.B.; Maher, R.; Kerherve, G.; Cohen, L.; Payne, D.J.; Kumar, R.V.; Glowacki, B.A. The synergistic effect of cobalt oxide and Gd-CeO2 dual infiltration in LSCF/CGO cathodes. J. Mater. Chem. A 2018, 6, 5071–5081. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Coll, D.; Marrero-López, D.; Núñez, P.; Piñol, S.; Frade, J.R. Grain boundary conductivity of Ce0.8Ln0.2O2−δ ceramics (Ln=Y, La, Gd, Sm) with and without Co-doping. Electrochim. Acta 2006, 51, 6463–6469. [Google Scholar] [CrossRef]
- Zhang, T.; Hing, P.; Huang, H.; Kilner, J. Sintering and grain growth of CoO-doped CeO2 ceramics. J. Eur. Ceram. Soc. 2002, 22, 27–34. [Google Scholar] [CrossRef]
- Tomov, R.I.; Mitchell-Williams, T.; Gao, C.; Kumar, R.V.; Glowacki, B.A. Performance optimization of LSCF/Gd:CeO2 composite cathodes via single-step inkjet printing infiltration. J. Appl. Electrochem. 2017, 47, 641–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venezia, E.; Viviani, M.; Presto, S.; Kumar, V.; Tomov, R.I. Inkjet Printing Functionalization of SOFC LSCF Cathodes. Nanomaterials 2019, 9, 654. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.T.; Yoon, H.S.; Ahn, J.S.; Wachsman, E.D. Bimodally integrated anode functional layer for lower temperature solid oxide fuel cells. J. Mater. Chem. 2012, 22, 17113–17120. [Google Scholar]
- Kiebach, R.; Zielke, P.; Høgh, J.V.T.; Thyden, K.; Wang, H.-J.; Barford, R.; Hendriksen, P.V. Infiltration of SOFC stacks: Evaluation of the electrochemical performance enhancement and the underlying changes in the microstructure. Fuel Cells 2016, 16, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Dowd, R.P., Jr.; Lee, S.; Fan, Y.; Gerdes, K. Engineering the solid oxide fuel cell electrocatalyst infiltration technique for industrial use. Int. J. Hydrog. Energy 2016, 41, 14971–14981. [Google Scholar] [CrossRef] [Green Version]
- Mitchel-Williams, T.B.; Tomov, R.I.; Saadabadi, S.A.; Krauz, M.; Aravind, P.V.; Glowacki, B.A.; Kumar, R.V. Infiltration of commercially available, anode supported SOFC’s via inkjet printing. Mater. Renew. Sustain. Energy. 2017, 6, 12. [Google Scholar] [CrossRef]
- Tomov, R.I.; Krauz, M.; Jewulski, J.; Hopkins, S.C.; Kluczowski, J.R.; Glowacka, D.M.; Glowacki, B.A. Direct ceramic inkjet printing of yttria-stabilized zirconia electrolyte layers for anode-supported solid oxide fuel cells. J. Power Sources 2010, 195, 7160–7167. [Google Scholar] [CrossRef]
- Wang, C.; Hopkins, S.C.; Tomov, R.I.; Kumar, R.V.; Glowacki, B.A. Optimisation of CGO suspensions for inkjet-printed SOFC electrolytes. J. Eur. Ceram. Soc. 2012, 32, 2317–2324. [Google Scholar] [CrossRef]
- Kluczowski, R.; Krauz, M.; Kawalec, M.; Ouweltjes, J.P. Near net shape manufacturing of planar anode supported solid oxide fuel cells by using ceramic injection molding and screen printing. J. Power Sources 2014, 268, 752–757. [Google Scholar] [CrossRef]
- Derby, B. Inkjet printing of functional and structural materials: Fluid property requirements, feature stability, and resolution. Annu. Rev. Mater. Res. 2010, 40, 395–414. [Google Scholar] [CrossRef]
- Derby, B. Inkjet printing ceramics: From drops to solid. J. Eur. Ceram. Soc. 2011, 31, 2543–2550. [Google Scholar] [CrossRef]
- Liu, Y.; Derby, B. Experimental study of the parameters for stable drop-on-demand inkjet performance. Phys. Fluids 2019, 31, 032004. [Google Scholar] [CrossRef] [Green Version]
- Reis, N.C.; Griffiths, R.F.; Santos, J.M. Parametric study of liquid droplets impinging on porous surfaces. Appl. Math. Model 2008, 32, 341–361. [Google Scholar] [CrossRef]
- Samson, A.J.; Søgaard, M.; Bonanos, N. Electrodes for solid oxide fuel cells based on infiltration of co-based materials. Electrochem. Solid-State Lett. 2012, 15, B54–B56. [Google Scholar] [CrossRef]
- Burye, T.E.; Nicholas, J.D. Nano-ceria pre-infiltration improves La0.6Sr0.4Co0.8Fe0.2O3-x infiltrated solid oxide fuel cell cathode performance. J. Power Sources 2015, 300, 402–412. [Google Scholar] [CrossRef] [Green Version]
- Burroughs, B.P.; Hamnett, A.; Orchard, A.F.; Thornton, G. Satellite structure in the X-ray photoelectron spectra of some binary and mixed oxides of lanthanum and cerium. J. Chem. Soc. Dalt. Trans. 1976, 17, 1686–1898. [Google Scholar] [CrossRef]
- Jud, E.; Gauckler, L.J. Sintering behavior of cobalt oxide doped ceria powders of different particle sizes. J. Electroceram. 2005, 14, 247–253. [Google Scholar] [CrossRef]
- Kleinlogel, C.; Gauckler, L.J. Sintering and properties of nanosized ceria solid solutions. Solid State Ion. 2000, 135, 567–573. [Google Scholar] [CrossRef]
- Jud, E.; Gauckler, L.; Halim, S.; Stark, W. Sintering behavior of in situ cobalt oxide-doped cerium-gadolinium oxide prepared by flame spray pyrolysis. J. Am. Ceram. Soc. 2006, 85, 2970–2973. [Google Scholar] [CrossRef]
- Prasad, D.H.; Park, S.Y.; Ji, H.; Kim, H.-R.; Son, J.-W.; Kim, B.-K.; Lee, H.-W.; Lee, J.-H. Cobalt oxide co-doping effect on the sinterability and electrical conductivity of nano-crystalline Gd-doped ceria. Ceram. Int. 2012, 38S, S497–S500. [Google Scholar] [CrossRef]
- Druce, J.; Kilner, J.A. Improvement of oxygen surface exchange kinetics for CGO with surface treatment. J. Electrochem. Soc. 2014, 161, F99–F104. [Google Scholar] [CrossRef]
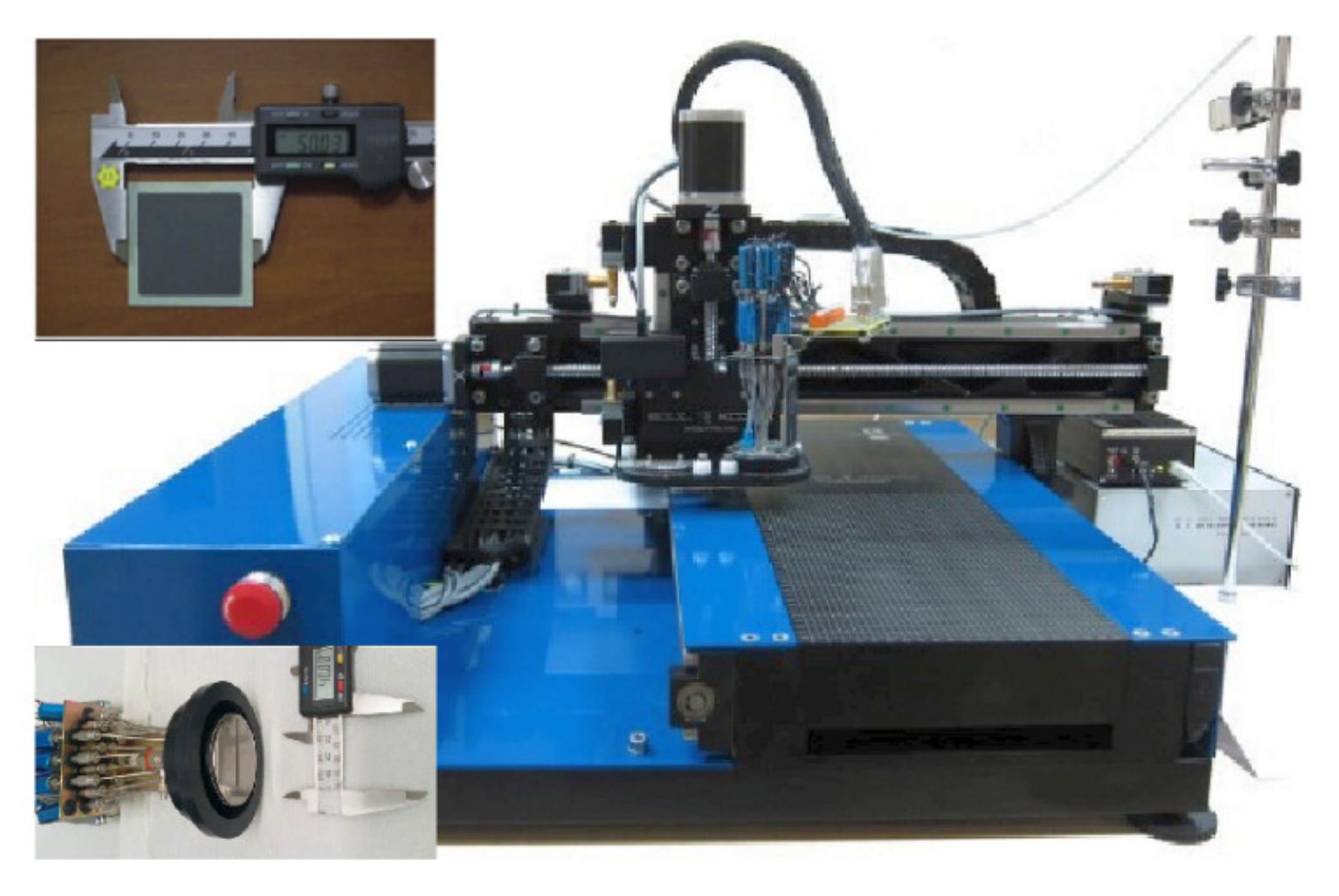
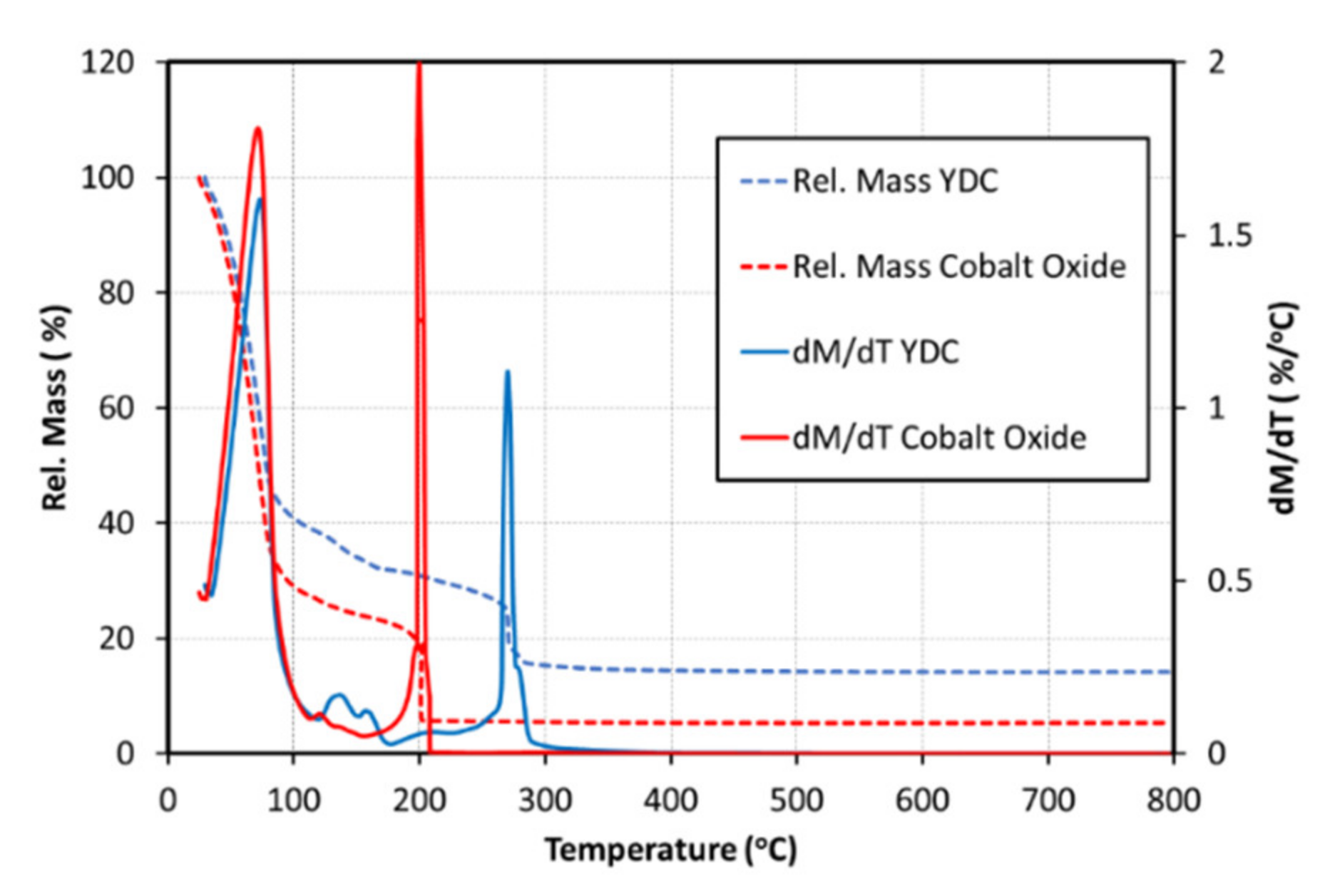
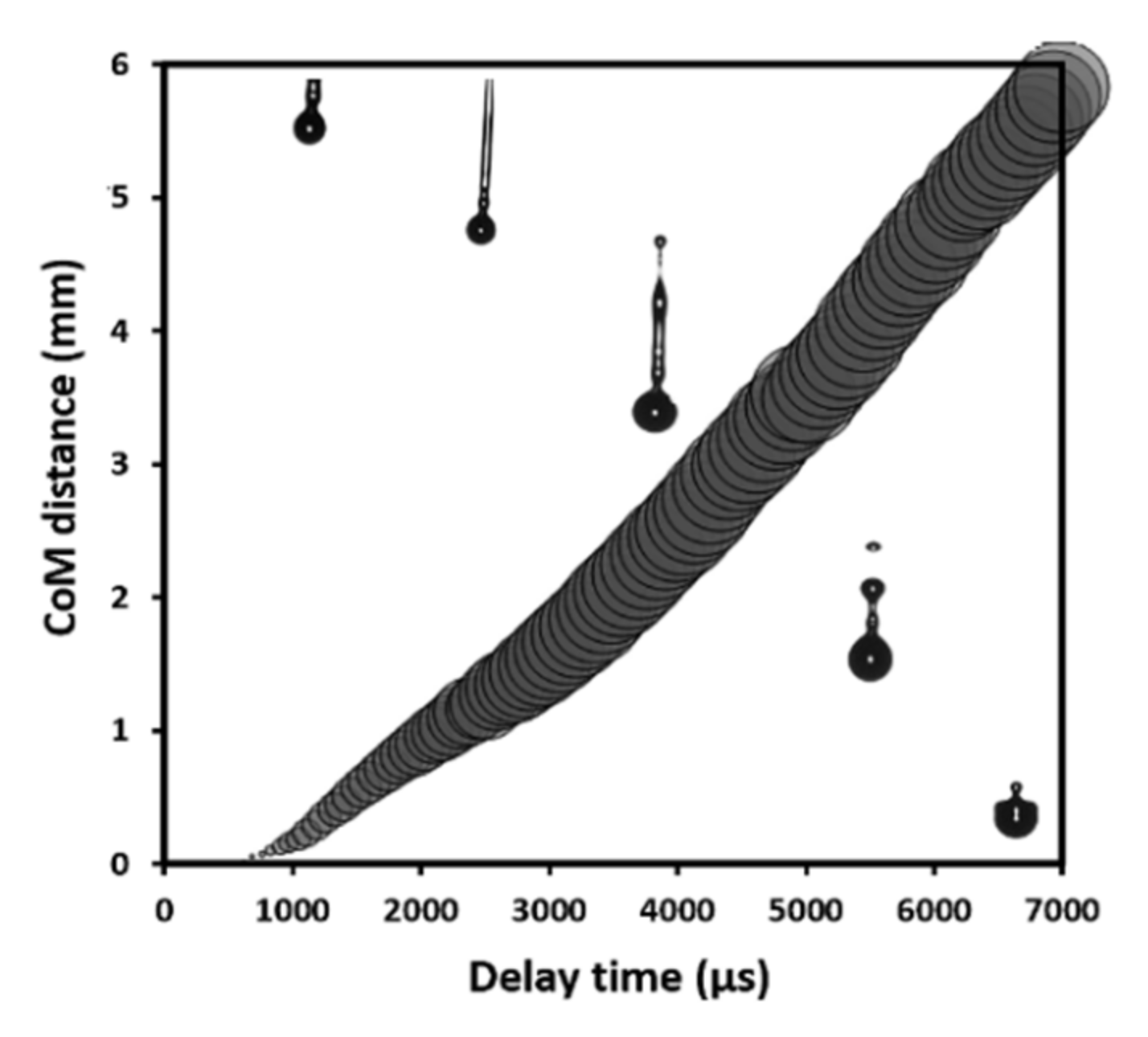
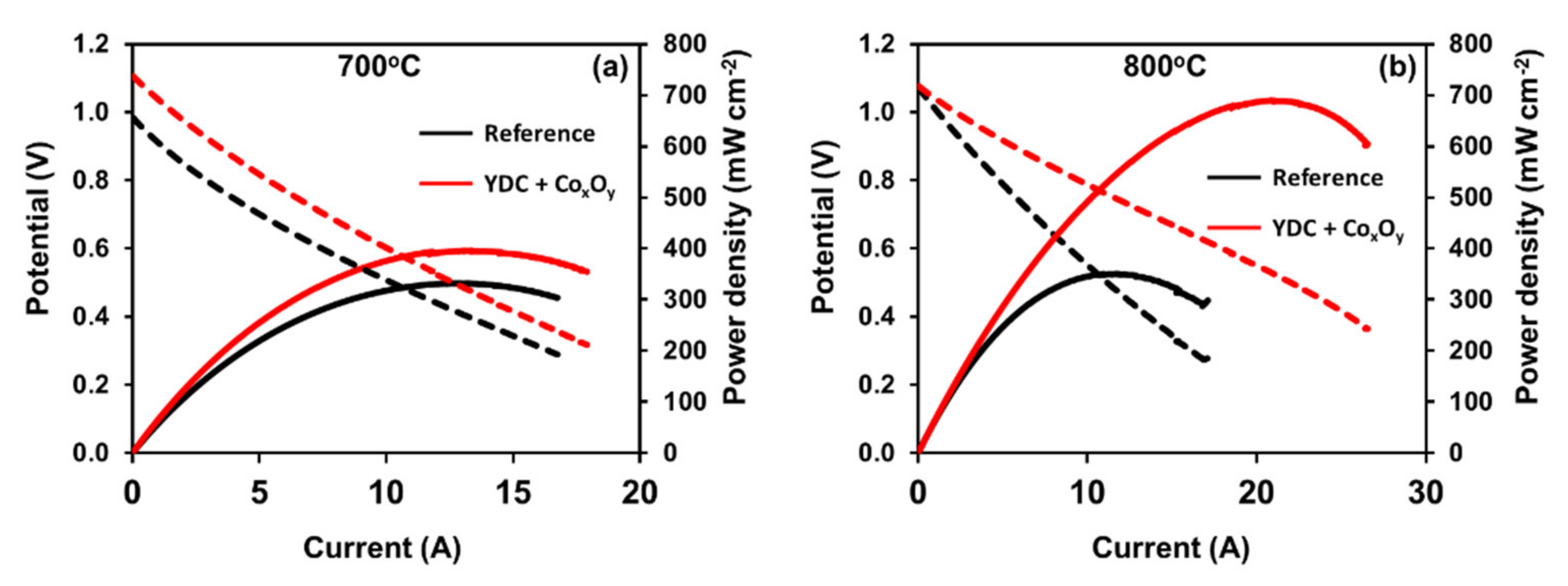
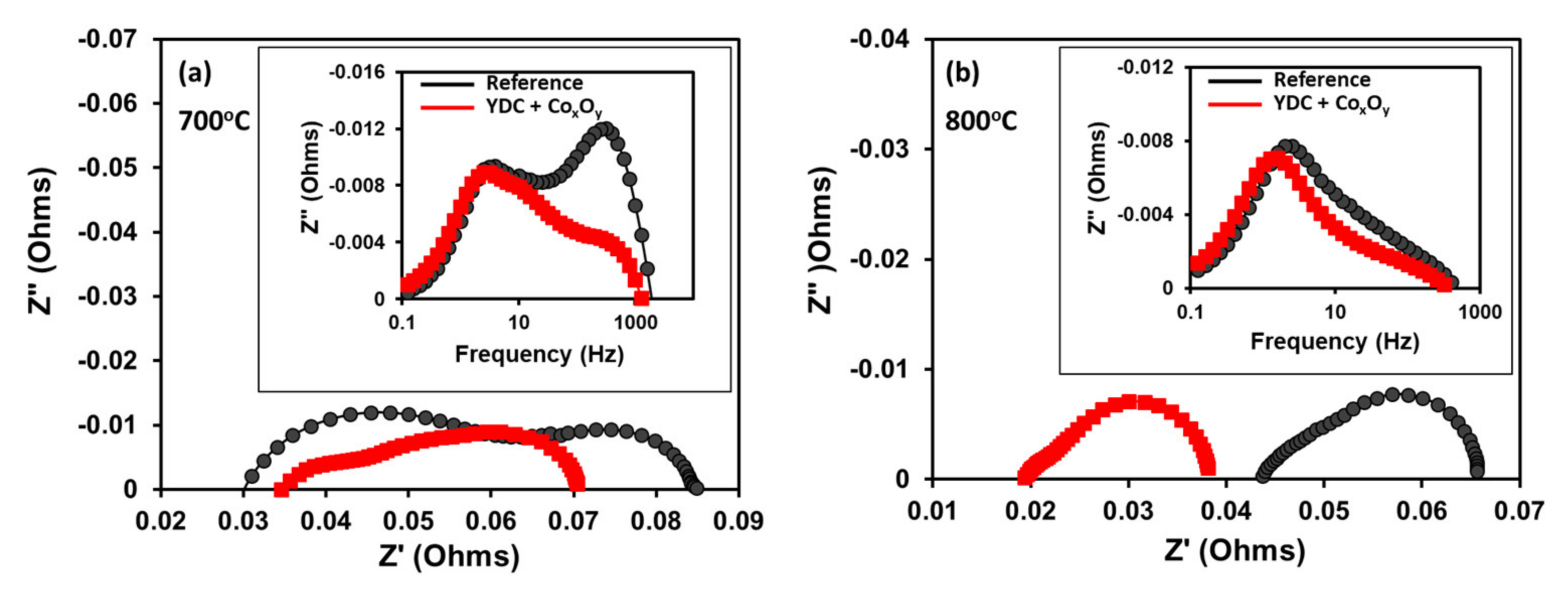
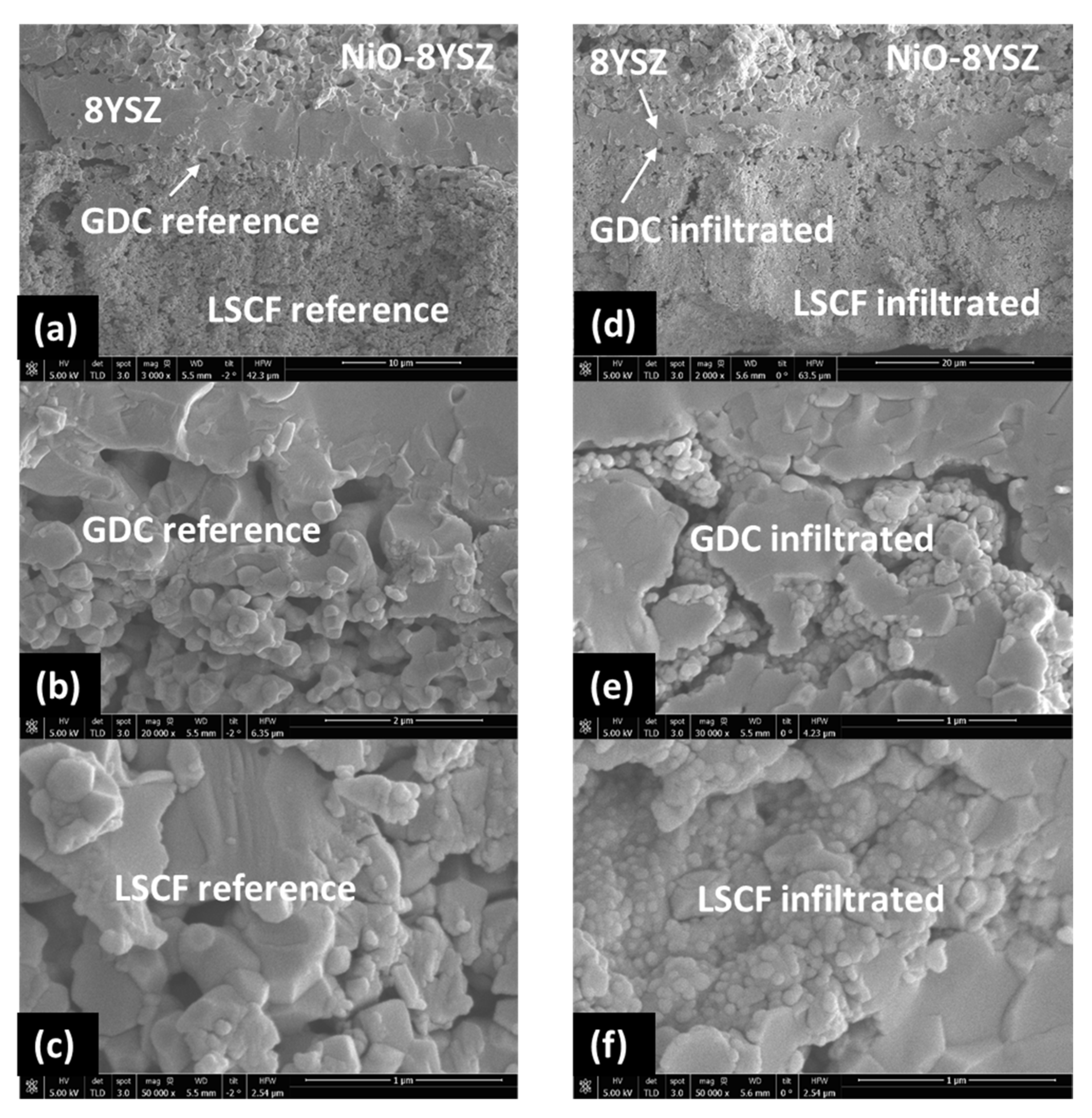

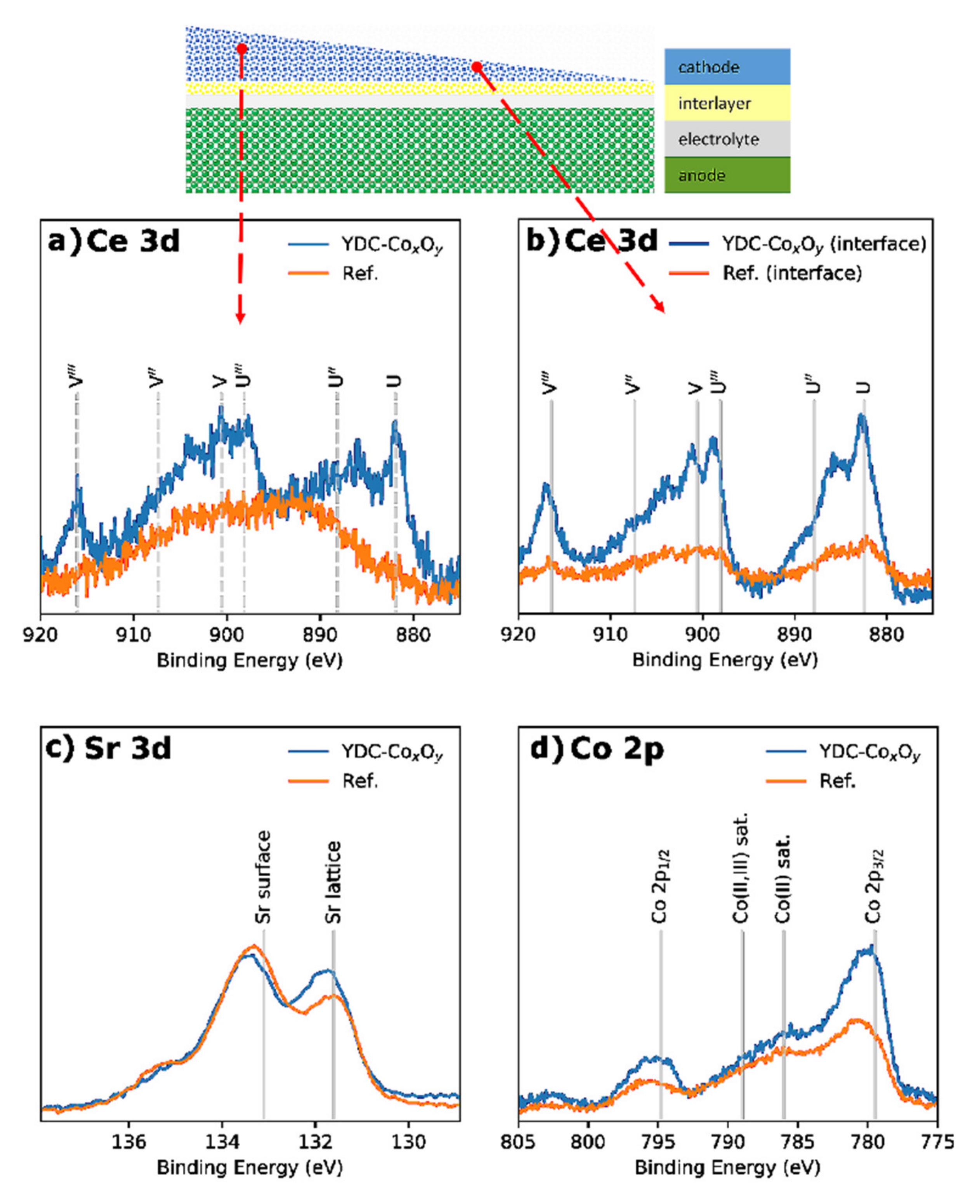
| Ink | Cation Concentration, M | Viscosity, cP | Opening Time, µs | Pressure, mbar | Drop Volume, nL | Drop Velocity, ms−1 |
|---|---|---|---|---|---|---|
| YDC | 0.75 | 4.8 | 240 | 200 | 57 | 1.5 |
| CoxOy | 0.75 | 4.3 | 230 | 225 | 62 | 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomov, R.I.; Mitchel-Williams, T.B.; Venezia, E.; Kawalec, M.; Krauz, M.; Kumar, R.V.; Glowacki, B.A. Inkjet Printing Infiltration of the Doped Ceria Interlayer in Commercial Anode-Supported SOFCs. Nanomaterials 2021, 11, 3095. https://doi.org/10.3390/nano11113095
Tomov RI, Mitchel-Williams TB, Venezia E, Kawalec M, Krauz M, Kumar RV, Glowacki BA. Inkjet Printing Infiltration of the Doped Ceria Interlayer in Commercial Anode-Supported SOFCs. Nanomaterials. 2021; 11(11):3095. https://doi.org/10.3390/nano11113095
Chicago/Turabian StyleTomov, Rumen I., Thomas B. Mitchel-Williams, Eleonora Venezia, Michal Kawalec, Mariusz Krauz, Ramachandran Vasant Kumar, and Bartek A. Glowacki. 2021. "Inkjet Printing Infiltration of the Doped Ceria Interlayer in Commercial Anode-Supported SOFCs" Nanomaterials 11, no. 11: 3095. https://doi.org/10.3390/nano11113095
APA StyleTomov, R. I., Mitchel-Williams, T. B., Venezia, E., Kawalec, M., Krauz, M., Kumar, R. V., & Glowacki, B. A. (2021). Inkjet Printing Infiltration of the Doped Ceria Interlayer in Commercial Anode-Supported SOFCs. Nanomaterials, 11(11), 3095. https://doi.org/10.3390/nano11113095






