Hybrid Molecularly Imprinted Polymers: The Future of Nanomedicine?
Abstract
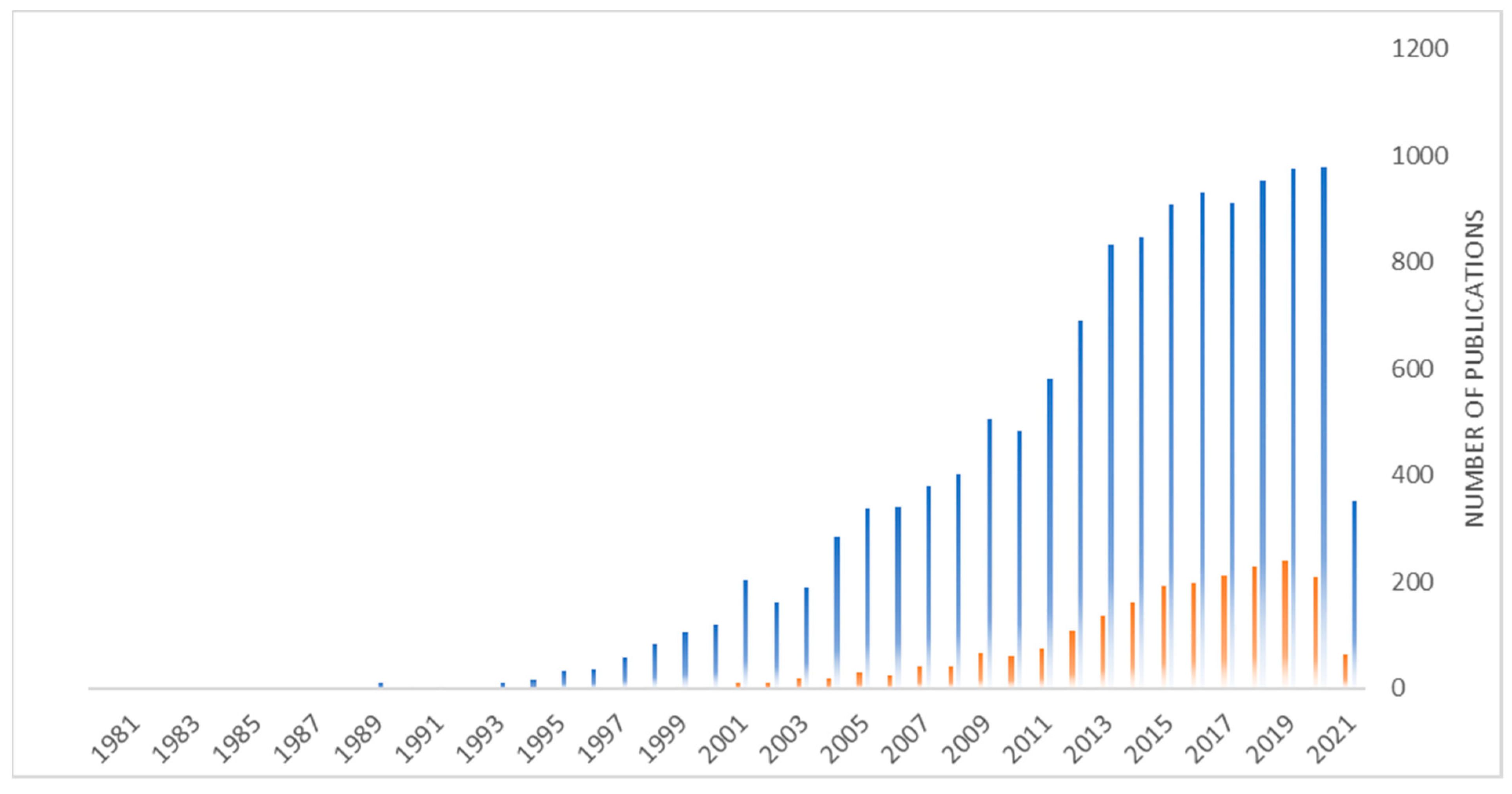
:1. Introduction
2. Fundamentals about MIPs and Their Adaptation for Nanomedicine
2.1. Historical Applications
2.2. Synthesis of MIPs
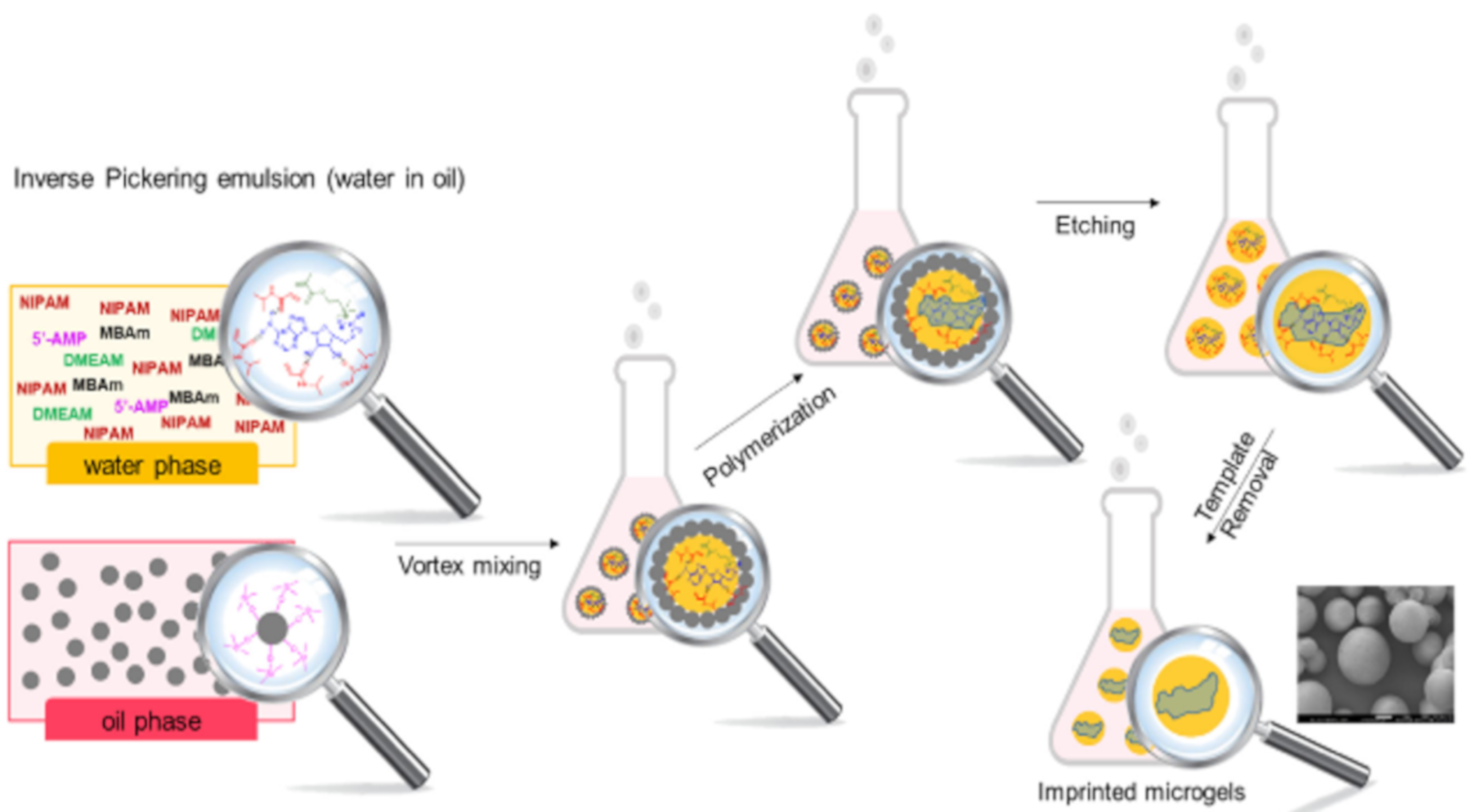
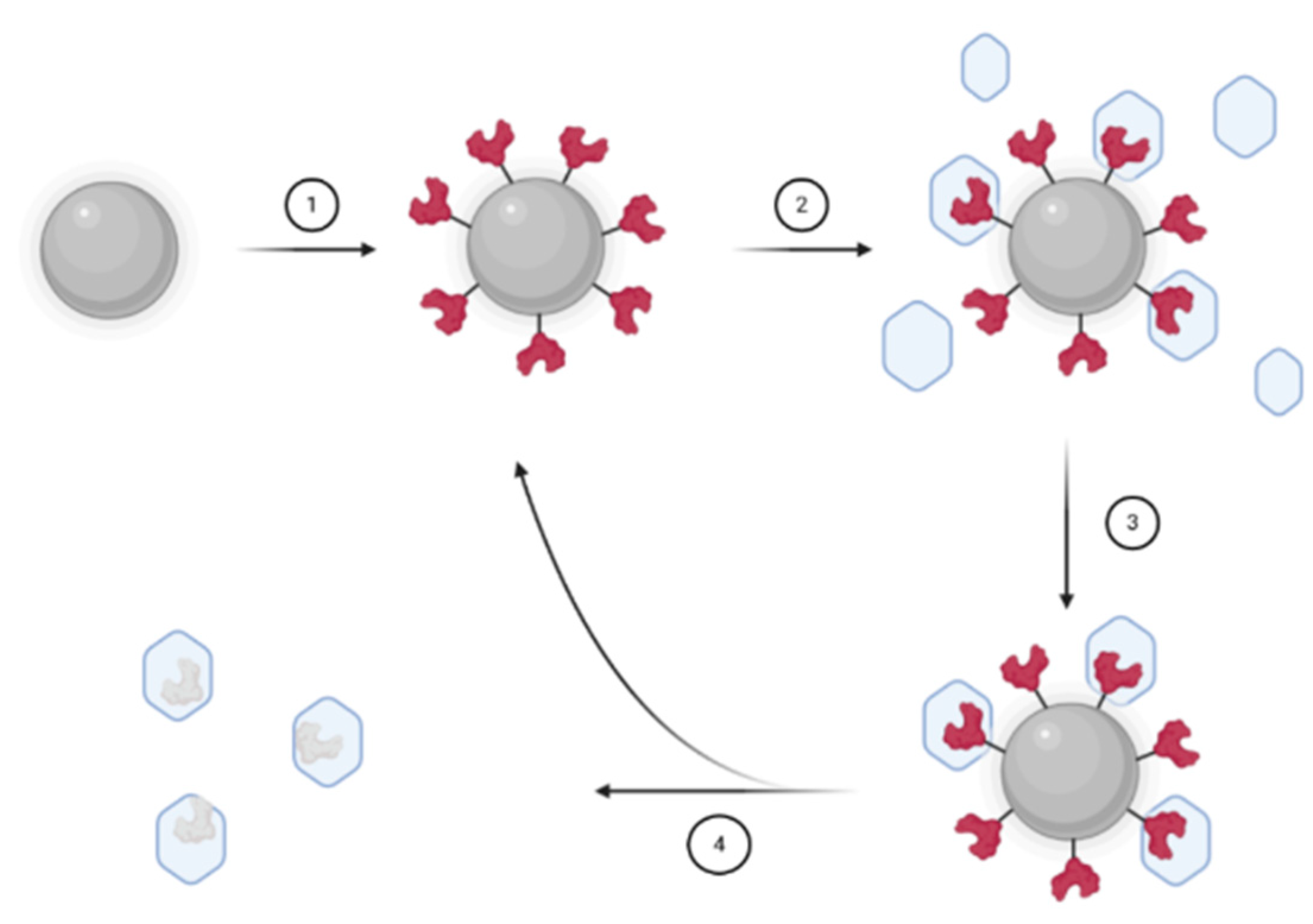
2.3. Synthesis of MIP Nanoparticles for Nanomedicine
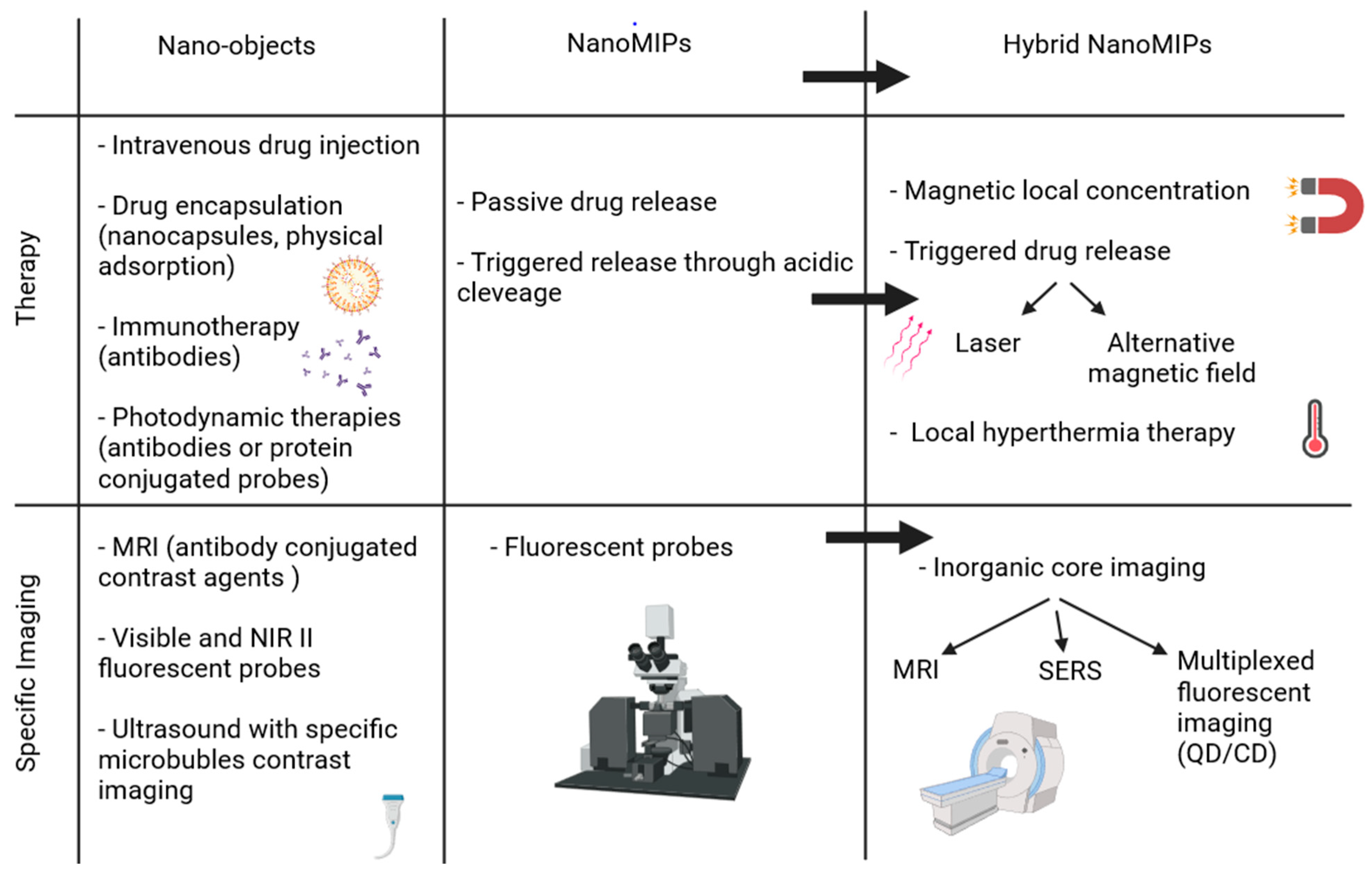
3. From MIPs to Hybrid MIPs
3.1. MIPs for Drug Delivery and Targeting
3.2. Properties of Inorganic Particles
3.3. Toxicity and Stealthiness
4. Application of Hybrids nanoMIPs for Medicine
4.1. Bioimaging
4.2. Therapy
4.3. Theranostic
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscope |
| AMF | Alternative magnetic field |
| ASP | Aspirin |
| CD | Carbon dot |
| DOX | Doxorubicin |
| EGDMA | Ethylene glycol dimethacrylate |
| EGFR | Epidermal growth factor |
| ELISA | Enzyme-linked immunosorbent assay |
| EPR effect | Endothelial permeability and retention effect |
| FTIR | Fourier transform infrared spectroscopy |
| hVEGF | Human vascular endothelial growth factor |
| LOD | Limit of detection |
| MAA | Methacrylic acid |
| MIP | Molecularly imprinted polymer |
| MRI | Magnetic resonance imaging |
| NIP | Non-imprinted polymer |
| NIR | Near infrared |
| NP | Nanoparticle |
| QCM | Quartz crystal microbalance |
| QD | Quantum dot |
| SAW | Surface acoustic wave |
| SERS | Surface enhanced Raman spectroscopy |
| SPIONs | Superparamagnetic iron oxide nanoparticles |
| SPR | Surface plasmon resonance |
| TEM | Transmission electronic microscopy |
References
- Overchuk, M.; Zheng, G. Overcoming obstacles in the tumor microenvironment: Recent advancements in nanoparticle delivery for cancer theranostics. Biomaterials 2018, 156, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, J.; Ferrari, M. Clinical Cancer Nanomedicine. Nano Today 2019, 25, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Van der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Haba, Y.; Kojima, C.; Harada, A.; Ura, T.; Horinaka, H.; Kono, K. Preparation of Poly(ethylene glycol)-Modified Poly(amido amine) Dendrimers Encapsulating Gold Nanoparticles and Their Heat-Generating Ability. Langmuir 2007, 23, 5243–5246. [Google Scholar] [CrossRef]
- Mofazzal Jahromi, M.A.; Sahandi Zangabad, P.; Moosavi Basri, S.M.; Sahandi Zangabad, K.; Ghamarypour, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2018, 123, 33–64. [Google Scholar] [CrossRef]
- Rudramurthy, G.R.; Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Nanoparticles: Alternatives Against Drug-Resistant Pathogenic Microbes. Molecules 2016, 21, 836. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Smith, C.C.; Yang, F.; Qi, Y.; Roche, K.C.; Serody, J.S.; Vincent, B.G.; Wang, A.Z. A Dual Immunotherapy Nanoparticle Improves T-Cell Activation and Cancer Immunotherapy. Adv. Mater. 2018, 30, 1706098. [Google Scholar] [CrossRef]
- Shi, Y.; Lammers, T. Combining Nanomedicine and Immunotherapy. Acc. Chem. Res. 2019, 52, 1543–1554. [Google Scholar] [CrossRef]
- Greene, M.K.M.; Richards, D.A.; Nogueira, J.C.F.; Campbell, K.; Smyth, P.; Fernández, M.; Scott, J.C.; Chudasama, V. Forming next-generation antibody–nanoparticle conjugates through the oriented installation of non-engineered antibody fragments. Chem. Sci. 2018, 9, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.X.; Danquah, M.K.; Pan, S.; Yon, L.S. Binding Characterization of Aptamer-Drug Layered Microformulations and In Vitro Release Assessment. J. Pharm. Sci. 2019, 108, 2934–2941. [Google Scholar] [CrossRef]
- Cerchia, L.; de Franciscis, V. Targeting cancer cells with nucleic acid aptamers. Trends Biotechnol. 2010, 28, 517–525. [Google Scholar] [CrossRef]
- Menger, M.; Yarman, A.; Erdőssy, J.; Yildiz, H.; Gyurcsányi, R.; Scheller, F. MIPs and Aptamers for Recognition of Proteins in Biomimetic Sensing. Biosensors 2016, 6, 35. [Google Scholar] [CrossRef]
- Hori, S.; Herrera, A.I.; Rossi, J.J.; Zhou, J. Current Advances in Aptamers for Cancer Diagnosis and Therapy. Cancers 2018, 10, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meir, R.; Shamalov, K.; Sadan, T.; Motiei, M.; Yaari, G.; Cohen, C.J.; Popovtzer, R. Fast Image-Guided Stratification Using Anti-Programmed Death Ligand 1 Gold Nanoparticles for Cancer Immunotherapy. ACS Nano 2017, 11, 11127–11134. [Google Scholar] [CrossRef] [PubMed]
- Meir, R.; Shamalov, K.; Cohen, C.J.; Popovtzer, R. Predicting treatment outcome and enhancing immunotherapy with anti-PDL1 gold nanoparticles. In Reporters, Markers, Dyes, Nanoparticles, and Molecular Probes for Biomedical Applications XI; International Society for Optics and Photonics: Bellingham, DC, USA, 2019; Volume 10893, p. 108930Z. [Google Scholar]
- Svenson, J.; Nicholls, I.A. On the thermal and chemical stability of molecularly imprinted polymers. Anal. Chim. Acta 2001, 435, 19–24. [Google Scholar] [CrossRef]
- Vlatakis, G.; Andersson, L.I.; Müller, R.; Mosbach, K. Drug assay using antibody mimics made by molecular imprinting. Nature 1993, 361, 645–647. [Google Scholar] [CrossRef]
- Ye, L.; Haupt, K. Molecularly imprinted polymers as antibody and receptor mimics for assays, sensors and drug discovery. Anal. Bioanal. Chem. 2004, 378, 1887–1897. [Google Scholar] [CrossRef]
- Canfarotta, F.; Waters, A.; Sadler, R.; McGill, P.; Guerreiro, A.; Papkovsky, D.; Haupt, K.; Piletsky, S. Biocompatibility and internalization of molecularly imprinted nanoparticles. Nano Res. 2016, 9, 3463–3477. [Google Scholar] [CrossRef]
- Ansell, R.J.; Mosbach, K. Magnetic molecularly imprinted polymer beads for drug radioligand binding assay. Analyst 1998, 123, 1611–1616. [Google Scholar] [CrossRef]
- Cazares-Cortes, E.; Cabana, S.; Boitard, C.; Nehlig, E.; Griffete, N.; Fresnais, J.; Wilhelm, C.; Abou-Hassan, A.; Ménager, C. Recent insights in magnetic hyperthermia: From the “hot-spot” effect for local delivery to combined magneto-photo-thermia using magneto-plasmonic hybrids. Adv. Drug Deliv. Rev. 2019, 138, 233–246. [Google Scholar] [CrossRef]
- Sanadgol, N.; Wackerlig, J. Developments of Smart Drug-Delivery Systems Based on Magnetic Molecularly Imprinted Polymers for Targeted Cancer Therapy: A Short Review. Pharmaceutics 2020, 12, 831. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, W.; Gu, Z.; Xing, R.; Ma, Y.; Zhang, Q.; Liu, Z. Inhibition of HER2-Positive Breast Cancer Growth by Blocking the HER2 Signaling Pathway with HER2-Glycan-Imprinted Nanoparticles. Angew. Chem. Int. Ed. 2019, 58, 10621–10625. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of carbon nanotubes. Chem Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef]
- Rossetti, R.; Nakahara, S.; Brus, L.E. Quantum size effects in the redox potentials, resonance Raman spectra, and electronic spectra of CdS crystallites in aqueous solution. J. Chem. Phys. 1983, 79, 1086–1088. [Google Scholar] [CrossRef]
- El-Schich, Z.; Zhang, Y.; Feith, M.; Beyer, S.; Sternbæk, L.; Ohlsson, L.; Stollenwerk, M.; Wingren, A.G. Molecularly imprinted polymers in biological applications. BioTechniques 2020, 69, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, L.; Liu, Z. Molecularly Imprinted Polymer Nanoparticles: An Emerging Versatile Platform for Cancer Therapy. Angew. Chem. Int. Ed. 2021, 60, 3858–3869. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K.; Medina Rangel, P.X.; Bui, B.T.S. Molecularly Imprinted Polymers: Antibody Mimics for Bioimaging and Therapy. Chem. Rev. 2020, 120, 9554–9582. [Google Scholar] [CrossRef]
- Chapuis, F.; Mullot, J.-U.; Pichon, V.; Tuffal, G.; Hennion, M.-C. Molecularly imprinted polymers for the clean-up of a basic drug from environmental and biological samples. J. Chromatogr. A 2006, 1135, 127–134. [Google Scholar] [CrossRef]
- De Sá, I.P.; de Higuera, J.M.; de Costa, V.C.; Costa, J.A.S.; da Silva, C.M.P.; Nogueira, A.R.A. Determination of Trace Elements in Meat and Fish Samples by MIP OES Using Solid-Phase Extraction. Food Anal. Methods 2019. [Google Scholar] [CrossRef]
- Zuo, H.G.; Yang, H.; Zhu, J.X.; Ding, Y. Preparation of a novel RAM-MIP for selective solid-phase extraction and gas chromatography determination of heptachlor, endosulfan and their metabolite residues in pork. Anal. Methods 2017, 9, 6009–6018. [Google Scholar] [CrossRef]
- Tan, S.; Yu, H.; He, Y.; Wang, M.; Liu, G.; Hong, S.; Yan, F.; Wang, Y.; Wang, M.; Li, T.; et al. A dummy molecularly imprinted solid-phase extraction coupled with liquid chromatography-tandem mass spectrometry for selective determination of four pyridine carboxylic acid herbicides in milk. J. Chromatography. B Anal. Technol. Biomed. Life Sci. 2019, 1108, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Climente, R.; Gómez-Caballero, A.; Halhalli, M.; Sellergren, B.; Goicolea, M.A.; Barrio, R.J. Iniferter-mediated grafting of molecularly imprinted polymers on porous silica beads for the enantiomeric resolution of drugs. J. Mol. Recognit. 2016, 29, 106–114. [Google Scholar] [CrossRef]
- Aboul-Enein, H.Y.; El-Awady, M.I.; Heard, C.M. Direct enantiomeric resolution of some cardiovascular agents using synthetic polymers imprinted with (-)-S-timolol as chiral stationary phase by thin layer chromatography. Die Pharmazie 2002, 53, 169–171. [Google Scholar]
- Conesa, A.; Palet, C. Molecularly imprinted membranes (MIM) for the enantioseparation of selenoaminoacid compounds. Desalination 2006, 200, 110–111. [Google Scholar] [CrossRef]
- Chapuis-Hugon, F.; Pichon, V. Utilisation d’outils sélectifs pour l’analyse de traces dans des échantillons complexes. Ann. Toxicol Anal. 2010, 22, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Jetzschmann, K.J.; Zhang, X.; Yarman, A.; Wollenberger, U.; Scheller, F.W. Label-Free MIP Sensors for Protein Biomarkers. In Label-Free Biosensing; Schöning, M.J., Poghossian, A., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; Volume 16, pp. 291–321. [Google Scholar]
- Whitcombe, M.J.; Kirsch, N.; Nicholls, I.A. Molecular imprinting science and technology: A survey of the literature for the years 2004-2011: Molecular imprinting science and technology: 2004–2011. J. Mol. Recognit. 2014, 27, 297–401. [Google Scholar]
- Servant, A. Synthesis and Characterisation of Molecularly Imprinted Nanoparticles with Enzyme-Like Catalytic Activity for the Kemp Elimination; Queen Mary, University of London: London, UK, 2010. [Google Scholar]
- Çakir, P.; Cutivet, A.; Resmini, M.; Bui, B.T.S.; Haupt, K. Protein-Size Molecularly Imprinted Polymer Nanogels as Synthetic Antibodies, by Localized Polymerization with Multi-initiators. Adv. Mater. 2013, 25, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Smolinska-Kempisty, K.K.; Guerreiro, A.; Canfarotta, F.; Cáceres, C.; Whitcombe, M.J.; Piletsky, S. A comparison of the performance of molecularly imprinted polymer nanoparticles for small molecule targets and antibodies in the ELISA format. Sci. Rep. 2016, 6, 37638. [Google Scholar] [CrossRef] [Green Version]
- García, Y.; Smolinska-Kempisty, K.; Pereira, E.; Piletska, E.V.; Piletsky, S.A. Development of competitive ‘pseudo’-ELISA assay for measurement of cocaine and its metabolites using molecularly imprinted polymer nanoparticles. Anal. Methods 2017, 9, 4592–4598. [Google Scholar] [CrossRef]
- Bossi, A.; Piletsky, S.A.; Piletska, E.V.; Righetti, P.G.; Turner, A.P.F. Surface-Grafted Molecularly Imprinted Polymers for Protein Recognition. Anal. Chem. 2001, 73, 5281–5286. [Google Scholar] [CrossRef] [PubMed]
- Chianella, I.; Guerreiro, A.R.; Moczko, E.; Caygill, J.S.; Piletska, E.V.; Sansalvador, I.M.P.D.V.; Whitcombe, M.J.; Piletsky, S. Direct replacement of antibodies with molecularly imprinted polymer nanoparticles in ELISA--development of a novel assay for vancomycin. Anal. Chem. 2013, 85, 8462–8468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chunta, S.; Suedee, R.; Lieberzeit, P.A. Low-Density Lipoprotein Sensor Based on Molecularly Imprinted Polymer. Anal. Chem. 2016, 88, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, M. Adsorption properties and structure of silica gel. Zhur Fiz Khim 1931, 2, 799–805. [Google Scholar]
- Wulf, G.; Sarhan, A. The Use of Polymers with Enzyme-Analogous Structures for the Resolution of Racemates. Angew. Chem. Int. Ed. 1972, 11, 341–344. [Google Scholar]
- Bodoki, A.E.; Iacob, B.-C.; Bodoki, E. Perspectives of Molecularly Imprinted Polymer-Based Drug Delivery Systems in Cancer Therapy. Polymers 2019, 11, 2085. [Google Scholar] [CrossRef] [Green Version]
- Holland, N.; Frisby, J.; Owens, E.; Hughes, H.; Duggan, P.; McLoughlin, P. The influence of polymer morphology on the performance of molecularly imprinted polymers. Polymer 2010, 51, 1578–1584. [Google Scholar] [CrossRef]
- Wulff, G. Molecular Imprinting in Cross-Linked Materials with the Aid of Molecular Templates—A Way towards Artificial Antibodies. Angew. Chem. Int. Ed. Engl. 1995, 34, 1812–1832. [Google Scholar] [CrossRef]
- Whitcombe, M.J.; Rodriguez, M.E.; Villar, P.; Vulfson, E.N. A New Method for the Introduction of Recognition Site Functionality into Polymers Prepared by Molecular Imprinting: Synthesis and Characterization of Polymeric Receptors for Cholesterol. J. Am. Chem. Soc. 1995, 117, 7105–7111. [Google Scholar] [CrossRef]
- Arshady, R.; Mosbach, K. Synthesis of substrate-selective polymers by host-guest polymerization. Die Makromol. Chem. 1981, 182, 687–692. [Google Scholar] [CrossRef]
- Ekberg, B.; Mosbach, K. Molecular imprinting: A technique for producing specific separation materials. Trends Biotechnol. 1989, 7, 92–96. [Google Scholar] [CrossRef]
- Vaihinger, D.; Landfester, K.; Kräuter, I.; Brunner, H.; Tovar, G.E.M. Molecularly imprinted polymer nanospheres as synthetic affinity receptors obtained by miniemulsion polymerisation. Macromol. Chem. Phys. 2002, 203, 1965–1973. [Google Scholar] [CrossRef]
- Ayari, M.G.; Kadhirvel, P.; Favetta, P.; Plano, B.; Dejous, C.; Carbonnier, B.; Agrofoglio, L.A. Synthesis of imprinted hydrogel microbeads by inverse Pickering emulsion to controlled release of adenosine 5’-monophosphate. Mater. Sci. Eng. C 2019, 101, 254–263. [Google Scholar] [CrossRef]
- Ye, L.; Cormack, P.A.G.; Mosbach, K. Molecularly imprinted monodisperse microspheres for competitive radioassay. Anal. Commun. 1999, 36, 35–38. [Google Scholar] [CrossRef]
- Ruela, A.L.M.; Figueiredo, E.; Carvalho, F.C.; de Araújo, M.B.; Pereira, G.R. Adsorption and release of nicotine from imprinted particles synthesised by precipitation polymerisation: Optimising transdermal formulations. Eur. Polym. J. 2018, 100, 67–76. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Titirici, M.M.; Hall, A.J.; Sellergren, B. Hierarchically Imprinted Stationary Phases: Mesoporous Polymer Beads Containing Surface-Confined Binding Sites for Adenine. Chem. Mater. 2002, 14, 21–23. [Google Scholar] [CrossRef]
- Ecevit, Y.; Haupt, K.; Mosbach, K. The Use of Immobilized Templates—A New Approach in Molecular Imprinting. Angew. Chem. Int. Ed. Engl. 2000, 39, 2115–2118. [Google Scholar]
- Nematollahzadeh, A.; Sun, W.; Aureliano, C.S.A.; Lütkemeyer, D.; Stute, J.; Abdekhodaie, M.J.; Shojaei, A.; Sellergren, B. High-Capacity Hierarchically Imprinted Polymer Beads for Protein Recognition and Capture. Angew. Chem. Int. Ed. 2011, 50, 495–498. [Google Scholar] [CrossRef]
- Rachkov, A.; Minoura, N. Recognition of oxytocin and oxytocin-related peptides in aqueous media using a molecularly imprinted polymer synthesized by the epitope approach. J. Chromatogr. A 2000, 889, 111–118. [Google Scholar] [CrossRef]
- Nishino, H.; Huang, C.-S.; Shea, K.J. Selective protein capture by epitope imprinting. Angew. Chem. Int. Ed. Engl. 2006, 45, 2392–2396. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-H.; Zhang, Y.; Tang, S.-F.; Fang, Z.-B.; Yang, H.-H.; Chen, X.; Chen, G.-N. Sensing HIV related protein using epitope imprinted hydrophilic polymer coated quartz crystal microbalance. Biosens. Bioelectron. 2012, 31, 439–444. [Google Scholar] [CrossRef]
- Ambrosini, S.; Beyazit, S.; Haupt, K.; Tse Sum Bui, B. Solid-phase synthesis of molecularly imprinted nanoparticles for protein recognition. Chem. Commun. 2013, 49, 6746. [Google Scholar] [CrossRef]
- Poma, A.; Guerreiro, A.; Caygill, S.; Moczko, E.; Piletsky, S. Automatic reactor for solid-phase synthesis of molecularly imprinted polymeric nanoparticles (MIP NPs) in water. RSC Adv. 2014, 4, 4203–4206. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Li, M.; Hong, X.; Du, D.; Ma, Y. Solid-phase interfacial synthesis of dual-imprinted colloid particles for multifunctional nanomedicine development. Colloid Interface Sci. Commun. 2020, 36, 100267. [Google Scholar] [CrossRef]
- Canfarotta, F.; Poma, A.; Guerreiro, A.; Piletsky, S. Solid-phase synthesis of molecularly imprinted nanoparticles. Nat. Protoc 2016, 11, 443–455. [Google Scholar] [CrossRef]
- Xu, J.; Medina-Rangel, P.X.; Haupt, K.; Tse Sum Bui, B. Guide to the Preparation of Molecularly Imprinted Polymer Nanoparticles for Protein Recognition by Solid-Phase Synthesis. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 590, pp. 115–141. [Google Scholar]
- Cáceres, C.; Moczko, E.; Basozabal, I.; Guerreiro, A.; Piletsky, S. Molecularly Imprinted Nanoparticles (NanoMIPs) Selective for Proteins: Optimization of a Protocol for Solid-Phase Synthesis Using Automatic Chemical Reactor. Polymers 2021, 13, 314. [Google Scholar] [CrossRef] [PubMed]
- Ashley, J.; Feng, X.; Halder, A.; Zhou, T.; Sun, Y. Dispersive solid-phase imprinting of proteins for the production of plastic antibodies. Chem. Commun. 2018, 54, 3355–3358. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Yin, D.; Wang, W.; Shen, X.; Zhu, J.-J.; Chen, H.-Y.; Liu, Z. Targeting and Imaging of Cancer Cells via Monosaccharide-Imprinted Fluorescent Nanoparticles. Sci. Rep. 2016, 6, 22757. [Google Scholar] [CrossRef] [Green Version]
- Hiratani, H.; Fujiwara, A.; Tamiya, Y.; Mizutani, Y.; Alvarez-Lorenzo, C. Ocular release of timolol from molecularly imprinted soft contact lenses. Biomaterials 2005, 26, 1293–1298. [Google Scholar] [CrossRef]
- Paul, P.K.; Treetong, A.; Suedee, R. Biomimetic insulin-imprinted polymer nanoparticles as a potential oral drug delivery system. Acta Pharm. 2017, 67, 149–168. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Kang, J.; Li, P.; Liu, L.; Wang, H.; Tang, B. Two-photon fluorescence imaging of sialylated glycans in vivo based on a sialic acid imprinted conjugated polymer nanoprobe. Chem. Commun. 2016, 52, 13991–13994. [Google Scholar] [CrossRef] [PubMed]
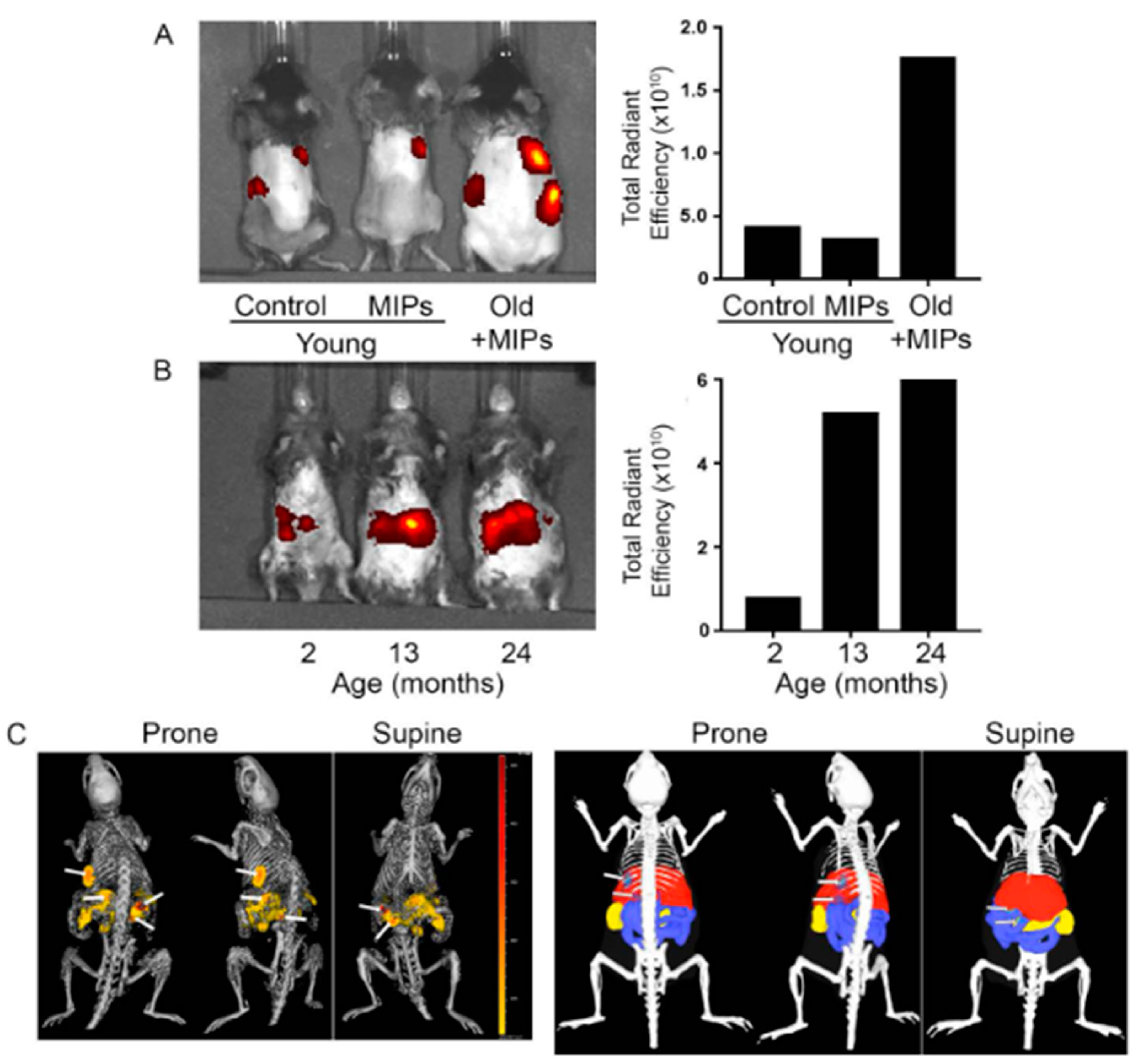
- Ekpenyong-Akiba, A.E.; Canfarotta, F.; Abd, H.B.; Poblocka, M.; Casulleras, M.; Castilla-Vallmanya, L.; Kocsis-Fodor, G.; Kelly, M.E.; Janus, J.; Althubiti, M.; et al. Detecting and targeting senescent cells using molecularly imprinted nanoparticles. Nanoscale Horiz. 2019, 4, 757–768. [Google Scholar] [CrossRef]
- Xu, Z.P.; Zeng, Q.H.; Lu, G.Q.; Yu, A.B. Inorganic nanoparticles as carriers for efficient cellular delivery. Chem. Eng. Sci. 2006, 61, 1027–1040. [Google Scholar] [CrossRef]
- Roa, W.; Zhang, X.; Guo, L.; Shaw, A.; Hu, X.; Xiong, Y.; Gulavita, S.; Patel, S.; Sun, X.; Chen, J.; et al. Gold nanoparticle sensitize radiotherapy of prostate cancer cells by regulation of the cell cycle. Nanotechnology 2009, 20, 375101. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.; Bhattacharya, R.; Wang, P.; Wang, L.; Basu, S.; Nagy, J.A.; Atala, A.; Mukhopadhyay, D.; Soker, S. Antiangiogenic properties of gold nanoparticles. Clin. Cancer Res. 2005, 11, 3530–3534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, S.; Nam, J.; Jung, S.; Song, J.; Doh, H.; Kim, S. Gold nanoparticle-mediated photothermal therapy: Current status and future perspective. Nanomedicine 2014, 9, 2003–2022. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Mackey, M.A.; Huang, X.; Kang, B.; El-Sayed, M.A. Beating cancer in multiple ways using nanogold. Chem. Soc. Rev. 2011, 40, 3391. [Google Scholar] [CrossRef] [Green Version]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1449. [Google Scholar] [CrossRef]
- Yadav, K.; Singh, M.R.; Rai, V.K.; Srivastava, N.; Prasad Yadav, N. Chapter 20—Commercial aspects and market potential of novel delivery systems for bioactives and biological agents. In Advances and Avenues in the Development of Novel Carriers for Bioactives and Biological Agents; Singh, M.R., Singh, D., Kanwar, J.R., Chauhan, N.S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 595–620. [Google Scholar] [CrossRef]
- Rosenthal, S.J.; Chang, J.C.; Kovtun, O.; McBride, J.R.; Tomlinson, I.D. Biocompatible Quantum Dots for Biological Applications. Chem. Biol. 2011, 18, 10–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef] [PubMed]
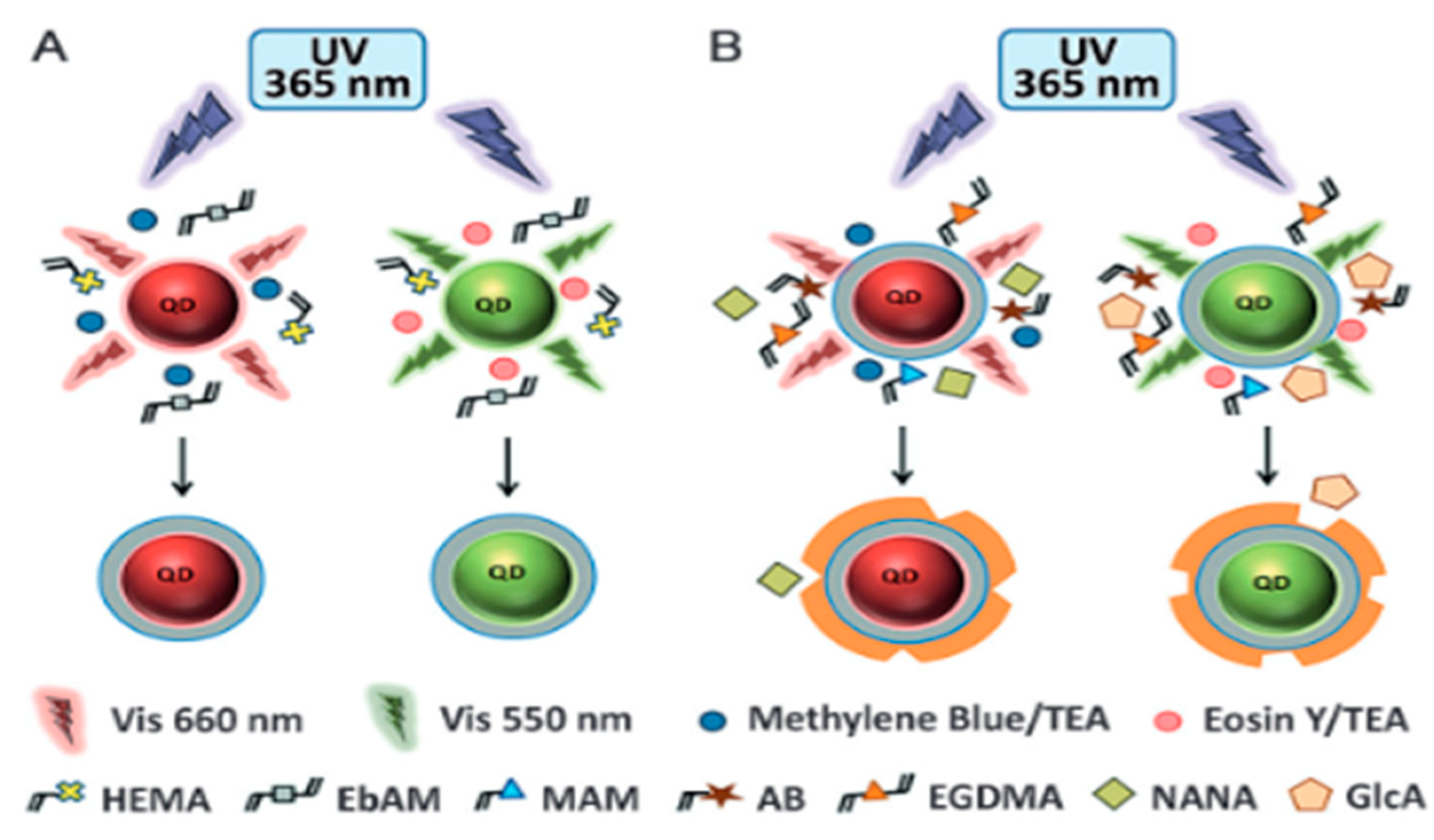
- Panagiotopoulou, M.; Salinas, Y.; Beyazit, S.; Kunath, S.; Duma, L.; Prost, E.; Mayes, A.G.; Resmini, M.; Tse Sum Bui, B.; Haupt, K. Molecularly Imprinted Polymer Coated Quantum Dots for Multiplexed Cell Targeting and Imaging. Angew. Chem. Int. Ed. 2016, 55, 8244–8248. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Guan, G.; Wang, S.; Zhang, Z. Core-shell nanostructured molecular imprinting fluorescent chemosensor for selective detection of atrazine herbicide. Analyst 2010, 136, 184–190. [Google Scholar] [CrossRef]
- Wei, F.; Xu, G.; Wu, Y.; Wang, X.; Yang, J.; Liu, L.; Zhou, P.; Hu, Q. Molecularly imprinted polymers on dual-color quantum dots for simultaneous detection of norepinephrine and epinephrine. Sens. Actuators B Chem. 2016, 229, 38–46. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, H.; Zhang, H. Direct and Highly Selective Drug Optosensing in Real, Undiluted Biological Samples with Quantum-Dot-Labeled Hydrophilic Molecularly Imprinted Polymer Microparticles. ACS Appl. Mater. Interfaces 2016, 8, 15741–15749. [Google Scholar] [CrossRef] [PubMed]
- Cumbo, A.; Lorber, B.; Corvini, P.F.-X.; Meier, W.; Shahgaldian, P. A synthetic nanomaterial for virus recognition produced by surface imprinting. Nat. Commun. 2013, 4, 1503. [Google Scholar] [CrossRef] [Green Version]
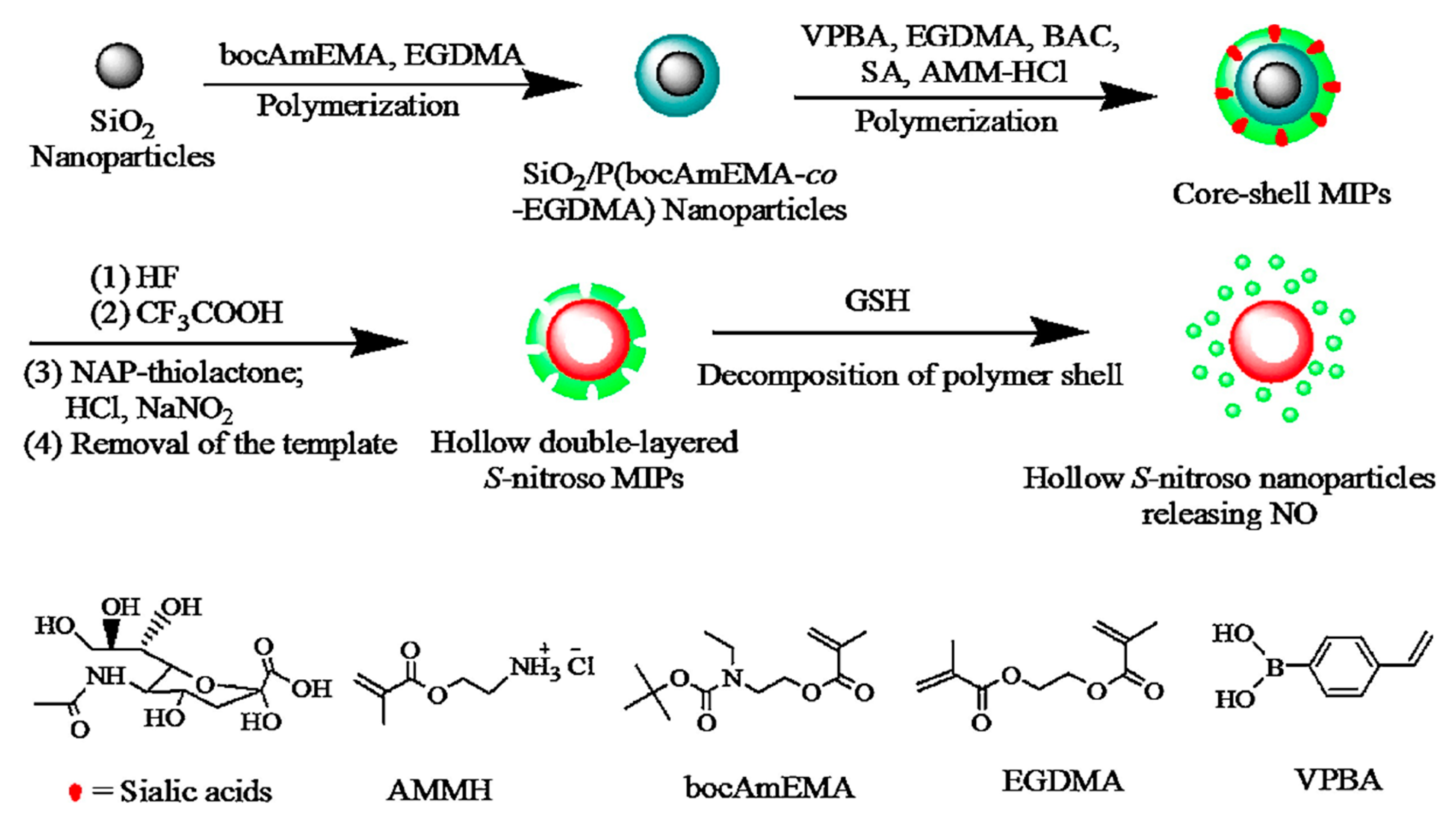
- Liu, T.; Qiao, Z.; Wang, J.; Zhang, P.; Zhang, Z.; Guo, D.-S.; Yang, X. Molecular imprinted S-nitrosothiols nanoparticles for nitric oxide control release as cancer target chemotherapy. Colloids Surfaces B Biointerfaces 2018, 173, 356–365. [Google Scholar] [CrossRef]
- Demir, B.; Lemberger, M.M.; Panagiotopoulou, M.; Medina Rangel, P.X.; Timur, S.; Hirsch, T.; Tse Sum Bui, B.; Wegener, J.; Haupt, K. Tracking Hyaluronan: Molecularly Imprinted Polymer Coated Carbon Dots for Cancer Cell Targeting and Imaging. ACS Appl. Mater. Interfaces 2018, 10, 3305–3313. [Google Scholar] [CrossRef]
- Zhang, T.; Qin, Y.; Tan, T.; Lv, Y. Targeted Live Cell Raman Imaging and Visualization of Cancer Biomarkers with Thermal-Stimuli Responsive Imprinted Nanoprobes. Part. Part. Syst. Charact. 2018, 35, 1800390. [Google Scholar] [CrossRef]
- Fadeel, B.; Garcia-Bennett, A.E. Better safe than sorry: Understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications. Adv. Drug Deliv. Rev. 2010, 62, 362–374. [Google Scholar] [CrossRef]
- Singh, N.; Jenkins, G.J.S.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef] [Green Version]
- Boitard, C.; Curcio, A.; Rollet, A.-L.; Wilhelm, C.; Ménager, C.; Griffete, N. Biological Fate of Magnetic Protein-Specific Molecularly Imprinted Polymers: Toxicity and Degradation. ACS Appl. Mater. Interfaces 2019, 11, 35556–35565. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Cui, Q.; Wang, C.; Wang, X.; Yang, Y.; Li, L. Preparation of Sialic Acid-Imprinted Fluorescent Conjugated Nanoparticles and Their Application for Targeted Cancer Cell Imaging. ACS Appl. Mater. Interfaces 2017, 9, 3006–3015. [Google Scholar] [CrossRef] [PubMed]
- Piletsky, S.; Canfarotta, F.; Poma, A.; Bossi, A.M.; Piletsky, S. Molecularly Imprinted Polymers for Cell Recognition. Trends Biotechnol. 2020, 38, 368–387. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, A.; Raffa, V.; Canfarotta, F.; Signore, G.; Piletsky, S.; MacDonald, M.P.; Cuschieri, A. In Vivo Recognition of Human Vascular Endothelial Growth Factor by Molecularly Imprinted Polymers. Nano Lett. 2017, 17, 2307–2312. [Google Scholar] [CrossRef] [Green Version]
- Panagiotopoulou, M.; Kunath, S.; Haupt, K.; Tse Sum Bui, B. Cell and Tissue Imaging with Molecularly Imprinted Polymers. In Synthetic Antibodies: Methods and Protocols; Tiller, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 399–415. [Google Scholar] [CrossRef]
- Panagiotopoulou, M.; Kunath, S.; Medina-Rangel, P.X.; Haupt, K.; Bui, B.T.S. Fluorescent molecularly imprinted polymers as plastic antibodies for selective labeling and imaging of hyaluronan and sialic acid on fixed and living cells. Biosens. Bioelectron. 2017, 88, 85–93. [Google Scholar] [CrossRef]
- Yin, D.; Wang, S.; He, Y.; Liu, J.; Zhou, M.; Ouyang, J.; Liu, B.; Chen, H.-Y.; Liu, Z. Surface-enhanced Raman scattering imaging of cancer cells and tissues via sialic acid-imprinted nanotags. Chem. Commun. 2015, 51, 17696–17699. [Google Scholar] [CrossRef]
- Zhang, K.; Guan, X.; Qiu, Y.; Wang, D.; Zhang, X.; Zhang, H. A pH/glutathione double responsive drug delivery system using molecular imprint technique for drug loading. Appl. Surf. Sci. 2016, 389, 1208–1213. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Shen, X. Janus molecularly imprinted polymer particles. Chem. Commun. 2014, 50, 2646–2649. [Google Scholar] [CrossRef]
- Asadi, E.; Abdouss, M.; Leblanc, R.M.; Ezzati, N.; Wilson, J.N.; Kordestani, D. Synthesis, characterization and in vivo drug delivery study of a biodegradable nano-structured molecularly imprinted polymer based on cross-linker of fructose. Polymer 2016, 97, 226–237. [Google Scholar] [CrossRef]
- Kan, X.; Geng, Z.; Zhao, Y.; Wang, Z.; Zhu, J.-J. Magnetic molecularly imprinted polymer for aspirin recognition and controlled release. Nanotechnology 2009, 20, 165601. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Jin, Z.; Cho, S.; Jeon, M.J.; Nguyen, V.D.; Park, J.-O.; Park, S. Folate-receptor-targeted NIR-sensitive polydopamine nanoparticles for chemo-photothermal cancer therapy. Nanotechnology 2017, 28, 425101. [Google Scholar] [CrossRef]
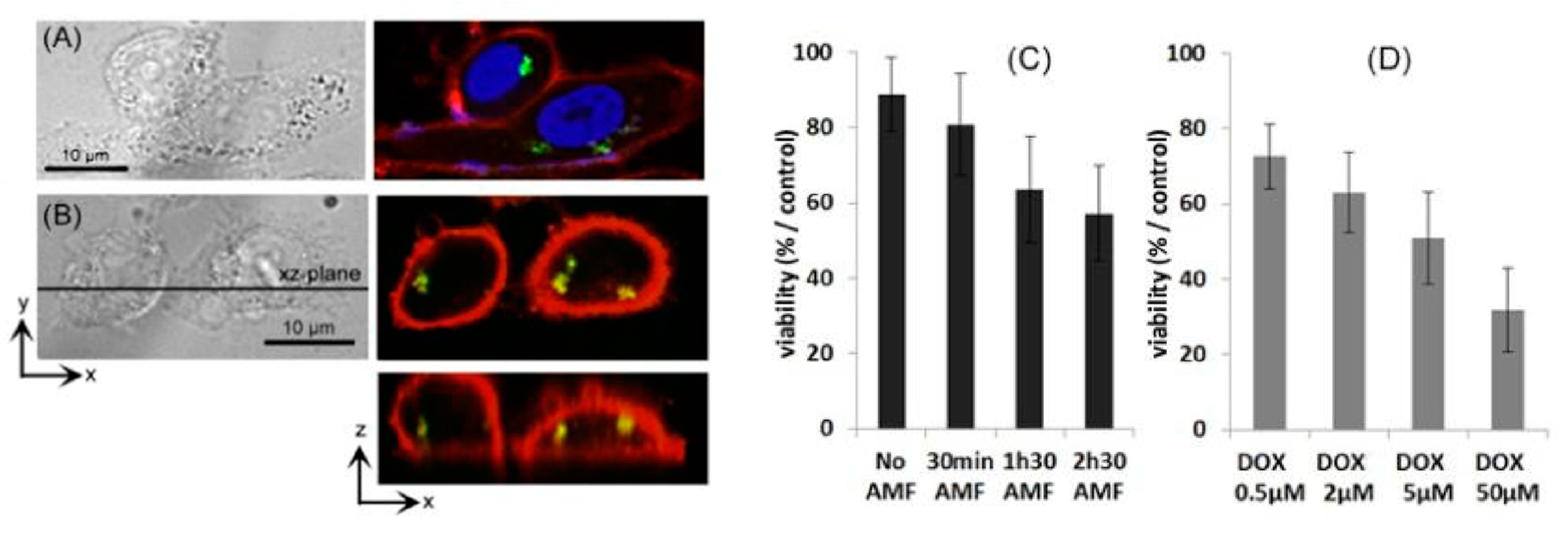
- Griffete, N.; Fresnais, J.; Espinosa, A.; Wilhelm, C.; Bée, A.; Ménager, C. Design of magnetic molecularly imprinted polymer nanoparticles for controlled release of doxorubicin under an alternative magnetic field in athermal conditions. Nanoscale 2015, 7, 18891–18896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazares-Cortes, E.; Nerantzaki, M.; Fresnais, J.; Wilhelm, C.; Griffete, N.; Ménager, C. Magnetic Nanoparticles Create Hot Spots in Polymer Matrix for Controlled Drug Release. Nanomaterials 2018, 8, 850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nerantzaki, M.; Michel, A.; Briot, E.; Siaugue, J.M.; Ménager, C.; Wilhelm, C.; Griffete, N. Controlled drug delivery for cancer cell treatment via magnetic doxorubicin imprinted silica nanoparticles. Chem. Commun. 2020, 56, 10255–10258. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, X.; Guan, P.; Du, C.; Tian, Y.; Ding, S.; Li, Z.; Yan, C. A novel controllable molecularly imprinted drug delivery system based on the photothermal effect of graphene oxide quantum dots. J. Mater. Sci. 2019, 54, 9124–9139. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, M.; Zhang, Y.; Ma, Z.-B.; Xiao, N.-N.; He, X.-W.; Li, W.-Y.; Zhang, Y.-K. Preparation of Dual-Template Epitope Imprinted Polymers for Targeted Fluorescence Imaging and Targeted Drug Delivery to Pancreatic Cancer BxPC-3 Cells. ACS Appl. Mater. Interfaces 2019, 11, 32431–32440. [Google Scholar] [CrossRef]
- Hashemi-Moghaddam, H.; Zavareh, S.; Karimpour, S.; Madanchi, H. Evaluation of molecularly imprinted polymer based on HER2 epitope for targeted drug delivery in ovarian cancer mouse model. React. Funct. Polym. 2017, 121, 82–90. [Google Scholar] [CrossRef]
- Tietze, R.; Lyer, S.; Dürr, S.; Struffert, T.; Engelhorn, T.; Schwarz, M.; Eckert, E.; Göen, T.; Vasylyev, S.; Peukert, W.; et al. Efficient drug-delivery using magnetic nanoparticles—Biodistribution and therapeutic effects in tumour bearing rabbits. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 961–971. [Google Scholar] [CrossRef]
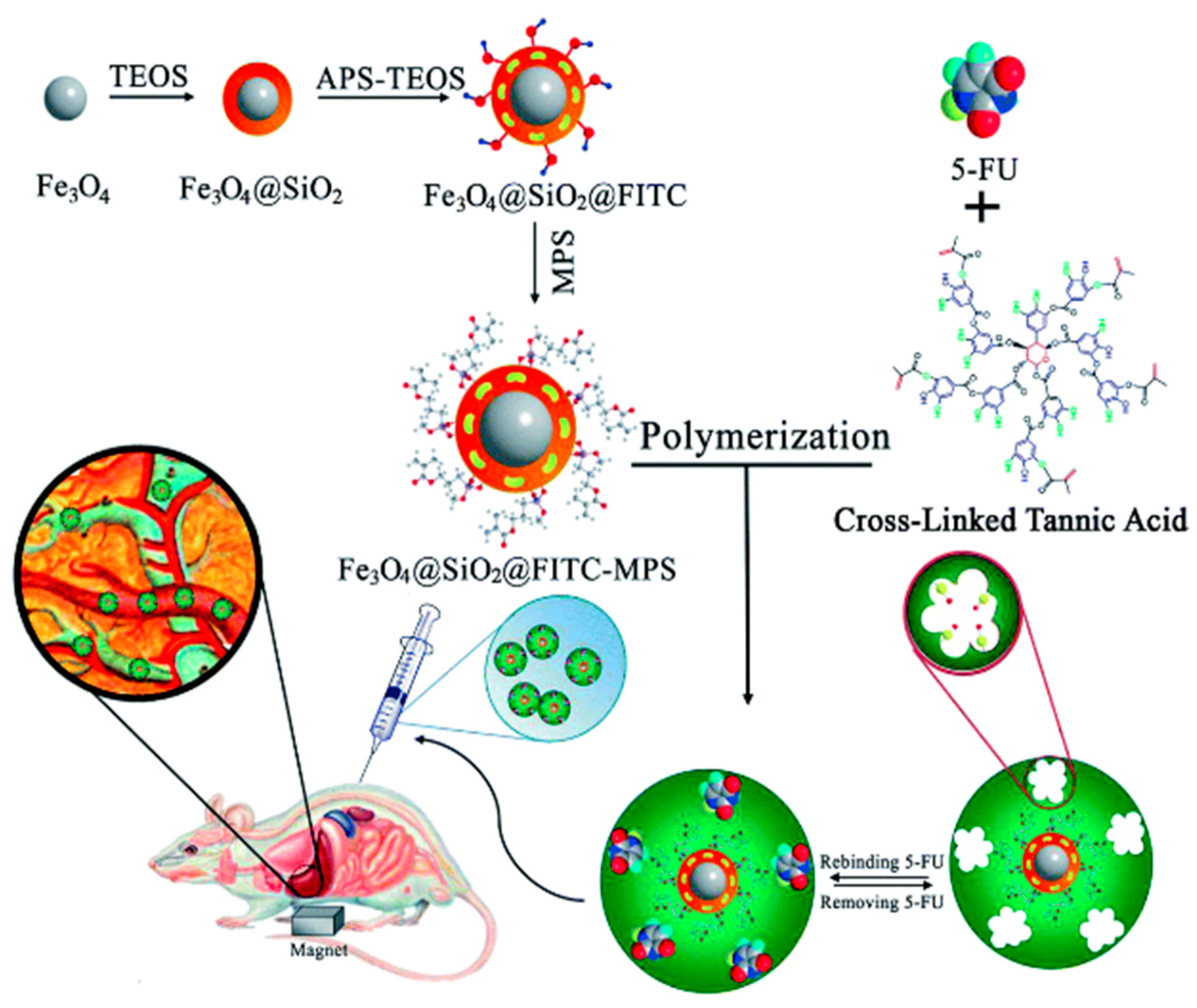
- Asadi, E.; Abdouss, M.; Leblanc, R.M.; Ezzati, N.; Wilson, J.N.; Azodi-Deilami, S. In vitro/in vivo study of novel anti-cancer, biodegradable cross-linked tannic acid for fabrication of 5-fluorouracil-targeting drug delivery nano-device based on a molecular imprinted polymer. RSC Adv. 2016, 6, 37308–37318. [Google Scholar] [CrossRef]
- Poma, A.; Guerreiro, A.; Whitcombe, M.J.; Piletska, E.V.; Turner, A.P.F.; Piletsky, S.A. Solid-Phase Synthesis of Molecularly Imprinted Polymer Nanoparticles with a Reusable Template—“Plastic Antibodies”. Adv. Funct. Mater. 2013, 23, 2821–2827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capriotti, A.; Piovesana, S.; Zenezini Chiozzi, R.; Montone, C.M.; Bossi, A.M.; Laganà, A. Does the protein corona take over the selectivity of molecularly imprinted nanoparticles? The biological challenges to recognition. J. Proteom. 2020, 219, 103736. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, G.; Zhang, Y.; Wu, F.; Wang, Q. Advanced Fluorescence Imaging Technology in the Near-Infrared-II Window for Biomedical Applications. J. Am. Chem. Soc. 2020, 142, 14789–14804. [Google Scholar] [CrossRef]
- Kang, M.S.; Lee, S.Y.; Kim, K.S.; Han, D.-W. State of the Art Biocompatible Gold Nanoparticles for Cancer Theragnosis. Pharmaceutics 2020, 12, 701. [Google Scholar] [CrossRef] [PubMed]





| Inorganic Material | Monomers | Synthesis Technique | Target | Application | Ref |
|---|---|---|---|---|---|
| CdTe QDs | N-isopropylacrylamide, N-tertbutylacrylamide, N(3-aminopropyl) methacrylamide hydrochloride acrylic acid N,N’methylenebisacrylamide | Solid phase synthesis of MIPs and chemical coupling to QDs | hVEGF | Bioimaging | [100] |
| Fe2O3 | acrylamide, ethylene glycol dimethacrylate | Bulk thermopolymerisation | Doxorubicin | dds | [109] |
| Fe2O3 | Acrylamide, N,N-methylene-bis-acrylamide | Bulk redox polymerization | GFP | Targeting/drug delivery | [97] |
| Fe3O4 | methacrylic acid, trimethylolpropane trimethacrylate | Bulk polymerization | Aspirin | dds | [107] |
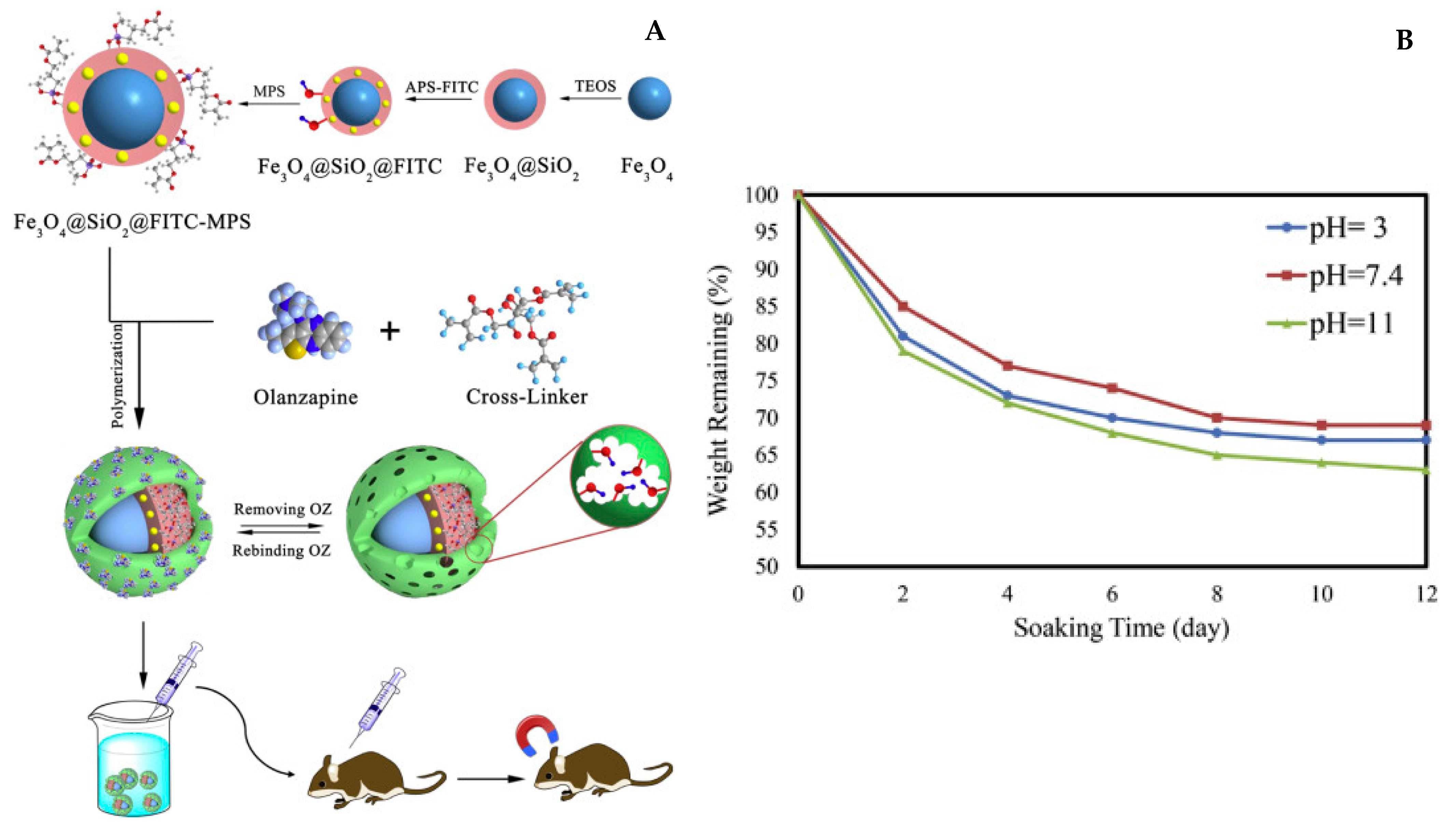
| Fe3O4/SiO2 | Fructose | Co-precipitation polymerization | Olanzapine | dds | [106] |
| Fe3O4/SiO2 | Tannic acid | Mini-emulsion polymerization | 5 fluorouracil | dds | [116] |
| FITC doped SiO2 NPs | TEOS | Bulk polymerization with pre-oriented template | HER2 | cancer therapy | [24] |
| FITC-doped SiO2 NPs | TEOS | Template immobilization (boronic acid) | Sialic acid, fucose or mannose | Fluorescence imaging (FITC) | [73] |
| gold nano rods | Poly(NIPAAm) template adsorption at 15 °C and colapsing at 37 °C generating an imprint | EGFR | Bioimaging | [94] | |
| Graphene oxide QD | Methylmetacrylate, ethylene glycol dimethacrylate | Mini-emulsion polymerization | Doxorubicin | dds | [112] |
| InP/ZnS QD | 4-acrylamidophenyl)(amino) methaniminium acetate, methacrylamide, ethylene glycol dimethacrylate or 2-hydroxyethyl methacrylate,N,N’-ethylenebis(acrylamide) | Bulk photopolymerization using QD’s emission | Glucuronic acid or N-acetylneuraminic acid | Bioimaging | [87] |
| silica | EGDMA | Bulk polymerization | Doxorubicin | dds | [104] |
| silica | Dopamine | Bulk dopamine condensation | HER2 + doxorubicin | dds/targeting | [114] |
| silicon nanoparticles | Ethylene glycol dimethacrylate, zinc acrylate, 4-Vinylbenzeneboronic acid | Bulk polymerization | Bleomycin + human fibroblast growth-factor-inducible 14 | dds/targeting | [113] |
| silver | TEOS | Boronate affinity-oriented surface imprinting approach/TEOS condensation | Sialic acid | Bioimaging (RAMAN) | [103] |
| silver | Methacrylic acid, N-isopropylacrylamide, N,N’-methylene-bis-acrylamide, allylamine | Wax in water Pickering emulsion for the Ag coating | propanolol | UV dds | [105] |
| Starch-based CD | 4-acrylamidophenyl)(amino)methaniminium acetate, methacrylamide, ethylene glycol dimethacrylate | Bulk photopolymerization | Glucuronic acid | Cancer cell targeting and imaging | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garnier, M.; Sabbah, M.; Ménager, C.; Griffete, N. Hybrid Molecularly Imprinted Polymers: The Future of Nanomedicine? Nanomaterials 2021, 11, 3091. https://doi.org/10.3390/nano11113091
Garnier M, Sabbah M, Ménager C, Griffete N. Hybrid Molecularly Imprinted Polymers: The Future of Nanomedicine? Nanomaterials. 2021; 11(11):3091. https://doi.org/10.3390/nano11113091
Chicago/Turabian StyleGarnier, Maylis, Michèle Sabbah, Christine Ménager, and Nébéwia Griffete. 2021. "Hybrid Molecularly Imprinted Polymers: The Future of Nanomedicine?" Nanomaterials 11, no. 11: 3091. https://doi.org/10.3390/nano11113091
APA StyleGarnier, M., Sabbah, M., Ménager, C., & Griffete, N. (2021). Hybrid Molecularly Imprinted Polymers: The Future of Nanomedicine? Nanomaterials, 11(11), 3091. https://doi.org/10.3390/nano11113091








