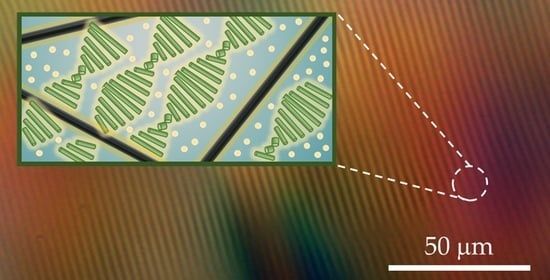
Surfactant-Mediated Co-Existence of Single-Walled Carbon Nanotube Networks and Cellulose Nanocrystal Mesophases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Cellulose Nanocrystals (CNCs)
2.1.2. Single-Walled Carbon Nanotubes (SWNTs)
2.1.3. Surfactants and Block Copolymers
2.2. Preparation Methods
2.2.1. Preparation of Solutions
2.2.2. Preparation of CNC Suspensions
2.2.3. Preparation of Dispersions of SWNTs
2.3. Characterization
2.3.1. Electrokinetic Mobility
2.3.2. Visual Inspection
2.3.3. Polarized Optical Microscopy (POM)
2.3.4. Small-Angle X-ray Scattering (SAXS)
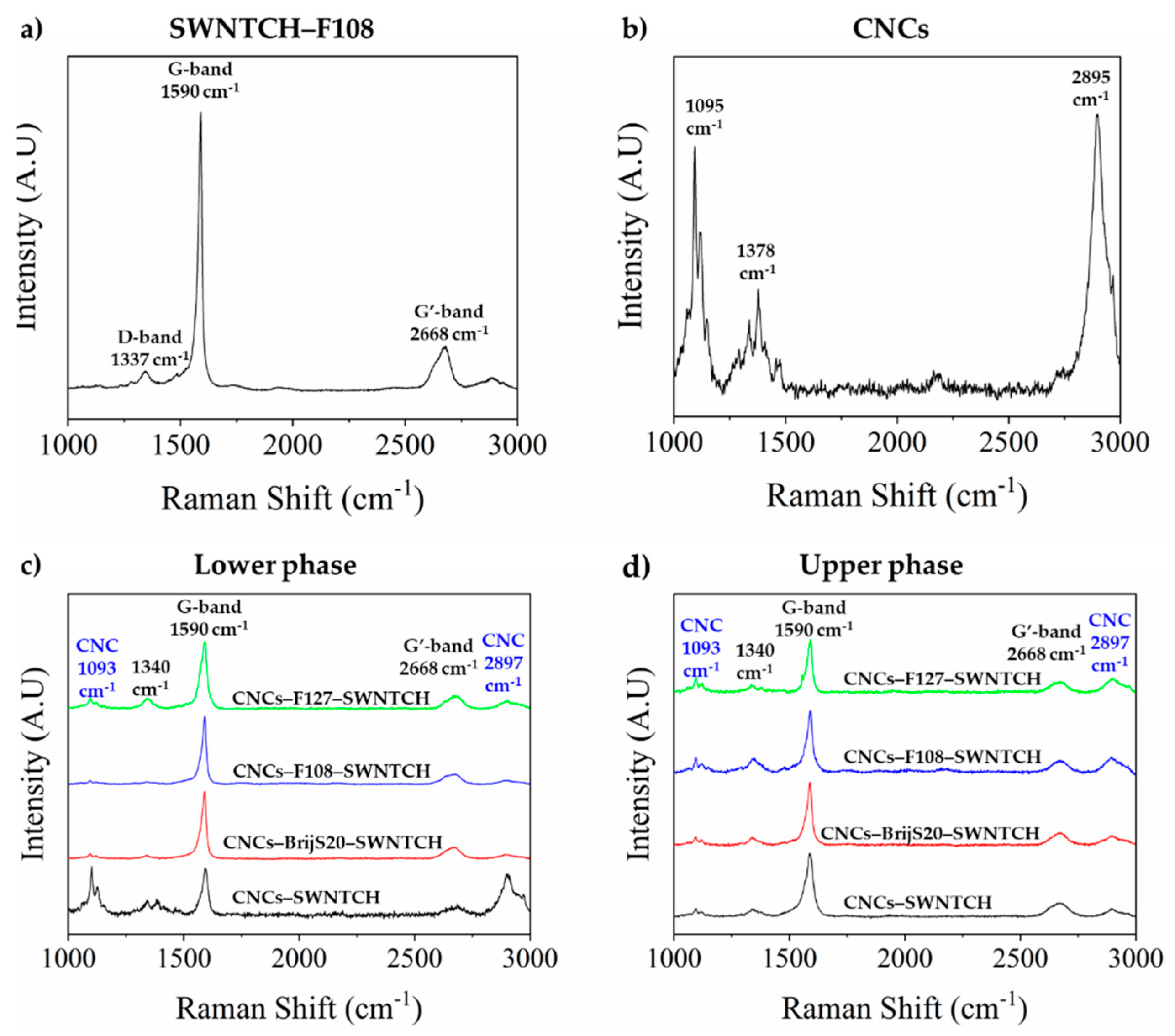
2.3.5. Raman Spectroscopy
2.3.6. Transmission Electron Microscopy (TEM) Imaging
3. Results
3.1. SWNTs Dispersions
3.2. CNs–Surfactant/Polymer Suspensions
3.3. CNC–Surfactant/Polymer–SWNTs Dispersions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, L.; Setyowati, K.; Li, A.; Gong, S.; Chen, J. Reversible Infrared Actuation of Carbon Nanotube-Liquid Crystalline Elastomer Nanocomposites. Adv. Mater. 2008, 20, 2271–2275. [Google Scholar] [CrossRef]
- Ben-David, O.; Nativ-Roth, E.; Yerushalmi-Rozen, R.; Gottlieb, M. Rheological investigation of single-walled carbon nanotubes—Induced structural ordering in CTAB solutions. Soft Matter 2009, 5, 1925–1930. [Google Scholar] [CrossRef]
- Kumar, S.; Bisoyi, H.K. Aligned Carbon Nanotubes in the Supramolecular Order of Discotic Liquid Crystals. Angew. Chem. Int. Ed. 2007, 46, 1501–1503. [Google Scholar] [CrossRef] [PubMed]
- Zakri, C. Carbon nanotubes and liquid crystalline phases. Liq. Cryst. Today 2007, 16, 1–11. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef]
- Schütz, C.; Bruckner, J.R.; Honorato-Rios, C.; Tosheva, Z.; Anyfantakis, M.; Lagerwall, J.P.F. From Equilibrium Liquid Crystal Formation and Kinetic Arrest to Photonic Bandgap Films Using Suspensions of Cellulose Nanocrystals. Crystals 2020, 10, 199. [Google Scholar] [CrossRef] [Green Version]
- Onsager, L. The Effects of Shape on the Interaction of Colloidal Particles. Ann. N. Y. Acad. Sci. 1949, 51, 627–659. [Google Scholar] [CrossRef]
- Flory, P.J.; Abe, A. Statistical Thermodynamics of Mixtures of Rodlike Particles. 1. Theory for Polydisperse Systems. Macromolecules 1978, 11, 1119–1122. [Google Scholar] [CrossRef]
- Flory, P.J. Phase equilibria in solutions of rod-like particles. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1956, 234, 73–89. [Google Scholar] [CrossRef]
- Stroobants, A.; Lekkerkerker, H.N.W.; Odijk, T. Effect of electrostatic interaction on the liquid crystal phase transition in solutions of rodlike polyelectrolytes. Macromolecules 1986, 19, 2232–2238. [Google Scholar] [CrossRef] [Green Version]
- Mendelson, O.; Chu, G.; Ziv, E.; Levi-Kalisman, Y.; Vasilyev, G.; Zussman, E.; Yerushalmi-Rozen, R. Exclusion and Trapping of Carbon Nanostructures in Nonisotropic Suspensions of Cellulose Nanostructures. J. Phys. Chem. B 2019, 123, 3535–3542. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.G.; Mu, X. Chiral Nematic Structure of Cellulose Nanocrystal Suspensions and Films; Polarized Light and Atomic Force Microscopy. Materials 2015, 8, 7873–7888. [Google Scholar] [CrossRef] [PubMed]
- Wilts, B.D.; Dumanli, A.G.; Middleton, R.; Vukusic, P.; Vignolini, S. Invited Article: Chiral optics of helicoidal cellulose nanocrystal films. APL Photon. 2017, 2, 040801. [Google Scholar] [CrossRef] [Green Version]
- Revol, J.-F.; Bradford, H.; Giasson, J.; Marchessault, R.H.; Gray, D.G. Helicoidal self-ordering of cellulose microfibrils in aqueous suspension. Int. J. Biol. Macromol. 1992, 14, 170–172. [Google Scholar] [CrossRef]
- Mu, X.; Gray, D.G. Formation of Chiral Nematic Films from Cellulose Nanocrystal Suspensions Is a Two-Stage Process. Langmuir 2014, 30, 9256–9260. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, C.; Yuan, Z.; Ji, X.; Fu, Y.; Li, H.; Qin, M. Composite Films with Ordered Carbon Nanotubes and Cellulose Nanocrystals. J. Phys. Chem. C 2017, 121, 8976–8981. [Google Scholar] [CrossRef]
- Olivier, C.; Moreau, C.; Bertoncini, P.; Bizot, H.; Chauvet, O.; Cathala, B. Cellulose Nanocrystal-Assisted Dispersion of Luminescent Single-Walled Carbon Nanotubes for Layer-by-Layer Assembled Hybrid Thin Films. Langmuir 2012, 28, 12463–12471. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-X.; Hamad, W.Y.; MacLachlan, M.J. Size-Selective Exclusion Effects of Liquid Crystalline Tactoids on Nanoparticles: A Separation Method. Angew. Chem. Int. Ed. 2018, 57, 3360–3365. [Google Scholar] [CrossRef]
- Chu, G.; Chen, F.; Zhao, B.; Zhang, X.; Zussman, E.; Rojas, O.J. Self-Assembled Nanorods and Microspheres for Functional Photonics: Retroreflector Meets Microlens Array. Adv. Opt. Mater. 2021, 9, 2002258. [Google Scholar] [CrossRef]
- Shvartzman-Cohen, R.; Levi-Kalisman, Y.; Nativ-Roth, A.E.; Yerushalmi-Rozen, R. Generic Approach for Dispersing Single-Walled Carbon Nanotubes: The Strength of a Weak Interaction. Langmuir 2004, 20, 6085–6088. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-S.; Kim, T.-H.; Do, C.; Lee, M.-J.; Choi, S.-M. Single-walled carbon nanotube induced re-entrant hexagonal phases in a Pluronic block copolymer system. Soft Matter 2013, 9, 3050–3056. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Physical and/or Chemical Compatibilization of Extruded Cellulose Nanocrystal Reinforced Polystyrene Nanocomposites. Macromolecules 2013, 46, 5570–5583. [Google Scholar] [CrossRef]
- Elazzouzi-Hafraoui, S.; Nishiyama, Y.; Putaux, J.-L.; Heux, L.; Dubreuil, F.; Rochas, C. The Shape and Size Distribution of Crystalline Nanoparticles Prepared by Acid Hydrolysis of Native Cellulose. Biomacromolecules 2008, 9, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.E.N.P.; de Moura, C.L.; Vieira, M.G.S.; Gramosa, N.V.; Chaibundit, C.; de Mattos, M.C.; Attwood, D.; Yeates, S.G.; Nixon, S.K.; Ricardo, N.M.P.S. Solubilisation capacity of Brij surfactants. Int. J. Pharm. 2012, 436, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Nativ-Roth, E.; Shvartzman-Cohen, R.; Bounioux, C.; Florent, M.; Zhang, D.; Szleifer, A.I.; Yerushalmi-Rozen, R. Physical Adsorption of Block Copolymers to SWNT and MWNT: A Nonwrapping Mechanism. Macromolecules 2007, 40, 3676–3685. [Google Scholar] [CrossRef]
- Bedrov, D.; Ayyagari, C.; Smith, G.D. Multiscale Modeling of Poly(ethylene oxide)−Poly(propylene oxide)−Poly(ethylene oxide) Triblock Copolymer Micelles in Aqueous Solution. J. Chem. Theory Comput. 2006, 2, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Martic, P.A.; Tan, J.S. Protein adsorption on pluronic copolymer-coated polystyrene particles. J. Colloid Interface Sci. 1989, 131, 252–266. [Google Scholar] [CrossRef]
- Manet, S.; Lecchi, A.; Impéror-Clerc, M.; Zholobenko, V.; Durand, D.; Oliveira, C.L.P.; Pedersen, J.S.; Grillo, I.; Meneau, F.; Rochas, C. Structure of Micelles of a Nonionic Block Copolymer Determined by SANS and SAXS. J. Phys. Chem. B 2011, 115, 11318–11329. [Google Scholar] [CrossRef] [PubMed]
- Valero, M.; Hu, W.; Houston, J.E.; Dreiss, C.A. Solubilisation of salicylate in F127 micelles: Effect of pH and temperature on morphology and interactions with cyclodextrin. J. Mol. Liq. 2021, 322, 114892. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Preparation and properties of cellulose nanocrystals: Rods, spheres, and network. Carbohydr. Polym. 2010, 82, 329–336. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Shvartzman-Cohen, R.; Nativ-Roth, E.; Baskaran, E.; Levi-Kalisman, Y.; Szleifer, I.; Yerushalmi-Rozen, R. Selective Dispersion of Single-Walled Carbon Nanotubes in the Presence of Polymers: The Role of Molecular and Colloidal Length Scales. J. Am. Chem. Soc. 2004, 126, 14850–14857. [Google Scholar] [CrossRef]
- Florent, M.; Shvartzman-Cohen, R.; Goldfarb, D.; Rozen, R.Y. Self-Assembly of Pluronic Block Copolymers in Aqueous Dispersions of Single-Wall Carbon Nanotubes as Observed by Spin Probe EPR. Langmuir 2008, 24, 3773–3779. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Noh, J.; Schütz, C.; Salazar-Alvarez, G.; Scalia, G.; Bergström, L.; Lagerwall, J.P.F. Macroscopic Control of Helix Orientation in Films Dried from Cholesteric Liquid-Crystalline Cellulose Nanocrystal Suspensions. ChemPhysChem 2014, 15, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Ilavsky, J.; Jemian, P.R. Irena: Tool suite for modeling and analysis of small-angle scattering. J. Appl. Crystallogr. 2009, 42, 347–353. [Google Scholar] [CrossRef]
- Mao, Y.; Bleuel, M.; Lyu, Y.; Zhang, X.; Henderson, D.; Wang, H.; Briber, R.M. Phase Separation and Stack Alignment in Aqueous Cellulose Nanocrystal Suspension under Weak Magnetic Field. Langmuir 2018, 34, 8042–8051. [Google Scholar] [CrossRef] [PubMed]
- Araki, J.; Wada, M.; Kuga, S. Steric Stabilization of a Cellulose Microcrystal Suspension by Poly(ethylene glycol) Grafting. Langmuir 2001, 17, 21–27. [Google Scholar] [CrossRef]
- Alexandridis, P.; Olsson, U.; Lindman, B. A Record Nine Different Phases (Four Cubic, Two Hexagonal, and One Lamellar Lyotropic Liquid Crystalline and Two Micellar Solutions) in a Ternary Isothermal System of an Amphiphilic Block Copolymer and Selective Solvents (Water and Oil). Langmuir 1998, 14, 2627–2638. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, K.; Zhan, C.; Geng, L.; Chu, B.; Hsiao, B.S. Characterization of Nanocellulose Using Small-Angle Neutron, X-ray, and Dynamic Light Scattering Techniques. J. Phys. Chem. B 2017, 121, 1340–1351. [Google Scholar] [CrossRef]
- Uhlig, M.; Fall, A.; Wellert, S.; Lehmann, M.; Prevost, S.; Wågberg, L.; Von Klitzing, R.; Nystrom, G. Two-Dimensional Aggregation and Semidilute Ordering in Cellulose Nanocrystals. Langmuir 2016, 32, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Hartschuh, A.; Sánchez, E.J.; Xie, X.S.; Novotny, L. High-Resolution Near-Field Raman Microscopy of Single-Walled Carbon Nanotubes. Phys. Rev. Lett. 2003, 90, 095503. [Google Scholar] [CrossRef] [Green Version]
- Lupoi, J.S.; Gjersing, E.; Davis, M.F. Evaluating Lignocellulosic Biomass, Its Derivatives, and Downstream Products with Raman Spectroscopy. Front. Bioeng. Biotechnol. 2015, 3, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanasekara, N.D.; Santos, R.P.O.; Douch, C.; Frollini, E.; Eichhorn, S.J. Orientation of cellulose nanocrystals in electrospun polymer fibres. J. Mater. Sci. 2015, 51, 218–227. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Luo, J.; Liu, H.C.; Davijani, A.A.B.; Wang, P.-H.; Kumar, S. Orientation and interfacial stress transfer of cellulose nanocrystal nanocomposite fibers. Polymer 2017, 110, 228–234. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Filho, A.G.S.; Saito, R. Defect characterization in graphene and carbon nanotubes using Raman spectroscopy. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 5355–5377. [Google Scholar] [CrossRef]
- Mortensen, K.; Talmon, Y. Cryo-TEM and SANS Microstructural Study of Pluronic Polymer Solutions. Macromolecules 1995, 28, 8829–8834. [Google Scholar] [CrossRef]
- Feng, K.; Gao, X.; Gu, Z.; Jin, Z. Improving Homogeneity of Iridescent Cellulose Nanocrystal Films by Surfactant-Assisted Spreading Self-Assembly. ACS Sustain. Chem. Eng. 2019, 7, 19062–19071. [Google Scholar] [CrossRef]
- Oguzlu, H.; Danumah, C.; Boluk, Y. Colloidal behavior of aqueous cellulose nanocrystal suspensions. Curr. Opin. Colloid Interface Sci. 2017, 29, 46–56. [Google Scholar] [CrossRef]
- Honorato-Rios, C.; Ekuhnhold, A.; Bruckner, J.R.; Edannert, R.; Eschilling, T.; Lagerwall, J.P.F. Equilibrium Liquid Crystal Phase Diagrams and Detection of Kinetic Arrest in Cellulose Nanocrystal Suspensions. Front. Mater. 2016, 3, 21. [Google Scholar] [CrossRef] [Green Version]
- Farahani, R.D.; Dubé, M.; Therriault, D. Three-Dimensional Printing of Multifunctional Nanocomposites: Manufacturing Techniques and Applications. Adv. Mater. 2016, 28, 5794–5821. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Akbulut, M.; Kristiansen, K.; Golan, Y.; Israelachvili, J.N. The role of interparticle and external forces in nanoparticle assembly. Nat. Mater. 2008, 7, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-H.; Métivier, R.; Wang, S.; Wang, H. Advanced Nanohybrid Materials: Surface Modification and Applications. J. Nanomater. 2012, 2012, 536405. [Google Scholar] [CrossRef]
- Farjana, S.; Toomadj, F.; Lundgren, P.; Sanz-Velasco, A.; Naboka, O.; Enoksson, P. Conductivity-Dependent Strain Response of Carbon Nanotube Treated Bacterial Nanocellulose. J. Sens. 2013, 2013, 741248. [Google Scholar] [CrossRef]
 ), in BrijS20 solution (0.5 wt%) (
), in BrijS20 solution (0.5 wt%) (  ), in F108 solution (1 wt%) (
), in F108 solution (1 wt%) (  ) and in F127 solution (1 wt%) (
) and in F127 solution (1 wt%) (  ). The curves are shifted for better visualization. SAXS I vs. q curves are presented in Figure S5 of the SI (d) Schematic illustration of the parallelepiped stacking model by Mao et al. [37].
). The curves are shifted for better visualization. SAXS I vs. q curves are presented in Figure S5 of the SI (d) Schematic illustration of the parallelepiped stacking model by Mao et al. [37].
 ), in BrijS20 solution (0.5 wt%) (
), in BrijS20 solution (0.5 wt%) (  ), in F108 solution (1 wt%) (
), in F108 solution (1 wt%) (  ) and in F127 solution (1 wt%) (
) and in F127 solution (1 wt%) (  ). The curves are shifted for better visualization. SAXS I vs. q curves are presented in Figure S5 of the SI (d) Schematic illustration of the parallelepiped stacking model by Mao et al. [37].
). The curves are shifted for better visualization. SAXS I vs. q curves are presented in Figure S5 of the SI (d) Schematic illustration of the parallelepiped stacking model by Mao et al. [37].
 ) and in CNC–BrijS20–SWNTCH dispersions (
) and in CNC–BrijS20–SWNTCH dispersions (  ).
).
 ) and in CNC–BrijS20–SWNTCH dispersions (
) and in CNC–BrijS20–SWNTCH dispersions (  ).
).
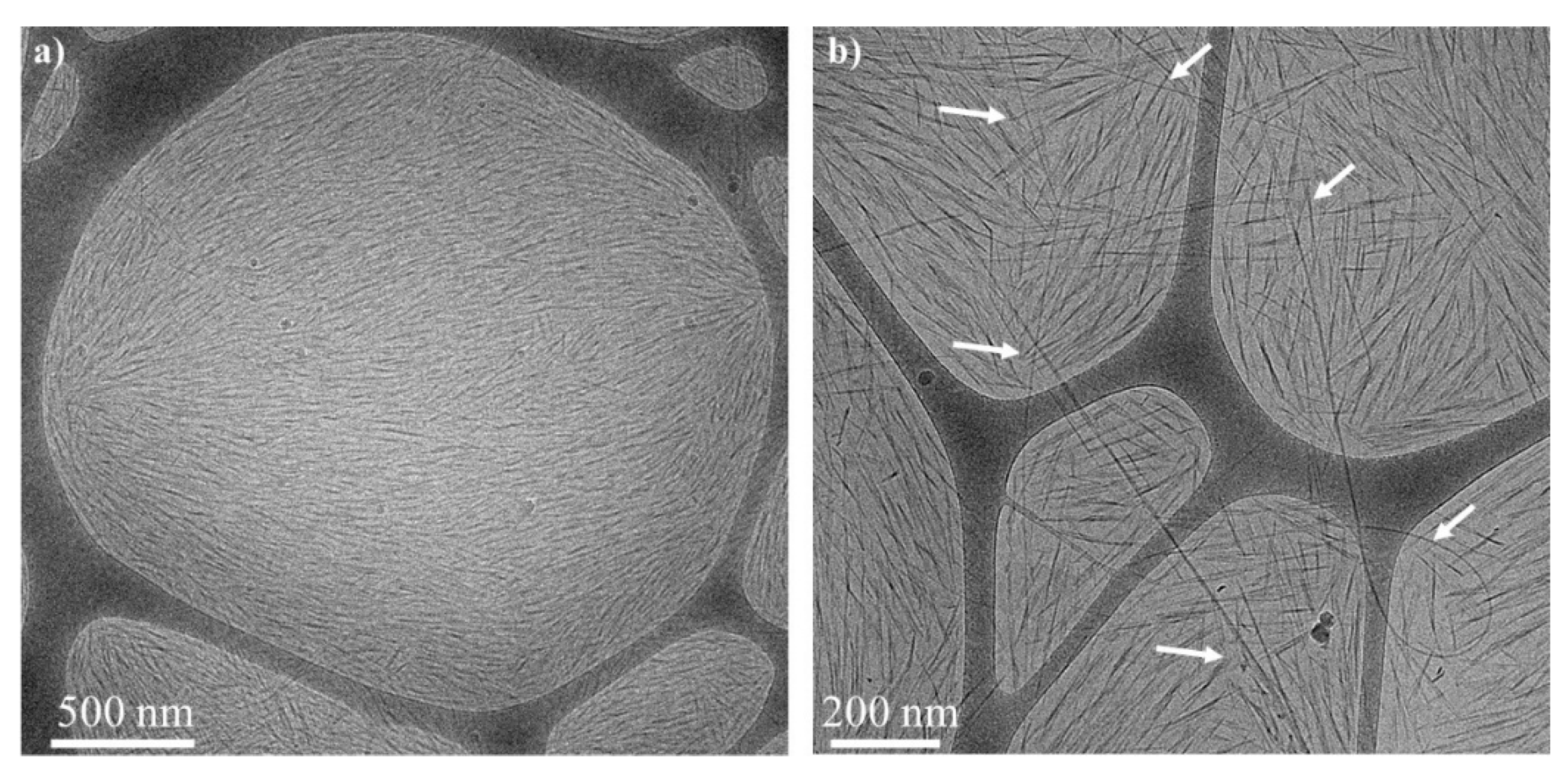
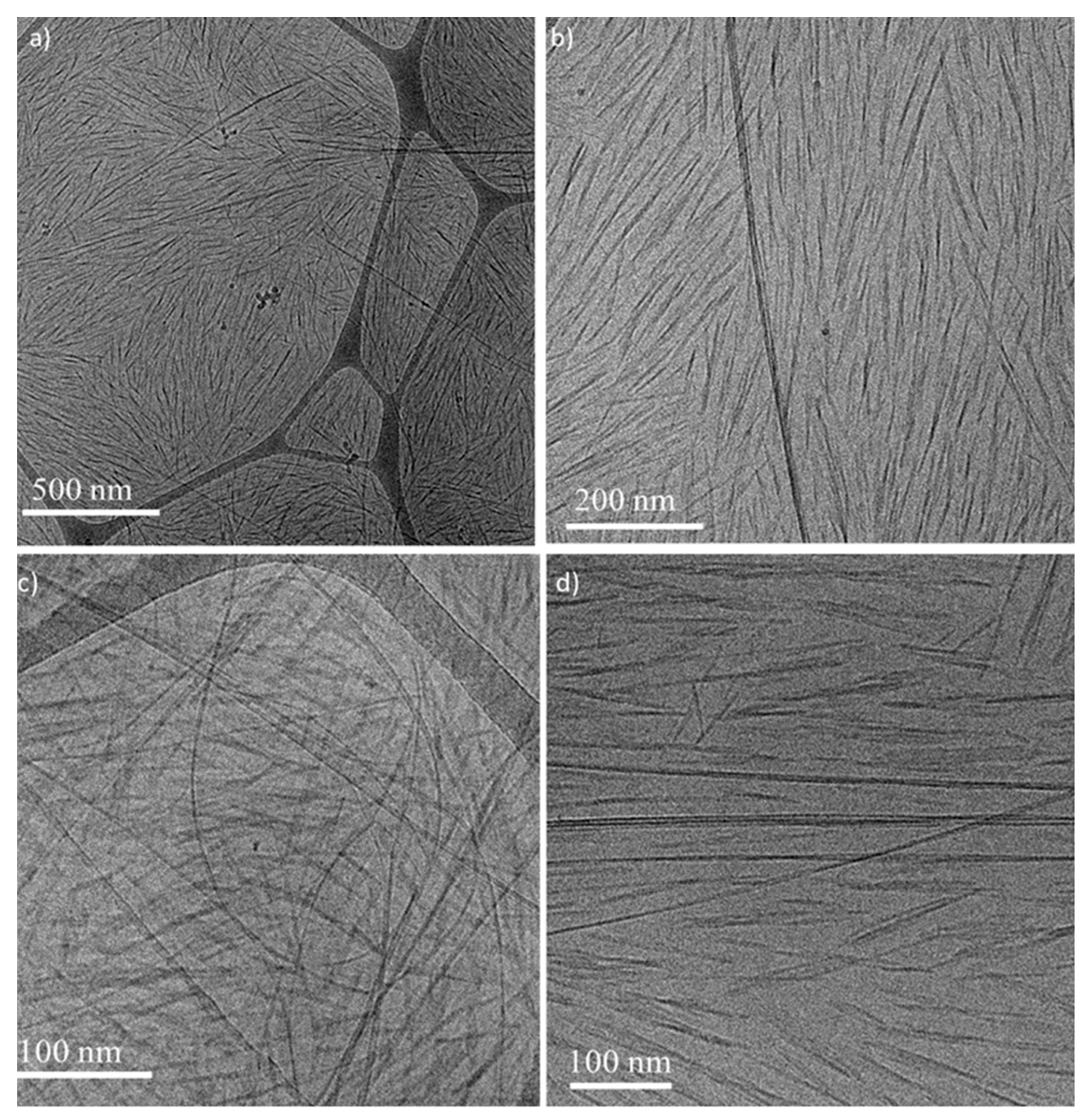
 ), 6 wt% CNCs–0.5 wt% BrijS20–0.1 wt% SWNTCH (
), 6 wt% CNCs–0.5 wt% BrijS20–0.1 wt% SWNTCH (  ), 6 wt% CNCs–1 wt% F108–0.1 wt% SWNTCH (
), 6 wt% CNCs–1 wt% F108–0.1 wt% SWNTCH (  ) and 6 wt% CNCs–1 wt% F127–0.1 wt% SWNTCH (
) and 6 wt% CNCs–1 wt% F127–0.1 wt% SWNTCH (  ). The curves are shifted for better visualization. (b) POM image of the N* phase of 6 wt% CNCs–0.5 wt% BrijS20–0.1 wt% SWNTCH mixture. Dried films of the (c) upper and (d) lower phases of 6 wt% CNCs–0.1 wt% SWNTCH, and (e) upper and (f) lower phases of 6 wt% CNCs–0.5 wt% BrijS20–0.1 wt% SWNTCH.
). The curves are shifted for better visualization. (b) POM image of the N* phase of 6 wt% CNCs–0.5 wt% BrijS20–0.1 wt% SWNTCH mixture. Dried films of the (c) upper and (d) lower phases of 6 wt% CNCs–0.1 wt% SWNTCH, and (e) upper and (f) lower phases of 6 wt% CNCs–0.5 wt% BrijS20–0.1 wt% SWNTCH.
 ), 6 wt% CNCs–0.5 wt% BrijS20–0.1 wt% SWNTCH (
), 6 wt% CNCs–0.5 wt% BrijS20–0.1 wt% SWNTCH (  ), 6 wt% CNCs–1 wt% F108–0.1 wt% SWNTCH (
), 6 wt% CNCs–1 wt% F108–0.1 wt% SWNTCH (  ) and 6 wt% CNCs–1 wt% F127–0.1 wt% SWNTCH (
) and 6 wt% CNCs–1 wt% F127–0.1 wt% SWNTCH (  ). The curves are shifted for better visualization. (b) POM image of the N* phase of 6 wt% CNCs–0.5 wt% BrijS20–0.1 wt% SWNTCH mixture. Dried films of the (c) upper and (d) lower phases of 6 wt% CNCs–0.1 wt% SWNTCH, and (e) upper and (f) lower phases of 6 wt% CNCs–0.5 wt% BrijS20–0.1 wt% SWNTCH.
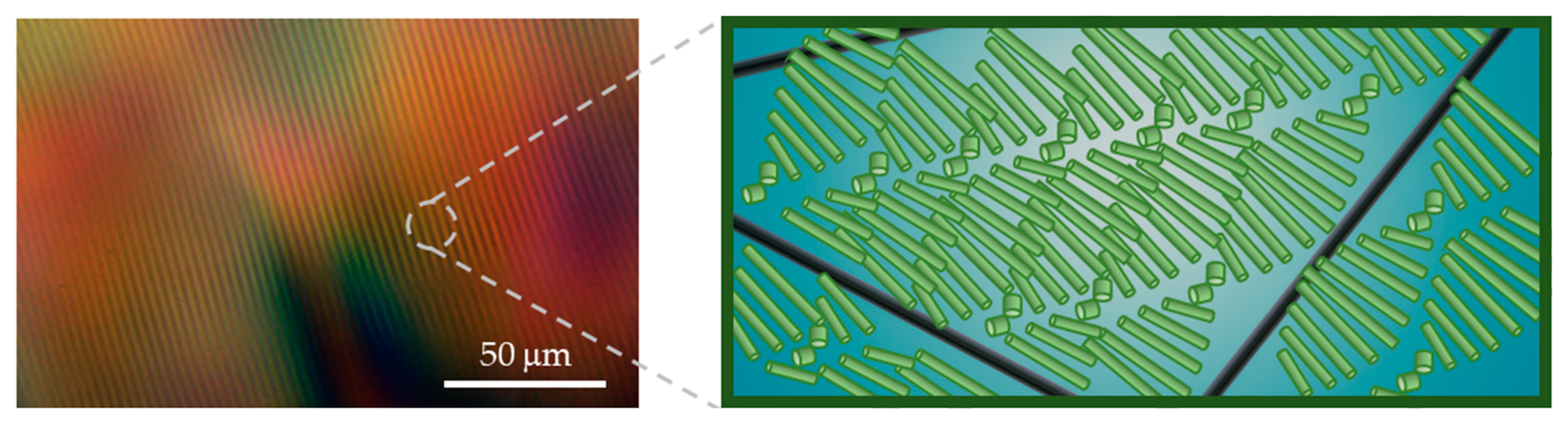
). The curves are shifted for better visualization. (b) POM image of the N* phase of 6 wt% CNCs–0.5 wt% BrijS20–0.1 wt% SWNTCH mixture. Dried films of the (c) upper and (d) lower phases of 6 wt% CNCs–0.1 wt% SWNTCH, and (e) upper and (f) lower phases of 6 wt% CNCs–0.5 wt% BrijS20–0.1 wt% SWNTCH.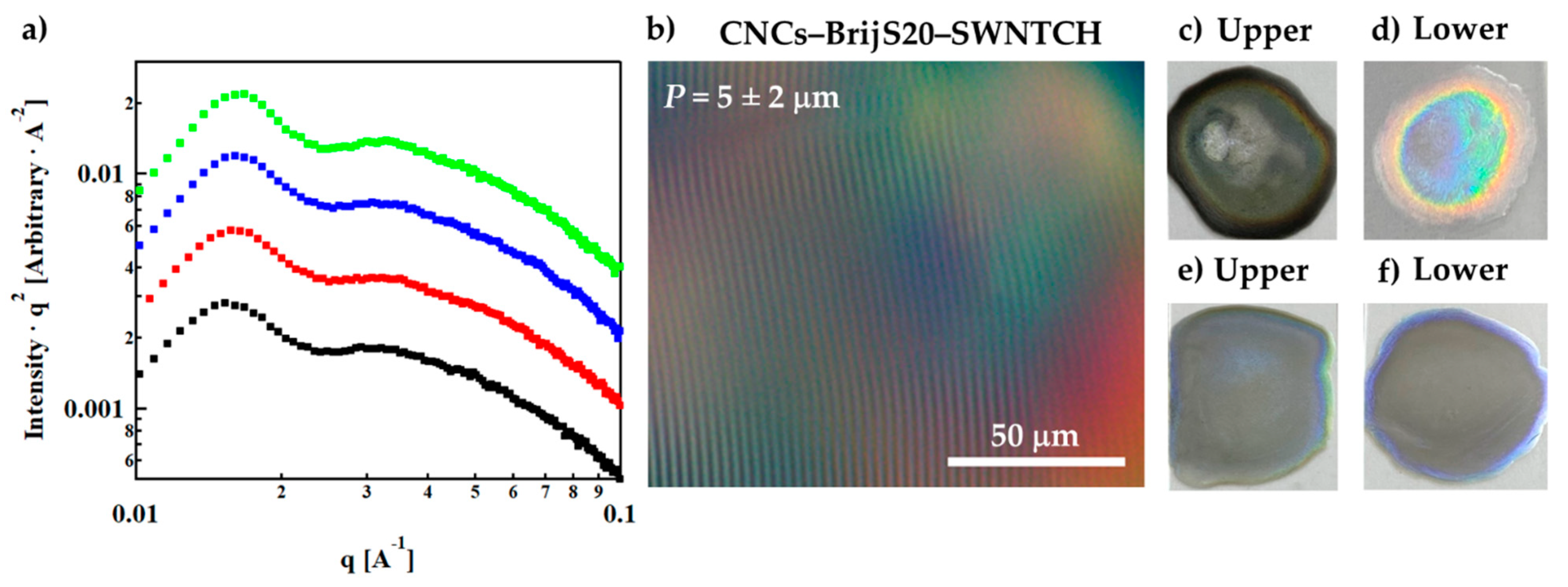
| Surfactant/Polymer | Mn | CMC (25 °C) (wt%) | Diameter of the Micelles (25 °C) (nm) |
|---|---|---|---|
| BrijS20 | 1152 | 7.6 × 10−4 | 5–8 |
| F108 | 14,600 | 4.5 * | 15–20 |
| F127 | 12,500 | 0.7 | 20–25 |
| Surfactant/Polymer (wt%) | Zeta Potential (mV) | ||
|---|---|---|---|
| BrijS20 | F108 | F127 | |
| 0.1 | −36 ± 1 | −44 ± 3 | −44 ± 3 |
| 0.2 | −29 ± 2 | −43± 1 | −44 ± 1 |
| Sample | |||
|---|---|---|---|
| 6 wt% CNCs | 2.6 | 20 | 35 ± 10 |
| 6 wt% CNCs–0.5 wt% BrijS20 | 3.1 | 39 | 36 ± 11 |
| 6 wt% CNCs–1 wt% F108 | 3.3 | 44 | 37 ± 12 |
| 6 wt% CNCs–1% F127 | 3.1 | 46 | 40 ± 13 |
| 6 wt% CNC–0.5 wt% BrijS20–0.1 wt% SWNTCH | 3.0 | 41 | 38 ± 12 |
| 6 wt% CNCs–1 wt% F108–0.1 wt% SWNTCH | 3.2 | 42 | 38 ± 12 |
| 6 wt% CNCs–1 wt% F127–0.1 wt% SWNTCH | 3.2 | 45 | 37 ± 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attia, D.; Yekymov, E.; Shmidov, Y.; Levi-Kalisman, Y.; Mendelson, O.; Bitton, R.; Yerushalmi-Rozen, R. Surfactant-Mediated Co-Existence of Single-Walled Carbon Nanotube Networks and Cellulose Nanocrystal Mesophases. Nanomaterials 2021, 11, 3059. https://doi.org/10.3390/nano11113059
Attia D, Yekymov E, Shmidov Y, Levi-Kalisman Y, Mendelson O, Bitton R, Yerushalmi-Rozen R. Surfactant-Mediated Co-Existence of Single-Walled Carbon Nanotube Networks and Cellulose Nanocrystal Mesophases. Nanomaterials. 2021; 11(11):3059. https://doi.org/10.3390/nano11113059
Chicago/Turabian StyleAttia, David, Evgenee Yekymov, Yulia Shmidov, Yael Levi-Kalisman, Orit Mendelson, Ronit Bitton, and Rachel Yerushalmi-Rozen. 2021. "Surfactant-Mediated Co-Existence of Single-Walled Carbon Nanotube Networks and Cellulose Nanocrystal Mesophases" Nanomaterials 11, no. 11: 3059. https://doi.org/10.3390/nano11113059
APA StyleAttia, D., Yekymov, E., Shmidov, Y., Levi-Kalisman, Y., Mendelson, O., Bitton, R., & Yerushalmi-Rozen, R. (2021). Surfactant-Mediated Co-Existence of Single-Walled Carbon Nanotube Networks and Cellulose Nanocrystal Mesophases. Nanomaterials, 11(11), 3059. https://doi.org/10.3390/nano11113059