Synthesis of Fullerenes from a Nonaromatic Chloroform through a Newly Developed Ultrahigh-Temperature Flash Vacuum Pyrolysis Apparatus
Abstract
:1. Introduction
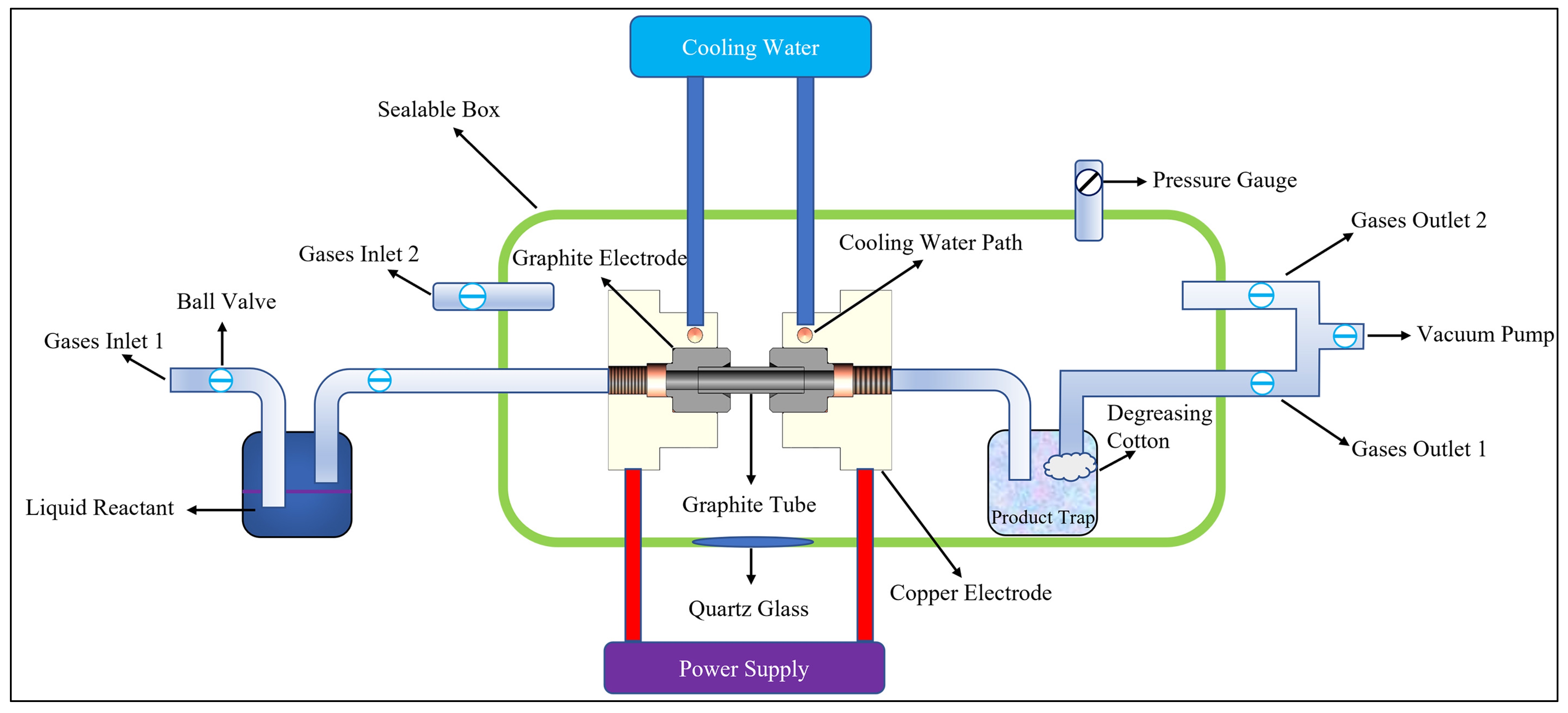
2. Materials and Methods
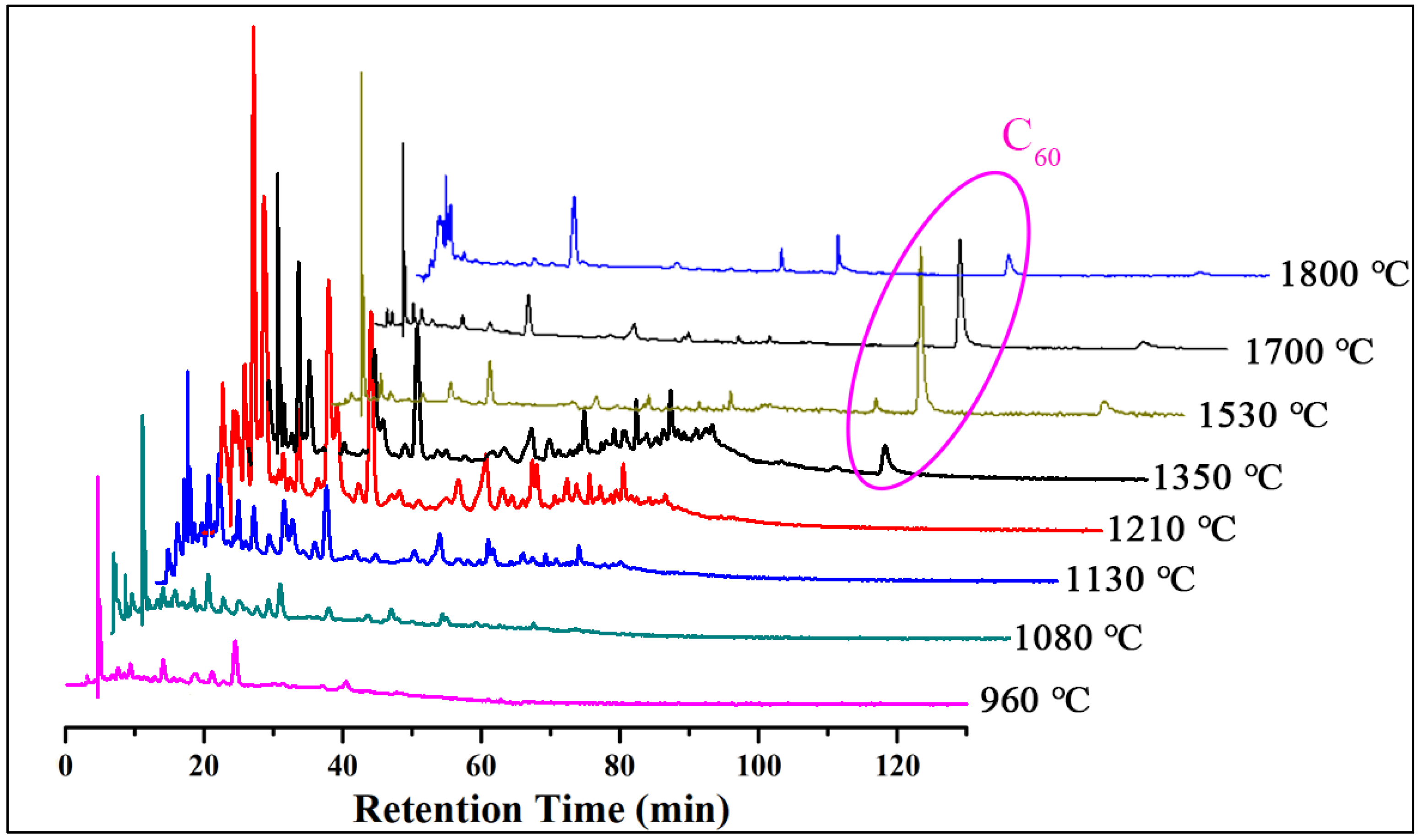
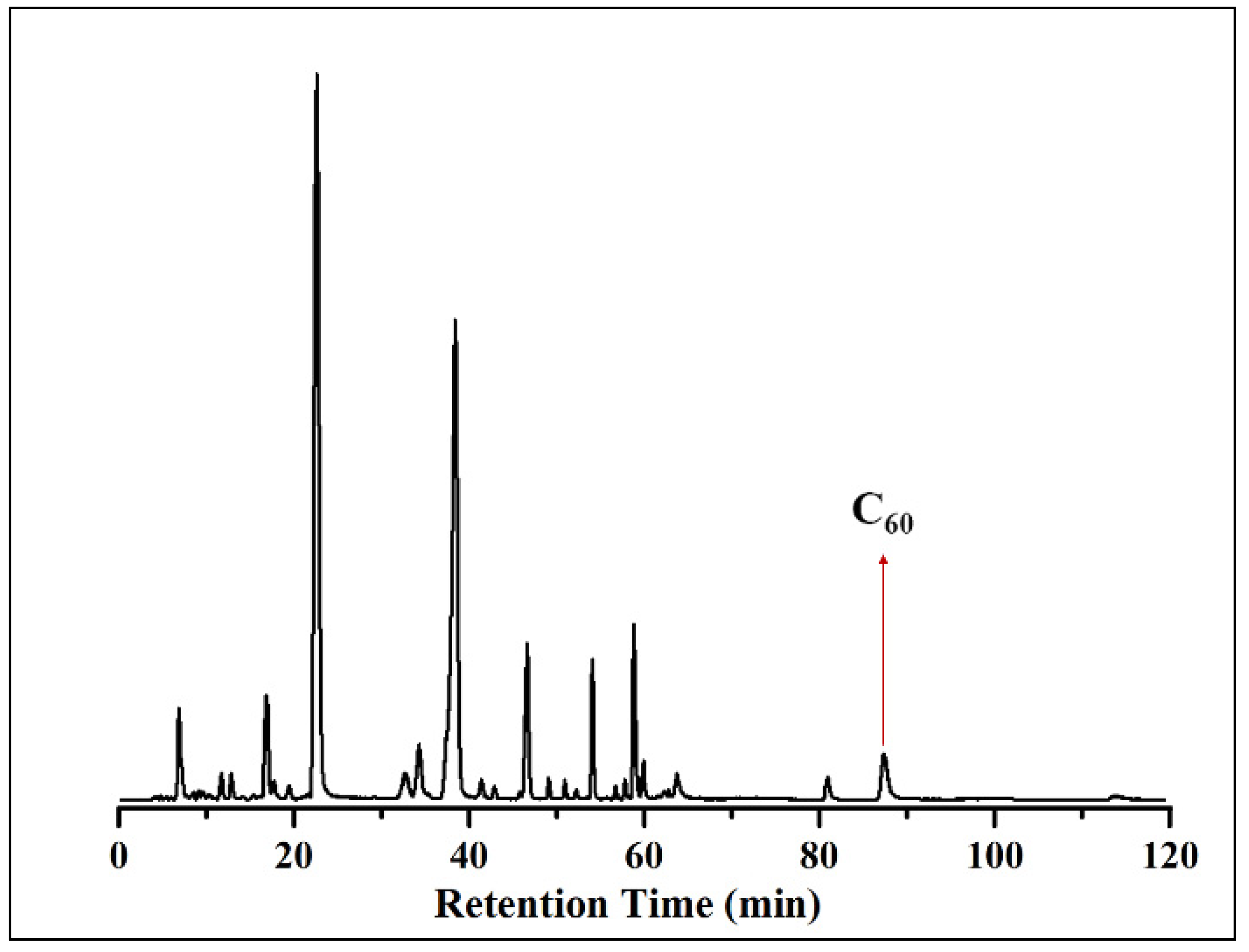
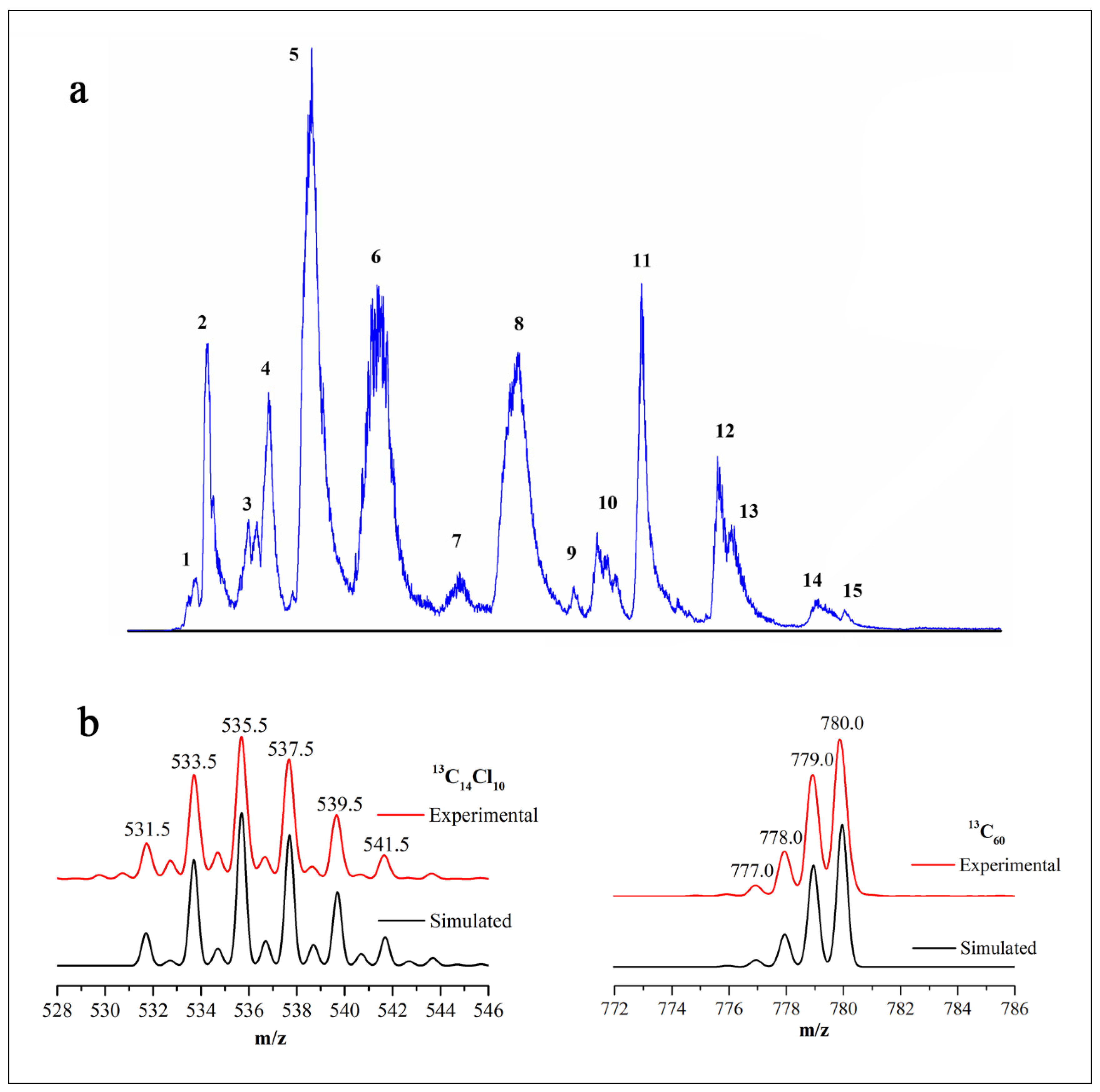
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Dai, L.; Chang, D.W.; Baek, J.; Lu, W. Carbon Nanomaterials for Advanced Energy Conversion and Storage. Small 2012, 8, 1130–1166. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.N.R.; Sood, A.K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene: The New Two-Dimensional Nanomaterial. Angew. Chem. Int. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef]
- Wajahat, M.; Kim, J.H.; Ahn, J.; Lee, S.; Bae, J.; Pyo, J.; Seol, S.K. 3D Printing of Fe3O4 Functionalized Graphene-Polymer (FGP) Composite Microarchitectures. Carbon 2020, 167, 278–284. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, J.; Chen, P.; Hong, D.; Wan, S.; Deng, X.; Tian, Y. Spectroscopic Study of Ensemble and Individual Graphene Quantum Dots. J. Phys. Chem. C 2020, 124, 12112–12119. [Google Scholar] [CrossRef]
- Chernyak, S.A.; Ivanov, A.S.; Stolbov, D.N.; Maksimov, S.V.; Maslakov, K.I.; Chernavskii, P.A.; Pokusaeva, Y.A.; Koklin, A.E.; Bogdan, V.I.; Savilov, S.V. Sintered Fe/CNT Framework Catalysts for CO2 Hydrogenation into Hydrocarbons. Carbon 2020, 168, 475–484. [Google Scholar] [CrossRef]
- Lu, X.; Akasaka, T.; Nagase, S. Carbide Cluster Metallofullerenes: Structure, Properties, and Possible Origin. Acc. Chem. Res. 2013, 46, 1627–1635. [Google Scholar] [CrossRef]
- Anthony, J.E.; Facchetti, A.; Heeney, M.; Marder, S.R.; Zhan, X.W. N-Type Organic Semiconductors in Organic Electronics. Adv. Mater. 2010, 22, 3876–3892. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.Q.; Fang, G.; Zeng, F.; Wang, X.D.; Wu, S.Z. Water-Dispersible Fullerene Aggregates as a Targeted Anticancer Prodrug with Both Chemo- and Photodynamic Therapeutic Actions. Small 2013, 4, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Mousavi MD, S.Z.; Nafisi, S.; Maibach, H.I. Fullerene Nanoparticle in Dermatological and Cosmetic Applications. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1071–1087. [Google Scholar] [CrossRef]
- Kumar, R.; Gleißner, E.H.; Tiu, E.G.V.; Yamakoshi, Y. C70 as a Photocatalyst for Oxidation of Secondary Benzylamines to Lmines. Org. Lett. 2016, 18, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Akada, M.; Hirai, T.; Takeuchi, J.; Yamamoto, T.; Kumashiro, R.; Tanigaki, K. Superconducting Phase Sequence in Rx C60 Fullerides (R = Sm and Yb). Phys. Rev. B 2006, 73, 094509. [Google Scholar] [CrossRef]
- Aloukos, P.; Iliopoulos, K.; Couris, S.; Guldi, D.M.; Sooambar, C.; Mateo-Alonso, A.; Nagaswaran, P.G.; Bonifazi, D.; Prato, M. Photophysics and Transient Nonlinear Optical Response of Donor–[60]Fullerene Hybrids. J. Mater. Chem. 2011, 21, 2524–2534. [Google Scholar] [CrossRef]
- Wu, B.S.; An, M.W.; Chen, J.M.; Xing, Z.; Chen, Z.C.; Deng, L.L.; Tian, H.R.; Yun, D.Q.; Xie, S.Y.; Zheng, L.S. Radiation-Processed Perovskite Solar Cells with Fullerene-Enhanced Performance and Stability. Cell Rep. Phys. Sci. 2021, in press. [Google Scholar] [CrossRef]
- Peumans, P.; Forrest, S.R.; Jin, F. Very-High-Efficiency Double-Heterostructure Copper Phthalocyanine/C60 Photovoltaic Cells. Appl. Phys. Lett. 2001, 79, 126–128. [Google Scholar] [CrossRef]
- Yoshimoto, Y.; Sugiyama, S.; Shimada, S.; Kaneko, T.; Takagi, S.; Kinefuchi, I. Molecular Insights into the Mechanical Properties of Polymer−Fullerene Bulk Heterojunctions for Organic Photovoltaic Applications. Macromolecules 2021, 54, 958–969. [Google Scholar] [CrossRef]
- Giancane, G.; Bettini, S.; Valli, L.; Bracamonte, V.; Carraro, M.; Bonchio, M.; Prato, M. Supramolecular Organic–Inorganic Domains Integrating Fullerene-Based Acceptors with Polyoxometalate-Bis-Pyrene Tweezers for Organic Photovoltaic Applications. J. Mater. Chem. C 2021, in press. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, L.; Shao, S. Emerging Electronic Applications of Fullerene Derivatives: An Era Beyond OPV. J. Mater. Chem. C 2021, in press. [Google Scholar] [CrossRef]
- Xie, S.Y.; Huang, R.B.; Yu, L.J.; Ding, J.; Zheng, L.S. Microwave Synthesis of Fullerenes from Chloroform. Appl. Phys. Lett. 1999, 75, 2764–2766. [Google Scholar] [CrossRef]
- Xie, S.Y.; Huang, R.B.; Deng, S.L.; Yu, L.J.; Zheng, L.S. Synthesis, Separation, and Characterization of Fullerenes and Their Chlorinated Fragments in the Glow Discharge Reaction of Chloroform. J. Phys. Chem. B 2001, 105, 1734–1738. [Google Scholar] [CrossRef]
- Krätschmer, W.; Lamb, D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A New Form of Carbon. Nature 1990, 347, 354. [Google Scholar] [CrossRef]
- Howard, J.B.; McKinnon, J.T.; Makarovsky, Y.; Lafleur, A.L.; Johnson, M.E. Fullerenes C60 and C70 in Flames. Nature 1991, 352, 139. [Google Scholar] [CrossRef]
- Tian, H.R.; Chen, M.M.; Wang, K.; Chen, Z.C.; Fu, C.Y.; Zhang, Q.; Li, S.H.; Deng, S.L.; Yao, Y.R.; Xie, S.Y.; et al. An Unconventional Hydrofullerene C66H4 with Symmetric Heptagons Retrieved in Low-Pressure Combustion. J. Am. Chem. Soc. 2019, 141, 6651–6657. [Google Scholar] [CrossRef]
- Guan, R.N.; Chen, M.Q.; Xin, J.P.; Xie, X.M.; Jin, F.; Zhang, Q.; Xie, S.Y.; Yang, S.F. Capturing the Missing Carbon Cage Isomer of C84 via Mutual Stabilization of a Triangular Monometallic Cyanide Cluster. J. Am. Chem. Soc. 2021, 143, 8078–8085. [Google Scholar] [CrossRef]
- Curl, R.F. On the Formation of the Fullerenes. Philos. Trans. R. Soc. Lond. A 1993, 343, 19–32. [Google Scholar]
- Chuvilin, A.; Kaiser, U.; Bichoutskaia, E.; Besley, N.A.; Khlobystov, A.N. Direct Transformation of Graphene to Fullerene. Nat. Chem. 2010, 2, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Cross, R.J.; Saunders, M. Transmutation of Fullerenes. J. Am. Chem. Soc. 2005, 127, 3044–3047. [Google Scholar] [CrossRef]
- Berné, O.; Tielens, A.G.G.M. Formation of Buckminsterfullerene (C60) in Interstellar Space. Proc. Natl. Acad. Sci. USA 2012, 109, 401–406. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.Y.; Bowles, F.L.; Bearden, D.W.; Ray, W.K.; Fuhrer, T.; Ye, Y.Q.; Dixon, C.; Harich, K.; Helm, R.F.; Olmstead, M.M.; et al. A Missing Link in the Transformation from Asymmetric to Symmetric Metallofullerene Cages Implies a Top-Down Fullerene Formation Mechanism. Nat. Chem. 2013, 5, 880–885. [Google Scholar] [CrossRef]
- Irle, S.; Zheng, G.; Wang, Z.; Morokuma, K. The C60 Formation Puzzle ‘Solved’: QM/MD Simulations Reveal the Shrinking Hot Giant Road of the Dynamic Fullerene Self-Assembly Mechanism. J. Phys. Chem. B 2006, 110, 14531–14545. [Google Scholar] [CrossRef]
- Wu, B.; Jiang, L.; Luo, Y.; Wang, C.R. The Effect of the Polyaromatic Hydrocarbon in the Formation of Fullerenes. Angew. Chem. Int. Ed. 2020, 59, 3942–3947. [Google Scholar] [CrossRef]
- Yamada, M.; Akasaka, T.; Nagase, S. Salvaging Reactive Fullerenes from Soot by Exohedral Derivatization. Angew. Chem. Int. Ed. 2018, 57, 13394–13405. [Google Scholar] [CrossRef]
- Karpf, M. Organic Synthesis at High Temperatures. Gas-Phase Flow Thermolysis. Angew. Chem. In. Ed. 1986, 25, 414–430. [Google Scholar] [CrossRef]
- Mcnab, H. Synthetic Applications of Flash Vacuum Pyrolysis. Contemp. Org. Synth. 1996, 3, 373–396. [Google Scholar] [CrossRef]
- Duffy, E.F.; Foot, J.S.; Mcnab, H.; Milligan, A.A. An Empirical Study of the Effect of the Variables in a Flash Vacuum Pyrolysis (FVP) Experiment. Org. Biomol. Chem. 2004, 2, 2677–2683. [Google Scholar] [CrossRef]
- Taylor, R.; Lanǵley, G.J.; Kroto, H.W.; Walton, D.R.M. Formation of C60 by Pyrolysis of Naphthalene. Nature 1993, 366, 728–731. [Google Scholar] [CrossRef]
- Crowley, C.; Kroto, H.W.; Taylor, R.; Walton, D.R.M.; Bratcher, M.S.; Cheng, P.C.; Scott, L.T. Formation of [60]Fullerene by Pyrolysis of Corannulene, 7,10-Bis(2,2’- Dibromovinyl)Fluoranthene, and 11,12 Benzofluoranthene. Tetrahedron Lett. 1995, 36, 9215–9218. [Google Scholar] [CrossRef]
- Scott, L.T.; Boorum, M.M.; McMahon, B.J.; Hagen, S.; Mack, J.; Blank, J.; Wegner, H.; Meijere, A.D. A Rational Chemical Synthesis of C60. Science 2002, 295, 1500–1503. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.Y.; Huang, R.B.; Chen, L.H.; Huang, W.J.; Zheng, L.S. Glow Discharge Synthesis and Molecular Structures of Perchlorofluoranthene and other Perchlorinated Fragments of Buckminsterfullerene. Chem. Commun. 1998, 18, 2045–2046. [Google Scholar] [CrossRef]
- Xie, S.Y.; Gao, F.; Xin, L.; Huang, R.B.; Wang, C.R.; Zhang, X.; Liu, M.L.; Deng, S.L.; Zheng, L.S. Capturing the Labile Fullerene[50] as C50Cl10. Science 2004, 304, 699. [Google Scholar] [CrossRef] [PubMed]
- Tohji, K.; Paul, A.; Malhotra, L.; Lorents, D.C.; Ruoff, R.S. Selective and High-Yield Synthesis of Higher Fullerenes. J. Phys. Chem. 1995, 99, 17785–17788. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.-G.; Zhuo, Y.-Q.; Zhang, X.-M.; Zhang, L.; Xu, P.-Y.; Tian, H.-R.; Lin, S.-C.; Zhang, Q.; Xie, S.-Y.; Zheng, L.-S. Synthesis of Fullerenes from a Nonaromatic Chloroform through a Newly Developed Ultrahigh-Temperature Flash Vacuum Pyrolysis Apparatus. Nanomaterials 2021, 11, 3033. https://doi.org/10.3390/nano11113033
Zhang H-G, Zhuo Y-Q, Zhang X-M, Zhang L, Xu P-Y, Tian H-R, Lin S-C, Zhang Q, Xie S-Y, Zheng L-S. Synthesis of Fullerenes from a Nonaromatic Chloroform through a Newly Developed Ultrahigh-Temperature Flash Vacuum Pyrolysis Apparatus. Nanomaterials. 2021; 11(11):3033. https://doi.org/10.3390/nano11113033
Chicago/Turabian StyleZhang, Hong-Gang, Ya-Qi Zhuo, Xiao-Min Zhang, Leng Zhang, Piao-Yang Xu, Han-Rui Tian, Shui-Chao Lin, Qianyan Zhang, Su-Yuan Xie, and Lan-Sun Zheng. 2021. "Synthesis of Fullerenes from a Nonaromatic Chloroform through a Newly Developed Ultrahigh-Temperature Flash Vacuum Pyrolysis Apparatus" Nanomaterials 11, no. 11: 3033. https://doi.org/10.3390/nano11113033
APA StyleZhang, H.-G., Zhuo, Y.-Q., Zhang, X.-M., Zhang, L., Xu, P.-Y., Tian, H.-R., Lin, S.-C., Zhang, Q., Xie, S.-Y., & Zheng, L.-S. (2021). Synthesis of Fullerenes from a Nonaromatic Chloroform through a Newly Developed Ultrahigh-Temperature Flash Vacuum Pyrolysis Apparatus. Nanomaterials, 11(11), 3033. https://doi.org/10.3390/nano11113033