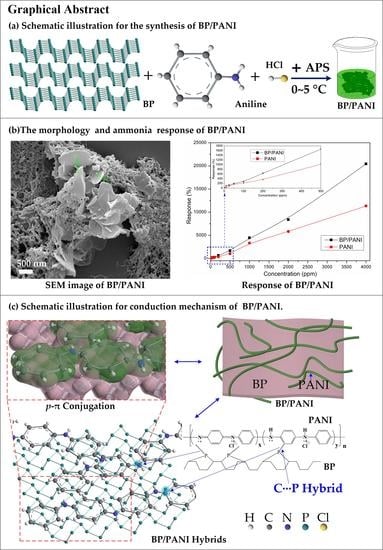
High Sensitivity of Ammonia Sensor through 2D Black Phosphorus/Polyaniline Nanocomposite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
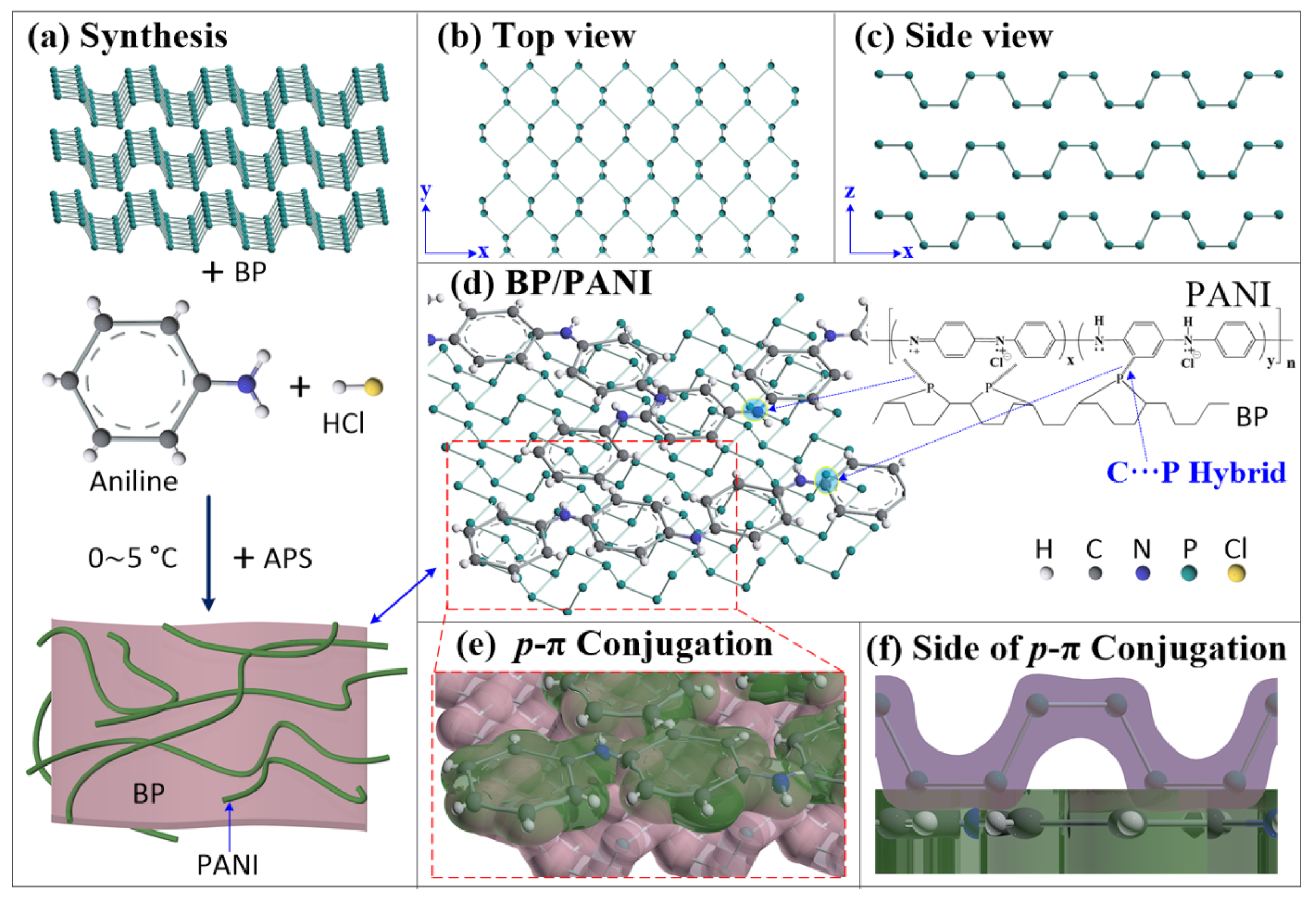
2.2. Synthesis of BP/PANI Nanocomposite
2.3. Device Fabrications and Apparatus
3. Results and Discussion
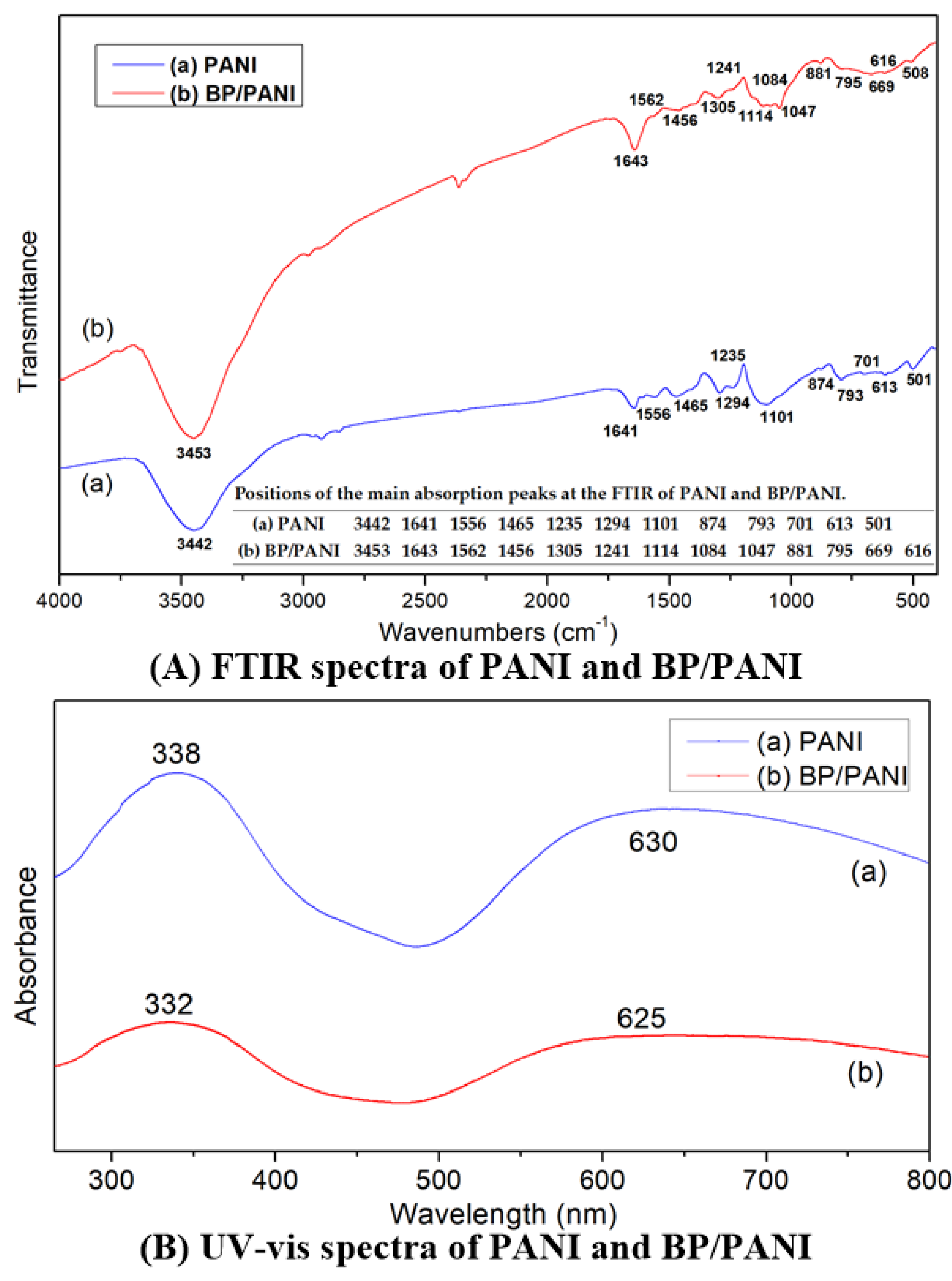
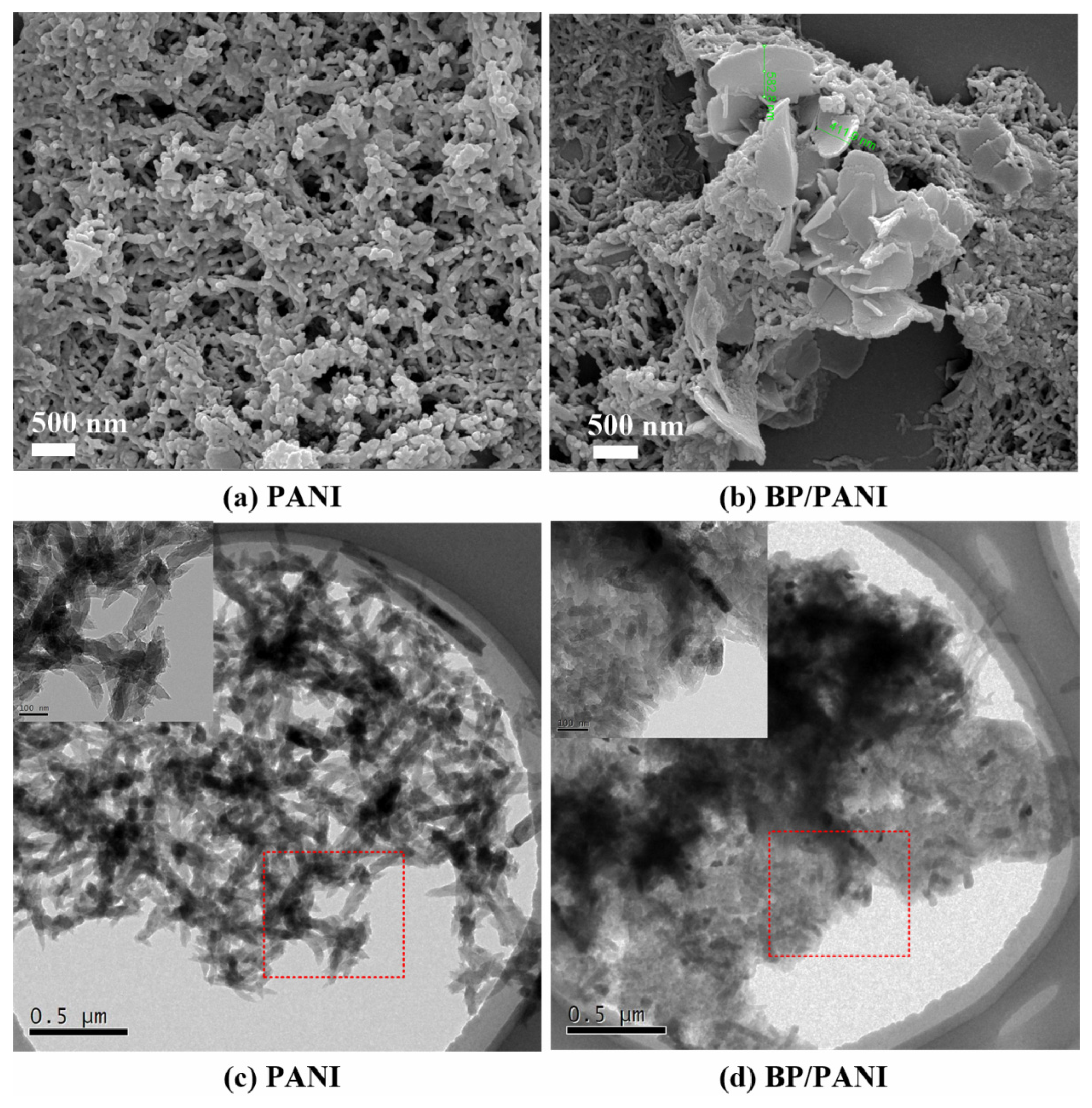
3.1. Characterization: FT-IR, UV−vis, SEM, and TEM of PANI and BP/PANI
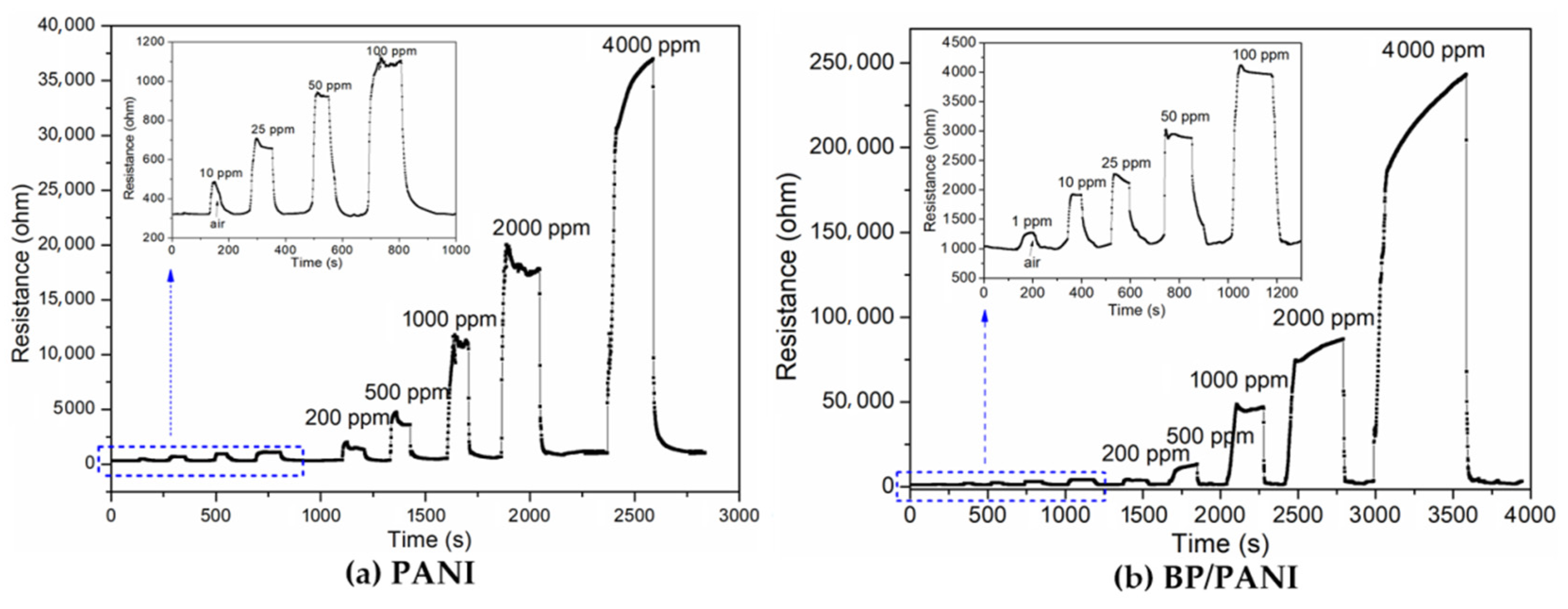
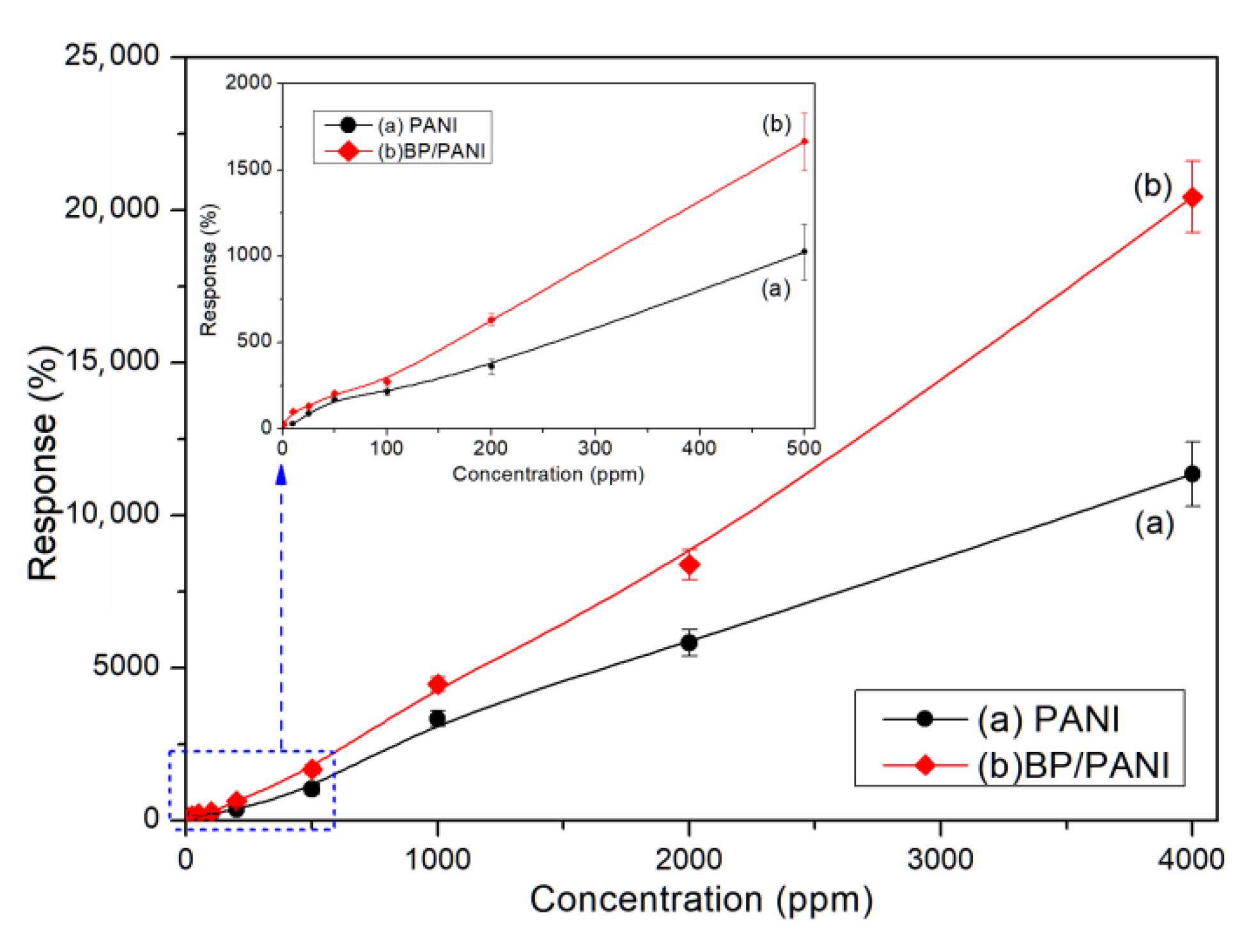
3.2. Gas Response of Sensors
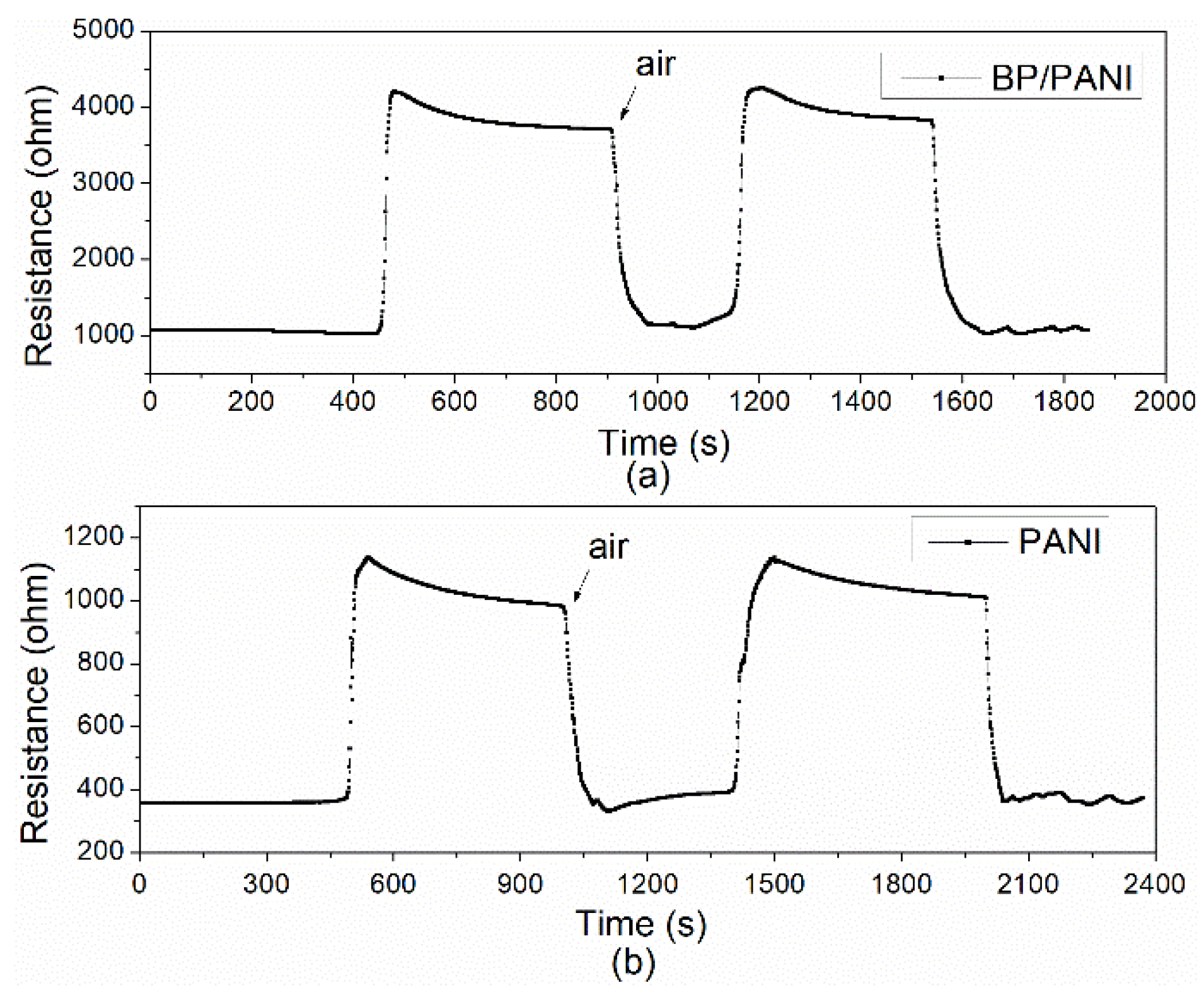
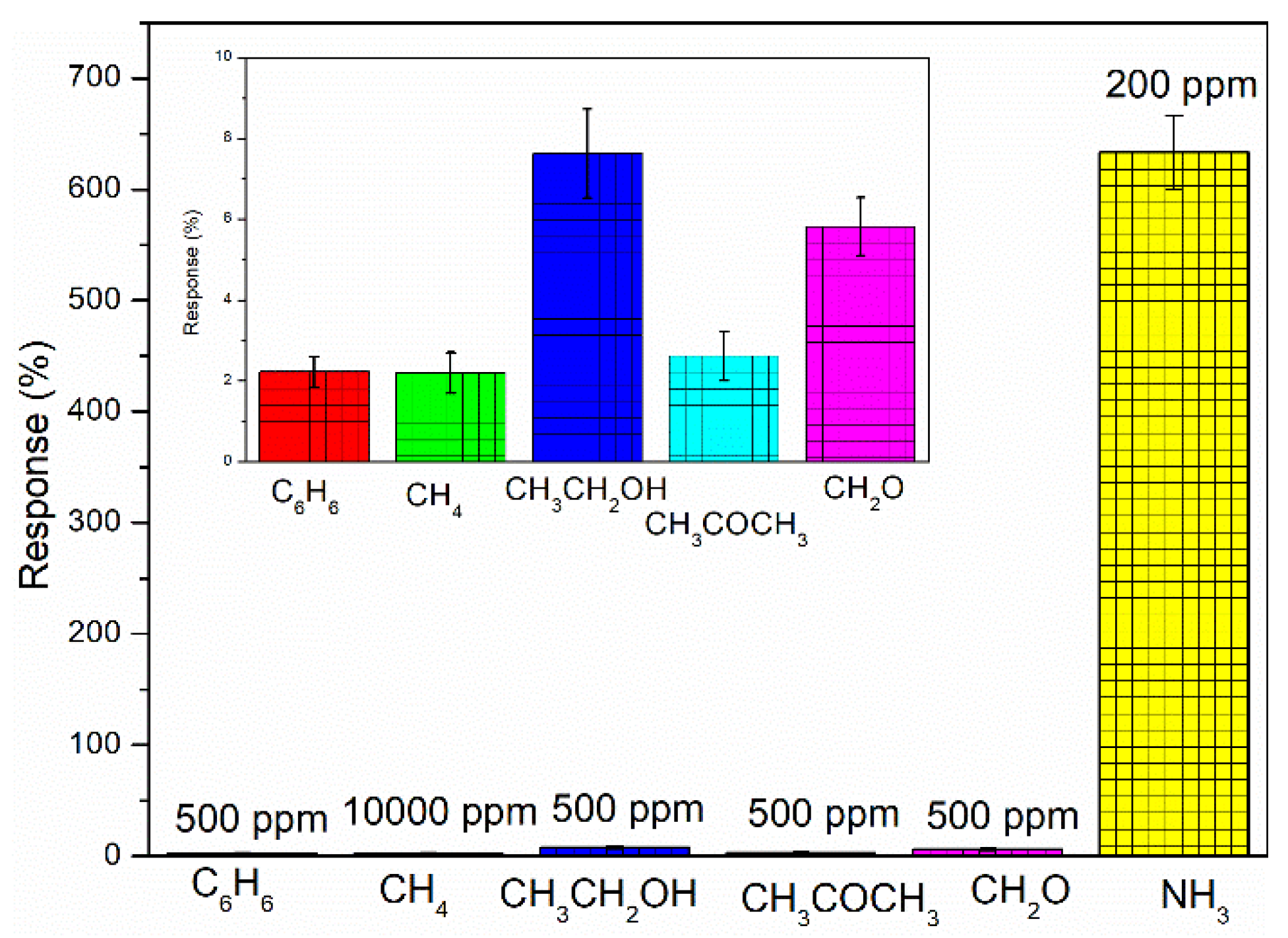
3.3. Response, Recovery, and Selectivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pushkarsky, M.; Webber, M.; Patel, C. Ultra-sensitive ambient ammonia detection using CO2-laser-based photoacoustic spectroscopy. Appl. Phys. A 2003, 77, 381–385. [Google Scholar] [CrossRef]
- Timmer, B.; Olthuis, W.; Berg, A.V.D. Ammonia sensors and their applications—A review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Yoo, K.-P.; Kwon, K.-H.; Min, N.-K.; Lee, M.J.; Lee, C.J. Effects of O2 plasma treatment on NH3 sensing characteristics of multiwall carbon nanotube/polyaniline composite films. Sens. Actuators B Chem. 2009, 143, 333–340. [Google Scholar] [CrossRef]
- Hsu, C.-S.; Chen, H.-I.; Lin, C.-W.; Chen, T.-Y.; Huang, C.-C.; Chou, P.-C.; Liu, W.-C.; Amalraj, F.; Talianker, M.; Markovsky, B.; et al. Ammonia Gas Sensing Performance of an Indium Tin Oxide (ITO) Based Device with an Underlying Au-Nanodot Layer. J. Electrochem. Soc. 2012, 160, B17–B22. [Google Scholar] [CrossRef]
- Wang, J.; Yang, P.; Wei, X. High-Performance, Room-Temperature, and No-Humidity-Impact Ammonia Sensor Based on Heterogeneous Nickel Oxide and Zinc Oxide Nanocrystals. ACS Appl. Mater. Interfaces 2015, 7, 3816–3824. [Google Scholar] [CrossRef]
- Huang, J.; Wang, J.; Gu, C.; Yu, K.; Meng, F.; Liu, J. A novel highly sensitive gas ionization sensor for ammonia detection. Sens. Actuators A Phys. 2009, 150, 218–223. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Singh, R.; Kashyap, R.; Kumar, R.; Kumar, D.; Sharma, S.; Kumar, M. Room temperature ammonia gas sensor using Meta Toluic acid functionalized graphene oxide. Mater. Chem. Phys. 2019, 240, 121922. [Google Scholar] [CrossRef]
- Kulkarni, S.B.; Navale, Y.H.; Navale, S.T.; Stadler, F.J.; Patil, V.B. Room temperature ammonia gas sensing properties of pol-yaniline nanofibers. J. Mater. Sci. Mater. Electron. 2019, 30, 1–10. [Google Scholar] [CrossRef]
- Tai, H.; Jiang, Y.; Xie, G.; Yu, J.; Chen, X. Fabrication and gas sensitivity of polyaniline–titanium dioxide nanocomposite thin film. Sens. Actuators B Chem. 2007, 125, 644–650. [Google Scholar] [CrossRef]
- Gong, J.; Li, Y.; Hu, Z.; Zhou, Z.; Deng, Y. Ultrasensitive NH3 Gas Sensor from Polyaniline Nanograin Enchased TiO2 Fibers. J. Phys. Chem. C 2010, 114, 9970–9974. [Google Scholar] [CrossRef]
- Zeng, F.-W.; Liu, X.-X.; Diamond, D.; Lau, K.T. Humidity sensors based on polyaniline nanofibres. Sens. Actuators B Chem. 2010, 143, 530–534. [Google Scholar] [CrossRef]
- Matsuguchi, M.; Io, J.; Sugiyama, G.; Sakai, Y. Effect of NH3 gas on the electrical conductivity of polyaniline blend films. Synth. Met. 2002, 128, 15–19. [Google Scholar] [CrossRef]
- Al-Mashat, L.; Shin, K.; Kalantar-zadeh, K.; Plessis, J.D.; Han, S.H.; Kojima, R.W.; Kaner, R.B.; Li, D.; Gou, X.; Ippolito, S.J.; et al. Graphene/Polyaniline Nanocomposite for Hydrogen Sensing. J. Phys. Chem. C 2010, 114, 16168–16173. [Google Scholar] [CrossRef]
- Srivastava, S.; Sharma, S.; Kumar, S.; Agrawal, S.; Singh, M.; Vijay, Y. Characterization of gas sensing behavior of multi walled carbon nanotube polyaniline composite films. Int. J. Hydrog. Energy 2009, 34, 8444–8450. [Google Scholar] [CrossRef]
- Sadek, A.Z.; Wlodarski, W.; Shin, K.; Kaner, R.B.; Kalantar-Zadeh, K. A layered surface acoustic wave gas sensor based on a polyaniline/In2O3 nanofibre composite. Nanotechnology 2006, 17, 4488–4492. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.-H.; Phiboolsirichit, N.; Mubeen, S.; A Deshusses, M.; Mulchandani, A.; Myung, N.V. Electrical and gas sensing properties of polyaniline functionalized single-walled carbon nanotubes. Nanotechnology 2010, 21, 75502. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Liu, A.; Yang, Z.; Zhao, L.; Wang, J.; Liu, F.; You, R.; He, J.; Wang, C.; Yan, X.; et al. Design and preparation of the WO3 hollow spheres@ PANI conducting films for room temperature flexible NH3 sensing device. Sens. Actuators B Chem. 2019, 289, 252–259. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, H.; Sun, J.; Ma, W.; Li, L.; Li, W.; Du, J. Novel QCM humidity sensors using stacked black phosphorus nanosheets as sensing film. Sens. Actuators B Chem. 2017, 244, 259–264. [Google Scholar] [CrossRef]
- Qiao, J.; Kong, X.; Hu, Z.-X.; Yang, F.; Ji, W. High-mobility transport anisotropy and linear dichroism in few-layer black phosphorus. Nat. Commun. 2014, 5, 4475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gusmão, R.; Sofer, Z.; Pumera, M. Black Phosphorus Rediscovered: From Bulk Material to Monolayers. Angew. Chem. Int. Ed. 2017, 56, 8052–8072. [Google Scholar] [CrossRef]
- Avsar, A.; Vera-Marun, I.J.; Tan, J.Y.; Watanabe, K.; Taniguchi, T.; Neto, A.H.C.; Özyilmaz, B. Air-Stable Transport in Graphene-Contacted, Fully Encapsulated Ultrathin Black Phosphorus-Based Field-Effect Transistors. ACS Nano 2015, 9, 4138–4145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, A.N.; Liu, B.; Chen, L.; Ma, Y.; Cong, S.; Aroonyadet, N.; Köpf, M.; Nilges, T.; Zhou, C. Black Phosphorus Gas Sensors. ACS Nano 2015, 9, 5618–5624. [Google Scholar] [CrossRef] [PubMed]
- Mayorga-Martinez, C.C.; Sofer, Z.; Pumera, M. Layered Black Phosphorus as a Selective Vapor Sensor. Angew. Chem. Int. Ed. 2015, 54, 14317–14320. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ren, R.; Pu, H.; Chang, J.; Mao, S.; Chen, J. Field-effect transistor biosensors with two-dimensional black phosphorus nanosheets. Biosens. Bioelectron. 2017, 89, 505–510. [Google Scholar] [CrossRef] [Green Version]
- Qian, C.; Wang, R.; Li, M.; Li, X.; Ge, B.; Bai, Z.; Jiao, T. Facile preparation of self-assembled black phosphorus-based composite LB films as new chemical gas sensors. Colloids Surf. A Physicochem. Eng. Asp. 2020, 608, 125616. [Google Scholar] [CrossRef]
- Wu, X.; Xu, Y.; Hu, Y.; Wu, G.; Cheng, H.; Yu, Q.; Zhang, K.; Chen, W.; Chen, S. Microfluidic-spinning construction of black-phosphorus-hybrid microfibres for non-woven fabrics toward a high energy density flexible supercapacitor. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Sun, B.; Li, W.; Gou, X.; Gou, Y.; Li, D.; Hu, F. Novel nanomaterial of porous graphene functionalized black phosphorus as electrochemical sensor platform for bisphenol A detection. J. Electroanal. Chem. 2019, 835, 1–9. [Google Scholar] [CrossRef]
- Sajedi-Moghaddam, A.; Mayorga-Martinez, C.C.; Sofer, Z.; Bouša, D.; Saievar-Iranizad, E.; Pumera, M. Black Phosphorus Nanoflakes/Polyaniline Hybrid Material for High-Performance Pseudocapacitors. J. Phys. Chem. C 2017, 121, 20532–20538. [Google Scholar] [CrossRef]
- Durai, L.; Gopalakrishnan, A.; Vishnu, N.; Badhulika, S. Polyaniline Sheathed Black Phosphorous: A Novel, Advanced Platform for Electrochemical Sensing Applications. Electroanalysis 2019, 32, 238–247. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Wang, Y.; Zhang, R.; Li, J.; Li, X.; Zang, Z. Conductometric room temperature ammonia sensors based on titanium dioxide nanoparticles decorated thin black phosphorus nanosheets. Sens. Actuators B Chem. 2021, 349, 130770. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, X.; Gou, Y.; Zhou, Z.; Jiang, M.; Lu, J.; Hui, D. Synthesis and mechanism of polyaniline nanotubes with rectangular cross section via in situ polymerization. Polym. Adv. Technol. 2011, 23, 796–802. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, S.; Dong, X.; Yao, Y.; Guo, Y.; Gu, S.; Zhou, Z. A facile method to graphene oxide/polyaniline nanocomposite with sandwich-like structure for enhanced electrical properties of humidity detection. Anal. Chim. Acta 2019, 1080, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Jing, X.; Wang, B.; Wang, F. Infrared spectra of soluble polyaniline. Synth. Met. 1988, 24, 231–238. [Google Scholar] [CrossRef]
- Trchová, M.; Šeděnková, I.; Tobolková, E.; Stejskal, J. FTIR spectroscopic and conductivity study of the thermal degradation of polyaniline films. Polym. Degrad. Stab. 2004, 86, 179–185. [Google Scholar] [CrossRef]
- Jiang, M.; Zhu, S.; Zhou, Z.; Zhao, A.; Lu, J. Effect of Ni2+ as a codopant on the structure, morphology, and conductivity of nanostructured polyaniline. J. Appl. Polym. Sci. 2011, 121, 3439–3445. [Google Scholar] [CrossRef]
- Monkman, A.; Bloor, D.; Stevens, G.; Stevens, J. Electronic energy levels of polyaniline. J. Phys. D Appl. Phys. 1987, 20, 1337–1345. [Google Scholar] [CrossRef]
- Chiou, N.R.; Epstein, A.J. Polyaniline nanofibers prepared by dilute polymerization. Adv. Mater. 2005, 17, 1679–1683. [Google Scholar] [CrossRef]
- Kebiche, H.; Debarnot, D.; Merzouki, A.; Poncin-Epaillard, F.; Haddaoui, N. Relationship between ammonia sensing properties of polyaniline nanostructures and their deposition and synthesis methods. Anal. Chim. Acta 2012, 737, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Shi, G. Gas Sensors Based on Conducting Polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef] [Green Version]
- Matsuguchi, M.; Okamoto, A.; Sakai, Y. Effect of humidity on NH3 gas sensitivity of polyaniline blend films. Sens. Actuators B Chem. 2003, 94, 46–52. [Google Scholar] [CrossRef]
- Liu, L.T.; Ye, X.Y.; Wu, K.; Zhou, Z.Y.; Lee, D.J.; Cui, T.H. Humidity Sensitivity of Carbon Nanotube and Poly (Dimethyldial-lylammonium Chloride) Composite Films. IEEE Sens. J. 2009, 9, 1308–1314. [Google Scholar] [CrossRef]
- Liu, A.; Lv, S.; Zhao, L.; Liu, F.; Wang, J.; You, R.; Yang, Z.; He, J.; Jiang, L.; Wang, C.; et al. Room temperature flexible NH3 sensor based on polyaniline coated Rh-doped SnO2 hollow nanotubes. Sens. Actuators B Chem. 2020, 330, 129313. [Google Scholar] [CrossRef]
- Tai, H.; Xu, X.; Ye, Z.; Liu, C.; Xie, G.; Jiang, Y. P–P heterojunction sensor of self-assembled polyaniline nano-thin film/microstructure silicon array for NH3 detection. Chem. Phys. Lett. 2015, 621, 58–64. [Google Scholar] [CrossRef]
- Joshi, S.; Lokhande, C. Fabrication of isotype (p-p) selenium–polyaniline heterojunction diode by electrochemical method. Appl. Surf. Sci. 2006, 252, 8539–8543. [Google Scholar] [CrossRef]
- Lei, W.; Zhang, T.; Liu, P.; Rodriguez, J.A.; Liu, G.; Liu, M. Bandgap- and Local Field-Dependent Photoactivity of Ag/Black Phosphorus Nanohybrids. ACS Catal. 2016, 6, 8009–8020. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, G.; Zhang, Y.-W. Layer-dependent Band Alignment and Work Function of Few-Layer Phosphorene. Sci. Rep. 2014, 4, 6677. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Liang, L.; Zhu, S.; Guo, Y.; Yao, Y.; Yang, Y.; Gu, S.; Zhou, Z. High Sensitivity of Ammonia Sensor through 2D Black Phosphorus/Polyaniline Nanocomposite. Nanomaterials 2021, 11, 3026. https://doi.org/10.3390/nano11113026
Wu Z, Liang L, Zhu S, Guo Y, Yao Y, Yang Y, Gu S, Zhou Z. High Sensitivity of Ammonia Sensor through 2D Black Phosphorus/Polyaniline Nanocomposite. Nanomaterials. 2021; 11(11):3026. https://doi.org/10.3390/nano11113026
Chicago/Turabian StyleWu, Zuquan, Lei Liang, Shibu Zhu, Yifan Guo, Yao Yao, Yong Yang, Shifu Gu, and Zuowan Zhou. 2021. "High Sensitivity of Ammonia Sensor through 2D Black Phosphorus/Polyaniline Nanocomposite" Nanomaterials 11, no. 11: 3026. https://doi.org/10.3390/nano11113026