Silver/Polypyrrole-Functionalized Polyurethane Foam Embedded Phase Change Materials for Thermal Energy Harvesting
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of PPy@PU
2.3. Preparation of Ag/PPy@PU
2.4. Preparation of the FSPCMs
2.5. Characterizations
3. Results and Discussion
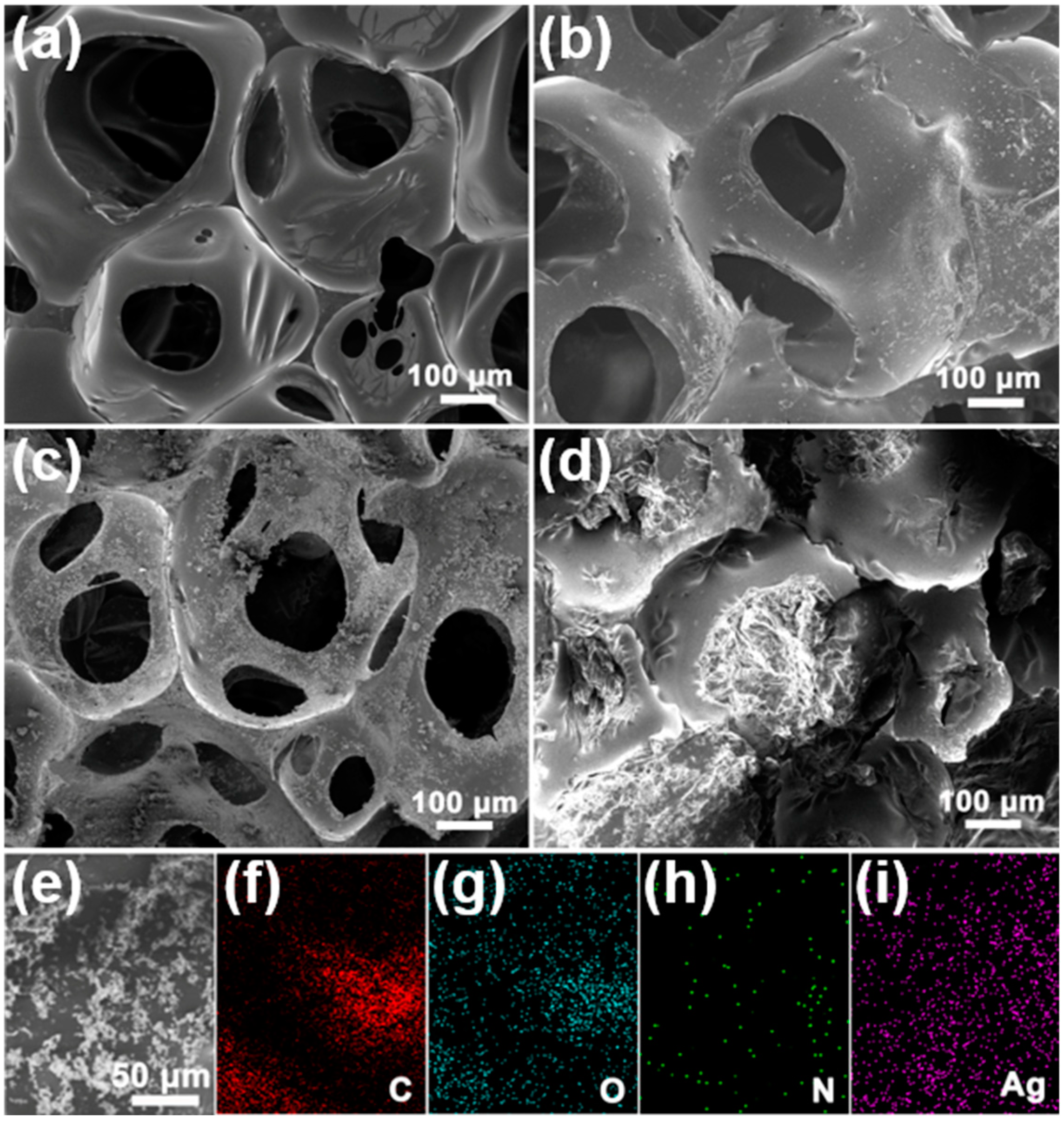
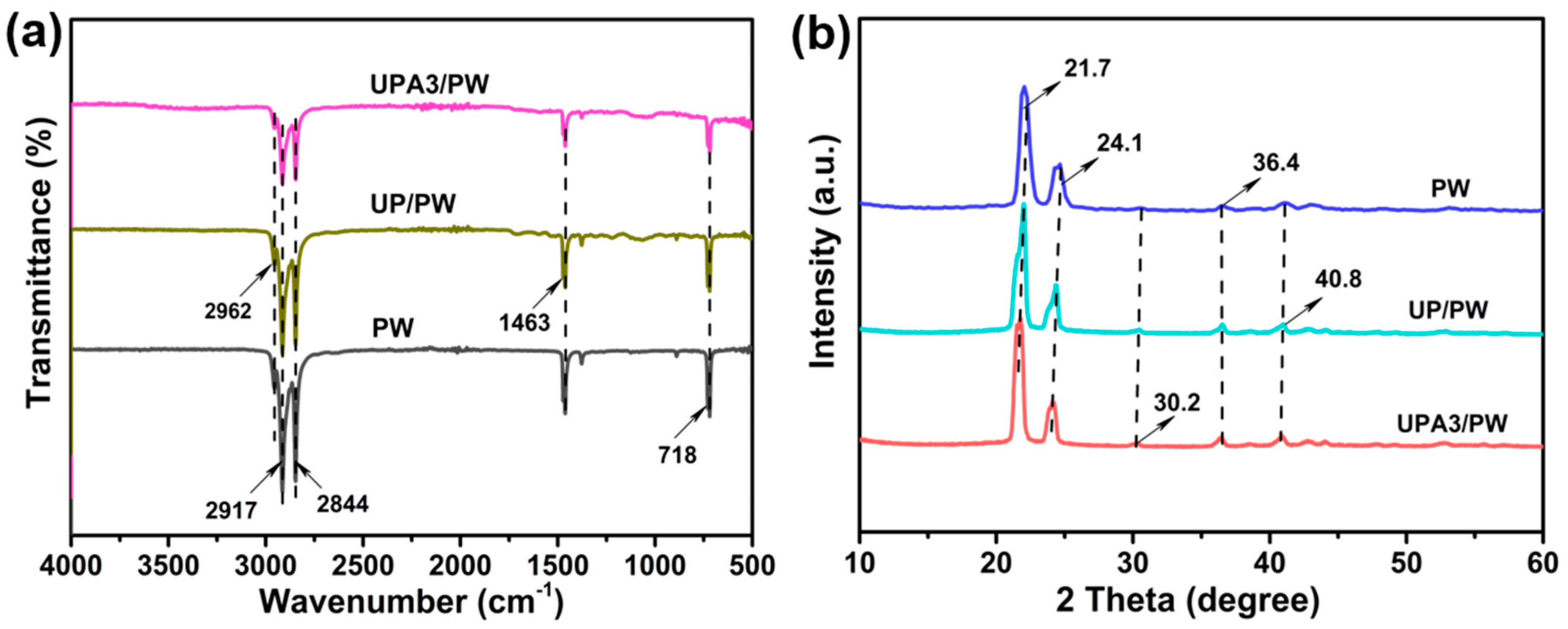
3.1. Morphology and Structure of the FSPCMs
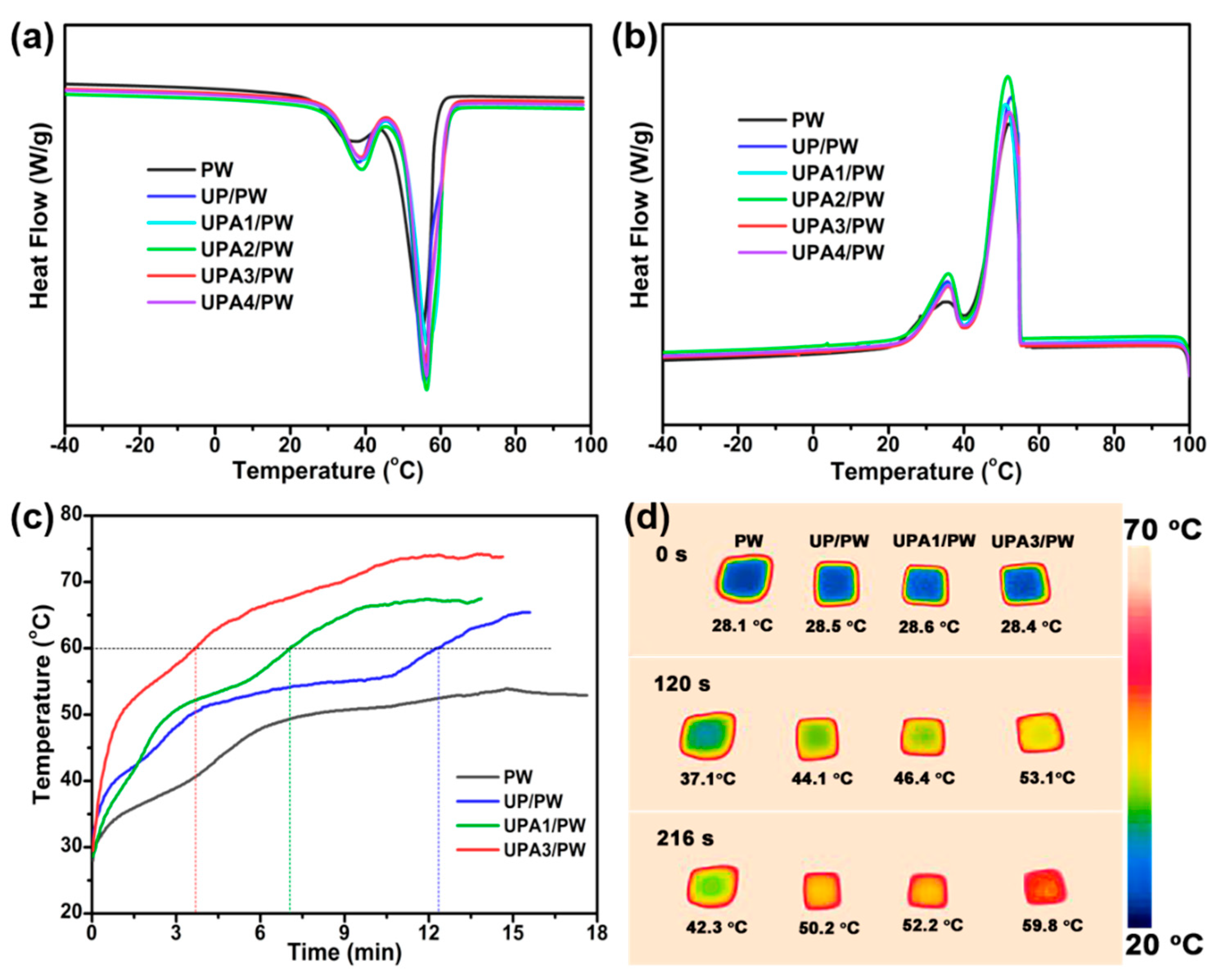
3.2. Thermal Properties of the FSPCMs
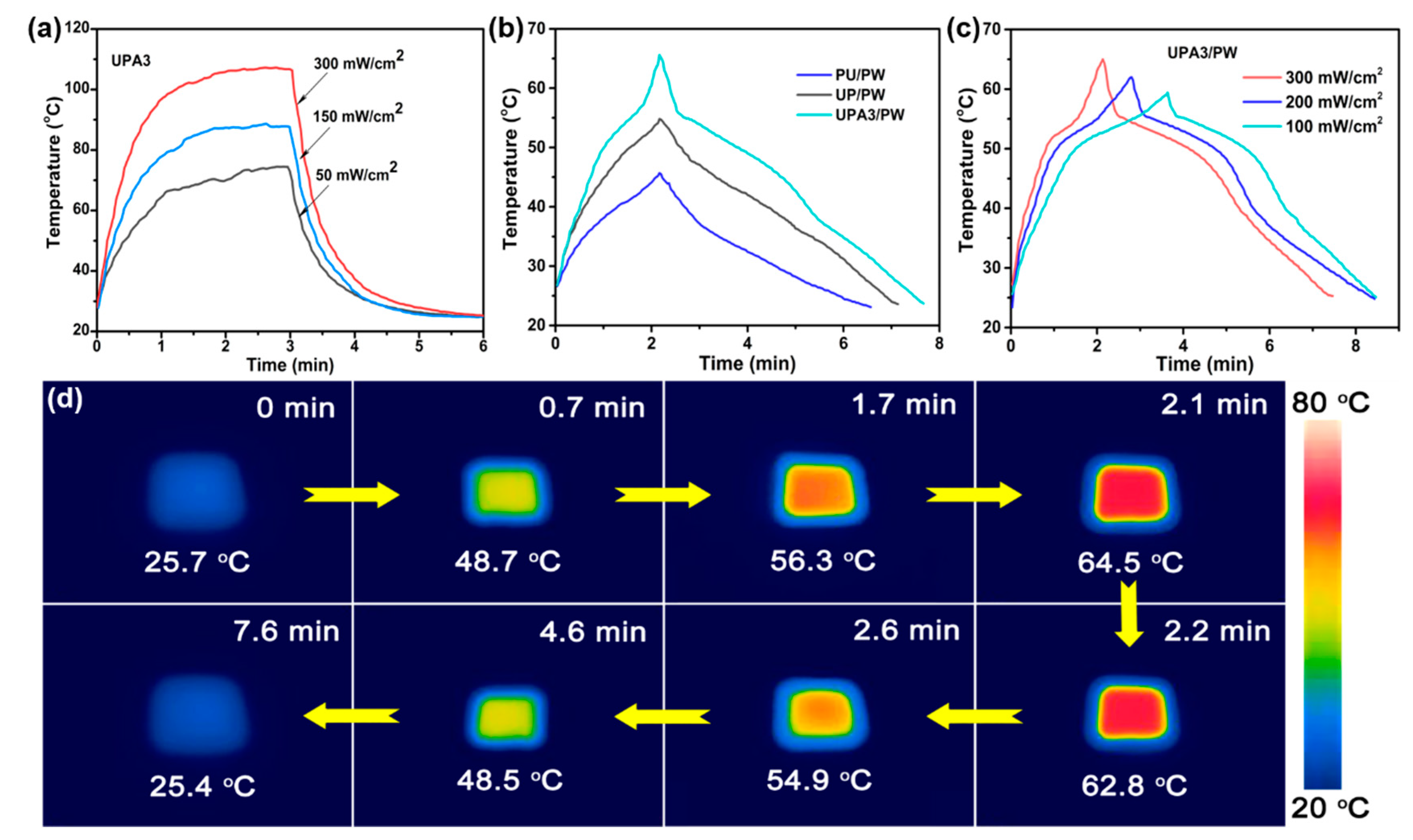
3.3. Solar–Thermal Energy Harvesting and Storage
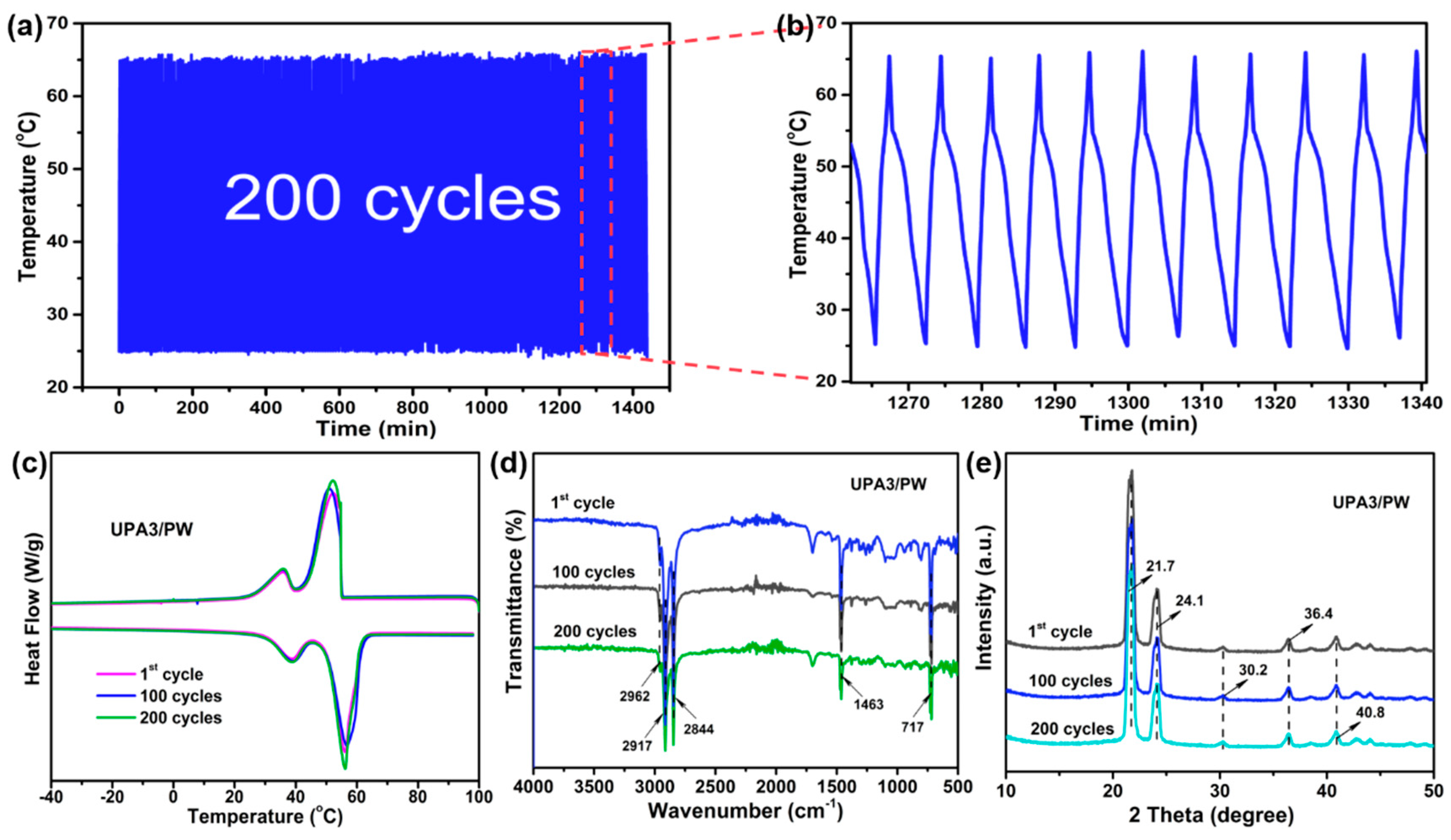
3.4. Cycling Durability of the FSPCMs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef]
- Yuan, K.; Shi, J.; Aftab, W.; Qin, M.; Usman, A.; Zhou, F.; Lv, Y.; Gao, S.; Zou, R. Engineering the thermal conductivity of functional phase-change materials for heat energy conversion, storage, and utilization. Adv. Funct. Mater. 2020, 30, 1904228. [Google Scholar] [CrossRef]
- Shi, T.; Zhang, X.; Qiao, J.; Wu, X.; Chen, G.; Leng, G.; Lin, F.; Min, X.; Huang, Z. Preparation and characterization of composite phase change materials based on paraffin and carbon foams derived from starch. Polymer 2021, 212, 123143. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, H.; Chen, X.; Zhang, Y.; Li, A.; Wang, G. Advanced multifunctional composite phase change materials based on photo-responsive materials. Nano Energy 2021, 80, 105454. [Google Scholar] [CrossRef]
- Wang, Z.; Tong, Z.; Ye, Q.; Hu, H.; Nie, X.; Yan, C.; Shang, W.; Song, C.; Wu, J.; Wang, J.; et al. Dynamic tuning of optical absorbers for accelerated solar-thermal energy storage. Nat. Commun. 2017, 8, 1478. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, B.; Zhang, S. Single-walled carbon nanotube/phase change Material composites: Sunlight-driven, reversible, form-stable phase transitions for solar thermal energy storage. Adv. Funct. Mater. 2013, 23, 4354–4360. [Google Scholar] [CrossRef]
- Zhang, Y.; Gurzadyan, G.; Umair, M.; Wang, W.; Lu, R.; Zhang, S.; Tang, B. Ultrafast and efficient photothermal conversion for sunlight-driven thermal-electric system. Chem. Eng. J. 2018, 344, 402–409. [Google Scholar] [CrossRef]
- Chen, L.; Zou, R.; Xia, W.; Liu, Z.; Shang, Y.; Zhu, J.; Wang, Y.; Lin, J.; Xia, D.; Cao, A. Electro- and photodriven phase change composites based on wax-infiltrated carbon nanotube sponges. ACS Nano 2012, 6, 10884–10892. [Google Scholar] [CrossRef]
- Chen, X.; Gao, H.; Tang, Z.; Dong, W.; Li, A.; Wang, G. Optimization strategies of composite phase change materials for thermal energy storage, transfer, conversion and utilization. Energy Environ. Sci. 2020, 13, 4498–4535. [Google Scholar] [CrossRef]
- Kashyap, V.; Sakunkaewkasem, S.; Jafari, P.; Nazari, M.; Eslami, B.; Nazifi, S.; Irajizad, P.; Marquez, M.; Lee, T.; Ghasemi, H. Full spectrum solar thermal energy harvesting and storage by a molecular and phase-change hybrid material. Joule 2020, 4, 1–12. [Google Scholar] [CrossRef]
- Shao, Y.; Hu, W.; Gao, M.; Xiao, Y.; Huang, T.; Zhang, N.; Yang, J.; Qi, X.; Wang, Y. Flexible MXene-coated melamine foam based phase change material composites for integrated solar-thermal energy conversion/storage, shape memory and thermal therapy functions. Compos. Part A 2021, 143, 106291. [Google Scholar] [CrossRef]
- Maithya, O.; Zhu, X.; Li, X.; Korir, S.; Feng, X.; Sui, X.; Wang, B. High-energy storage graphene oxide modified phase change microcapsules from regenerated chitin Pickering Emulsion for photothermal conversion. Sol. Energy Mater. Sol. Cells 2021, 222, 110924. [Google Scholar] [CrossRef]
- Mehrali, M.; Elshof, J.; Shahia, M.; Mahmoudi, A. Simultaneous solar-thermal energy harvesting and storage via shape stabilized salt hydrate phase change material. Chem. Eng. J. 2021, 405, 126624. [Google Scholar] [CrossRef]
- Li, G.; Hong, G.; Dong, D.; Song, W.; Zhang, X. Multiresponsive graphene-aerogel-directed phase-change smart fibers. Adv. Mater. 2018, 30, 1801754. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, H.; Mao, H.; Li, J.; Shi, H. Light-to-thermal conversion and thermoregulated capability of coaxial fibers with a combined influence from comb-like polymeric phase change material and carbon nanotube. ACS Appl. Mater. Interfaces 2019, 11, 14150–14158. [Google Scholar] [CrossRef]
- Lu, Y.; Xiao, X.; Fu, J.; Huan, C.; Qi, S.; Zhan, Y.; Zhu, Y.; Xu, G. Novel smart textile with phase change materials encapsulated core-sheath structure fabricated by coaxial electrospinning. Chem. Eng. J. 2019, 355, 532–539. [Google Scholar] [CrossRef]
- Xue, F.; Jin, X.; Xie, X.; Qi, X.; Yang, J.; Wang, Y. Constructing reduced graphene oxide/boron nitride frameworks in melamine foam towards synthesizing phase change materials applied in thermal management of microelectronic devices. Nanoscale 2019, 11, 18691–18701. [Google Scholar] [CrossRef]
- Jing, J.; Wu, H.; Shao, Y.; Qi, X.; Yang, J.; Wang, Y. Melamine foam-supported form-stable phase change materials with simultaneous thermal energy storage and shape memory properties for thermal management of electronic devices. ACS Appl. Mater. Interfaces 2019, 11, 19252–19259. [Google Scholar] [CrossRef]
- Chen, X.; Gao, H.; Hai, G.; Jia, D.; Xing, L.; Chen, S.; Cheng, P.; Han, M.; Dong, W.; Wang, G. Carbon nanotube bundles assembled flexible hierarchical framework based phase change material composites for thermal energy harvesting and thermotherapy. Energy Storage Mater. 2020, 26, 129–137. [Google Scholar] [CrossRef]
- Qiu, J.; Huo, D.; Xue, J.; Zhu, G.; Liu, H.; Xia, Y. Encapsulation of a phase-change material in nanocapsules with a well-defined hole in the wall for the controlled release of drugs. Angew. Chem. Int. Ed. 2019, 58, 10606–10611. [Google Scholar] [CrossRef]
- Aftab, J.S.W.; Liang, Z.; Yuan, K.; Maqbool, M.; Jiang, H.; Xiong, F.; Qin, M.; Gao, S.; Zou, R. Tuning the flexibility and thermal storage capacity of solid-solid phase change materials towards wearable applications. J. Mater. Chem. A 2020, 8, 20133. [Google Scholar]
- Cao, Y.; Fan, D.; Lin, S.; Mu, L.; Ng, F.T.; Pan, Q. Phase change materials based on comb-like polynorbornenes and octadecylamine-functionalized graphene oxide nanosheets for thermal energy storage. Chem. Eng. J. 2020, 389, 124318. [Google Scholar] [CrossRef]
- Wu, S.; Li, T.; Tong, Z.; Chao, J.; Zhai, T.; Xu, J.; Yan, T.; Wu, M.; Xu, Z.; Bao, H.; et al. High-performance thermally conductive phase change composites by large-size oriented graphite sheets for scalable thermal energy harvesting. Adv. Mater. 2019, 31, 1905099. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Qiu, J.; Deng, S.; Du, Z.; Cheng, X.; Wang, H. Flame-retardant and solid-solid phase change composites based on dopamine-decorated BP nanosheets/polyurethane for efficient solar-to-thermal energy storage. Renew. Energy 2021, 164, 1–10. [Google Scholar] [CrossRef]
- Lu, X.; Huang, H.; Zhang, X.; Lin, P.; Huang, J.; Sheng, X.; Zhang, L.; Qu, J. Novel light-driven and electro-driven polyethylene glycol/two-dimensional MXene form-stable phase change material with enhanced thermal conductivity and electrical conductivity for thermal energy storage. Compos. B Eng. 2019, 177, 107372. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Shi, L.; Wang, P. MXene Ti3C2: An effective 2D light-to-heat conversion material. ACS Nano 2017, 11, 3752–3759. [Google Scholar] [CrossRef] [Green Version]
- George, M.; Pandey, A.; Rahim, N.; Tyagi, V.; Shahabuddin, S.; Saidur, R. A novel polyaniline (PANI)/paraffin wax nano composite phase change material: Superior transition heat storage capacity, thermal conductivity and thermal reliability. Sol. Energy 2020, 204, 448–458. [Google Scholar] [CrossRef]
- George, M.; Pandey, A.; Rahim, N.; Tyagi, V.; Shahabuddin, S.; Saidur, R. Long-term thermophysical behavior of paraffin wax and paraffin wax/polyaniline (PANI) composite phase change materials. J. Energy Storage 2020, 31, 101568. [Google Scholar] [CrossRef]
- Wu, H.; Chen, R.; Shao, Y.; Qi, X.; Yang, J.; Wang, Y. Novel flexible phase change materials with mussel-inspired modification of melamine foam for simultaneous light-actuated shape memory and light-to-thermal energy storage capability. ACS Sustain. Chem. Eng. 2019, 7, 13532–13542. [Google Scholar] [CrossRef]
- Xie, Y.; Li, W.; Huang, H.; Dong, D.; Zhang, X.; Zhang, L.; Chen, Y.; Sheng, X.; Lu, X. Bio-based radish@PDA/PEG sandwich composite with high efficiency solar thermal energy storage. ACS Sustain. Chem. Eng. 2020, 8, 8448–8457. [Google Scholar] [CrossRef]
- Ge, J.; Wang, Y.; Wang, H.; Mao, H.; Li, J.; Shi, H. Thermal properties and shape stabilization of epoxidized methoxy polyethylene glycol composite PCMs tailored by polydopamine-functionalized graphene oxide. Sol. Energy Mater. Sol. Cells 2020, 208, 110388. [Google Scholar] [CrossRef]
- Xu, J.; Tan, Y.; Du, X.; Du, Z.; Cheng, X.; Wang, H. Cellulose nanofibril/polypyrrole hybrid aerogel supported form-stable phase change composites with superior energy storage density and improved photothermal conversion efficiency. Cellulose 2020, 27, 9547–9558. [Google Scholar] [CrossRef]
- Silakhori, M.; Fauzi, H.; Mahmoudian, M.; Metselaar, H.; Mahlia, T.; Khanlou, H. Preparation and thermal properties of form-stable phase change materials composed of palmitic acid/polypyrrole/graphene nanoplatelets. Energy Build. 2015, 99, 189–195. [Google Scholar] [CrossRef]
- Wei, Q.; Oribayo, O.; Feng, X.; Rempel, G.; Pan, Q. Synthesis of polyurethane foams loaded with TiO2 nanoparticles and their modification for enhanced performance in oil spill cleanup. Ind. Eng. Chem. Res. 2018, 57, 8918–8926. [Google Scholar] [CrossRef]
- Bober, P.; Liu, J.; Mikkonen, K.; Ihalainen, P.; Pesonen, M.; Plumed-Ferrer, C.; Wright, A.; Lindfors, T.; Xu, C.; Latonen, R. Biocomposites of nanofibrillated cellulose, polypyrrole, and silver nanoparticles with electroconductive and antimicrobial properties. Biomacromolecules 2014, 15, 3655–3663. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Qiu, J.; Jin, X.; Umair, M.; Lu, R.; Zhang, S.; Tang, B. Ag-graphene/PEG composite phase change materials for enhancing solar thermal energy conversion and storage capacity. Appl. Energy 2019, 237, 83–90. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Fang, J.W.J. From anisotropic graphene aerogels to electron and photo-driven phase change composites. J. Mater. Chem. A 2016, 4, 17042. [Google Scholar] [CrossRef]
- Kalidasan, B.; Pandey, A.K.; Shahabuddin, S.; George, M.; Sharma, K.; Samykano, M.; Tyagi, V.V.; Saidur, R. Synthesis and characterization of conducting polyaniline@cobalt-paraffin wax nanocomposite as nano-phase change material: Enhanced thermophysical properties. Renew. Energy 2021, 173, 1057–1069. [Google Scholar]
- Zuo, X.; Li, J.; Zhao, X.; Yang, H.; Chen, D. Emerging paraffin/carbon-coated nanoscroll composite phase change material for thermal energy storage. Renew. Energy 2020, 152, 579–589. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, Y.; Zhao, R.; Cheng, W. A high heat storage capacity form-stable composite phase change material with enhanced flame retardancy. Appl. Energy 2020, 262, 114536. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, W.; Jiang, Z.; He, F.; Zhang, K.; He, R.; Wu, J.; Fan, J. Silicone rubber/paraffin@silicon dioxide form-stable phase change materials with thermal energy storage and enhanced mechanical property. Sol. Energy Mater. Sol. Cells 2019, 196, 16–24. [Google Scholar] [CrossRef]
- Fan, D.; Lu, Y.; Cao, Y.; Liu, J.; Lin, S.; Xiong, D.; Pan, Q. Phase change materials confined into sunlight capturer sponge towards thermal energy harvesting and storage. Sol. Energy 2021, 226, 147–153. [Google Scholar]
- Zuo, X.; Zhao, X.; Li, J.; Hu, Y.; Yang, H.; Chen, D. Enhanced thermal conductivity of form-stable composite phase-change materials with graphite hybridizing expanded perlite/paraffin. Sol. Energy 2020, 209, 85–95. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Sheng, D.; Lin, C.; Ji, F.; Dong, L.; Xu, S.; Wu, H.; Yang, Y. Polyurethane-based solid-solid phase change materials with in situ reduced graphene oxide for light-thermal energy conversion and storage. Chem. Eng. J. 2018, 338, 117–125. [Google Scholar] [CrossRef]
- Umair, M.; Zhang, Y.; Tehrim, A.; Zhang, S.; Tang, B. Form-stable phase-change composites supported by a biomass derived carbon scaffold with multiple energy conversion abilities. Ind. Eng. Chem. Res. 2020, 59, 1393–1401. [Google Scholar] [CrossRef]
- Cheng, G.; Wang, X.; He, Y. 3D graphene paraffin composites based on sponge skeleton for photo thermal conversion and energy storage. Appl. Therm. Eng. 2020, 178, 115560. [Google Scholar] [CrossRef]
- Cao, Y.; Fan, D.; Lin, S.; Ng, F.; Pan, Q. Branched alkylated polynorbornene and 3D flower-like MoS2 nanospheres reinforced phase change composites with high thermal energy storage capacity and photothermal conversion efficiency. Renew. Energy 2021, 179, 687–695. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, D.; Meng, Y.; Jiang, Y.; Qian, S.; Liu, J.; Xu, Y.; Xiong, D.; Cao, Y. Silver/Polypyrrole-Functionalized Polyurethane Foam Embedded Phase Change Materials for Thermal Energy Harvesting. Nanomaterials 2021, 11, 3011. https://doi.org/10.3390/nano11113011
Fan D, Meng Y, Jiang Y, Qian S, Liu J, Xu Y, Xiong D, Cao Y. Silver/Polypyrrole-Functionalized Polyurethane Foam Embedded Phase Change Materials for Thermal Energy Harvesting. Nanomaterials. 2021; 11(11):3011. https://doi.org/10.3390/nano11113011
Chicago/Turabian StyleFan, Dongli, Yuan Meng, Yuzhuo Jiang, Siyi Qian, Jie Liu, Yuzhi Xu, Dangsheng Xiong, and Yufeng Cao. 2021. "Silver/Polypyrrole-Functionalized Polyurethane Foam Embedded Phase Change Materials for Thermal Energy Harvesting" Nanomaterials 11, no. 11: 3011. https://doi.org/10.3390/nano11113011
APA StyleFan, D., Meng, Y., Jiang, Y., Qian, S., Liu, J., Xu, Y., Xiong, D., & Cao, Y. (2021). Silver/Polypyrrole-Functionalized Polyurethane Foam Embedded Phase Change Materials for Thermal Energy Harvesting. Nanomaterials, 11(11), 3011. https://doi.org/10.3390/nano11113011







