Fluorescent Biosensors Based on Silicon Nanowires
Abstract
:1. Introduction
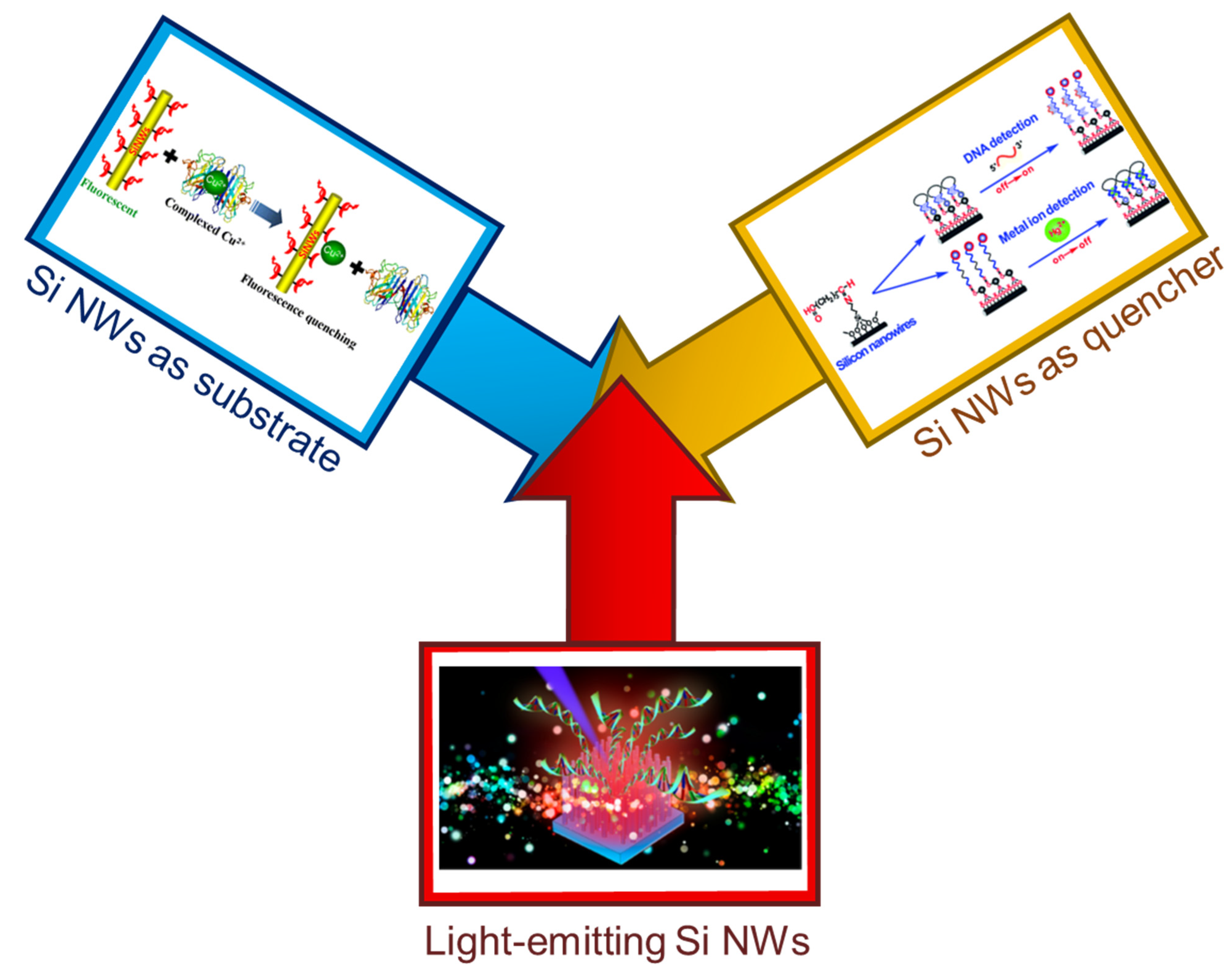
Silicon Nanowire Sensing Platform
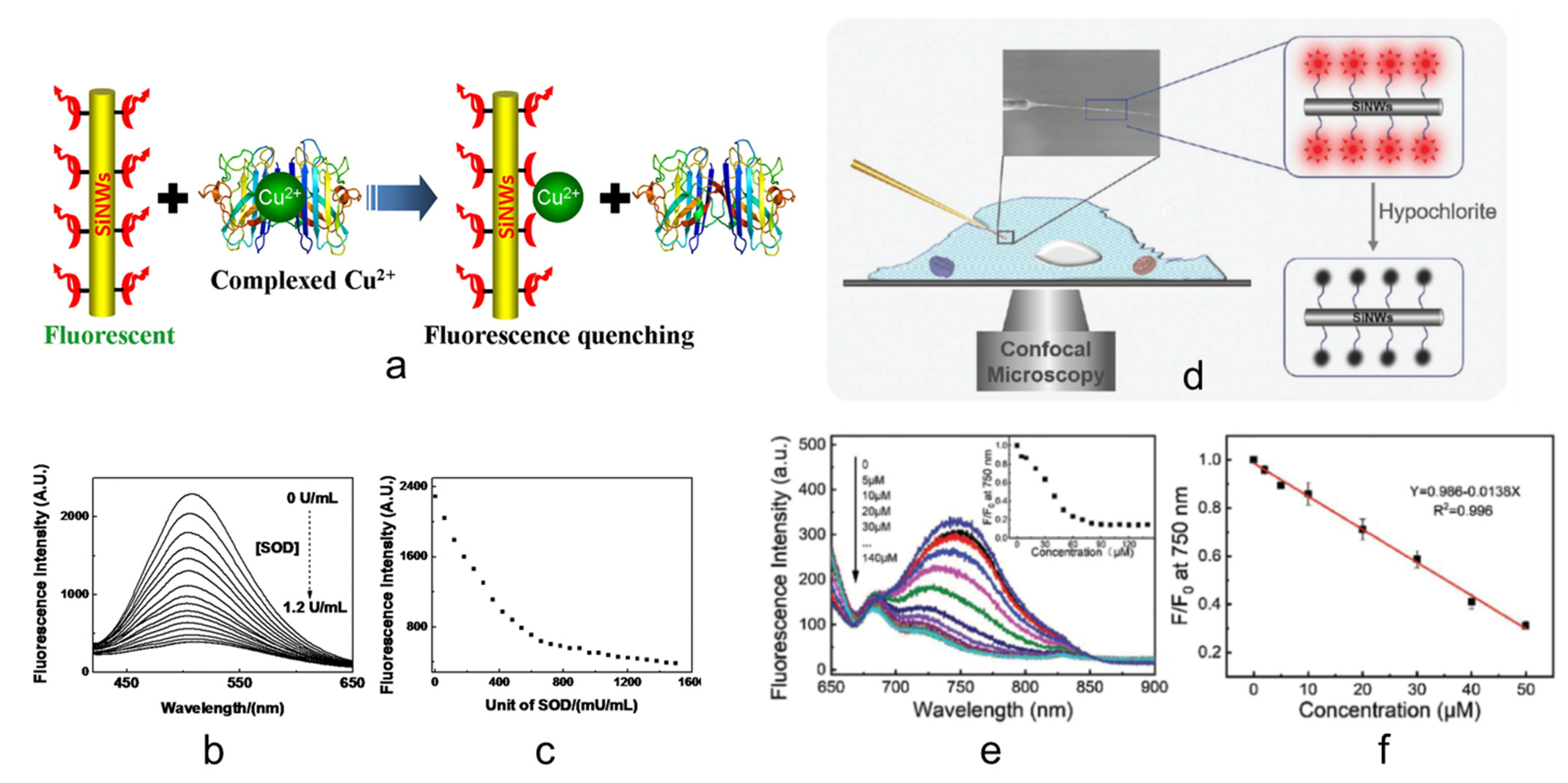
2. Fluorescent Sensors with Si NW as a Substrate
Systems for Macro- and Micro-Sensing
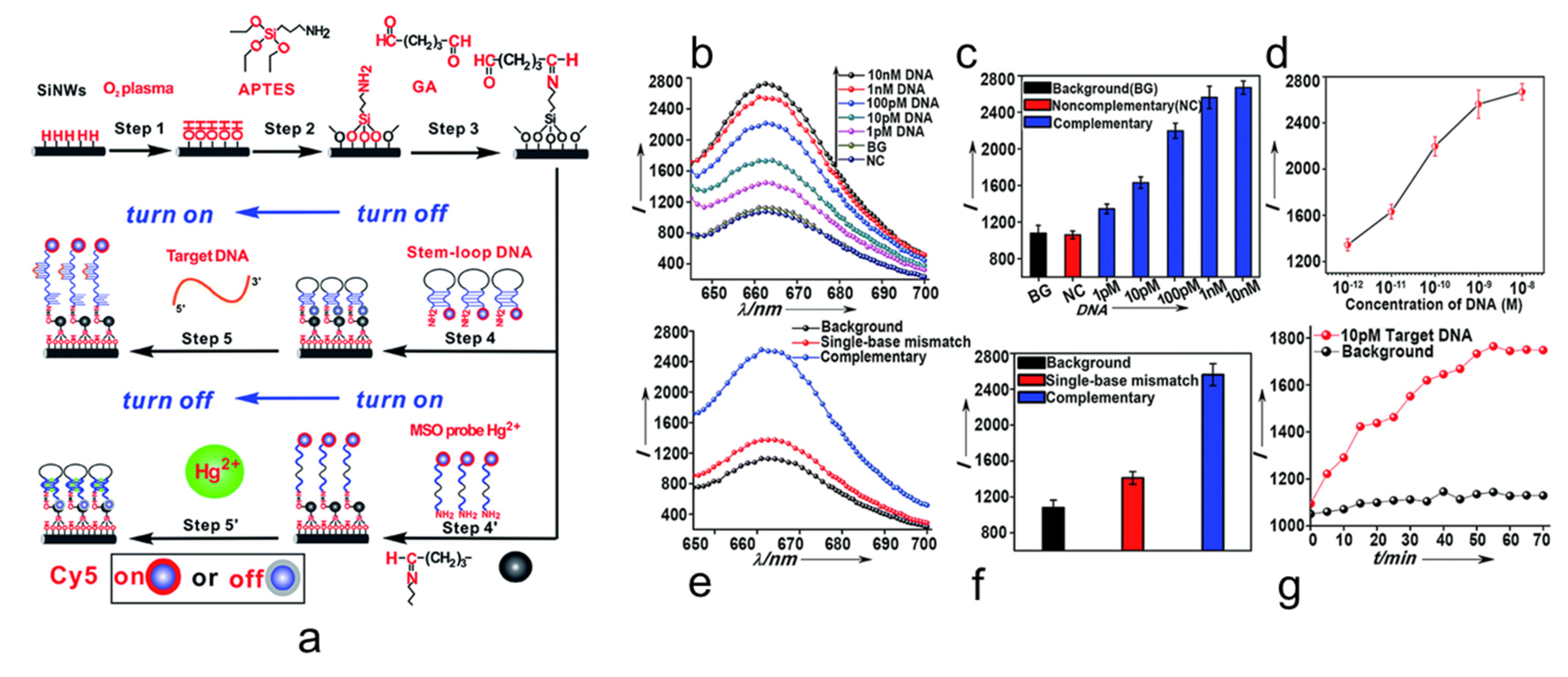
3. Fluorescent Sensors with Si NW as a Quencher
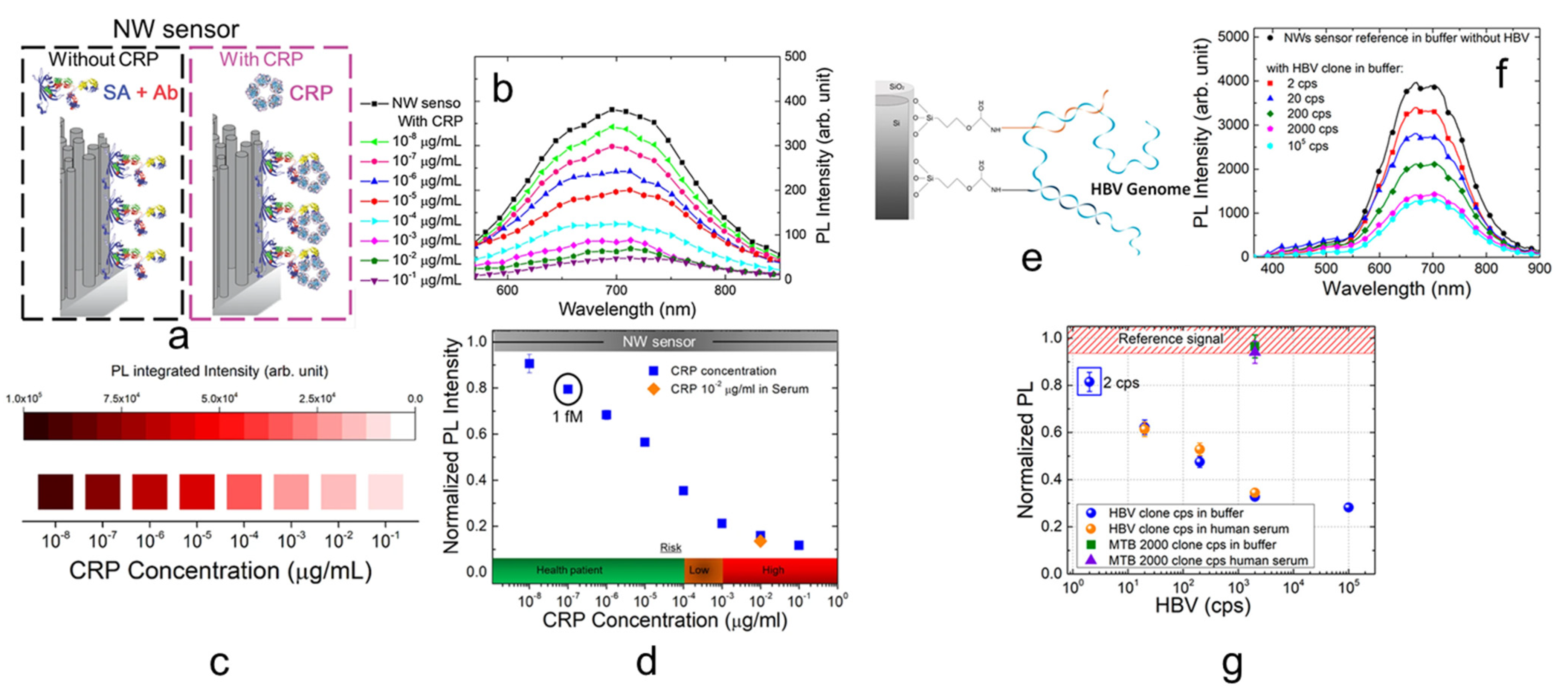
4. Fluorescent Si NW Sensors
5. Future Perspective
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mabey, D.; Peeling, R.W.; Ustianowski, A.; Perkins, M.D. Diagnostics for the Developing World. Nat. Rev. Microbiol. 2004, 2, 231–240. [Google Scholar] [CrossRef]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.Y.; Chen, J.; Guo, X.; et al. Characterization of MicroRNAs in Serum: A Novel Class of Biomarkers for Diagnosis of Cancer and Other Diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef] [Green Version]
- Morrow, D.A.; Braunwald, E. Future of Biomarkers in Acute Coronary Syndromes: Moving toward a Multimarker Strategy. Circulation 2003, 108, 250–252. [Google Scholar] [CrossRef] [Green Version]
- Jhala, N.; Jhala, D.; Vickers, S.M.; Eltoum, I.; Batra, S.K.; Manne, U.; Eloubeidi, M.; Jones, J.J.; Grizzle, W.E. Biomarkers in Diagnosis of Pancreatic Carcinoma in Fine-Needle Aspirates. Am. J. Clin. Pathol. 2006, 126, 572–579. [Google Scholar] [CrossRef]
- Fremont, R.D.; Koyama, T.; Calfee, C.S.; Wu, W.; Dossett, L.A.; Bossert, F.R.; Mitchell, D.; Wickersham, N.; Bernard, G.R.; Matthay, M.A.; et al. Acute Lung Injury in Patients with Traumatic Injuries: Utility of a Panel of Biomarkers for Diagnosis and Pathogenesis. J. Trauma Inj. Infect. Crit. Care 2010, 68, 1121–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuetz, P.; Aujesky, D.; Mueller, C.; Mueller, B. Biomarker-Guided Personalised Emergency Medicine for All-Hope for Another Hype? Swiss Med Wkly. 2015, 145, w14079. [Google Scholar] [CrossRef]
- Gouma, P.; Kalyanasundaram, K.; Bishop, A. Electrospun Single-Crystal MoO3 Nanowires for Biochemistry Sensing Probes. J. Mater. Res. 2020, 21, 2904–2910. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Song, J.; Liu, J.; Xu, N.; Wang, Z.L. Piezoelectric Field Effect Transistor and Nanoforce Sensor Based on a Single ZnO Nanowire. Nano Lett. 2006, 6, 2768–2772. [Google Scholar] [CrossRef]
- Lawrence, N.S.; Davis, J.; Jiang, L.; Jones, T.G.J.; Davies, S.N.; Compton, R.G. The Electrochemical Analog of the Methylene Blue Reaction: A Novel Amperometric Approach to the Detection of Hydrogen Sulfide. Electroanalysis 2000, 12, 1453–1460. [Google Scholar] [CrossRef]
- Dhanekar, S.; Sharma, I.; Islam, S.S. Optical Measurement of Trace Level Water Vapours Using Functionalized Porous Silicon: Selectivity Studies. RSC Adv. 2016, 6, 72371–72377. [Google Scholar] [CrossRef]
- Jiménez, D.; Martínez-Máñez, R.; Sancenón, F.; Ros-Lis, J.V.; Benito, A.; Soto, J. A New Chromo-Chemodosimeter Selective for Sulfide Anion. J. Am. Chem. Soc. 2003, 125, 9000–9001. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.G.; Cha, S.; Lee, H.; Jeon, H.L.; Chang, S.-K. Sulfide-Selective Chemosignaling by a Cu2+ Complex of Dipicolylamine Appended Fluorescein. Chem. Commun. 2009, 7390–7392. [Google Scholar] [CrossRef] [PubMed]
- Elghanian, R.; Storhoff, J.J.; Mucic, R.C.; Letsinger, R.L.; Mirkin, C.A. Selective Colorimetric Detection of Polynucleotides Based on the Distance-Dependent Optical Properties of Gold Nanoparticles. Science 1997, 277, 1078–1081. [Google Scholar] [CrossRef] [Green Version]
- Furne, J.; Saeed, A.; Levitt, M.D. Whole Tissue Hydrogen Sulfide Concentrations Are Orders of Magnitude Lower than Presently Accepted Values. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 295, 1479–1485. [Google Scholar] [CrossRef] [Green Version]
- Yi, Y.; Zhu, G.; Liu, C.; Huang, Y.; Zhang, Y.; Li, H.; Zhao, J.; Yao, S. A Label-Free Silicon Quantum Dots-Based Photoluminescence Sensor for Ultrasensitive Detection of Pesticides. Anal. Chem. 2013, 85, 11464–11470. [Google Scholar] [CrossRef]
- Barone, P.W.; Baik, S.; Heller, D.A.; Strano, M.S. Near-Infrared Optical Sensors Based on Single-Walled Carbon Nanotubes. Nat. Mater. 2004, 4, 86–92. [Google Scholar] [CrossRef]
- Kumar, N.; Dorfman, A.; Hahm, J. Ultrasensitive DNA Sequence Detection Using Nanoscale ZnO Sensor Arrays. Nanotechnology 2006, 17, 2875. [Google Scholar] [CrossRef]
- Wang, H.; Mu, L.; She, G.; Shi, W. Silicon Nanowires-Based Fluorescent Sensor for in Situ Detection of Hydrogen Sulfide in Extracellular Environment. RSC Adv. 2015, 5, 65905–65908. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of Hazardous Volatile Organic Compounds (VOCs) by Metal Oxide Nanostructures-Based Gas Sensors: A Review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Morganti, D.; Leonardi, A.A.; Faro, M.J.L.; Leonardi, G.; Salvato, G.; Fazio, B.; Musumeci, P.; Livreri, P.; Conoci, S.; Neri, G.; et al. Ultrathin Silicon Nanowires for Optical and Electrical Nitrogen Dioxide Detection. Nanomaterials 2021, 11, 1767. [Google Scholar] [CrossRef]
- Acha, N.D.; Elosúa, C.; Corres, J.M.; Arregui, F.J. Fluorescent Sensors for the Detection of Heavy Metal Ions in Aqueous Media. Sensors 2019, 19, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent Sensors for Measuring Metal Ions in Living Systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef]
- Domaille, D.W.; Que, E.L.; Chang, C.J. Synthetic Fluorescent Sensors for Studying the Cell Biology of Metals. Nat. Chem. Biol. 2008, 4, 168–175. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Malkovskiy, A.; Sun, S.; Pang, Y. Aggregation Control of Squaraines and Their Use as Near-Infrared Fluorescent Sensors for Protein. J. Phys. Chem. B 2010, 114, 8574–8580. [Google Scholar] [CrossRef] [PubMed]
- O’Banion, C.P.; Yasuda, R. Fluorescent Sensors for Neuronal Signaling. Curr. Opin. Neurobiol. 2020, 63, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Dai, X.; Zhao, S.; Cui, X.; Gong, T.; Song, Z.; Meng, H.; Zhang, X.; Yu, B. Aptamer-Based Fluorescent Sensors for the Detection of Cancer Biomarkers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119038. [Google Scholar] [CrossRef] [PubMed]
- Sparano, B.A.; Koide, K. Fluorescent Sensors for Specific RNA: A General Paradigm Using Chemistry and Combinatorial Biology. J. Am. Chem. Soc. 2007, 129, 4785–4794. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Wang, Z.; Xing, H.; Wong, N.Y.; Lu, Y. Label-Free Fluorescent Functional DNA Sensors Using Unmodified DNA: A Vacant Site Approach. Anal. Chem. 2010, 82, 4122–4129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurdekar, A.D.; Chunduri, L.A.A.; Manohar, C.S.; Haleyurgirisetty, M.K.; Hewlett, I.K.; Venkataramaniah, K. Streptavidin-Conjugated Gold Nanoclusters as Ultrasensitive Fluorescent Sensors for Early Diagnosis of HIV Infection. Sci. Adv. 2018, 4. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.-I.; Kim, H.; Choi, Y.; Kim, Y. A Fluorescent Turn-on Probe for the Detection of Alkaline Phosphatase Activity in Living Cells. Chem. Commun. 2011, 47, 9825–9827. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Zhao, S.; Gao, W.; Chen, B.; He, L.; Zhu, S. A Unique Approach to Development of Near-Infrared Fluorescent Sensors for in Vivo Imaging. J. Am. Chem. Soc. 2012, 134, 13510–13523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cheng, X.; Zhao, Y.; Yang, Y. Lighting Up Live-Cell and In Vivo Central Carbon Metabolism with Genetically Encoded Fluorescent Sensors. Annu. Rev. Anal. Chem. 2020, 13, 293–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Mu, L.; She, G.; Xu, H.; Shi, W. Fluorescent Biosensor for Alkaline Phosphatase Based on Fluorescein Derivatives Modified Silicon Nanowires. Sens. Actuators B Chem. 2014, 203, 774–781. [Google Scholar] [CrossRef]
- Cao, X.; Mu, L.; Chen, M.; Bu, C.; Liang, S.; She, G.; Shi, W. Single Silicon Nanowire-Based Fluorescent Sensor for Endogenous Hypochlorite in an Individual Cell. Adv. Biosyst. 2018, 2, 1800213. [Google Scholar] [CrossRef]
- Pansare, V.J.; Hejazi, S.; Faenza, W.J.; Prud’homme, R.K. Review of Long-Wavelength Optical and NIR Imaging Materials: Contrast Agents, Fluorophores, and Multifunctional Nano Carriers. Chem. Mater. 2012, 24, 812–827. [Google Scholar] [CrossRef] [Green Version]
- Alford, R.; Simpson, H.M.; Duberman, J.; Hill, G.C.; Ogawa, M.; Regino, C.; Kobayashi, H.; Choyke, P.L. Toxicity of Organic Fluorophores Used in Molecular Imaging: Literature Review. Mol. Imaging 2009, 8, 341–354. [Google Scholar] [CrossRef] [Green Version]
- Gan, S.D.; Patel, K.R. Enzyme Immunoassay and Enzyme-Linked Immunosorbent Assay. J. Investig. Dermatol. 2013, 133, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, H.; Jang, J. Conducting-Polymer Nanomaterials for High-Performance Sensor Applications: Issues and Challenges. Adv. Funct. Mater. 2009, 19, 1567–1576. [Google Scholar] [CrossRef]
- Xu, K.; Huang, J.; Ye, Z.; Ying, Y.; Li, Y. Recent Development of Nano-Materials Used in DNA Biosensors. Sensors 2009, 9, 5534–5557. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire Nanosensors for Highly Sensitive and Selective Detection of Biological and Chemical Species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef]
- Patolsky, F.; Zheng, G.; Lieber, C.M. Fabrication of Silicon Nanowire Devices for Ultrasensitive, Label-Free, Real-Time Detection of Biological and Chemical Species. Nat. Protoc. 2006, 1, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Alivisatos, P. The Use of Nanocrystals in Biological Detection. Nat. Biotechnol. 2003, 22, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S. In Vivo Cancer Targeting and Imaging with Semiconductor Quantum Dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum Dots for Live Cells, In Vivo Imaging, and Diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, R.; Willner, I. Optical Molecular Sensing with Semiconductor Quantum Dots (QDs). Chem. Soc. Rev. 2012, 41, 4067–4085. [Google Scholar] [CrossRef] [PubMed]
- Maity, D.; Manna, A.K.; Karthigeyan, D.; Kundu, T.K.; Pati, S.K.; Govindaraju, T. Visible–Near-Infrared and Fluorescent Copper Sensors Based on Julolidine Conjugates: Selective Detection and Fluorescence Imaging in Living Cells. Chem.—A Eur. J. 2011, 17, 11152–11161. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, Y.N.; Montes-Bayón, M.; Blanco-González, E.; Sanz-Medel, A. Quantitative Analysis and Simultaneous Activity Measurements of Cu, Zn-Superoxide Dismutase in Red Blood Cells by HPLC−ICPMS. Anal. Chem. 2010, 82, 2387–2394. [Google Scholar] [CrossRef]
- Ordóñez, Y.N.; Deitrich, C.L.; Montes-Bayón, M.; Blanco-González, E.; Feldmann, J.; Sanz-Medel, A. Species Specific Isotope Dilution versus Internal Standardization Strategies for the Determination of Cu, Zn-Superoxide Dismutase in Red Blood Cells. J. Anal. At. Spectrom. 2010, 26, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Dodani, S.C.; Leary, S.C.; Cobine, P.A.; Winge, D.R.; Chang, C.J. A Targetable Fluorescent Sensor Reveals That Copper-Deficient SCO1 and SCO2 Patient Cells Prioritize Mitochondrial Copper Homeostasis. J. Am. Chem. Soc. 2011, 133, 8606–8616. [Google Scholar] [CrossRef] [Green Version]
- Sapsford, K.; Pons, T.; Medintz, I.; Mattoussi, H. Biosensing with Luminescent Semiconductor Quantum Dots. Sensors 2006, 6, 925–953. [Google Scholar] [CrossRef] [Green Version]
- Lieber, C.M. Semiconductor Nanowires: A Platform for Nanoscience and Nanotechnology. MRS Bull. 2011, 36, 1052–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Yan, R.; Fardy, M. Semiconductor Nanowire: What’s Next? Nano Lett. 2010, 10, 1529–1536. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Da, P.; Xu, M.; Wu, H.; Zheng, G. Silicon Nanowires for Biosensing, Energy Storage, and Conversion. Adv. Mater. 2013, 25, 5177–5195. [Google Scholar] [CrossRef]
- Lopez, F.J.; Givan, U.; Connell, J.G.; Lauhon, L.J. Silicon Nanowire Polytypes: Identification by Raman Spectroscopy, Generation Mechanism, and Misfit Strain in Homostructures. ACS Nano 2011, 5, 8958–8966. [Google Scholar] [CrossRef]
- Mu, L.; Shi, W.; Chang, J.C.; Lee, S.T. Silicon Nanowires-Based Fluorescence Sensor for Cu(II). Nano Lett. 2007, 8, 104–109. [Google Scholar] [CrossRef]
- Chen, M.; Mu, L.; Wang, S.; Cao, X.; Liang, S.; Wang, Y.; She, G.; Yang, J.; Wang, Y.; Shi, W. A Single Silicon Nanowire-Based Ratiometric Biosensor for Ca2+ at Various Locations in a Neuron. ACS Chem. Neurosci. 2020, 11, 1283–1290. [Google Scholar] [CrossRef]
- Jung, Y.; Tong, L.; Tanaudommongkon, A.; Cheng, J.-X.; Yang, C. In Vitro and In Vivo Nonlinear Optical Imaging of Silicon Nanowires. Nano Lett. 2009, 9, 2440–2444. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Song, F.; Zhang, B.; Ning, H.; Cui, J.; Peng, X. Improving the Brightness and Photostability of NIR Fluorescent Silica Nanoparticles through Rational Fine-Tuning of the Covalent Encapsulation Methods. J. Mater. Chem. B 2017, 5, 5278–5283. [Google Scholar] [CrossRef]
- Svechkarev, D.; Mohs, A.M. Organic Fluorescent Dye-Based Nanomaterials: Advances in the Rational Design for Imaging and Sensing Applications. Curr. Med. Chem. 2019, 26, 4042. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Mu, L.; Zhang, H.; She, G.; Zhou, B.; Xu, H.; Wang, P.; Shi, W. Silicon Nanowire-Based Fluorescent Nanosensor for Complexed Cu2+ and Its Bioapplications. Nano Lett. 2014, 14, 3124–3129. [Google Scholar] [CrossRef]
- Song, S.; Liang, Z.; Zhang, J.; Wang, L.; Li, G.; Fan, C. Gold-Nanoparticle-Based Multicolor Nanobeacons for Sequence-Specific DNA Analysis. Angew. Chem. Int. Ed. 2009, 48, 8670–8674. [Google Scholar] [CrossRef]
- Wang, W.; Kong, T.; Zhang, D.; Zhang, J.; Cheng, G. Label-Free MicroRNA Detection Based on Fluorescence Quenching of Gold Nanoparticles with a Competitive Hybridization. Anal. Chem. 2015, 87, 10822–10829. [Google Scholar] [CrossRef]
- He, S.; Song, B.; Li, D.; Zhu, C.; Qi, W.; Wen, Y.; Wang, L.; Song, S.; Fang, H.; Fan, C. A Graphene Nanoprobe for Rapid, Sensitive, and Multicolor Fluorescent DNA Analysis. Adv. Funct. Mater. 2010, 20, 453–459. [Google Scholar] [CrossRef]
- Lu, C.-H.; Yang, H.-H.; Zhu, C.-L.; Chen, X.; Chen, G.-N. A Graphene Platform for Sensing Biomolecules. Angew. Chem. 2009, 121, 4879–4881. [Google Scholar] [CrossRef]
- Maxwell, D.J.; Taylor, J.R.; Nie, S. Self-Assembled Nanoparticle Probes for Recognition and Detection of Biomolecules. J. Am. Chem. Soc. 2002, 124, 9606–9612. [Google Scholar] [CrossRef]
- Schmidt, V.; Riel, H.; Senz, S.; Karg, S.; Riess, W.; Gösele, U. Realization of a Silicon Nanowire Vertical Surround-Gate Field-Effect Transistor. Small 2006, 2, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Nah, J.; Liu, E.S.; Shahrjerdi, D.; Varahramyan, K.M.; Banerjee, S.K.; Tutuc, E. Realization of Dual-Gated Ge- SixGe1−x Core-Shell Nanowire Field Effect Transistors with Highly Doped Source and Drain. Appl. Phys. Lett. 2009, 94, 063117. [Google Scholar] [CrossRef] [Green Version]
- Koo, S.M.; Edelstein, M.D.; Li, Q.; Richter, C.A.; Vogel, E.M. Silicon Nanowires as Enhancement-Mode Schottky Barrier Field-Effect Transistors. Nanotechnology 2005, 16, 1482–1485. [Google Scholar] [CrossRef]
- Walavalkar, S.S.; Hofmann, C.E.; Homyk, A.P.; Henry, M.D.; Atwater, H.A.; Scherer, A. Tunable Visible and Near-IR Emission from Sub-10 Nm Etched Single-Crystal Si Nanopillars. Nano Lett. 2010, 10, 4423–4428. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.A.; Nastasi, F.; Morganti, D.; Lo Faro, M.J.; Picca, R.A.; Cioffi, N.; Franzò, G.; Serroni, S.; Priolo, F.; Puntoriero, F.; et al. New Hybrid Light Harvesting Antenna Based on Silicon Nanowires and Metal Dendrimers. Adv. Opt. Mater. 2020, 2001070. [Google Scholar] [CrossRef]
- Lo Faro, M.J.; Ruello, G.; Leonardi, A.A.; Morganti, D.; Irrera, A.; Priolo, F.; Gigan, S.; Volpe, G.; Fazio, B. Visualization of Directional Beaming of Weakly Localized Raman from a Random Network of Silicon Nanowires. Adv. Sci. 2021, 2100139. [Google Scholar] [CrossRef]
- Lo Faro, M.J.; Leonardi, A.A.; Priolo, F.; Fazio, B.; Miritello, M.; Irrera, A. Erbium Emission in Er:Y2O3 Decorated Fractal Arrays of Silicon Nanowires. Sci. Rep. 2020, 10, 12854. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.-Q.; Lee, S.-T. Silicon Nanowires for Photovoltaic Solar Energy Conversion. Adv. Mater. 2011, 23, 198–215. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Misra, S.; Wang, J.; Qian, S.; Foldyna, M.; Xu, J.; Shi, Y.; Johnson, E.; Cabarrocas, P.R.I. Understanding Light Harvesting in Radial Junction Amorphous Silicon Thin Film Solar Cells. Sci. Rep. 2014, 4, 4357. [Google Scholar] [CrossRef]
- Kempa, T.J.; Cahoon, J.F.; Kim, S.K.; Day, R.W.; Bell, D.C.; Park, H.G.; Lieber, C.M. Coaxial Multishell Nanowires with High-Quality Electronic Interfaces and Tunable Optical Cavities for Ultrathin Photovoltaics. Proc. Natl. Acad. Sci. USA 2012, 109, 1407–1412. [Google Scholar] [CrossRef] [Green Version]
- Lo Faro, M.J.; Leonardi, A.A.; Morganti, D.; Fazio, B.; Vasi, C.; Musumeci, P.; Priolo, F.; Irrera, A. Low Cost Fabrication of Si NWs/CuI Heterostructures. Nanomaterials 2018, 8, 569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, M.; Su, S.; He, Y.; Huang, Q.; Hu, W.; Li, D.; Fan, C.; Lee, S.-T. Long-Term Antimicrobial Effect of Silicon Nanowires Decorated with Silver Nanoparticles. Adv. Mater. 2010, 22, 5463–5467. [Google Scholar] [CrossRef]
- He, Y.; Su, S.; Xu, T.; Zhong, Y.; Zapien, J.A.; Li, J.; Fan, C.; Lee, S.T. Silicon Nanowires-Based Highly-Efficient SERS-Active Platform for Ultrasensitive DNA Detection. Nano Today 2011, 6, 122–130. [Google Scholar] [CrossRef]
- He, Y.; Zhong, Y.; Peng, F.; Wei, X.; Su, Y.; Su, S.; Gu, W.; Liao, L.; Lee, S.-T. Highly Luminescent Water-Dispersible Silicon Nanowires for Long-Term Immunofluorescent Cellular Imaging. Angew. Chem. Int. Ed. 2011, 50, 3080–3083. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Ng, J.K.; Kunitake, M.E.; Conklin, B.R.; Yang, P. Interfacing Silicon Nanowires with Mammalian Cells. J. Am. Chem. Soc. 2007, 129, 7228–7229. [Google Scholar] [CrossRef]
- He, Y.; Zhong, Y.; Peng, F.; Wei, X.; Su, Y.; Lu, Y.; Su, S.; Gu, W.; Liao, L.; Lee, S.-T. One-Pot Microwave Synthesis of Water-Dispersible, Ultraphoto- and PH-Stable, and Highly Fluorescent Silicon Quantum Dots. J. Am. Chem. Soc. 2011, 133, 14192–14195. [Google Scholar] [CrossRef]
- Ding, Z.; Quinn, B.M.; Haram, S.K.; Pell, L.E.; Korgel, B.A.; Bard, A.J. Electrochemistry and Electrogenerated Chemiluminescence from Silicon Nanocrystal Quantum Dots. Science 2002, 296, 1293–1297. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Su, Y.; Yang, X.; Kang, Z.; Xu, T.; Zhang, R.; Fan, C.; Lee, S.-T. Photo and PH Stable, Highly-Luminescent Silicon Nanospheres and Their Bioconjugates for Immunofluorescent Cell Imaging. J. Am. Chem. Soc. 2009, 131, 4434–4438. [Google Scholar] [CrossRef]
- He, Y.; Kang, Z.-H.; Li, Q.-S.; Tsang, C.H.A.; Fan, C.-H.; Lee, S.-T. Ultrastable, Highly Fluorescent, and Water-Dispersed Silicon-Based Nanospheres as Cellular Probes. Angew. Chem. Int. Ed. 2009, 48, 128–132. [Google Scholar] [CrossRef]
- Irrera, A.; Magazzù, A.; Artoni, P.; Simpson, S.H.; Hanna, S.; Jones, P.H.; Priolo, F.; Gucciardi, P.G.; Maragò, O.M. Photonic Torque Microscopy of the Nonconservative Force Field for Optically Trapped Silicon Nanowires. Nano Lett. 2016, 16, 4181–4188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; von dem Bussche, A.; Hurt, R.H.; Kane, A.B.; Gao, H. Cell Entry of One-Dimensional Nanomaterials Occurs by Tip Recognition and Rotation. Nat. Nanotechnol. 2011, 6, 714–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kam, N.W.S.; Liu, Z.; Dai, H. Carbon Nanotubes as Intracellular Transporters for Proteins and DNA: An Investigation of the Uptake Mechanism and Pathway. Angew. Chem. Int. Ed. 2006, 45, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Yi, C.; Ji, S.; Fong, C.-C.; Yang, M. Cell Adhesion and Spreading Behavior on Vertically Aligned Silicon Nanowire Arrays. ACS Appl. Mater. Interfaces 2008, 1, 30–34. [Google Scholar] [CrossRef]
- Myndrul, V.; Iatsunskyi, I. Nanosilicon-Based Composites for (Bio)Sensing Applications: Current Status, Advantages, and Perspectives. Materials 2019, 12, 2880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-H.; Heller, D.A.; Jin, H.; Barone, P.W.; Song, C.; Zhang, J.; Trudel, L.J.; Wogan, G.N.; Tannenbaum, S.R.; Strano, M.S. The Rational Design of Nitric Oxide Selectivity in Single-Walled Carbon Nanotube near-Infrared Fluorescence Sensors for Biological Detection. Nat. Chem. 2009, 1, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Miao, R.; Mu, L.; Zhang, H.; Xu, H.; She, G.; Wang, P.; Shi, W. Modified Silicon Nanowires: A Fluorescent Nitric Oxide Biosensor with Enhanced Selectivity and Stability. J. Mater. Chem. 2012, 22, 3348–3353. [Google Scholar] [CrossRef]
- Na, Y.-R.; Kim, S.Y.; Gaublomme, J.T.; Shalek, A.K.; Jorgolli, M.; Park, H.; Yang, E.G. Probing Enzymatic Activity inside Living Cells Using a Nanowire–Cell “Sandwich” Assay. Nano Lett. 2012, 13, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Shalek, A.K.; Robinson, J.T.; Karp, E.S.; Lee, J.S.; Ahn, D.-R.; Yoon, M.-H.; Sutton, A.; Jorgolli, M.; Gertner, R.S.; Gujral, T.S.; et al. Vertical Silicon Nanowires as a Universal Platform for Delivering Biomolecules into Living Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 1870–1875. [Google Scholar] [CrossRef] [Green Version]
- Shalek, A.K.; Gaublomme, J.T.; Wang, L.; Yosef, N.; Chevrier, N.; Andersen, M.S.; Robinson, J.T.; Pochet, N.; Neuberg, D.; Gertner, R.S.; et al. Nanowire-Mediated Delivery Enables Functional Interrogation of Primary Immune Cells: Application to the Analysis of Chronic Lymphocytic Leukemia. Nano Lett. 2012, 12, 6498–6504. [Google Scholar] [CrossRef]
- Robinson, J.T.; Jorgolli, M.; Shalek, A.K.; Yoon, M.-H.; Gertner, R.S.; Park, H. Vertical Nanowire Electrode Arrays as a Scalable Platform for Intracellular Interfacing to Neuronal Circuits. Nat. Nanotechnol. 2012, 7, 180–184. [Google Scholar] [CrossRef] [Green Version]
- Su, S.; Wei, X.; Zhong, Y.; Guo, Y.; Su, Y.; Huang, Q.; Lee, S.-T.; Fan, C.; He, Y. Silicon Nanowire-Based Molecular Beacons for High-Sensitivity and Sequence-Specific DNA Multiplexed Analysis. ACS Nano 2012, 6, 2582–2590. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, X.; Zhong, Y.; Lu, Y.; Wang, S.; Wei, X.; Su, Y.; He, Y. Stem-Loop DNA-Assisted Silicon Nanowires-Based Biochemical Sensors with Ultra-High Sensitivity, Specificity, and Multiplexing Capability. Nanoscale 2014, 6, 9215–9222. [Google Scholar] [CrossRef]
- Li, J.; He, G.; Ueno, H.; Jia, C.; Noji, H.; Qi, C.; Guo, X. Direct Real-Time Detection of Single Proteins Using Silicon Nanowire-Based Electrical Circuits. Nanoscale 2016, 8, 16172–16176. [Google Scholar] [CrossRef]
- Patolsky, F.; Zheng, G.; Lieber, C.M. Nanowire Sensors for Medicine and the Life Sciences. Nanomedicine 2006, 1, 51–65. [Google Scholar] [CrossRef] [Green Version]
- Leonardi, A.A.; Lo Faro, M.J.; Irrera, A. Biosensing Platforms Based on Silicon Nanostructures: A Critical Review. Anal. Chim. Acta 2021, 338393. [Google Scholar] [CrossRef]
- Irrera, A.; Leonardi, A.A.; di Franco, C.; lo Faro, M.J.; Palazzo, G.; D’Andrea, C.; Manoli, K.; Franzò, G.; Musumeci, P.; Fazio, B.; et al. New Generation of Ultrasensitive Label-Free Optical Si Nanowire-Based Biosensors. ACS Photonics 2018, 5, 471–479. [Google Scholar] [CrossRef]
- Leonardi, A.A.; lo Faro, M.J.; Petralia, S.; Fazio, B.; Musumeci, P.; Conoci, S.; Irrera, A.; Priolo, F. Ultrasensitive Label- and PCR-Free Genome Detection Based on Cooperative Hybridization of Silicon Nanowires Optical Biosensors. ACS Sens. 2018, 3, 1690–1697. [Google Scholar] [CrossRef]
- Leonardi, A.A.; Lo Faro, M.J.; Di Franco, C.; Palazzo, G.; D’Andrea, C.; Morganti, D.; Manoli, K.; Musumeci, P.; Fazio, B.; Lanza, M.; et al. Silicon Nanowire Luminescent Sensor for Cardiovascular Risk in Saliva. J. Mater. Sci. Mater. Electron. 2020, 31, 10–17. [Google Scholar] [CrossRef]
- Linder, M.C.; Hazegh-Azam, M. Copper Biochemistry and Molecular Biology. Am. J. Clin. Nutr. 1996, 63, 797S–811S. [Google Scholar] [CrossRef]
- Rae, T.D.; Schmidt, P.J.; Pufahl, R.A.; Culotta, V.C.; O’Halloran, T.V. Undetectable Intracellular Free Copper: The Requirement of a Copper Chaperone for Superoxide Dismutase. Science 1999, 284, 805–808. [Google Scholar] [CrossRef] [Green Version]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative Diseases and Oxidative Stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Kim, B.E.; Turski, M.L.; Nose, Y.; Casad, M.; Rockman, H.A.; Thiele, D.J. Cardiac Copper Deficiency Activates a Systemic Signaling Mechanism That Communicates with the Copper Acquisition and Storage Organs. Cell Metab. 2010, 11, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.C.; Gray, H.B.; Winkler, J.R. Copper(II) Binding to α-Synuclein, the Parkinson’s Protein. J. Am. Chem. Soc. 2008, 130, 6898–6899. [Google Scholar] [CrossRef] [Green Version]
- Collins, J.F.; Prohaska, J.R.; Knutson, M.D. Metabolic Crossroads of Iron and Copper. Nutr. Rev. 2010, 68, 133–147. [Google Scholar] [CrossRef] [Green Version]
- Banci, L.; Bertini, I.; Ciofi-Baffoni, S.; Kozyreva, T.; Zovo, K.; Palumaa, P. Affinity Gradients Drive Copper to Cellular Destinations. Nature 2010, 465, 645–648. [Google Scholar] [CrossRef]
- Etienne, M.; Walcarius, A. Analytical Investigation of the Chemical Reactivity and Stability of Aminopropyl-Grafted Silica in Aqueous Medium. Talanta 2003, 59, 1173–1188. [Google Scholar] [CrossRef]
- Yang, J.K.; Davis, A.P. Competitive Adsorption of Cu(II)–EDTA and Cd(II)–EDTA onto TiO2. J. Colloid Interface Sci. 1999, 216, 77–85. [Google Scholar] [CrossRef]
- Kabil, O.; Banerjee, R. Redox Biochemistry of Hydrogen Sulfide. J. Biol. Chem. 2010, 285, 21903–21907. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, C.; Navarra, P.; Preziosi, P. Roles of Nitric Oxide, Carbon Monoxide, and Hydrogen Sulfide in the Regulation of the Hypothalamic–Pituitary–Adrenal Axis. J. Neurochem. 2010, 113, 563–575. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Buga, G.M.; Wood, K.S.; Byrns, R.E.; Chaudhuri, G. Endothelium-Derived Relaxing Factor Produced and Released from Artery and Vein Is Nitric Oxide. Proc. Natl. Acad. Sci. USA 1987, 84, 9265–9269. [Google Scholar] [CrossRef] [Green Version]
- Palmer, R.M.J.; Ferrige, A.G.; Moncada, S. Nitric Oxide Release Accounts for the Biological Activity of Endothelium-Derived Relaxing Factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef]
- Ooi, K.; Shiraki, K.; Morishita, Y.; Nobori, T. High-Molecular Intestinal Alkaline Phosphatase in Chronic Liver Diseases. J. Clin. Lab. Anal. 2007, 21, 133–139. [Google Scholar] [CrossRef]
- Gyurcsányi, R.E.; Bereczki, A.; Nagy, G.; Neuman, M.R.; Lindner, E. Amperometric Microcells for Alkaline Phosphatase Assay. Analyst 2002, 127, 235–240. [Google Scholar] [CrossRef]
- Koide, Y.; Urano, Y.; Kenmoku, S.; Kojima, H.; Nagano, T. Design and Synthesis of Fluorescent Probes for Selective Detection of Highly Reactive Oxygen Species in Mitochondria of Living Cells. J. Am. Chem. Soc. 2007, 129, 10324–10325. [Google Scholar] [CrossRef]
- Chen, P.; Zheng, Z.; Zhu, Y.; Dong, Y.; Wang, F.; Liang, G. Bioluminescent Turn-On Probe for Sensing Hypochlorite in Vitro and in Tumors. Anal. Chem. 2017, 89, 5693–5696. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, J.; Hilderbrand, S.A.; Waterman, P.; Heinecke, J.W.; Weissleder, R.; Libby, P. A Fluorescent Probe for the Detection of Myeloperoxidase Activity in Atherosclerosis-Associated Macrophages. Chem. Biol. 2007, 14, 1221–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, B.; Ren, H.; Lv, X.; Zhao, Y.; Yu, B.; He, Y.; Ma, Y.; Niu, C.; Kong, J.; Yu, F.; et al. Hypochlorite-Induced Oxidative Stress Elevates the Capability of HDL in Promoting Breast Cancer Metastasis. J. Transl. Med. 2012, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbeck, M.J.; Nesti, L.J.; Sharkey, P.F.; Parvizi, J. Myeloperoxidase and Chlorinated Peptides in Osteoarthritis: Potential Biomarkers of the Disease. J. Orthop. Res. 2007, 25, 1128–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sand, C.; Peters, S.L.; Pfaffendorf, M.; Zwieten, P.A. Van Effects of Hypochlorite and Hydrogen Peroxide on Cardiac Autonomic Receptors and Vascular Endothelial Function. Clin. Exp. Pharmacol. Physiol. 2003, 30, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Oushiki, D.; Kojima, H.; Terai, T.; Arita, M.; Hanaoka, K.; Urano, Y.; Nagano, T. Development and Application of a Near-Infrared Fluorescence Probe for Oxidative Stress Based on Differential Reactivity of Linked Cyanine Dyes. J. Am. Chem. Soc. 2010, 132, 2795–2801. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Yang, Y.; Chen, H. A Unique Class of Near-Infrared Functional Fluorescent Dyes with Carboxylic-Acid-Modulated Fluorescence ON/OFF Switching: Rational Design, Synthesis, Optical Properties, Theoretical Calculations, and Applications for Fluorescence Imaging in Living Animals. J. Am. Chem. Soc. 2012, 134, 1200–1211. [Google Scholar] [CrossRef]
- Panizzi, P.; Nahrendorf, M.; Wildgruber, M.; Waterman, P.; Figueiredo, J.-L.; Aikawa, E.; McCarthy, J.; Weissleder, R.; Hilderbrand, S.A. Oxazine Conjugated Nanoparticle Detects in Vivo Hypochlorous Acid and Peroxynitrite Generation. J. Am. Chem. Soc. 2009, 131, 15739–15744. [Google Scholar] [CrossRef] [Green Version]
- Stringer, C.; Pachitariu, M. Computational Processing of Neural Recordings from Calcium Imaging Data. Curr. Opin. Neurobiol. 2019, 55, 22–31. [Google Scholar] [CrossRef]
- Heredia, D.J.; Feng, C.-Y.; Agarwal, A.; Nennecker, K.; Hennig, G.W.; Gould, T.W. Postnatal Restriction of Activity-Induced Ca2+ Responses to Schwann Cells at the Neuromuscular Junction Are Caused by the Proximo-Distal Loss of Axonal Synaptic Vesicles during Development. J. Neurosci. 2018, 38, 8650–8665. [Google Scholar] [CrossRef]
- Tyagi, S.; Kramer, F.R. Molecular Beacons in Diagnostics. F1000 Med. Rep. 2012, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Farrell, R.E. Quantitative PCR Techniques. In RNA Methodologies; Academic Press: Cambridge, MA, USA, 2017; pp. 283–328. ISBN 978-0-12-804678-4. [Google Scholar]
- Song, S.; Qin, Y.; He, Y.; Huang, Q.; Fan, C.; Chen, H.-Y. Functional Nanoprobes for Ultrasensitive Detection of Biomolecules. Chem. Soc. Rev. 2010, 39, 4234–4243. [Google Scholar] [CrossRef]
- He, Y.; Fan, C.; Lee, S.T. Silicon Nanostructures for Bioapplications. Nano Today 2010, 5, 282–295. [Google Scholar] [CrossRef]
- Yang, R.; Jin, J.; Chen, Y.; Shao, N.; Kang, H.; Xiao, Z.; Tang, Z.; Wu, Y.; Zhu, Z.; Tan, W. Carbon Nanotube-Quenched Fluorescent Oligonucleotides: Probes That Fluoresce upon Hybridization. J. Am. Chem. Soc. 2008, 130, 8351–8358. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Kramer, F.R. Molecular Beacons: Probes That Fluoresce upon Hybridization. Nat. Biotechnol. 1996, 14, 303–308. [Google Scholar] [CrossRef]
- Sidransky, D. Nucleic Acid-Based Methods for the Detection of Cancer. Science 1997, 278, 1054–1058. [Google Scholar] [CrossRef]
- Yokota, J. Tumor Progression and Metastasis. Carcinogenesis 2000, 21, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Ko, F.H.; Yang, Y.S.; Hsia, D.L.; Lee, B.S.; Su, T.S. Label-Free Biosensing of a Gene Mutation Using a Silicon Nanowire Field-Effect Transistor. Biosens. Bioelectron. 2009, 25, 820–825. [Google Scholar] [CrossRef]
- Miyake, Y.; Togashi, H.; Tashiro, M.; Yamaguchi, H.; Oda, S.; Kudo, M.; Tanaka, Y.; Kondo, Y.; Sawa, R.; Fujimoto, T.; et al. MercuryII-Mediated Formation of Thymine−HgII−Thymine Base Pairs in DNA Duplexes. J. Am. Chem. Soc. 2006, 128, 2172–2173. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Su, Y.; Li, J.; Li, D.; Zhang, J.; Song, S.; Zhao, Y.; Li, G.; Fan, C. Highly Sensitive Electrochemical Sensor for Mercury(II) Ions by Using a Mercury-Specific Oligonucleotide Probe and Gold Nanoparticle-Based Amplification. Anal. Chem. 2009, 81, 7660–7666. [Google Scholar] [CrossRef]
- Liu, X.; Tang, Y.; Wang, L.; Zhang, J.; Song, S.; Fan, C.; Wang, S. Optical Detection of Mercury(II) in Aqueous Solutions by Using Conjugated Polymers and Label-Free Oligonucleotides. Adv. Mater. 2007, 19, 1471–1474. [Google Scholar] [CrossRef]
- Leonardi, A.A.; Lo Faro, M.J.; Irrera, A. Silicon Nanowires Synthesis by Metal-Assisted Chemical Etching: A Review. Nanomaterials 2021, 11, 383. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wei, X.; Peng, F.; Zhong, Y.; Lu, Y.; Su, S.; Xu, T.; Lee, S.-T.; He, Y. Gold Nanoparticles-Decorated Silicon Nanowires as Highly Efficient Near-Infrared Hyperthermia Agents for Cancer Cells Destruction. Nano Lett. 2012, 12, 1845–1850. [Google Scholar] [CrossRef]
- Peng, F.; Su, Y.; Wei, X.; Lu, Y.; Zhou, Y.; Zhong, Y.; Lee, S.-T.; He, Y. Silicon-Nanowire-Based Nanocarriers with Ultrahigh Drug-Loading Capacity for In Vitro and In Vivo Cancer Therapy. Angew. Chem. Int. Ed. 2013, 52, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Dohnalová, K.; Poddubny, A.N.; Prokofiev, A.A.; De Boer, W.D.; Umesh, C.P.; Paulusse, J.M.; Zuilhof, H.; Gregorkiewicz, T. Surface Brightens up Si Quantum Dots: Direct Bandgap-like Size-Tunable Emission. Light Sci. Appl. 2013, 2, e47. [Google Scholar] [CrossRef]
- Timmerman, D.; Izeddin, I.; Gregorkiewicz, T. Saturation of Luminescence from Si Nanocrystals Embedded in SiO2. Phys. Status Solidi (a) 2010, 207, 183–187. [Google Scholar] [CrossRef]
- Leonardi, A.A.; Lo Faro, M.J.; Irrera, A. CMOS-Compatible and Low-Cost Thin Film MACE Approach for Light-Emitting Si NWs Fabrication. Nanomaterials 2020, 10, 966. [Google Scholar] [CrossRef]
- Leonardi, A.A.; Battaglia, R.; Morganti, D.; Faro, M.J.L.; Fazio, B.; Pascali, C.d.; Francioso, L.; Palazzo, G.; Mallardi, A.; Purrello, M.; et al. A Novel Silicon Platform for Selective Isolation, Quantification, and Molecular Analysis of Small Extracellular Vesicles. Int. J. Nanomed. 2021, 16, 5153–5165. [Google Scholar] [CrossRef]
- Eckersall, P.D.; Conner, J.G.; Harvie, J. An Immunoturbidimetric Assay for Canine C-Reactive Protein. Vet. Res. Commun. 1991, 15, 17–24. [Google Scholar] [CrossRef]
- Collaboration, T.E.R.F. C-Reactive Protein, Fibrinogen, and Cardiovascular Disease Prediction. N. Engl. J. Med. 2012, 367, 1310–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Punyadeera, C.; Dimeski, G.; Kostner, K.; Beyerlein, P.; Cooper-White, J. One-Step Homogeneous C-Reactive Protein Assay for Saliva. J. Immunol. Methods 2011, 373, 19–25. [Google Scholar] [CrossRef]
- Chan, S.; Fauchet, P.M.; Li, Y.; Rothberg, L.J.; Miller, B.L. Porous Silicon Microcavities for Biosensing Applications. Phys. Status Solidi A 2000, 182, 541–546. [Google Scholar] [CrossRef]
- Shekhar Bandhavkar, S.; Bandhavkar, S. Developing Strategies for Early Detection of Hepatitis B Infection. Clin. Microbiol. 2016, 5, 234–236. [Google Scholar] [CrossRef]
- Gitlin, N. Hepatitis B: Diagnosis, Prevention, and Treatment. Clin. Chem. 1997, 43, 1500–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Probe | Target | Matrix | LOD | Refs. |
|---|---|---|---|---|
| N-(quinoline-8-yl)-2-(3-triethoxysilyl-propylamino)-acetamide (QlOEt) | Cu2+ | Buffer | 10 nM | [55] |
| 3-[2-(2-aminoethylamino)-ethylamino] propyl-trimethoxysilane (3-A) as receptor and a dansyl group (D) as a fluorophore | Cu2+ | Buffer, liver extract, cell culture | 30 nM | [60] |
| Naphthalimide azide derivative | H2S | Buffer, cell culture | mM | [18] |
| reduced-fluoresceamine | NO | Buffer, liver extract, cell culture | mM | [91] |
| reduced-fluoresceamine | alkaline phosphatase (ALP) | Buffer, cell culture | 0.0175 U/mL | [33] |
| IR780 | HClO | Buffer, cell culture | µM | [34] |
| Ratiometric detection with ruthenium-based dye as the reference molecule and Fluo-3 as the probe | Ca2+ | Buffer, cell culture | - | [56] |
| Probe | Target | Matrix | LOD | Ref. |
|---|---|---|---|---|
| DNA probe–FAM | Complementary DNA–tumor suppressor genes (p16, p21, p53) | Buffer | 50 pM | [96] |
| DNA probe–CY5 | Complementary DNA–tumor suppressor genes (p16, p21, p53) | Buffer | 50 pM | [96] |
| DNA probe–ROX | Complementary DNA–tumor suppressor genes (p16, p21, p53) | Buffer | 50 pM | [96] |
| DNA probe–FAM | Complementary DNA–tumor suppressor genes (p16, p21, p53) | Buffer | 10 pM | [97] |
| DNA probe–ROX | Complementary DNA–tumor suppressor genes (p16, p21, p53) | Buffer | 10 pM | [97] |
| DNA probe–CY5 | Complementary DNA–tumor suppressor genes (p16, p21, p53) | Buffer | 10 pM | [97] |
| T-rich mercury-specific oligonucleotide (MSO) tagged with Cy5 | Mg2+ | Buffer | 5 pM | [97] |
| Probe | Target | Matrix | LOD | Refs. |
|---|---|---|---|---|
| Antibody | C-reactive protein | Buffer/Serum | fM | [101] |
| Primer | Hepatitis B Virus (DNA) | Buffer/Serum | 2–20 copies | [102] |
| Antibody | Small extracellular vesicles | Buffer | 105 Ex/mL | [148] |
| Sensor | Target | Expertise Required | Average LOD | Note | Ref. |
|---|---|---|---|---|---|
| Si NWs as a substrate | Metal ions, endogenous gases | Low in macro/High in micro | µM-nM | Can be used for cell imaging | Table 1 |
| Si NW nanoMBs | DNAs | High | nM-pM | Distinguish a single base mismatch | Table 2 |
| Light-emitting Si NWs | Proteins, DNAs, vesicles | Medium | fM for protein, few DNA cps | Does not need other fluoroscent molecules | Table 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonardi, A.A.; Lo Faro, M.J.; Fazio, B.; Spinella, C.; Conoci, S.; Livreri, P.; Irrera, A. Fluorescent Biosensors Based on Silicon Nanowires. Nanomaterials 2021, 11, 2970. https://doi.org/10.3390/nano11112970
Leonardi AA, Lo Faro MJ, Fazio B, Spinella C, Conoci S, Livreri P, Irrera A. Fluorescent Biosensors Based on Silicon Nanowires. Nanomaterials. 2021; 11(11):2970. https://doi.org/10.3390/nano11112970
Chicago/Turabian StyleLeonardi, Antonio Alessio, Maria José Lo Faro, Barbara Fazio, Corrado Spinella, Sabrina Conoci, Patrizia Livreri, and Alessia Irrera. 2021. "Fluorescent Biosensors Based on Silicon Nanowires" Nanomaterials 11, no. 11: 2970. https://doi.org/10.3390/nano11112970
APA StyleLeonardi, A. A., Lo Faro, M. J., Fazio, B., Spinella, C., Conoci, S., Livreri, P., & Irrera, A. (2021). Fluorescent Biosensors Based on Silicon Nanowires. Nanomaterials, 11(11), 2970. https://doi.org/10.3390/nano11112970











