Ga2O3(Sn) Oxides for High-Temperature Gas Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Materials Characterization
3. Results and Discussion
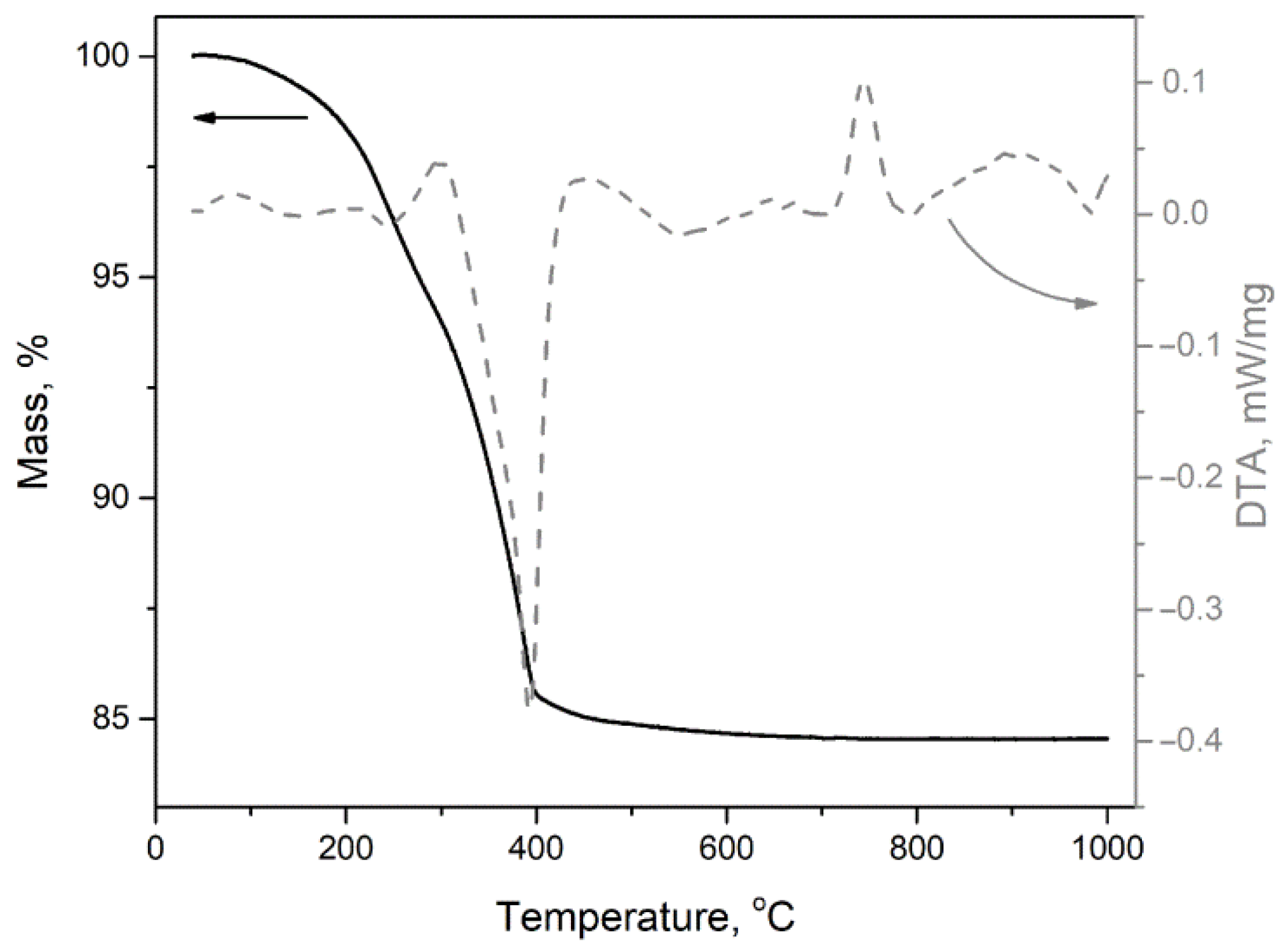
3.1. Precursor Characterization
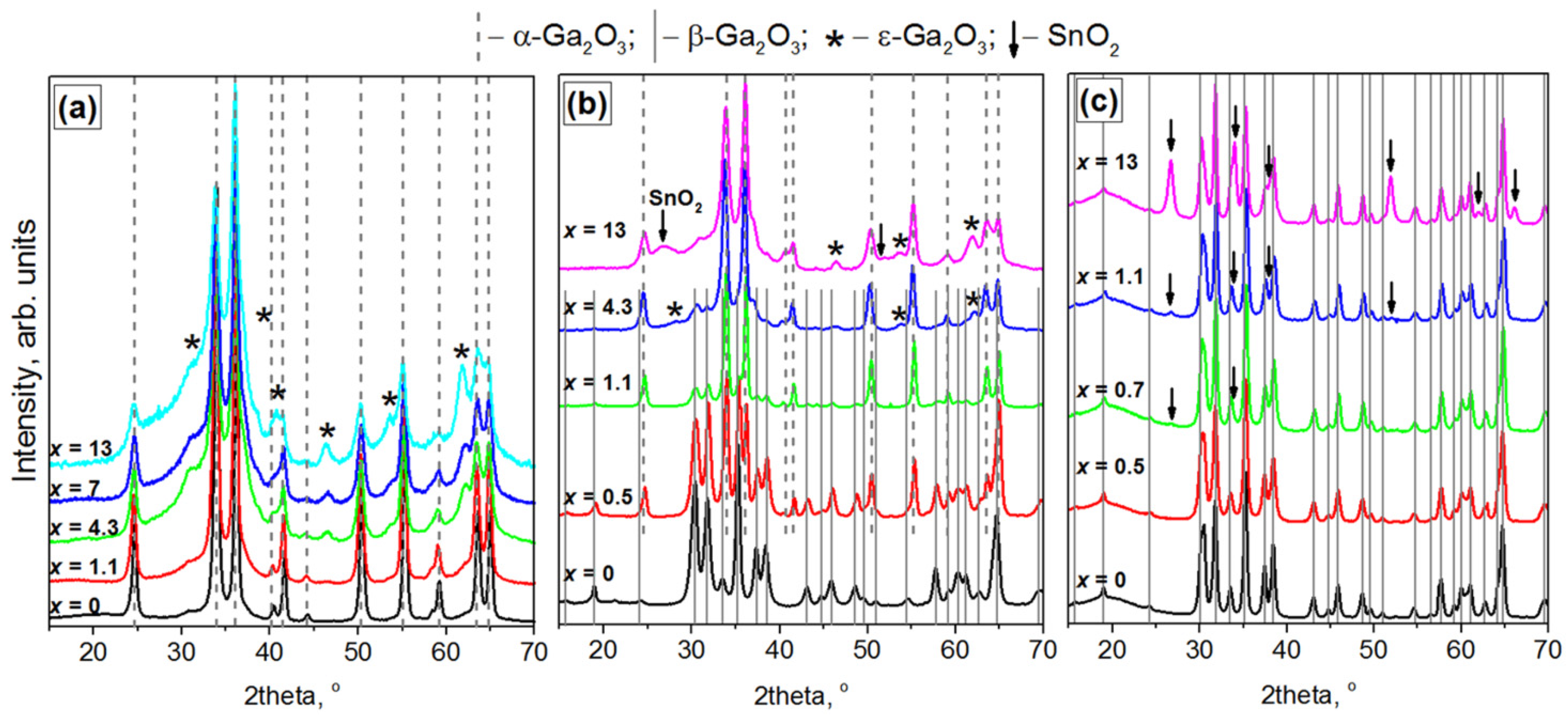
3.2. Phase Composition of Ga2O3(Sn)
3.2.1. Annealing Temperature 500 °C
3.2.2. Annealing Temperature 750 °C
3.2.3. Annealing Temperature 1000 °C
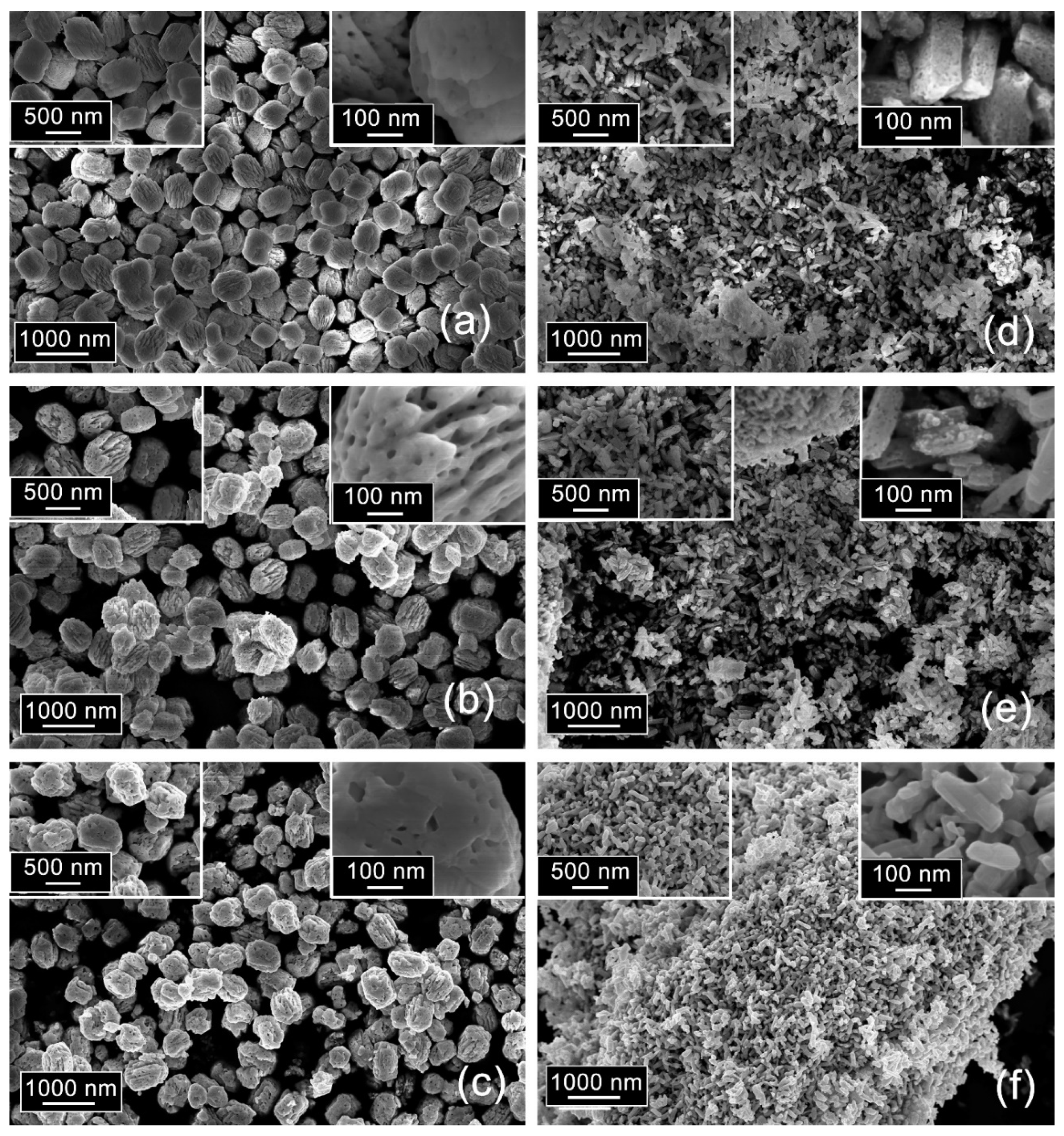
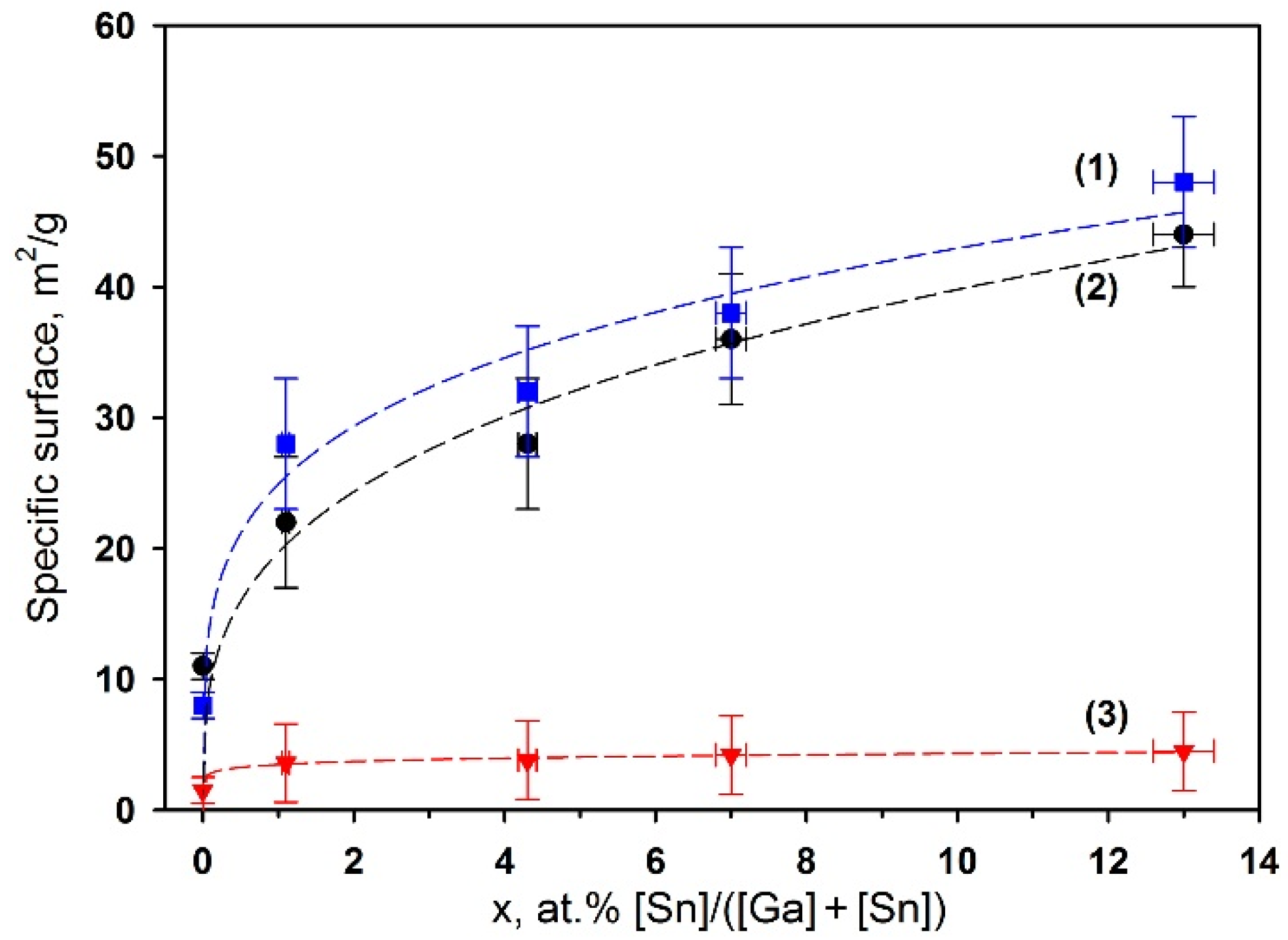
3.3. Microstructure of Ga2O3(Sn)
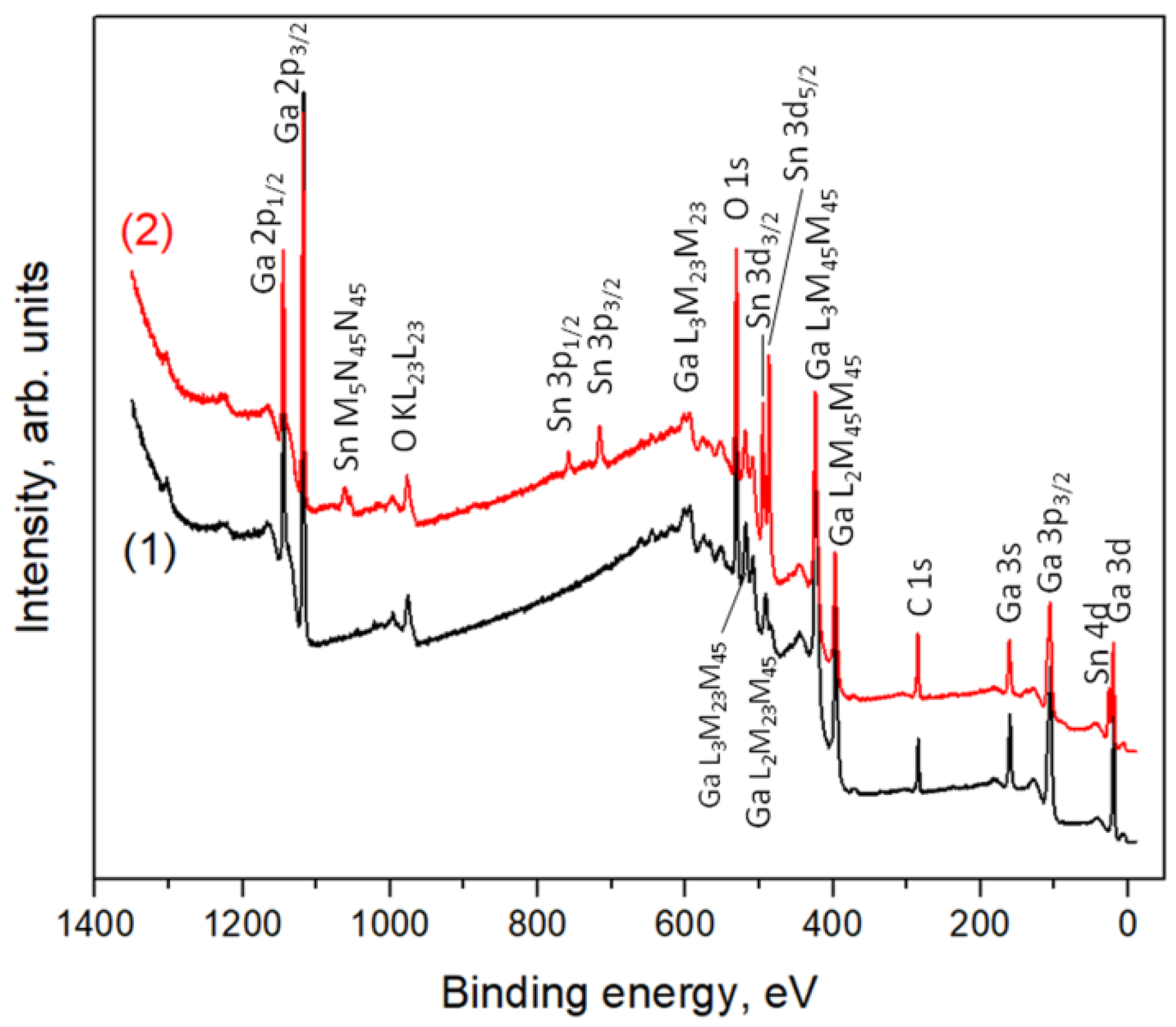
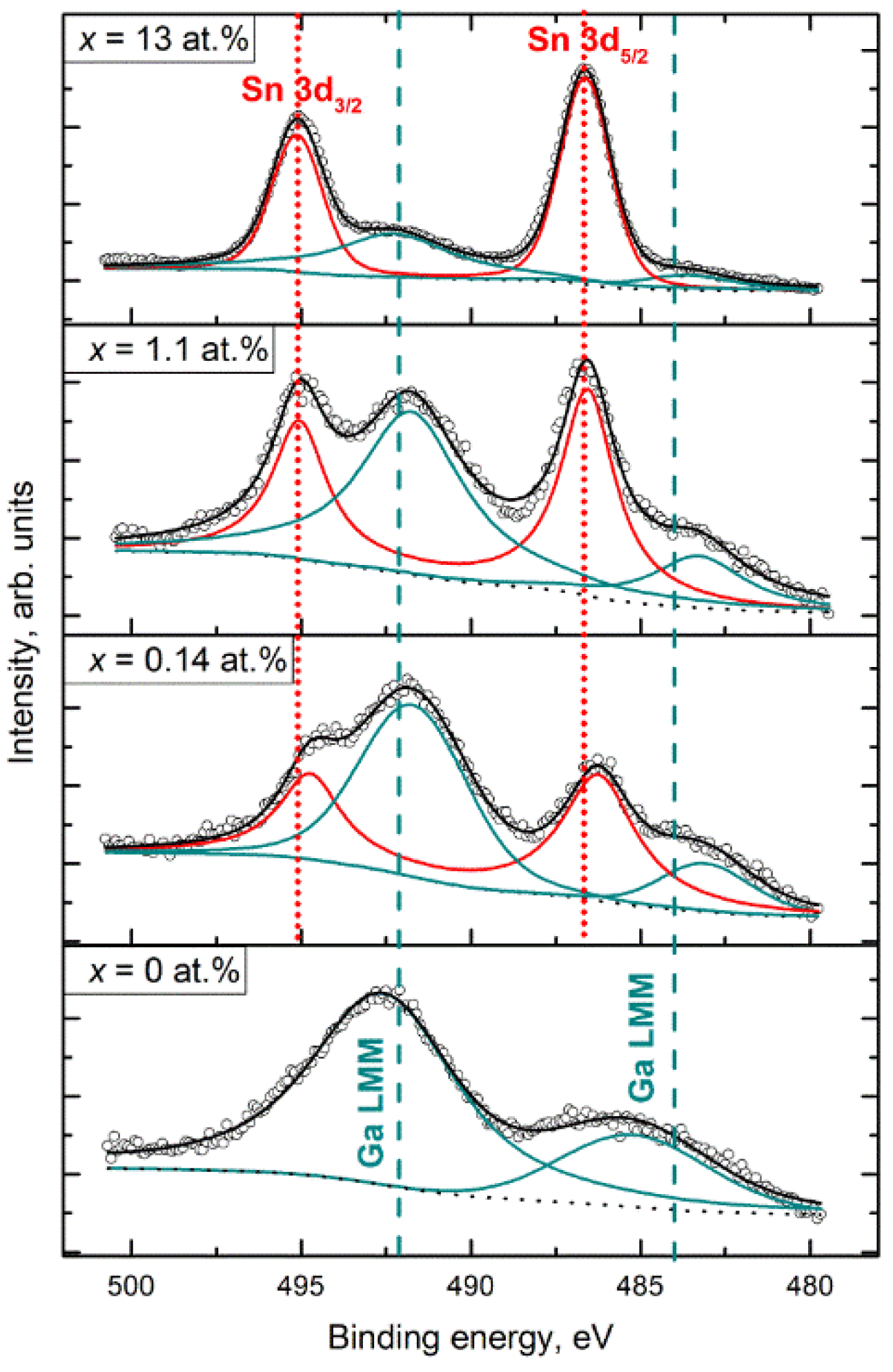
3.4. XPS Study
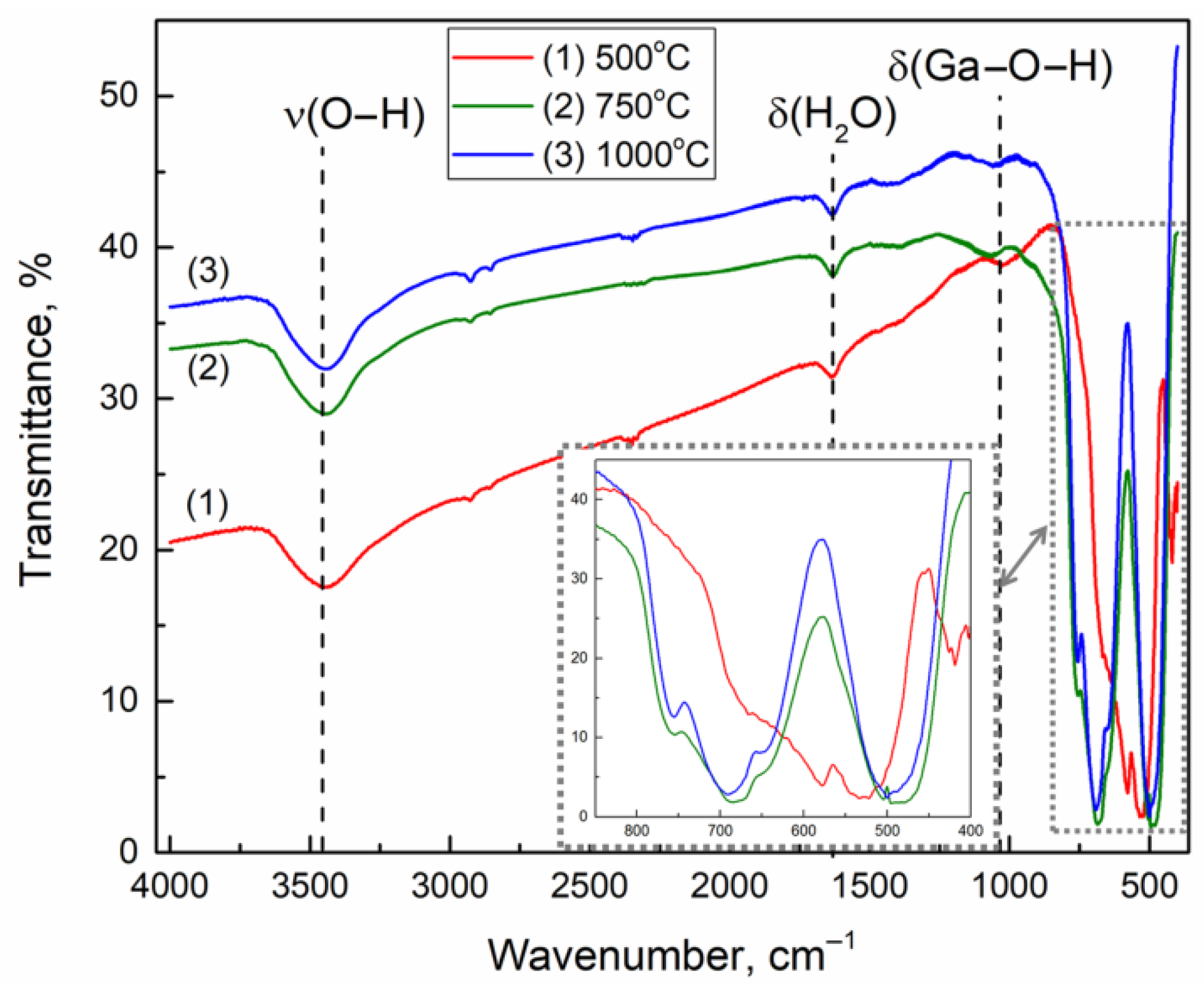
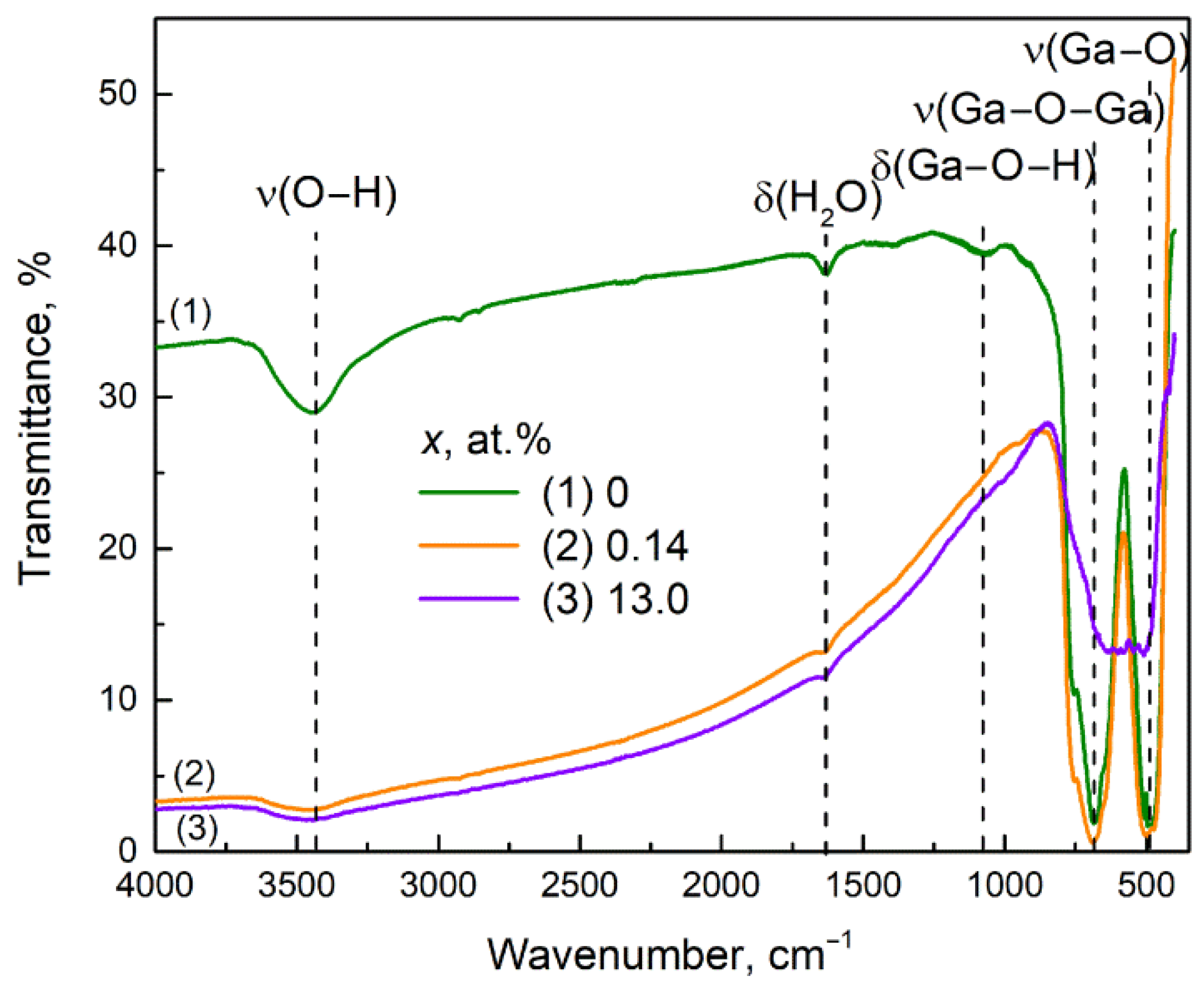
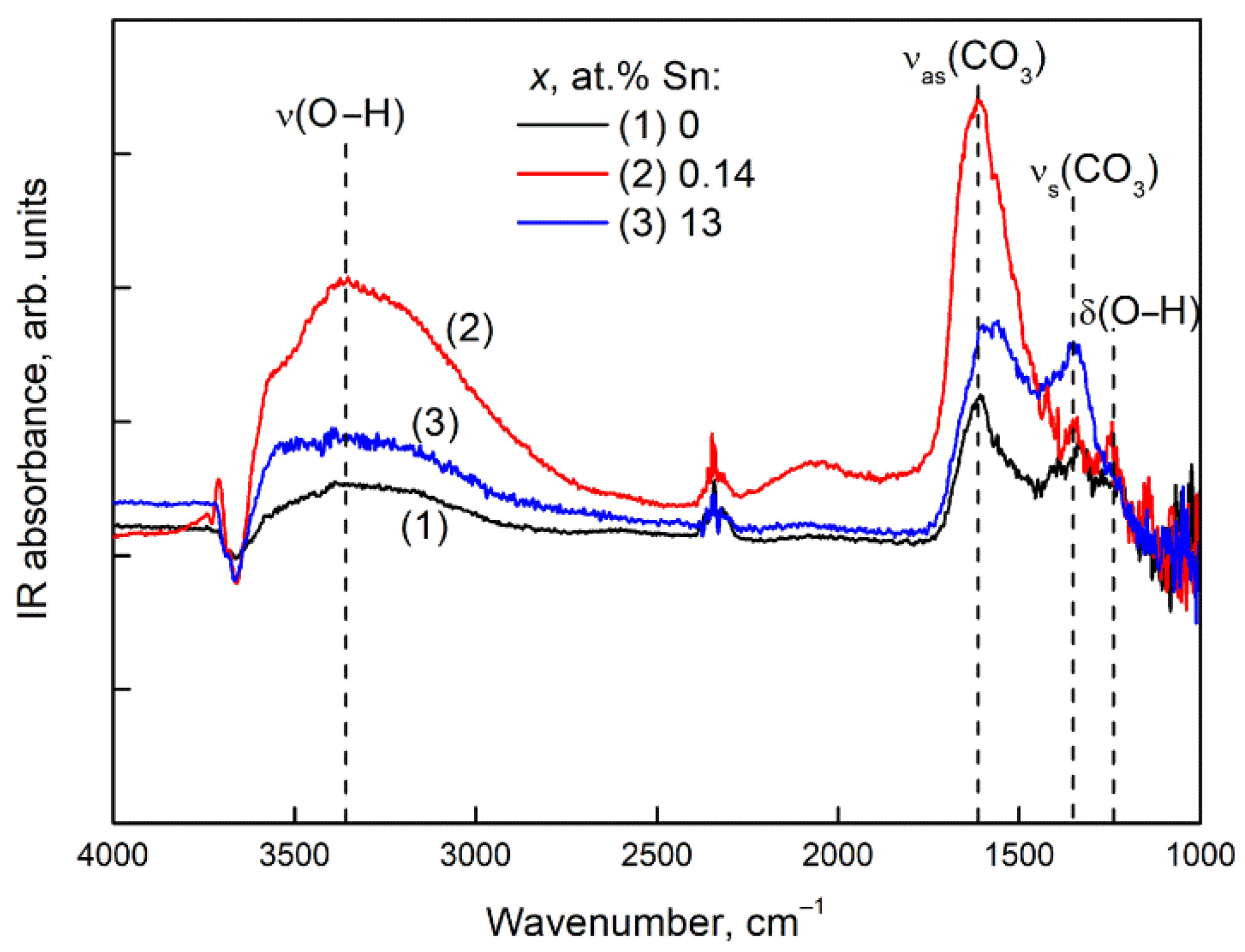
3.5. FTIR Spectroscopy Study
3.6. DRIFT Spectroscopy
3.6.1. CO2 Adsorption
3.6.2. NH3 Adsorption
3.7. Sensor Properties
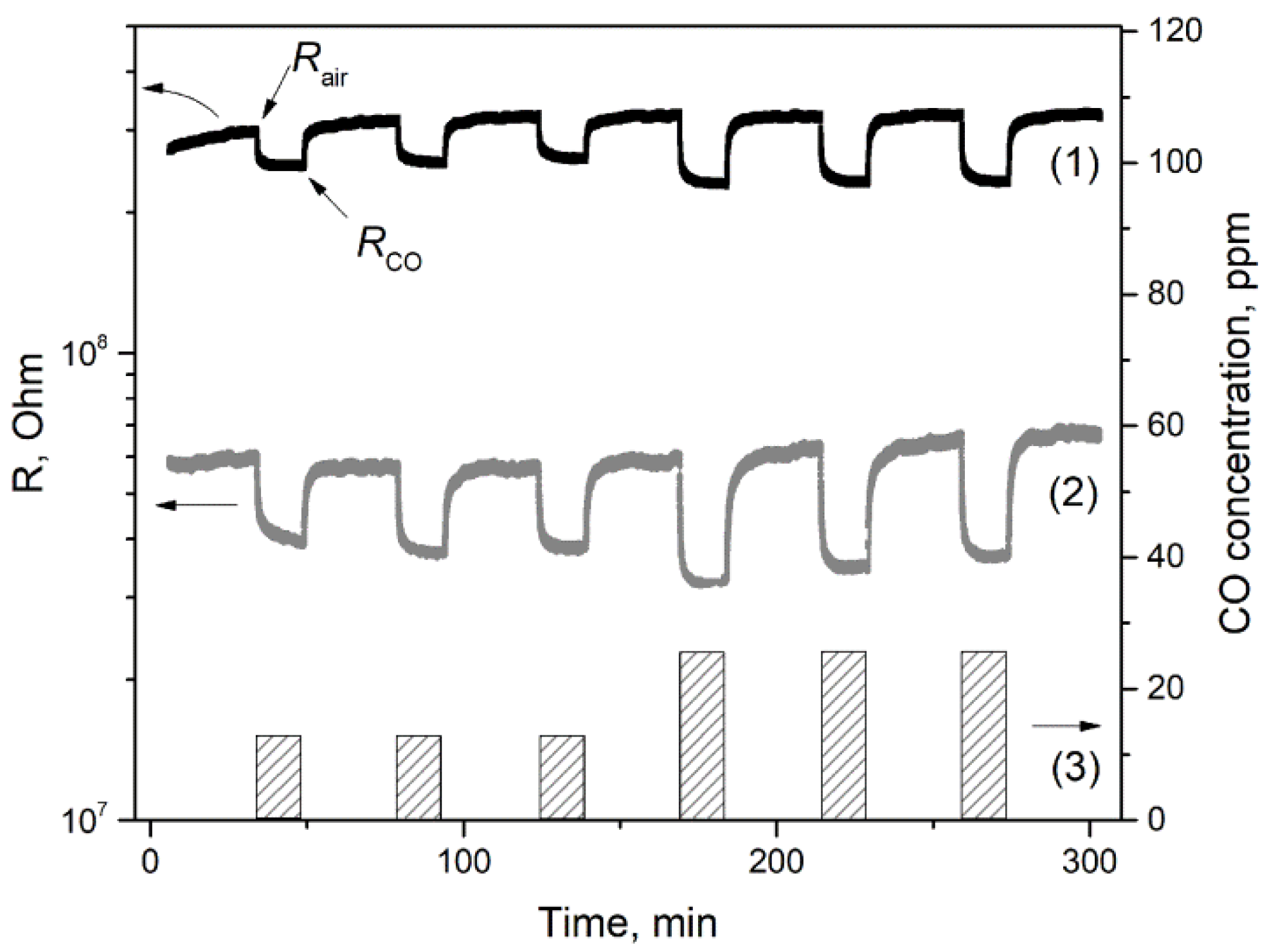
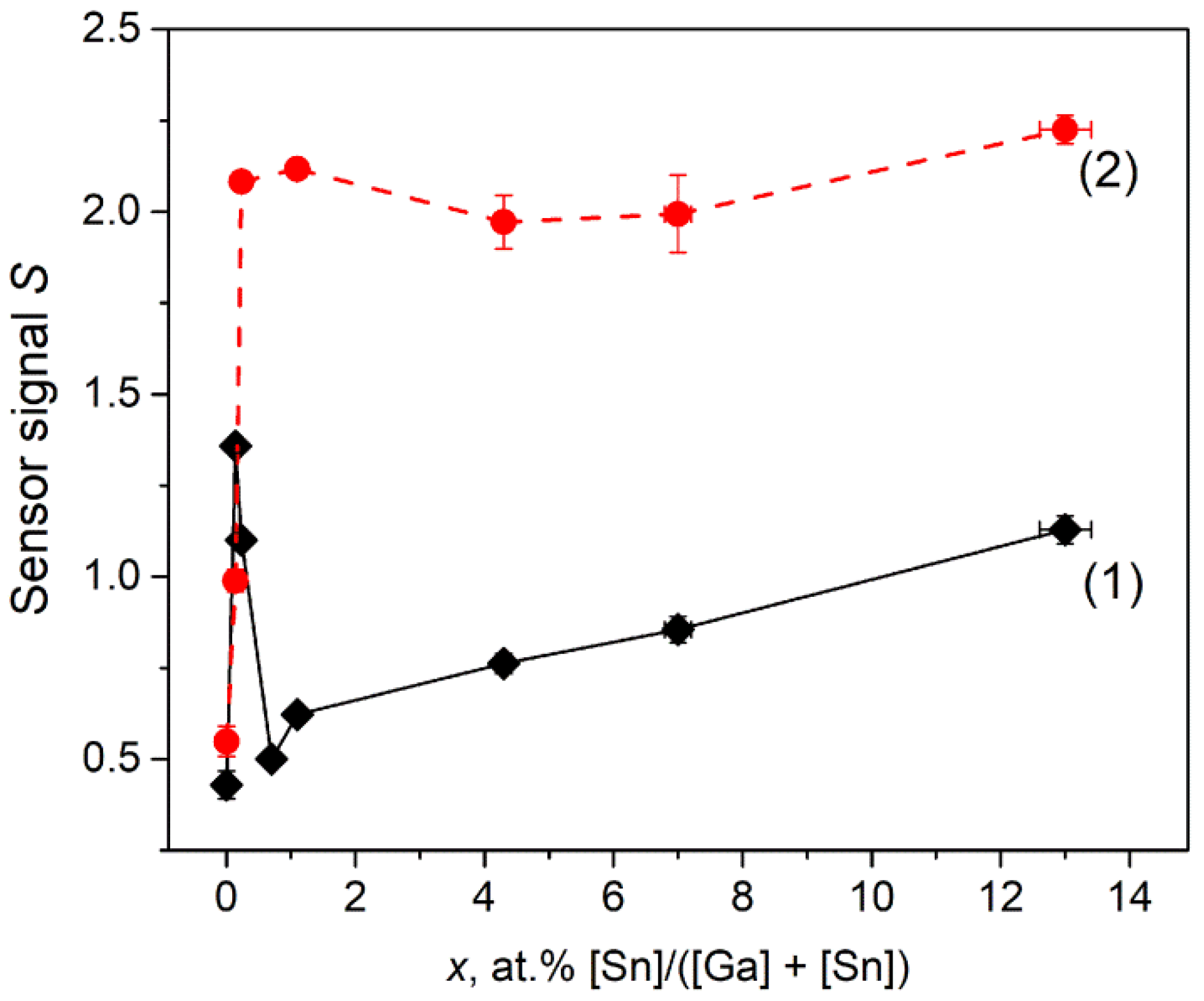
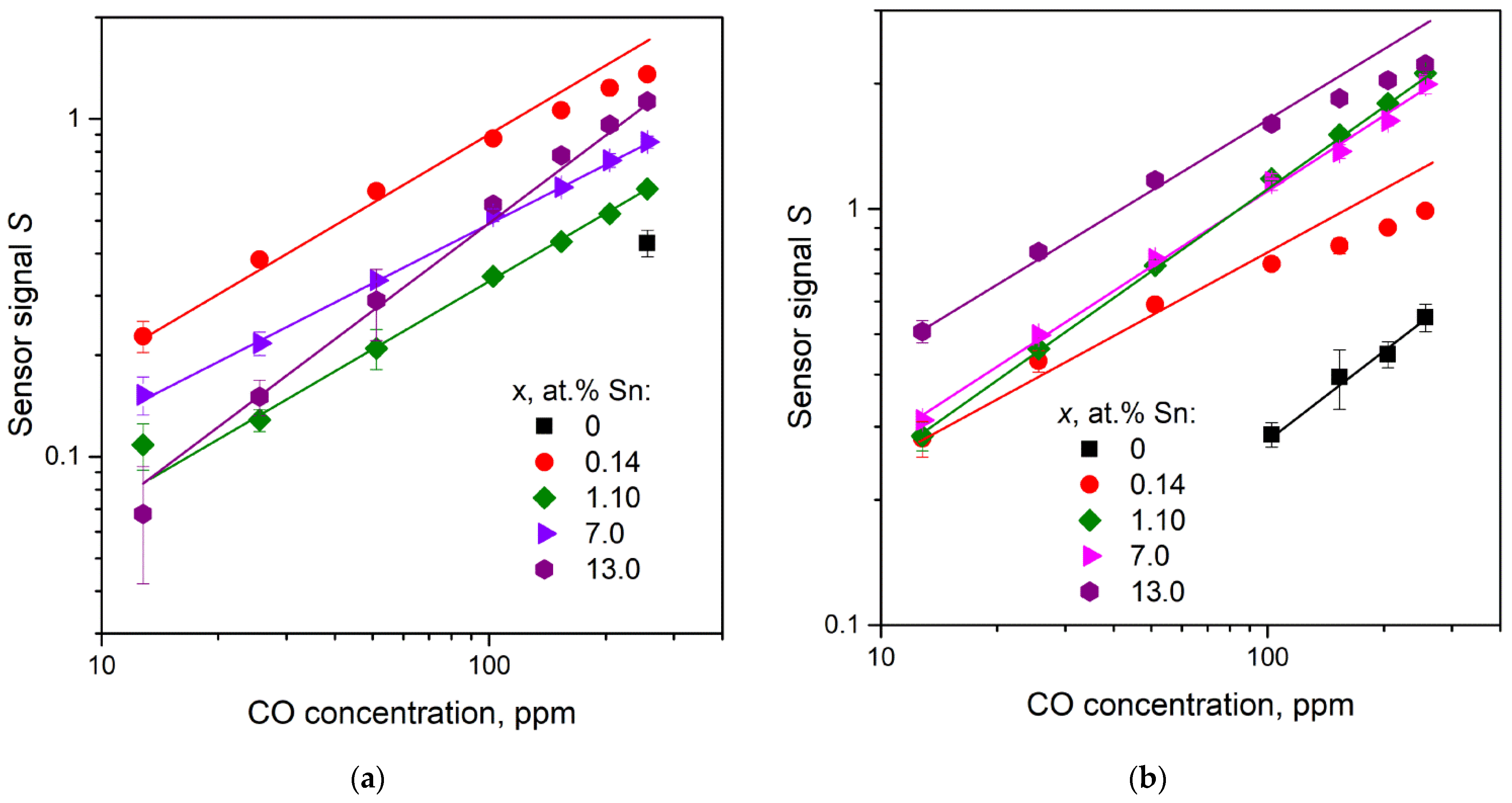
3.7.1. CO Gas Response
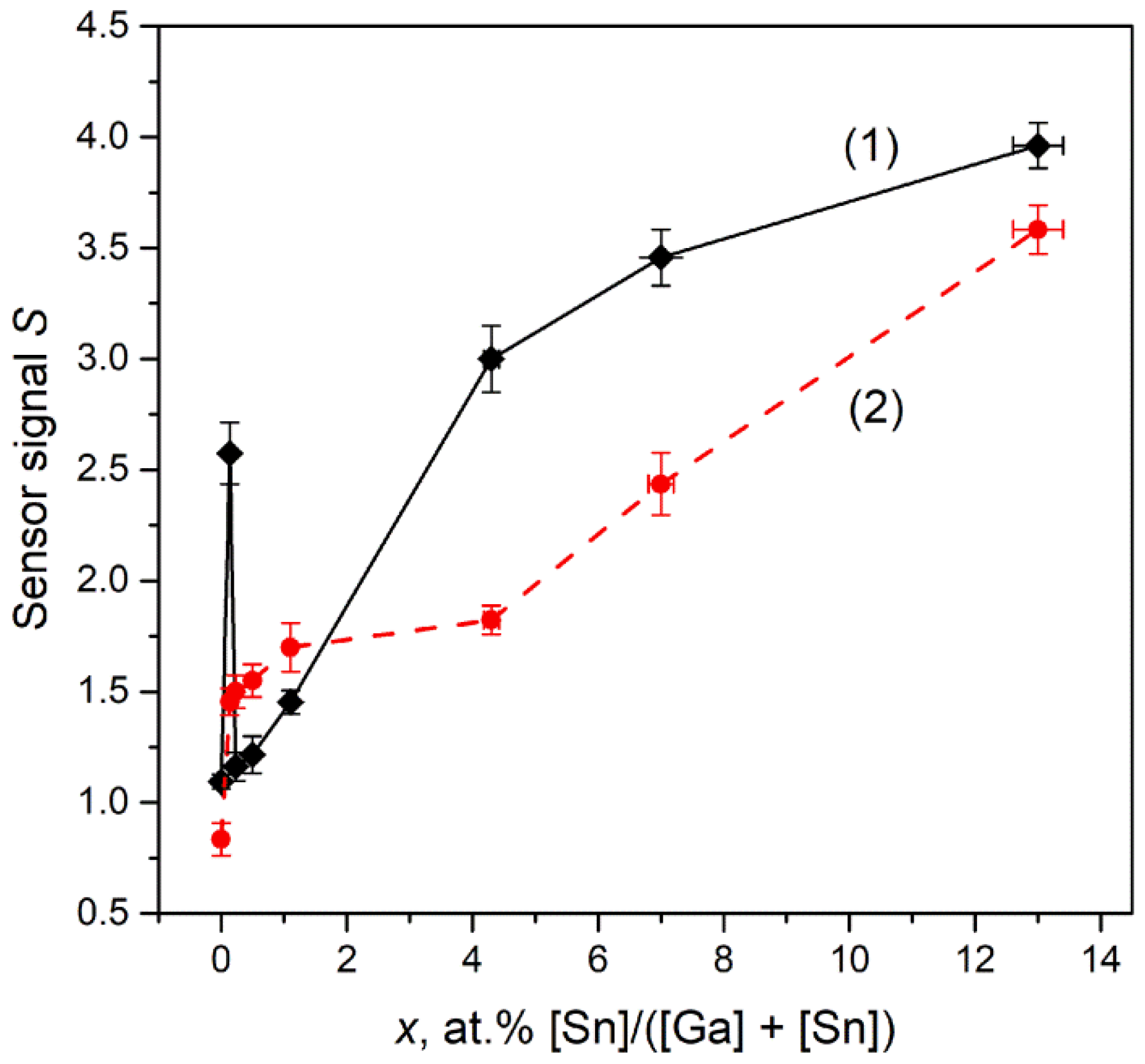
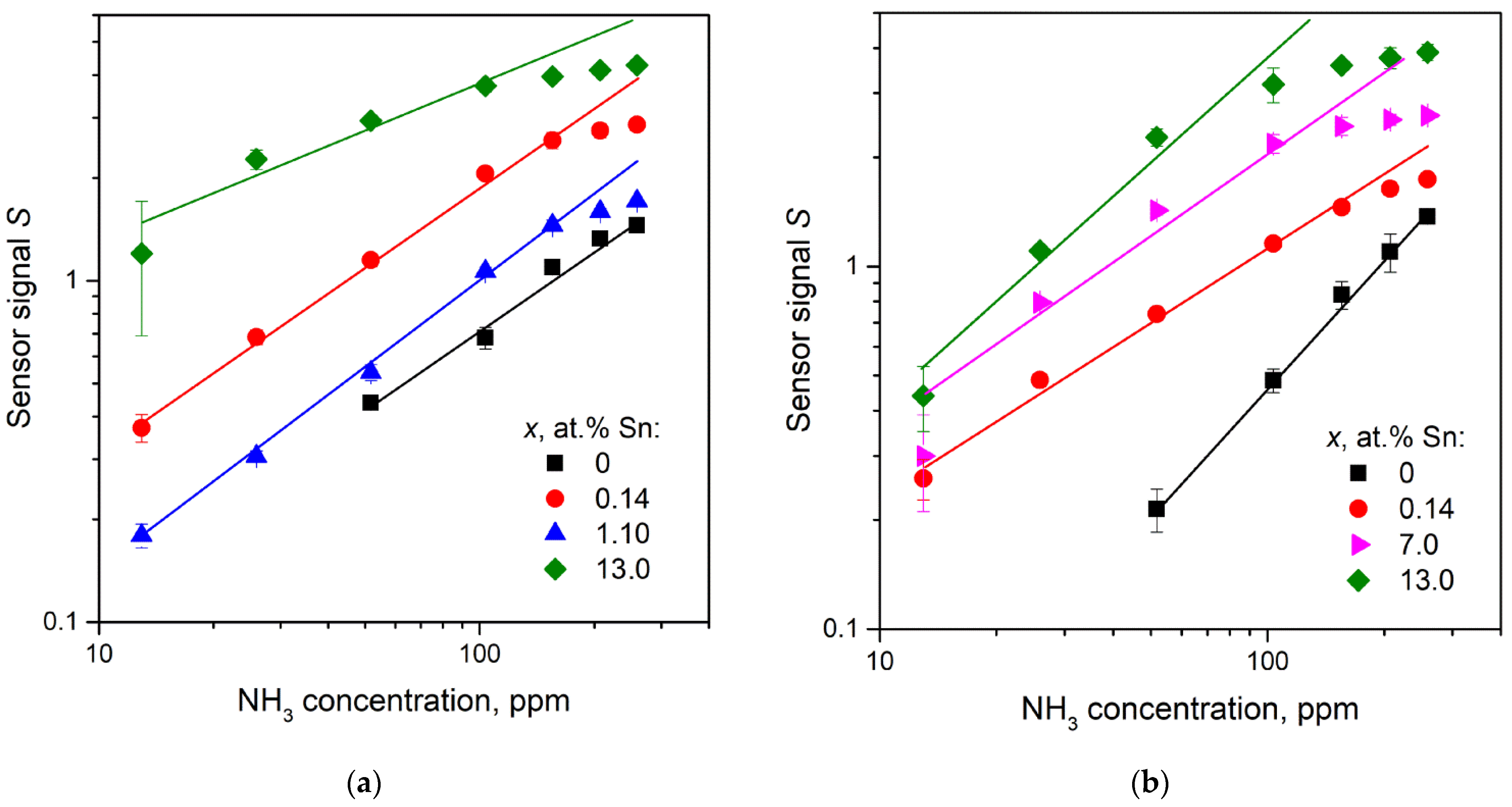
3.7.2. NH3 Gas Response
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pearton, S.J.; Yang, J.; Cary, P.H.; Ren, F.; Kim, J.; Tadjer, M.J.; Mastro, M.A. A review of Ga2O3 materials, processing, and devices. Appl. Phys. Rev. 2018, 5, 011301. [Google Scholar] [CrossRef] [Green Version]
- Stepanov, S.I.; Nikolaev, V.I.; Bougrov, V.E.; Romanov, A.E. Gallium oxide: Properties and applications—A review. Rev. Adv. Mater. Sci. 2016, 44, 63–86. [Google Scholar]
- Afzal, A. β-Ga2O3 nanowires and thin films for metal oxide semiconductor gas sensors: Sensing mechanisms and performance enhancement strategies. J. Mater. 2019, 5, 542–557. [Google Scholar] [CrossRef]
- Richter, D.; Fritze, H. High-temperature gas Sensors. In Gas Sensing Fundamentals; Springer Series on Chemical Sensors and Biosensors (Methods and Applications), 1st ed.; Kohl, C.-D., Wagner, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 15, pp. 1–46. [Google Scholar] [CrossRef]
- Liu, Y.; Parisi, J.; Sun, X.; Lei, Y. Solid-state gas sensors for high temperature application—A review. J. Mater. Chem. A 2014, 2, 9919–9943. [Google Scholar] [CrossRef]
- Manandhar, S.; Battu, A.K.; Devaraj, A.; Shutthanandan, V.; Thevuthasan, S.; Ramana, C.V. Rapid response high temperature oxygen sensor based on titanium doped gallium oxide. Sci. Rep. 2020, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Jang, S.; Baik, K.H. 19—Ga2O3-based gas sensors. In Gallium Oxide: Technology, Devices and Applications. A Volume in Metal Oxides, 1st ed.; Pearton, S., Ren, F., Mastro, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 439–464. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, S.H.; Park, S.Y.; Lee, C. Synthesis and CO gas sensing properties of surfacenitridated Ga2O3 nanowires. RSC Adv. 2014, 4, 63402–63407. [Google Scholar] [CrossRef]
- Krawczyk, M.; Suchorska-Woźniak, P.; Szukiewicz, R.; Kuchowicz, M.; Korbutowicz, R.; Teterycz, H. Morphology of Ga2O3 nanowires and their sensitivity to volatile organic compounds. Nanomaterials 2021, 11, 456. [Google Scholar] [CrossRef]
- Ojha, B.; Illyaskutty, N.; Knoblauch, J.; Balachandran, M.R.; Kohler, H. High-temperature CO/HC gas sensors to optimize firewood combustion in low-power fireplaces. J. Sens. Sens. Syst. 2017, 6, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Playford, H.Y.; Hannon, A.C.; Barney, E.R.; Walton, R.I. Structures of uncharacterised polymorphs of gallium oxide from total neutron diffraction. Chem. Eur. J. 2013, 19, 2803–2813. [Google Scholar] [CrossRef]
- Yoshioka, S.; Hayashi, H.; Kuwabara, A.; Oba, F.; Matsunaga, K.; Tanaka, I. Structures and energetics of Ga2O3 polymorphs. J. Phys. Condens. Matter 2007, 19, 346211. [Google Scholar] [CrossRef]
- Zinkevich, M.; Aldinger, F. Thermodynamic assessment of the gallium-oxygen system. J. Am. Ceram. Soc. 2004, 87, 683–691. [Google Scholar] [CrossRef]
- Penner, S.; Zhuo, C.; Thalinger, R.; Grünbacher, M.; Hejny, C.; Vanicek, S.; Noisternig, M. Physico-chemical properties of unusual Ga2O3 polymorphs. Monatsh. Chem. 2016, 147, 289–300. [Google Scholar] [CrossRef]
- Kracht, M.; Karg, A.; Schörmann, J.; Weinhold, M.; Zink, D.; Michel, F.; Rohnke, M.; Schowalter, M.; Gerken, B.; Rosenauer, A.; et al. Tin-assisted synthesis of ϵ-Ga2O3 by molecular beam epitaxy. Phys. Rev. Appl. 2017, 8, 054002. [Google Scholar] [CrossRef]
- Mazeina, L.; Picard, Y.N.; Maximenko, S.I.; Perkins, F.K.; Glaser, E.R.; Twigg, M.E.; Freitas, J.A., Jr.; Prokes, S.M. Growth of Sn-doped β-Ga2O3 nanowires and Ga2O3-SnO2 heterostructures for gas sensing applications. Cryst. Growth Des. 2009, 9, 4471–4479. [Google Scholar] [CrossRef]
- Yao, Y.; Okur, S.; Lyle, L.A.M.; Tompa, G.S.; Salagaj, T.; Sbrockey, N.; Davis, R.F.; Porter, L.M. Growth and characterization of α-, β-, and ε-phases of Ga2O3 using MOCVD and HVPE techniques. Mater. Res. Lett. 2018, 6, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Fornari, R.; Pavesi, M.; Montedoro, V.; Klimm, D.; Mezzadri, F.; Cora, I.; Pecz, B.; Boschi, F.; Parisini, A.; Baraldi, A.; et al. Thermal stability of ε-Ga2O3 polymorph. Acta Mater. 2017, 140, 411–416. [Google Scholar] [CrossRef]
- Ma, H.P.; Lu, H.L.; Wang, T.; Yang, J.G.; Li, X.; Chen, J.X.; Tao, J.J.; Guo, Q.; Zhang, D.W. Precise control of the microstructural, optical, and electrical properties of ultrathin Ga2O3 film through nanomixing with few atom-thick SiO2 interlayer via plasma enhanced atomic layer deposition. J. Mater. Chem. C 2018, 6, 12518. [Google Scholar] [CrossRef]
- Bermudez, V.M.; Prokes, S.M. Infrared spectroscopy and surface chemistry of β-Ga2O3 nanoribbons. Langmuir 2007, 23, 12566–12576. [Google Scholar] [CrossRef]
- Krehula, S.; Ristić, M.; Kubuki, S.; Iida, Y.; Fabián, M.; Musić, S. The formation and microstructural properties of uniform a-GaOOH particles and their calcination products. J. Alloys Comp. 2015, 620, 217–227. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Vayssilov, G.N. Characterization of oxide surfaces and zeolites by carbon monoxide as an IR probe molecule. Adv. Catal. 2002, 47, 307–511. [Google Scholar] [CrossRef]
- Pan, Y.-X.; Kuai, P.; Liu, Y.; Ge, Q.; Liu, C.-J. Promotion effects of Ga2O3 on CO2 adsorption and conversion over a SiO2-supported Ni catalyst. Energy Environ. Sci. 2010, 3, 1322–1325. [Google Scholar] [CrossRef]
- Collins, S.E.; Baltanás, M.A.; Bonivardi, A.L. Infrared spectroscopic study of the carbon dioxide adsorption on the surface of Ga2O3 polymorphs. J. Phys. Chem. B 2006, 110, 5498–5507. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I. Identification of neutral and charged NxOy surface species by IR spectroscopy. Catal. Rev. Sci. Eng. 2000, 42, 71–144. [Google Scholar] [CrossRef]
- Marikutsa, A.; Rumyantseva, M.; Konstantinova, E.A.; Gaskov, A. The key role of active sites in the development of selective metal oxide sensor materials. Sensors 2021, 21, 2554. [Google Scholar] [CrossRef]
- Mahmoud, W.E. Solar blind avalanche photodetector based on the cation exchange growth of β-Ga2O3/SnO2 bilayer heterostructure thin film. Sol. Energy Mater. Sol. Cells 2016, 152, 65–72. [Google Scholar] [CrossRef]
- Ginley, D.S.; Perkins, J.D. Transparent conductors. In Handbook of Transparent Conductors; Ginley, D., Ed.; Springer: Boston, MA, USA, 2011. [Google Scholar] [CrossRef]
- Pan, Y.-X.; Liu, C.-J.; Mei, D.; Ge, Q. Effects of hydration and oxygen vacancy on CO2 adsorption and activation on β-Ga2O3(100). Langmuir 2010, 26, 5551–5558. [Google Scholar] [CrossRef] [PubMed]
- Ducéré, J.-M.; Hemeryck, A.; Estève, A.; Rouhani, M.D.; Landa, G.; Ménini, P.; Tropis, C.; Maisonnat, A.; Fau, P.; Chaudret, B. A computational chemist approach to gas sensors: Modeling the response of SnO2 to CO, O2, and H2O gases. J. Comput. Chem. 2012, 33, 247–258. [Google Scholar] [CrossRef] [PubMed]

| Sn Content x, at.% [Sn]/([Ga] + [Sn]) (ICP-MS) | Annealing Temperature, °C | ||
|---|---|---|---|
| 500 | 750 | 1000 | |
| 0 ≤ x ≤ 0.23 | α-Ga2O3 | β-Ga2O3 | β-Ga2O3 |
| 0.50 ± 0.04 | α-Ga2O3 | β-Ga2O3, α-Ga2O3 | β-Ga2O3 |
| 0.70 ± 0.02 | α-Ga2O3 | β-Ga2O3, α-Ga2O3, ε-Ga2O3 | β-Ga2O3, SnO2 |
| 1.10 ± 0.05 | α-Ga2O3 | α-Ga2O3, β-Ga2O3, ε-Ga2O3 | β-Ga2O3, SnO2 |
| 4.30 ± 0.12 | α-Ga2O3, ε-Ga2O3 | α-Ga2O3, β-Ga2O3, ε-Ga2O3 | β-Ga2O3, SnO2 |
| 7.0 ± 0.2 | α-Ga2O3, ε-Ga2O3 | α-Ga2O3, β-Ga2O3, ε-Ga2O3, SnO2 | β-Ga2O3, SnO2 |
| 13.0 ± 0.4 | α-Ga2O3, ε-Ga2O3, SnO2 | α-Ga2O3, ε-Ga2O3, SnO2 | β-Ga2O3, SnO2 |
| Sn Content x, at.% [Sn]/([Ga] + [Sn]) (ICP-MS) | Annealing Temperature, °C | |||||
|---|---|---|---|---|---|---|
| 500 | 750 | 1000 | ||||
| Sn Content, at.% (XPS) | [Ga]:[O] Ratio | Sn Content, at.% (XPS) | [Ga]:[O] Ratio | Sn Content, at.% (XPS) | [Ga]:[O] Ratio | |
| 0 | 0 | 1:1.02 | 0 | 1:1.11 | 0 | 1:1.15 |
| 0.14 ± 0.02 | 1.4 ± 0.2 | 1:1.24 | 3.1 ± 0.3 | 1:1.33 | ||
| 1.10 ± 0.05 | 3.6 ± 0.4 | 1:1.30 | 3.2 ± 0.3 | 1:1.24 | 4.5 ± 0.5 | 1:1.28 |
| 13.0 ± 0.4 | 20 ± 2 | 1:1.62 | 17 ± 2 | 1:1.54 | 15 ± 2 | 1:1.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorobyeva, N.; Rumyantseva, M.; Platonov, V.; Filatova, D.; Chizhov, A.; Marikutsa, A.; Bozhev, I.; Gaskov, A. Ga2O3(Sn) Oxides for High-Temperature Gas Sensors. Nanomaterials 2021, 11, 2938. https://doi.org/10.3390/nano11112938
Vorobyeva N, Rumyantseva M, Platonov V, Filatova D, Chizhov A, Marikutsa A, Bozhev I, Gaskov A. Ga2O3(Sn) Oxides for High-Temperature Gas Sensors. Nanomaterials. 2021; 11(11):2938. https://doi.org/10.3390/nano11112938
Chicago/Turabian StyleVorobyeva, Nataliya, Marina Rumyantseva, Vadim Platonov, Darya Filatova, Artem Chizhov, Artem Marikutsa, Ivan Bozhev, and Alexander Gaskov. 2021. "Ga2O3(Sn) Oxides for High-Temperature Gas Sensors" Nanomaterials 11, no. 11: 2938. https://doi.org/10.3390/nano11112938






