Interaction between ZnO Nanoparticles and Albumin and Its Effect on Cytotoxicity, Cellular Uptake, Intestinal Transport, Toxicokinetics, and Acute Oral Toxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and NP Preparation
2.2. Characterization
2.3. Dissolution Properties of ZnO NPs
2.4. ICP–AES Analysis
2.5. Fluorescence Quenching of Food Proteins by ZnO NPs
2.6. SDS–Polyacrylamide Gel Electrophoresis (PAGE) Analysis
2.7. Cytotoxicity
2.7.1. Cell Culture
2.7.2. Cell Proliferation
2.7.3. Lactate Dehydrogenase Leakage (LDH) Assay
2.7.4. Reactive Oxygen Species (ROS) Generation
2.8. Cellular Uptake
2.9. Intestinal Transport
2.10. Animals
2.11. Toxicokinetic Study
2.12. 14-Day Repeated Oral Toxicity Study
2.13. Statistical Analysis
3. Results and Discussion
3.1. Characterization of ZnO NPs in Food Proteins
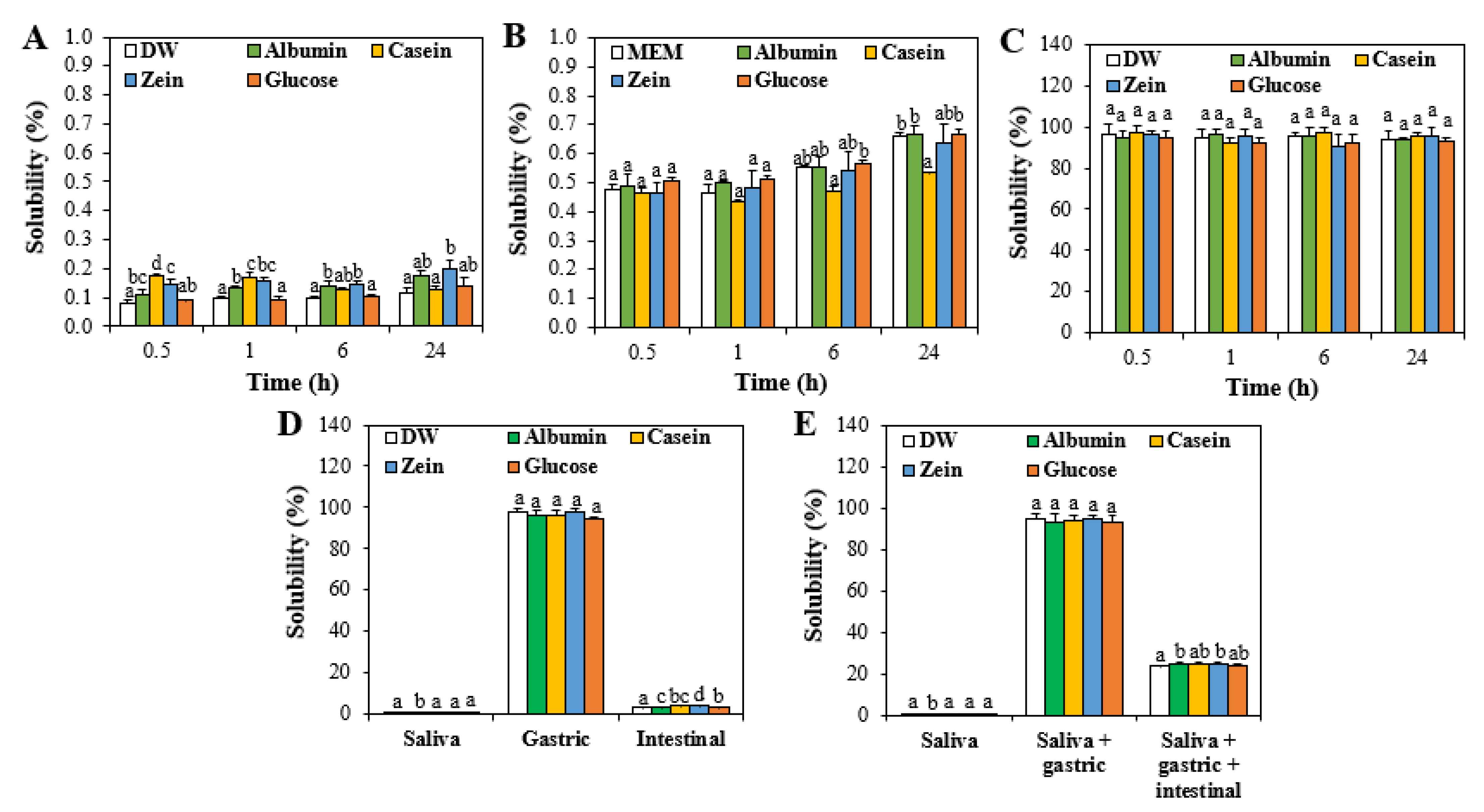
3.2. Solubility of ZnO NPs in Food Proteins
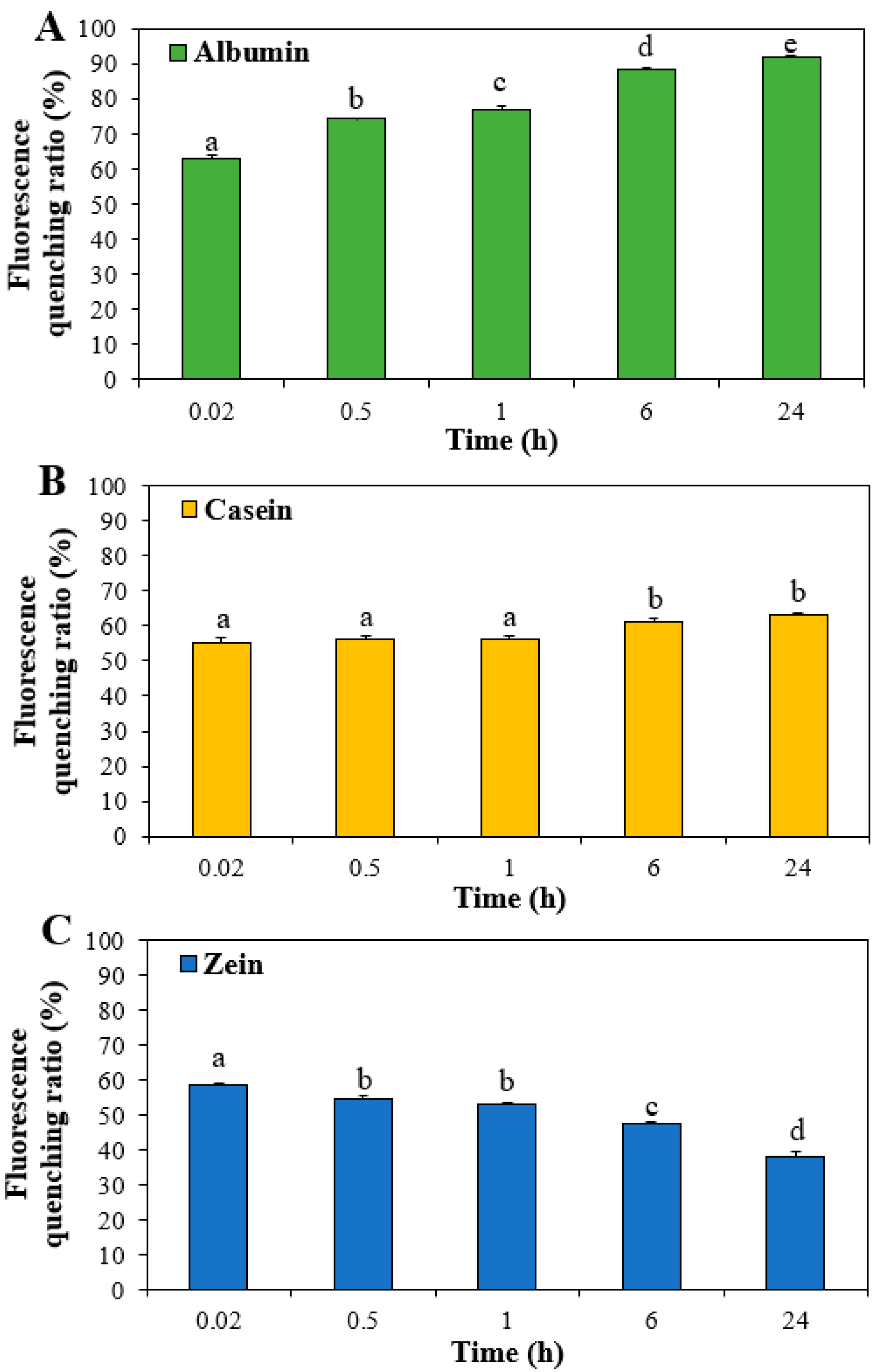
3.3. Interactions between ZnO NPs and Food Proteins
3.4. Primary Structural Stability and Digestion of Food Proteins by Interactions with ZnO NPs
3.5. Cytotoxicity of ZnO NPs by Interactions with Food Proteins
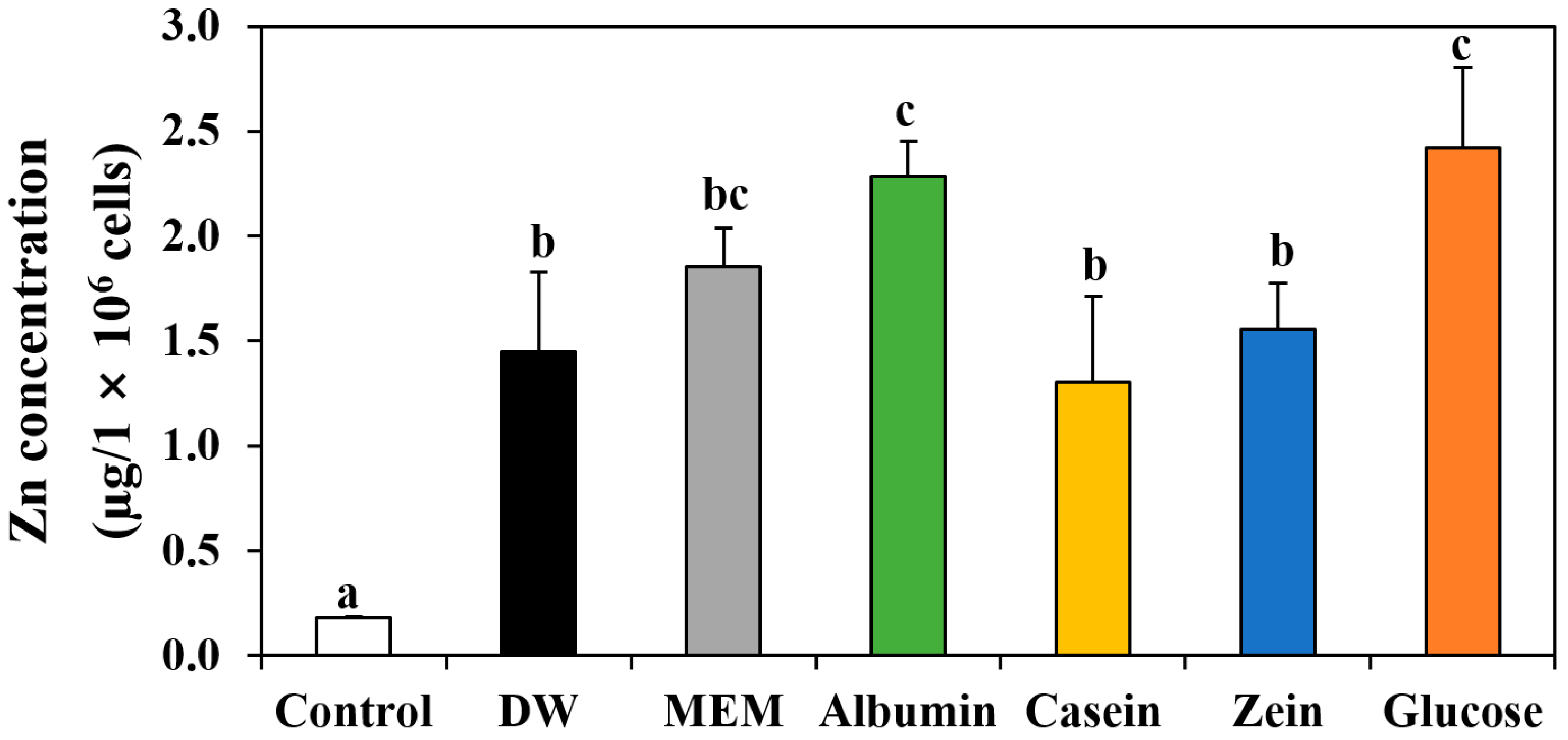
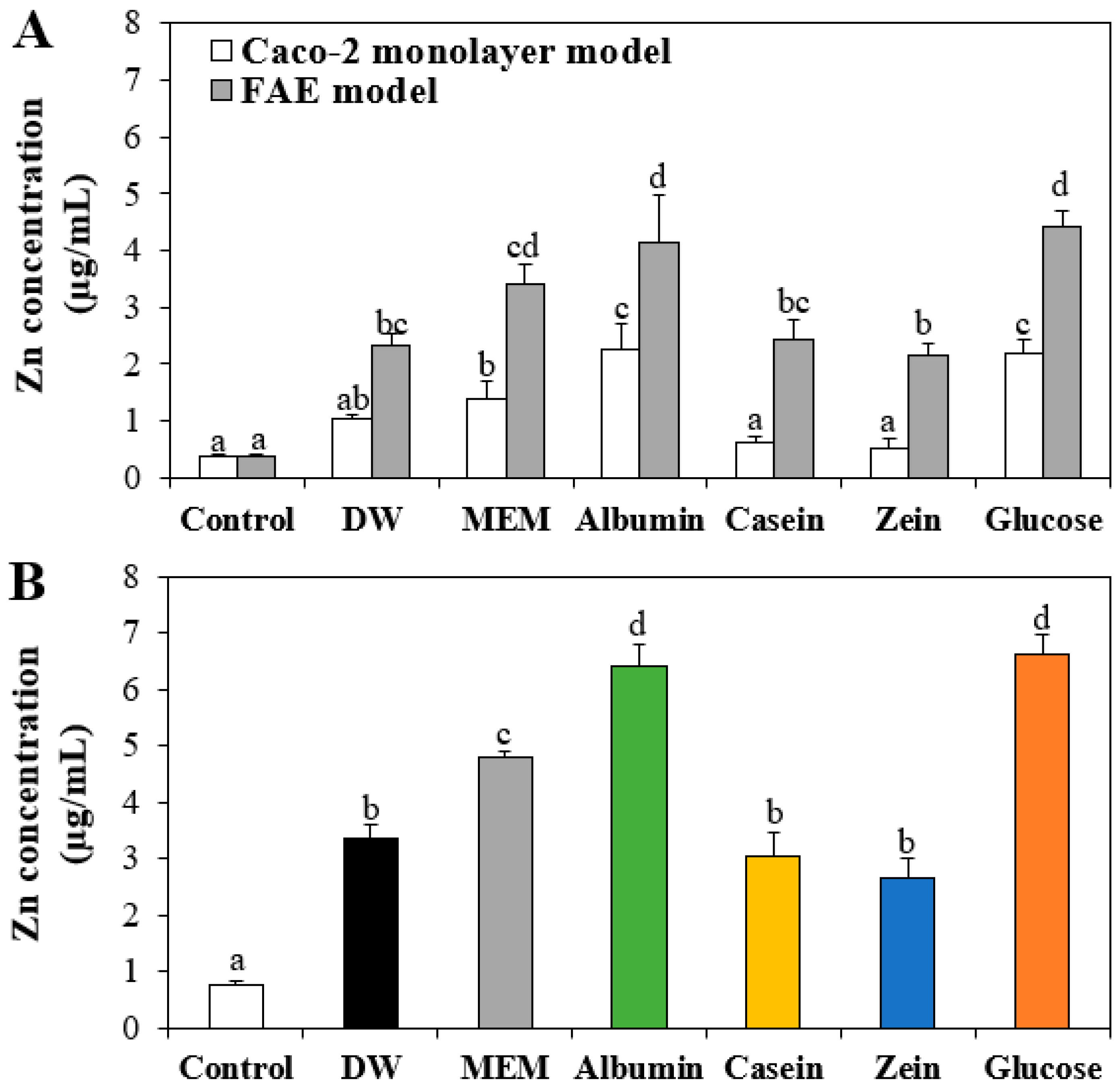
3.6. Cellular Uptake of ZnO NPs by Interactions with Food Proteins
3.7. Intestinal Transport of ZnO NPs by Interactions with Food Proteins
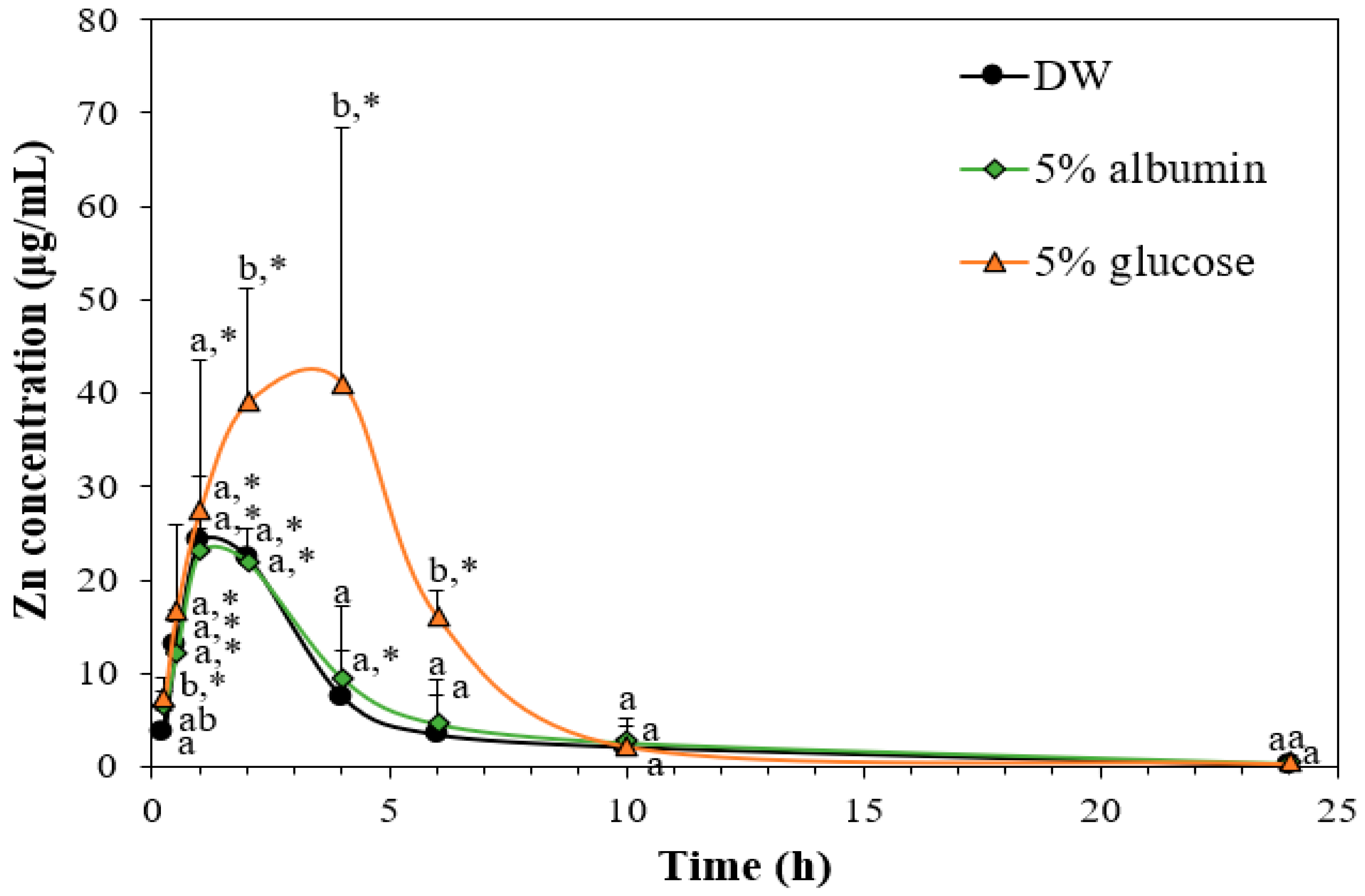
3.8. Toxicokinetics of ZnO NPs by Interactions with Food Matrices
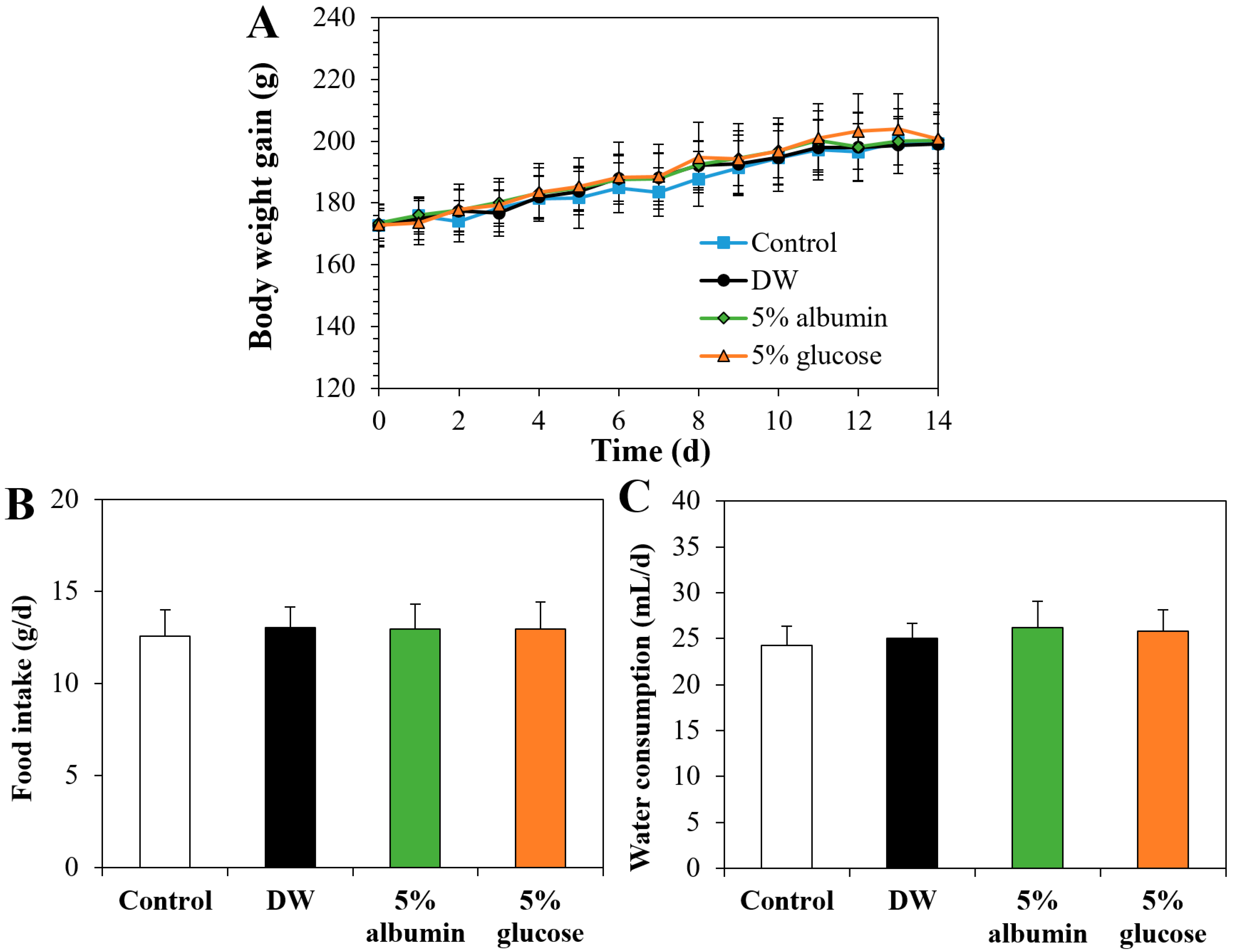
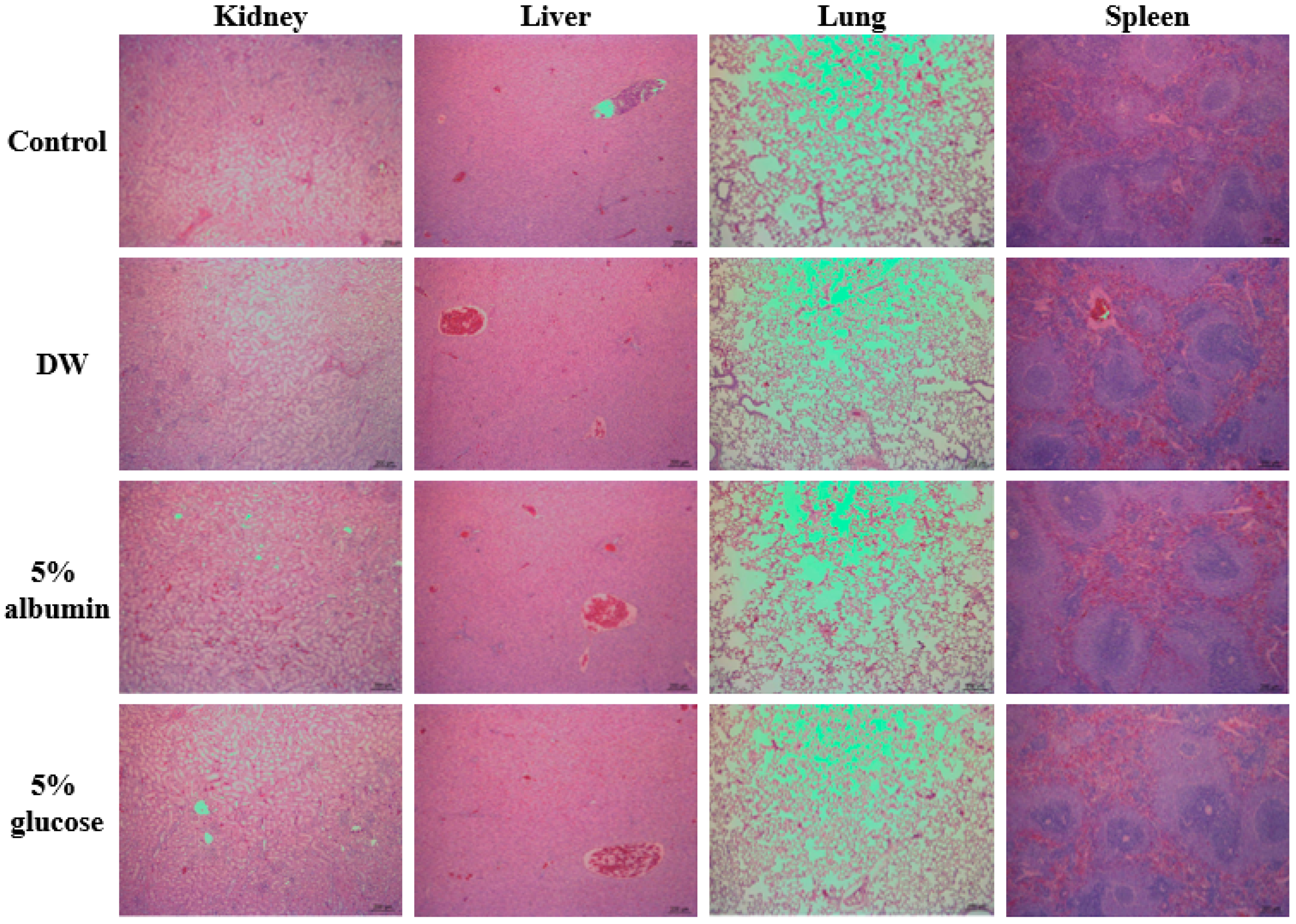
3.9. Oral Toxicity of ZnO NPs by Interactions with Food Matrices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A review of microwave synthesis of zinc oxide nanomaterials: Reactants, process parameters and morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef]
- MacDonald, R.S. The role of zinc in growth and cell proliferation. J. Nutr. 2000, 130, 1500S–1508S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dardenne, M. Zinc and immune function. Eur. J. Clin. Nutr. 2002, 56, S20–S23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafeez, B.; Khanif, Y.; Saleem, M. Role of zinc in plant nutrition-a review. J. Exp. Agric. Int. 2013, 3, 374–391. [Google Scholar] [CrossRef]
- Faizan, M.; Hayat, S.; Pichtel, J. Effects of zinc oxide nanoparticles on crop plants: A perspective analysis. In Sustainable Agriculture Reviews 41; Springer: New York, NY, USA, 2020; pp. 83–99. [Google Scholar]
- Fosmire, G.J. Zinc toxicity. Am. J. Clin. Nutr. 1990, 51, 225–227. [Google Scholar] [CrossRef]
- Nriagu, J. Zinc toxicity in humans. In Encyclopedia of Environmental Health, 1st ed.; Nriagu, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 801–807. [Google Scholar]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef]
- Go, M.-R.; Yu, J.; Bae, S.-H.; Kim, H.-J.; Choi, S.-J. Effects of interactions between ZnO nanoparticles and saccharides on biological responses. Int. J. Mol. Sci. 2018, 19, 486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, S.-H.; Yu, J.; Lee, T.G.; Choi, S.-J. Protein food matrix—ZnO nanoparticle interactions affect protein conformation, but may not be biological responses. J. Mol. Sci. 2018, 19, 3926. [Google Scholar] [CrossRef] [Green Version]
- Jung, E.B. Interaction between Food Additive ZnO Nanoparticles and Food Proteins. Master’s Thesis, Seoul Women’s University, Seoul, Korea, 16 June 2021. [Google Scholar]
- Lee, J.-A.; Kim, M.-K.; Song, J.H.; Jo, M.-R.; Yu, J.; Kim, K.-M.; Kim, Y.-R.; Oh, J.-M.; Choi, S.-J. Biokinetics of food additive silica nanoparticles and their interactions with food components. Colloids Surf. B 2017, 150, 384–392. [Google Scholar] [CrossRef]
- Jo, M.-R.; Yu, J.; Kim, H.-J.; Song, J.H.; Kim, K.-M.; Oh, J.-M.; Choi, S.-J. Titanium dioxide nanoparticle-biomolecule interactions influence oral absorption. Nanomaterials 2016, 6, 225. [Google Scholar] [CrossRef] [Green Version]
- Dobrovolskaia, M.A.; Shurin, M.; Shvedova, A.A. Current understanding of interactions between nanoparticles and the immune system. Toxicol. Appl. Pharmacol. 2016, 299, 78–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parveen, R.; Shamsi, T.N.; Fatima, S. Nanoparticles-protein interaction: Role in protein aggregation and clinical implications. Int. J. Biol. Macromol. 2017, 94, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Barbero, F.; Russo, L.; Vitali, M.; Piella, J.; Salvo, I.; Borrajo, M.L.; Busquets-Fité, M.; Grandori, R.; Bastús, N.G.; Casals, E. Formation of the protein corona: The interface between nanoparticles and the immune system. Semin. Immunol. 2017, 34, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Yu, J. Effects of Interactions between ZnO Nanoparticles and Biological/Food Matrices on Toxicological Responses. Ph.D. Thesis, Seoul Women’s University, Seoul, Korea, 21 August 2020. [Google Scholar]
- Coreas, R.; Cao, X.; DeLoid, G.M.; Demokritou, P.; Zhong, W. Lipid and protein corona of food-grade TiO2 nanoparticles in simulated gastrointestinal digestion. NanoImpact 2020, 20, 100272. [Google Scholar] [CrossRef] [PubMed]
- Geitner, N.K.; Hendren, C.O.; Cornelis, G.; Kaegi, R.; Lead, J.R.; Lowry, G.V.; Lynch, I.; Nowack, B.; Petersen, E.; Bernhardt, E. Harmonizing across environmental nanomaterial testing media for increased comparability of nanomaterial datasets. Environ. Sci. Nano 2020, 7, 13–36. [Google Scholar] [CrossRef] [Green Version]
- Peters, R.; Kramer, E.; Oomen, A.G.; Herrera Rivera, Z.E.; Oegema, G.; Tromp, P.C.; Fokkink, R.; Rietveld, A.; Marvin, H.J.; Weigel, S. Presence of nano-sized silica during in vitro digestion of foods containing silica as a food additive. ACS Nano 2012, 6, 2441–2451. [Google Scholar] [CrossRef]
- des Rieux, A.; Ragnarsson, E.G.; Gullberg, E.; Préat, V.; Schneider, Y.-J.; Artursson, P. Transport of nanoparticles across an in vitro model of the human intestinal follicle associated epithelium. Eur. J. Pharm. Sci. 2005, 25, 455–465. [Google Scholar] [CrossRef]
- Jo, M.-R.; Bae, S.-H.; Go, M.-R.; Kim, H.-J.; Hwang, Y.-G.; Choi, S.-J. Toxicity and biokinetics of colloidal gold nanoparticles. Nanomaterials 2015, 5, 835–850. [Google Scholar] [CrossRef] [Green Version]
- Anders, C.B.; Chess, J.J.; Wingett, D.G.; Punnoose, A. Serum proteins enhance dispersion stability and influence the cytotoxicity and dosimetry of ZnO nanoparticles in suspension and adherent cancer cell models. Nanoscale Res. Lett. 2015, 10, 448. [Google Scholar] [CrossRef] [Green Version]
- Jo, M.-R.; Chung, H.-E.; Kim, H.-J.; Bae, S.-H.; Go, M.-R.; Yu, J.; Choi, S.-J. Effects of zinc oxide nanoparticle dispersants on cytotoxicity and cellular uptake. Mol. Cell. Toxicol. 2016, 12, 281–288. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Elgohary, M.M.; Kamel, N.M. Implications of protein-and peptide-based nanoparticles as potential vehicles for anticancer drugs. Adv. Protein Chem. Struct. Biol. 2015, 98, 169–221. [Google Scholar]
- Shukla, R.; Cheryan, M. Zein: The industrial protein from corn. Ind. Crops Prod. 2001, 13, 171–192. [Google Scholar] [CrossRef]
- Patil, S.; Sandberg, A.; Heckert, E.; Self, W.; Seal, S. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials 2007, 28, 4600–4607. [Google Scholar] [CrossRef] [Green Version]
- Fukuzaki, S.; Urano, H.; Nagata, K. Adsorption of bovine serum albumin onto metal oxide surfaces. J. Ferment. Bioeng. 1996, 81, 163–167. [Google Scholar] [CrossRef]
- Yu, J.; Kim, H.-J.; Go, M.-R.; Bae, S.-H.; Choi, S.-J. ZnO interactions with biomatrices: Effect of particle size on ZnO-protein corona. Nanomaterials 2017, 7, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatehah, M.O.; Aziz, H.A.; Stoll, S. Stability of ZnO nanoparticles in solution. Influence of pH, dissolution, aggregation and disaggregation effects. J. Colloid Sci. Biotechnol. 2014, 3, 75–84. [Google Scholar] [CrossRef]
- Adamcakova-Dodd, A.; Stebounova, L.V.; Kim, J.S.; Vorrink, S.U.; Ault, A.P.; O’Shaughnessy, P.T.; Grassian, V.H.; Thorne, P.S. Toxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation models. Part. Fibre Toxicol. 2014, 11, 15. [Google Scholar] [CrossRef] [Green Version]
- Bian, S.-W.; Mudunkotuwa, I.A.; Rupasinghe, T.; Grassian, V.H. Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: Influence of pH, ionic strength, size, and adsorption of humic acid. Langmuir 2011, 27, 6059–6068. [Google Scholar] [CrossRef] [PubMed]
- Voss, L.; Saloga, P.E.; Stock, V.; Böhmert, L.; Braeuning, A.; Thünemann, A.F.; Lampen, A.; Sieg, H. Environmental impact of ZnO nanoparticles evaluated by in vitro simulated digestion. ACS Appl. Nano Mater. 2019, 3, 724–733. [Google Scholar] [CrossRef]
- Zhu, M.; Nie, G.; Meng, H.; Xia, T.; Nel, A.; Zhao, Y. Physicochemical properties determine nanomaterial cellular uptake, transport, and fate. Acc. Chem. Res. 2013, 46, 622–631. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Baek, M.; Chung, H.; Choi, S. Effects of physicochemical properties of zinc oxide nanoparticles on cellular uptake. J. Phys. Conf. Ser. 2011, 304, 012007. [Google Scholar] [CrossRef]
- Gamboa, J.M.; Leong, K.W. In vitro and in vivo models for the study of oral delivery of nanoparticles. Adv. Drug Del. Rev. 2013, 65, 800–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Choi, S.-J. Particle Size and Biological Fate of ZnO Do Not Cause Acute Toxicity, but Affect Toxicokinetics and Gene Expression Profiles in the Rat Livers after Oral Administration. Int. J. Mol. Sci. 2021, 22, 1698. [Google Scholar] [CrossRef] [PubMed]
- Ramaiah, S.K. A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem. Toxicol. 2007, 45, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Gowda, S.; Desai, P.B.; Kulkarni, S.S.; Hull, V.V.; Math, A.A.; Vernekar, S.N. Markers of renal function tests. N. Am. J. Med. Sci. 2010, 2, 170. [Google Scholar] [PubMed]
- McInnes, E.F. Wistar and sprague-dawley rats. In Background Lesions in Laboratory Animals: A Color Atlas, 1st ed.; Mclnnes, E., Mann, P., Eds.; Elsevier: Amsterdam, The Netherlnds, 2012; pp. 17–36. [Google Scholar]
- Frazier, K.S.; Seely, J.C.; Hard, G.C.; Betton, G.; Burnett, R.; Nakatsuji, S.; Nishikawa, A.; Durchfeld-Meyer, B.; Bube, A. Proliferative and nonproliferative lesions of the rat and mouse urinary system. Toxicol. Pathol. 2012, 40, 14S–86S. [Google Scholar] [CrossRef]
- McInnes, E. Common Spontaneous and Background Lesions in Laboratory Animals. In Pathology for Toxicologists: Principles and Practices of Laboratory Animal Pathology for Study Personnel, 1st ed.; Mclnnes, E., Ed.; Wiley-blackwell: Hoboken, NJ, USA, 2017; pp. 59–71. [Google Scholar]



| Toxicokinetic Parameters | DW | 5% Albumin | 5% Glucose |
|---|---|---|---|
| Cmax (µg/mL) | 25.3 ± 3.1 a | 25.3 ± 2.1 a | 41.9 ± 4.6 b |
| Tmax (h) | 1.3 ± 0.5 a | 1.0 ± 0.0 a | 3.5 ± 1.0 b |
| AUC (h × µg/mL) | 132.8 ± 27.1 a | 126.5 ± 18.2 a | 280.9 ± 49.8 b |
| T1/2 (h) | 2.5 ± 0.0 a | 2.7 ± 0.2 a | 4.5 ± 0.4 b |
| MRT (h) | 4.0 ± 0.4 a | 4.3 ± 0.2 a | 5.8 ± 0.7 b |
| CL/F (mL/h) | 625.2 ± 151.1 a | 641.1 ± 82.3 a | 256.6 ± 24.0 b |
| Absorption (%) | 8.9 ± 1.9 a | 8.4 ± 0.3 a | 18.8 ± 3.1 b |
| Organ | Control | DW | 5% Albumin | 5% Glucose |
|---|---|---|---|---|
| Brain | 0.8 ± 0.0 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| Heart | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 |
| Kidney | 0.8 ± 0.1 | 0.8 ± 0.0 | 0.8 ± 0.1 | 0.8 ± 0.0 |
| Large intestine | 1.9 ± 0.3 | 1.6 ± 0.3 | 1.7 ± 0.3 | 1.5 ± 0.6 |
| Liver | 3.7 ± 0.2 | 3.8 ± 0.3 | 3.9 ± 0.3 | 3.9 ± 0.2 |
| Lung | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 |
| Ovary | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Small intestine | 4.1 ± 0.4 | 4.2 ± 0.3 | 4.3 ± 0.3 | 4.1 ± 0.3 |
| Spleen | 0.3 ± 0.1 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.1 |
| Stomach | 1.7 ± 0.3 | 1.7 ± 0.6 | 1.5 ± 0.4 | 1.3 ± 0.3 |
| Groups | WBC | WBC Differential Counting (%) | RBC | Hb | HCT | MCV | MCH | MCHC | RETI | PLT | PT | APTT | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (103/μL) | NE | LY | MO | EO | BA | (106/μL) | (g/dL) | (%) | (fL) | (pg) | (g/dL) | (%) | (103/μL) | (s) | (s) | |
| Control | 6.89 ± 0.86 | 10.3 ± 1.9 | 85.0 ± 1.9 | 1.9 ± 0.3 | 1.3 ± 0.4 | 0.6 ± 0.1 | 7.08 ± 0.19 | 13.8 ± 0.5 | 44.4 ± 1.7 | 62.6 ± 1.4 | 19.5 ± 0.4 | 31.1 ± 0.5 | 3.25 ± 0.88 | 950 ± 151 | 17.4 ± 0.5 | 38.5 ± 2.7 |
| DW | 6.47 ± 1.21 | 14.6 ± 5.0 | 78.3 ± 5.4 | 2.0 ± 0.3 | 3.1 ± 0.9 ** | 0.8 ± 0.3 | 7.04 ± 0.41 | 13.6 ± 1.0 | 43.8 ± 2.9 | 62.2 ± 1.6 | 19.3 ± 0.5 | 31.0 ± 0.6 | 2.72 ± 0.22 | 902 ± 300 | 17.3 ± 0.9 | 39.0 ± 7.3 |
| 5% albumin | 6.74 ± 1.22 | 7.9 ± 1.8 | 86.4 ± 1.5 | 2.0 ± 0.2 | 1.8 ± 0.4 | 0.7 ± 0.4 | 7.27 ± 0.34 | 14.0 ± 0.5 | 45.4 ± 1.3 | 62.5 ± 1.3 | 19.2 ± 0.4 | 30.8 ± 0.4 | 2.65 ± 0.55 | 1056 ± 76 | 17.1 ± 0.3 | 46.2 ± 4.8 |
| 5% glucose | 6.79 ± 1.37 | 9.5 ± 1.2 | 83.7 ± 1.1 | 2.1 ± 0.5 | 2.2 ± 0.4 | 0.8 ± 0.4 | 7.25 ± 0.33 | 13.9 ± 0.6 | 44.5 ± 2.0 | 61.5 ± 1.1 | 19.2 ± 0.5 | 31.2 ± 0.6 | 2.97 ± 0.60 | 1171 ± 121 | 16.7 ± 0.3 | 40.4 ± 13.1 |
| Groups | TP | ALB | A/G | T-BIL | ALP | AST | ALT | CREA | BUN | CHOL | TG | GLU | CA | IP | CK | Na | K | Cl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g/dL) | (g/dL) | (mg/dL) | (U/L) | (U/L) | (U/L) | (g/dL) | (g/dL) | (g/dL) | (g/dL) | (g/dL) | (g/dL) | (g/dL) | (IU/L) | (mmol/L) | (mmol/L) | (mmol/L) | ||
| Control | 6.8 ± 0.2 | 4.4 ± 0.1 | 1.9 ± 0.1 | 0.0 ± 0.0 | 783 ± 131 | 77 ± 6 | 49 ± 5 | 0.54 ± 0.04 | 26.3 ± 4.7 | 88 ± 14 | 82 ± 9 | 299 ± 33 | 12.7 ± 0.3 | 9.4 ± 0.7 | 185 ± 86 | 147.7 ± 0.7 | 5.60 ± 0.30 | 97.4 ± 0.9 |
| DW | 6.2 ± 0.1 ** | 4.1 ± 0.1 ** | 2.0 ± 0.1 | 0.0 ± 0.0 | 550 ± 63 * | 72 ± 5 | 48 ± 4 | 0.48 ± 0.02 * | 19.6 ± 2.8 * | 90 ± 8 | 66 ± 15 | 289 ± 18 | 12.1 ± 0.3 * | 10.9 ± 0.8 * | 278 ± 229 | 147.2 ± 1.0 | 6.22 ± 0.22 | 97.9 ± 1.2 |
| 5% albumin | 6.2 ± 0.2 ** | 4.1 ± 0.2 ** | 1.9 ± 0.1 | 0.0 ± 0.0 | 542 ± 128 * | 74 ± 7 | 56 ± 9 | 0.49 ± 0.03 | 25.4 ± 2.5 | 83 ± 8 | 51 ± 13 * | 247 ± 39 | 12.1 ± 0.3 * | 10.3 ± 0.8 | 191 ± 45 | 148.0 ± 1.6 | 5.84 ± 0.46 | 98.8 ± 1.2 |
| 5% glucose | 6.1 ± 0.2 ** | 3.9 ± 0.2 ** | 1.8 ± 0.2 | 0.0 ± 0.0 | 592 ± 114 | 71 ± 10 | 48 ± 6 | 0.50 ± 0.02 | 20.0 ± 2.9 | 85 ± 8 | 62 ± 16 | 259 ± 17 | 12.0 ± 0.2 * | 10.4 ± 0.8 | 209 ± 39 | 147.3 ± 1.7 | 5.88 ± 0.40 | 98.7 ± 2.1 |
| Organs | Numbers of Animals | Histopathological Findings | Control | DW | 5% Albumin | 5% Glucose |
|---|---|---|---|---|---|---|
| Liver | 5 | No abnormalities detected | 5 | 5 | 5 | 5 |
| Kidney | 5 | No abnormalities detected | 5 | 5 | 4 | 4 |
| Tubular degeneration and inflammatory cell infiltration minimal | 0 | 0 | 0 | 1 | ||
| Scar, cortical minimal | 0 | 0 | 1 | 0 | ||
| Lung | 5 | No abnormalities detected | 5 | 5 | 5 | 5 |
| Spleen | 5 | No abnormalities detected | 5 | 5 | 5 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, E.-B.; Yu, J.; Choi, S.-J. Interaction between ZnO Nanoparticles and Albumin and Its Effect on Cytotoxicity, Cellular Uptake, Intestinal Transport, Toxicokinetics, and Acute Oral Toxicity. Nanomaterials 2021, 11, 2922. https://doi.org/10.3390/nano11112922
Jung E-B, Yu J, Choi S-J. Interaction between ZnO Nanoparticles and Albumin and Its Effect on Cytotoxicity, Cellular Uptake, Intestinal Transport, Toxicokinetics, and Acute Oral Toxicity. Nanomaterials. 2021; 11(11):2922. https://doi.org/10.3390/nano11112922
Chicago/Turabian StyleJung, Eun-Been, Jin Yu, and Soo-Jin Choi. 2021. "Interaction between ZnO Nanoparticles and Albumin and Its Effect on Cytotoxicity, Cellular Uptake, Intestinal Transport, Toxicokinetics, and Acute Oral Toxicity" Nanomaterials 11, no. 11: 2922. https://doi.org/10.3390/nano11112922
APA StyleJung, E.-B., Yu, J., & Choi, S.-J. (2021). Interaction between ZnO Nanoparticles and Albumin and Its Effect on Cytotoxicity, Cellular Uptake, Intestinal Transport, Toxicokinetics, and Acute Oral Toxicity. Nanomaterials, 11(11), 2922. https://doi.org/10.3390/nano11112922