Facile Synthesis, Characterization, Photocatalytic Activity, and Cytotoxicity of Ag-Doped MgO Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pure and Ag-Doped MgO NPs
2.3. Characterization of Pure and Ag-Doped MgO NPs
2.4. Photocatalytic Activity Measurement
2.5. Cell Culture and Cytotoxicity Assay
3. Results and Discussion
3.1. X-ray Diffraction (XRD) Analysis
3.2. Transmission Electron Microscopy (TEM) Analysis
3.3. Energy-Dispersive X-ray (EDX) Analysis
3.4. Scanning Electron Microscopy (SEM) Analysis
3.5. X-ray Photoelectron Spectroscopy (XPS) Analysis
3.6. Fourier-Transform Infrared (FTIR) Analysis
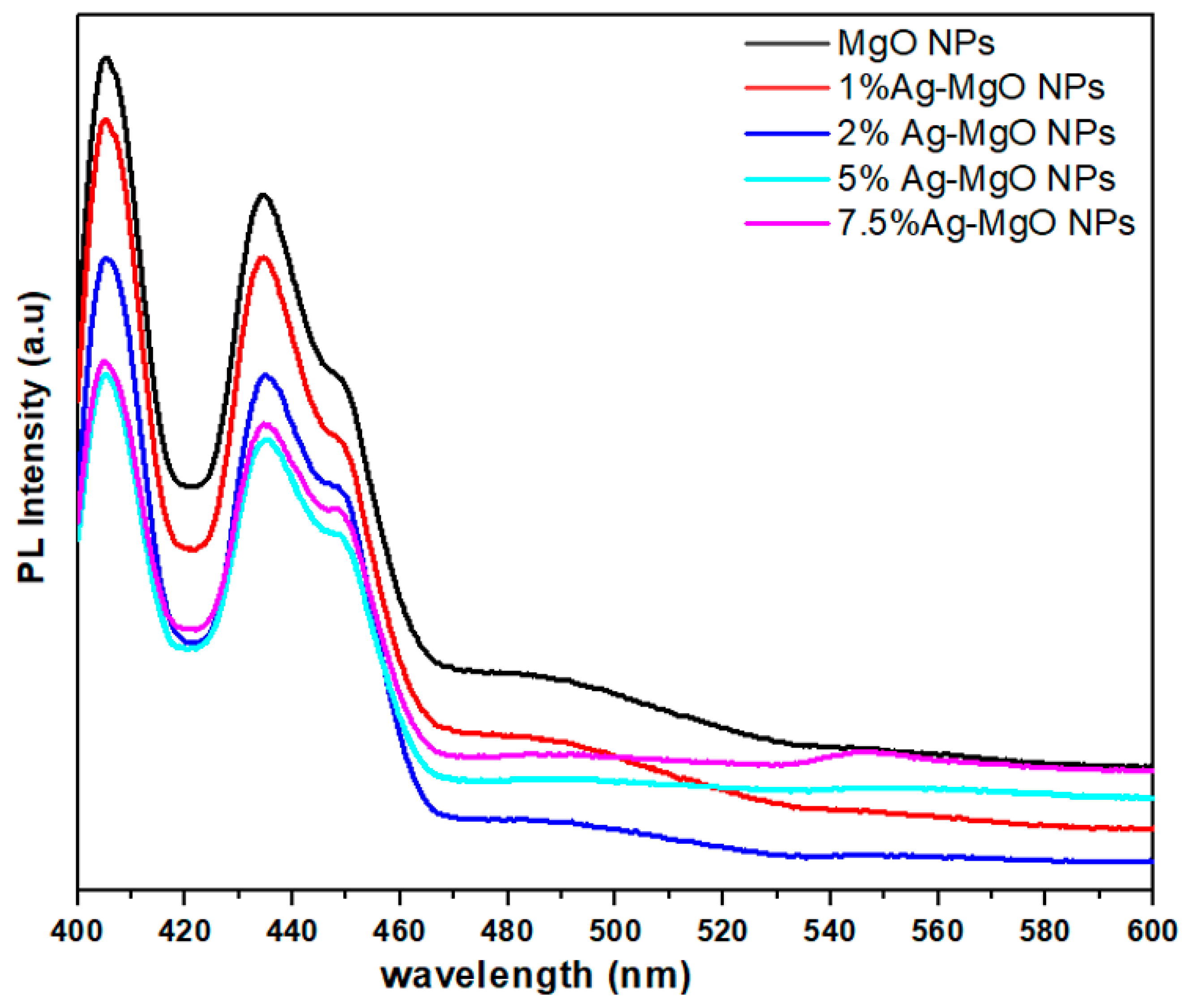
3.7. Photoluminescence (PL) Spectroscopy Analysis
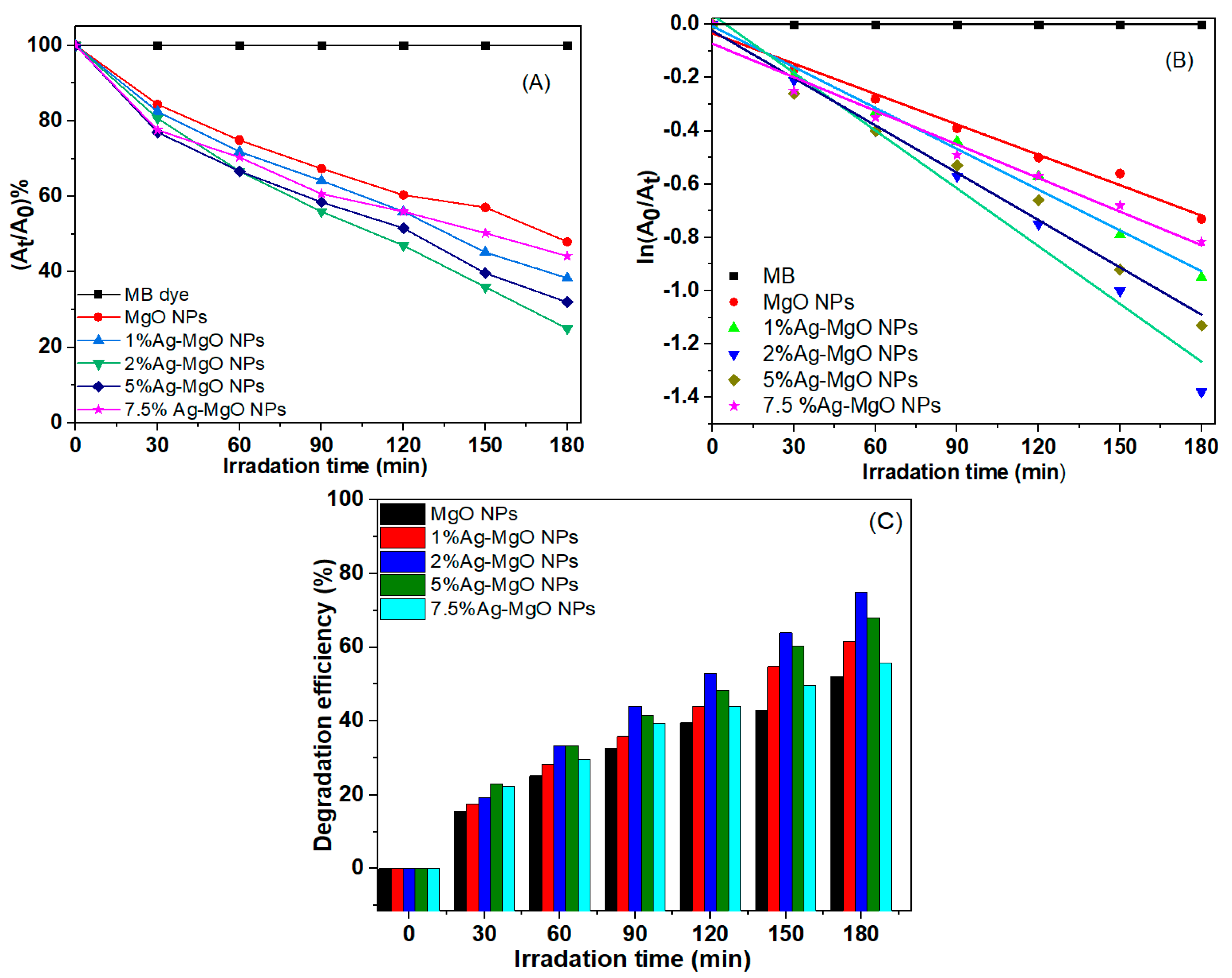
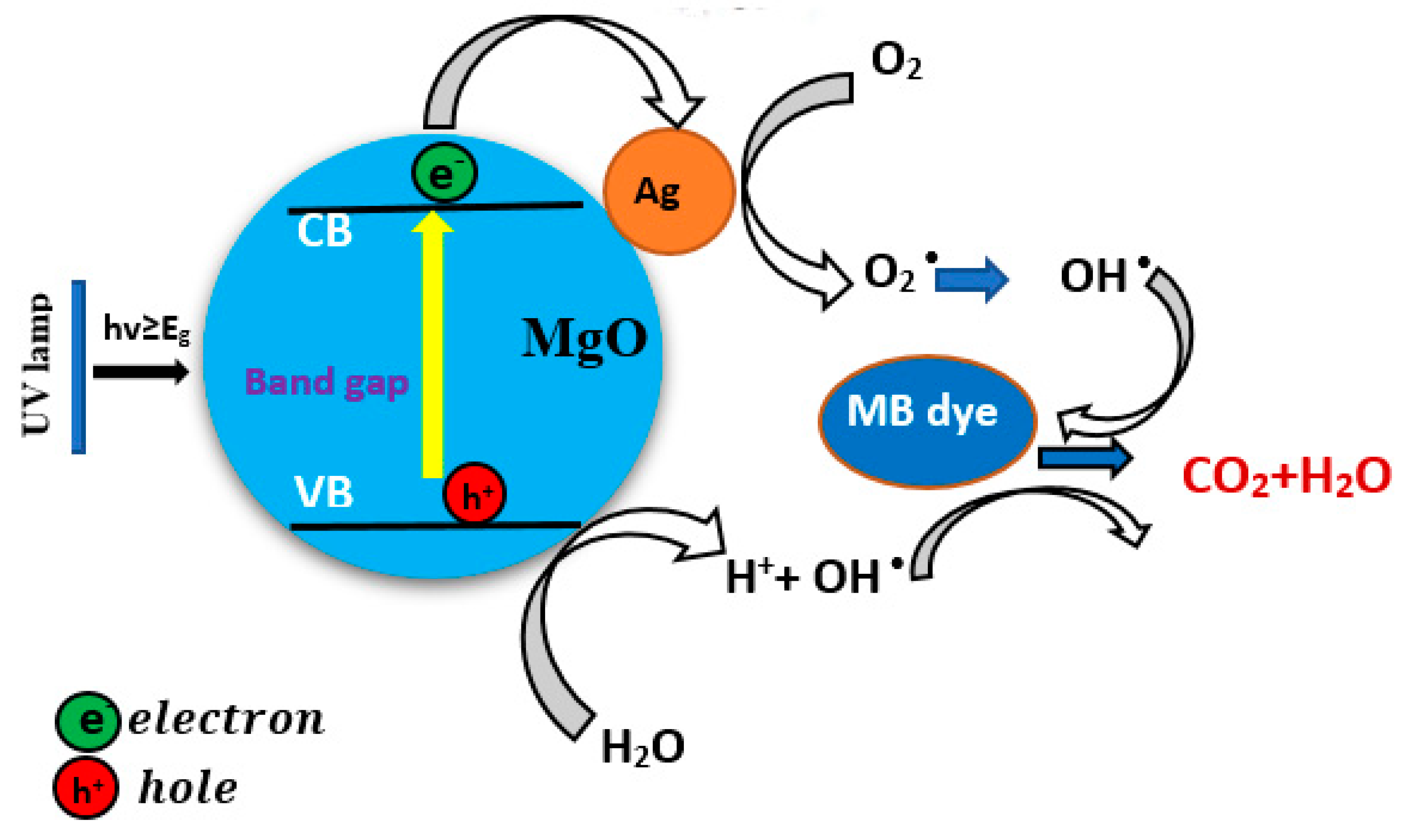
3.8. Photocatalytic Study
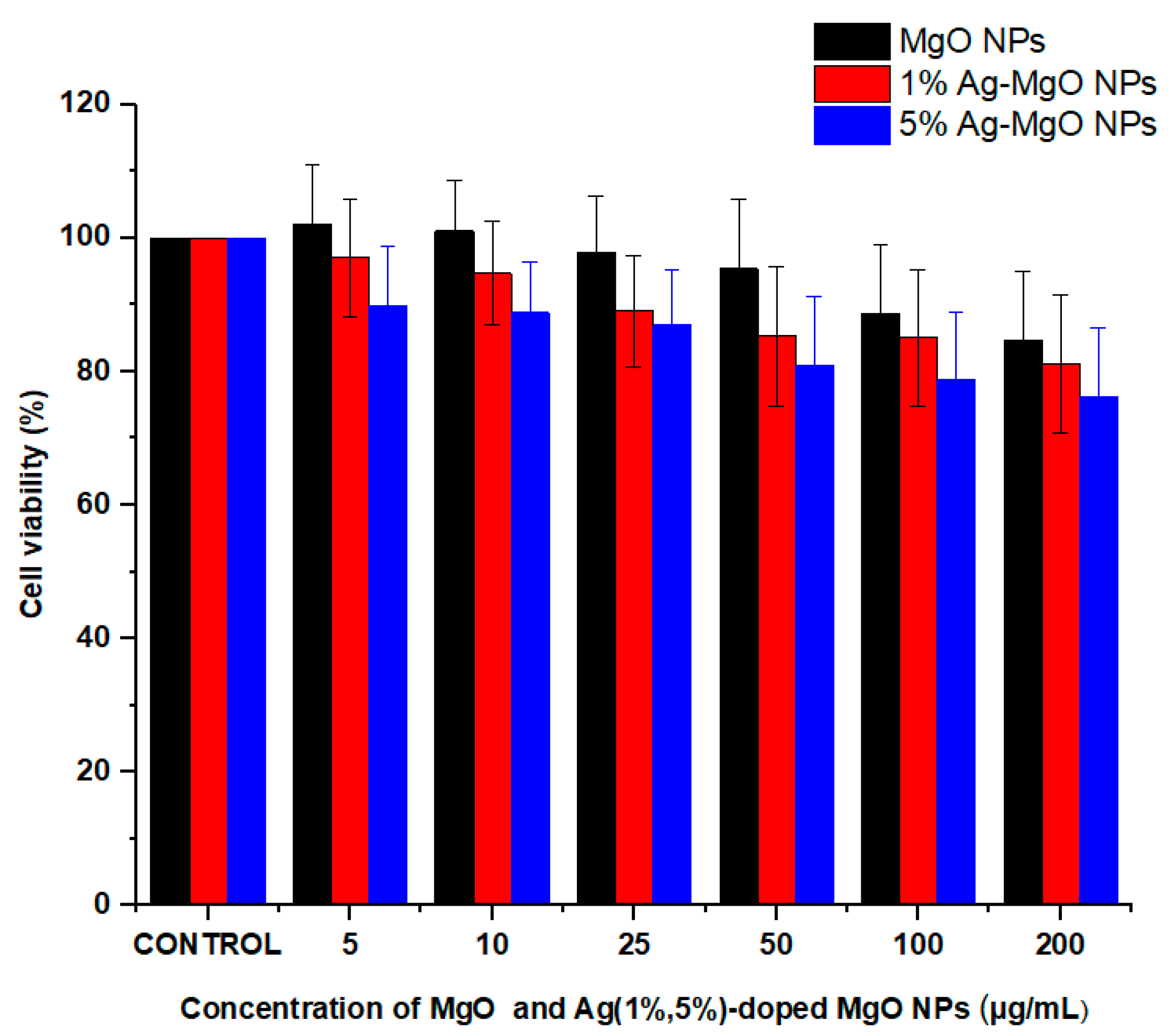
3.9. Cytotoxicity Assay
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, S.; Wang, Z.; Zha, Z.; Wang, S.; Wang, Z. Metal nanoparticles or metal oxide nanoparticles, an efficient and promising family of novel heterogeneous catalysts in organic synthesis. Dalton Trans. 2009, 43, 9363–9373. [Google Scholar] [CrossRef] [PubMed]
- Teske, S.S.; Detweiler, C.S. The Biomechanisms of Metal and Metal-Oxide Nanoparticles’ Interactions with Cells. Int. J. Environ. Res. Public Health 2015, 12, 1112–1134. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.M.; Liu, W.S. The roles of surface-doped metal ions (V, Mn, Fe, Cu, Ce, and W) in the interfacial behavior of TiO2 photocatalysts. Appl. Catal. B Environ. 2014, 156–157, 466–475. [Google Scholar] [CrossRef]
- Wu, H.; Huang, X.; Gao, M.; Liao, X.; Shi, B. Polyphenol-grafted collagen fiber as reductant and stabilizer for one-step synthesis of size-controlled gold nanoparticles and their catalytic application to 4-nitrophenol reduction. Green Chem. 2011, 13, 651–658. [Google Scholar] [CrossRef]
- Ge, S.; Wang, G.; Shen, Y.; Zhang, Q.; Jia, D.; Wang, H.; Dong, Q.; Yin, T. Cytotoxic effects of MgO nanoparticles on human umbilical vein endothelial cells in vitro. IET Nanobiotechnol. 2011, 5, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Bouafia, A.; Laouini, S.E.; Ahmed, A.S.A.; Soldatov, A.V.; Algarni, H.; Chong, K.F.; Ali, G.A.M. The Recent Progress on Silver Nanoparticles: Synthesis and Electronic Applications. Nanomater. Rev. 2021, 11, 2318. [Google Scholar] [CrossRef]
- Rao, Y.; Wang, W.; Tan, F.; Cai, Y.; Lu, J.; Qiao, X. Influence of different ions doping on the antibacterial properties of MgO nanopowders. Appl. Surf. Sci. 2013, 284, 726–731. [Google Scholar] [CrossRef]
- Alinejad, A.; Akbari, H.; Ghaderpoori, M.; Jeihooni, A.K.; Adibzadeh, A. Catalytic ozonation process using a MgO nano-catalyst to degrade methotrexate from aqueous solutions and cytotoxicity studies in human lung epithelial cells (A549) after treatment. RSC Adv. 2019, 9, 8204–8214. [Google Scholar] [CrossRef] [Green Version]
- Güney, H.; İskenderoğlu, D. The effect of Ag dopant on MgO nanocrystallites grown by SILAR method. Mater. Sci. Semicond. Process. 2018, 84, 151–156. [Google Scholar] [CrossRef]
- Srisuvetha, V.T.; Rayar, S.L.; Shanthi, G. Role of cerium (Ce) dopant on structural, optical and photocatalytic properties of MgO nanoparticles by wet chemical route. J. Mater. Sci. Mater. Electron. 2020, 31, 2799–2808. [Google Scholar] [CrossRef]
- Nor Fadilah, C.; Norlida, K.; Nurhanna, B.; Kelimah, E. Effect of cu doped in mgo on nanostructures and their band gap energies. Solid State Phenom. 2019, 290, 323–328. [Google Scholar] [CrossRef]
- Parvizi, E.; Tayebee, R.; Koushki, E. Mg-Doped ZnO and Zn-Doped MgO Semiconductor Nanoparticles; Synthesis and Catalytic, Optical and Electro-Optical Characterization. Semiconductors 2019, 53, 1769–1783. [Google Scholar] [CrossRef]
- Abed, C.; Ali, M.B.; Addad, A.; Elhouichet, H. Growth, structural and optical properties of ZnO-ZnMgO-MgO nanocomposites and their photocatalytic activity under sunlight irradiation. Mater. Res. Bull. 2019, 110, 230–238. [Google Scholar] [CrossRef]
- Mantilaka, M.P.G.; De Silva, R.T.; Ratnayake, S.P.; Amaratunga, G.; de Silva, K.M.N. Photocatalytic activity of electrospun MgO nanofibres: Synthesis, characterization and applications. Mater. Res. Bull. 2018, 99, 204–210. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, D.; Wang, W.; Tan, F.; Wong, P.K.; Wang, X.; Qiu, X.; Qiao, X. Highly effective antibacterial activity and synergistic effect of Ag-MgO nanocomposite against Escherichia coli. J. Alloys Compd. 2016, 684, 282–290. [Google Scholar] [CrossRef]
- Nigam, A.; Saini, S.; Rai, A.K.; Pawar, S.J. Structural, optical, cytotoxicity, and antimicrobial properties of MgO, ZnO and MgO/ZnO nanocomposite for biomedical applications. Ceram. Int. 2021, 47, 19515–19525. [Google Scholar] [CrossRef]
- Patil, H.R.; Murthy, Z.V.P. Vanadium-doped magnesium oxide nanoparticles formation in presence of ionic liquids and their use in photocatalytic degradation of methylene blue. Acta Metall. Sin. Engl. Lett. 2016, 29, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Anilkumar, M.R.; Nagaswarupa, H.P.; Nagabhushana, H.; Sharma, S.C.; Vidya, Y.S.; Anantharaju, K.S.; Prashantha, S.C.; Shivakuamra, C.; Gurushantha, K. Bio-inspired route for the synthesis of spherical shaped MgO:Fe3+ nanoparticles: Structural, photoluminescence and photocatalytic investigation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 703–713. [Google Scholar] [CrossRef]
- Senthilkumar, V.; Ramesh, S. Evaluation of Cytotoxicity of Magnesium Oxide Nanoparticles—An In vitro Study. Int. J. Dent. Oral Sci. 2021, 8, 1739–1743. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Prabhu, R.; Muruganantham, K.; Shanmuganathan, R.; Natarajan, S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J. Photochem. Photobiol. B Biol. 2019, 190, 86–97. [Google Scholar] [CrossRef]
- Ethiraj, A.S.; Uttam, P.; Varunkumar, K.; Chong, K.F.; Ali, G.A. Photocatalytic performance of a novel semiconductor nanocatalyst: Copper doped nickel oxide for phenol degradation. Mater. Chem. Phys. 2020, 242, 122520. [Google Scholar] [CrossRef]
- Sackey, J.; Bashir, A.K.H.; Ameh, A.E.; Nkosi, M.; Kaonga, C.; Maaza, M. Date pits extracts assisted synthesis of magnesium oxides nanoparticles and its application towards the photocatalytic degradation of methylene blue. J. King Saud Univ. Sci. 2020, 32, 2767–2776. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Siddiqui, M.A.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; AlSalhi, M.S.; Alrokayan, S.A. Oxidative stress mediated apoptosis induced by nickel ferrite nanoparticles in cultured A549 cells. Toxicology 2011, 283, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X. Ag-doping improving the detection sensitivity of bolometer based on ZnO thin films. Vacuum 2015, 117, 47–49. [Google Scholar] [CrossRef]
- Kant, R.; Singh, A.K.; Arora, A. Tuning of MgO nanoparticles on Ag dopant additions for charge storage applications. Vacuum 2021, 189, 110247. [Google Scholar] [CrossRef]
- Cai, Y.; Wu, D.; Zhu, X.; Wang, W.; Tan, F.; Chen, J.; Qiao, X.; Qiu, X. Sol-gel preparation of Ag-doped MgO nanoparticles with high efficiency for bacterial inactivation. Ceram. Int. 2017, 43, 1066–1072. [Google Scholar] [CrossRef]
- Patterson, A. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Nasrin, S.; Hoque, S.M.; Chowdhury, F.; Hossen, M.M. Influence of Zn substitution on the structural and magnetic properties of Co 1-x Zn x Fe 2 O 4 nano-ferrites. IOSR J. Appl. Phys. 2014, 6, 58–65. [Google Scholar] [CrossRef]
- Munir, T.; Kashif, M.; Mahmood, K.; Imran, M.; Ali, A.; Sabir, N.; Amin, N.; Mahmood, A.; Ali, H.; Ahmed, N. Impact of silver dopant on structural, optical and electrical properties of ZnO nanoparticles. J. Ovonic Res. 2019, 15, 173–179. [Google Scholar]
- Ivanova, T.; Harizanova, A.; Koutzarova, T.; Vertruyen, B. Sol-gel nanocrystalline ZnO:Ag films: Structural and optical properties. Superlattices Microstruct. 2014, 70, 1–6. [Google Scholar] [CrossRef]
- Albiter, E.; Valenzuela, M.A.; Alfaro, S.; Valverde-Aguilar, G.; Martínez-Pallares, F.M. Photocatalytic deposition of Ag nanoparticles on TiO2: Metal precursor effect on the structural and photoactivity properties. J. Saudi Chem. Soc. 2015, 19, 563–573. [Google Scholar] [CrossRef] [Green Version]
- Majeed Khan, M.A.; Kumar, S.; Ahamed, M.; Alrokayan, S.A. Fe-doping induced tailoring in the microstructure and optical properties of ZnO nanoparticles synthesized via sol–gel route. J. Mater. Sci. Mater. Electron. 2015, 26, 6113–6118. [Google Scholar] [CrossRef]
- Nigussie, G.Y.; Tesfamariam, G.M.; Tegegne, B.M.; Weldemichel, Y.A.; Gebreab, T.W.; Gebrehiwot, D.G.; Gebremichel, G.E. Antibacterial activity of Ag-Doped TiO2 and Ag-Doped ZnO nanoparticles. Int. J. Photoenergy 2018, 2018, 5927485. [Google Scholar] [CrossRef] [Green Version]
- Sagadevan, S.; Pal, K.; Chowdhury, Z.Z.; Hoque, M.E. Structural, dielectric and optical investigation of chemically synthesized Ag-doped ZnO nanoparticles composites. J. Sol. Gel Sci. Technol. 2017, 83, 394–404. [Google Scholar] [CrossRef]
- Ayinde, W.B.; Gitari, M.W.; Muchindu, M.; Samie, A. Biosynthesis of Ultrasonically Modified Ag-MgO Nanocomposite and Its Potential for Antimicrobial Activity. J. Nanotechnol. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Vilayurganapathy, S.; Devaraj, A.; Colby, R.; Pandey, A.; Varga, T.; Shutthanandan, V.; Manandhar, S.; El-Khoury, P.Z.; Kayani, A.; Hess, W.P.; et al. Subsurface synthesis and characterization of Ag nanoparticles embedded in MgO. Nanotechnology 2013, 24, 095707. [Google Scholar] [CrossRef]
- Rani, N.; Chahal, S.; Kumar, P.; Shukla, R.; Singh, S.K. Role of Oxygen Vacancies for Mediating Ferromagnetic Ordering in La-Doped MgO Nanoparticles. J. Supercond. Nov. Magn. 2020, 33, 1473–1480. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Wang, M.H. Biogenic silver embedded magnesium oxide nanoparticles induce the cytotoxicity in human prostate cancer cells. Adv. Powder Technol. 2019, 30, 786–794. [Google Scholar] [CrossRef]
- Patil, S.S.; Mali, M.G.; Tamboli, M.S.; Patil, D.R.; Kulkarni, M.V.; Yoon, H.; Kim, H.; Al-Deyab, S.S.; Yoon, S.S.; Kolekar, S.S.; et al. Green approach for hierarchical nanostructured Ag-ZnO and their photocatalytic performance under sunlight. Catal. Today 2016, 260, 126–134. [Google Scholar] [CrossRef]
- Singh, R.; Barman, P.B.; Sharma, D. Synthesis, structural and optical properties of Ag doped ZnO nanoparticles with enhanced photocatalytic properties by photo degradation of organic dyes. J. Mater. Sci. Mater. Electron. 2017, 28, 5705–5717. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Sarsari, I.A.; Kameli, P.; Salamati, H. Effect of Ag doping on structural, optical, and photocatalytic properties of ZnO nanoparticles. J. Alloys Compd. 2015, 640, 408–415. [Google Scholar] [CrossRef] [Green Version]
- Ali, T.; Ahmed, A.; Alam, U.; Uddin, I.; Tripathi, P.; Muneer, M. Enhanced photocatalytic and antibacterial activities of Ag-doped TiO2 nanoparticles under visible light. Mater. Chem. Phys. 2018, 212, 325–335. [Google Scholar] [CrossRef]
- Balamurugan, S.; Ashna, L.; Parthiban, P. Synthesis of nanocrystalline MgO particles by combustion followed by annealing method using hexamine as a fuel. J. Nanotechnol. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Pimpliskar, P.V.; Motekar, S.C.; Umarji, G.G.; Lee, W.; Arbuj, S.S. Synthesis of silver-loaded ZnO nanorods and their enhanced photocatalytic activity and photoconductivity study. Photochem. Photobiol. Sci. 2019, 18, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, G.; Velavan, R.; Batoo, K.M.; Raslan, E.H. Results in Physics Microstructure, optical and photocatalytic properties of MgO nanoparticles. Results Phys. 2020, 16, 103013. [Google Scholar] [CrossRef]
- Khan, M.I.; Akhtar, M.N.; Ashraf, N.; Najeeb, J.; Munir, H.; Awan, T.I.; Tahir, M.B.; Kabli, M.R. Green synthesis of magnesium oxide nanoparticles using Dalbergia sissoo extract for photocatalytic activity and antibacterial efficacy. Appl. Nanosci. 2020, 10, 2351–2364. [Google Scholar] [CrossRef]
- Diana, P.; Saravanakumar, S.; Prasad, K.H.; Sivaganesh, D.; Chidhambaram, N.; Isaac, R.S.R.; Alshahrani, T.; Shkir, M.; Aifaify, S.; Ali, K.S.S. Enhanced Photocatalytic Decomposition Efficacy of Novel MgO NPs: Impact of Annealing Temperatures. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3027–3036. [Google Scholar] [CrossRef]
- Sahni, G.; Panwar, A.; Kaur, B. Controlled green synthesis of silver nanoparticles by Allium cepa and Musa acuminata with strong antimicrobial activity. Int. Nano Lett. 2015, 5, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Panchal, P.; Paul, D.R.; Sharma, A.; Hooda, D.; Yadav, R.; Meena, P.; Nehra, S.P. Phytoextract mediated ZnO/MgO nanocomposites for photocatalytic and antibacterial activities. J. Photochem. Photobiol. A Chem. 2019, 385, 112049. [Google Scholar] [CrossRef]
- Mageshwari, K.; Mali, S.S.; Sathyamoorthy, R.; Patil, P.S. Template-free synthesis of MgO nanoparticles for effective photocatalytic applications. Powder Technol. 2013, 249, 456–462. [Google Scholar] [CrossRef]
- Moradi, M.; Moussavi, G.; Yaghmaeian, K.; Yazdanbakhsh, A.; Srivastava, V.; Sillanpää, M. Synthesis of novel Ag-doped S-MgO nanosphere as an efficient UVA/LED-activated photocatalyst for non-radical oxidation of diclofenac: Catalyst preparation and characterization and photocatalytic mechanistic evaluation. Appl. Catal. B Environ. 2020, 260, 118128. [Google Scholar] [CrossRef]
- Azfar, A.K.; Kasim, M.F.; Lokman, I.M.; Rafaie, H.A.; Mastuli, M.S. Comparative study on photocatalytic activity of transition metals (Ag and Ni)doped ZnO nanomaterials synthesized via sol–gel method. R. Soc. Open Sci. 2020, 7, 191590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abirami, R.; Kalaiselvi, C.R.; Kungumadevi, L.; Senthil, T.S.; Kang, M. Synthesis and characterization of ZnTiO3 and Ag doped ZnTiO3 perovskite nanoparticles and their enhanced photocatalytic and antibacterial activity. J. Solid State Chem. 2020, 281, 121019. [Google Scholar] [CrossRef]
- Laouini, S.E.; Bouafia, A.; Soldatov, A.V.; Algarni, H.; Tedjani, M.L.; Ali, G.A.M.; Barhoum, A. Green synthesized of Ag/Ag2O nanoparticles using aqueous leaves extracts of phoenix dactylifera l. And their azo dye photodegradation. Membranes 2021, 11, 468. [Google Scholar] [CrossRef]
- Mendoza-Mendoza, E.; Nuñez-Briones, A.G.; García-Cerda, L.A.; Peralta-Rodríguez, R.D.; Montes-Luna, A.J. One-step synthesis of ZnO and Ag/ZnO heterostructures and their photocatalytic activity. Ceram. Int. 2018, 44, 6176–6180. [Google Scholar] [CrossRef]
- Maruthai, J.; Muthukumarasamy, A.; Baskaran, B. Optical, biological and catalytic properties of ZnO/MgO nanocomposites derived via Musa paradisiaca bract extract. Ceram. Int. 2018, 44, 13152–13160. [Google Scholar] [CrossRef]
- Koo, Y.; Littlejohn, G.; Collins, B.; Yun, Y.; Shanov, V.N.; Schulz, M.; Pai, D.; Sankar, J. Synthesis and characterization of Ag-TiO2-CNT nanoparticle composites with high photocatalytic activity under artificial light. Compos. Part. B Eng. 2014, 57, 105–111. [Google Scholar] [CrossRef]
- Khan, M.A.M.; Siwach, R.; Kumar, S.; Ahmed, J.; Ahamed, M. Hydrothermal preparation of Zn-doped In2O3 nanostructure and its microstructural, optical, magnetic, photocatalytic and dielectric behaviour. J. Alloys Compd. 2020, 846, 156479. [Google Scholar] [CrossRef]
- Xiang, X.; Xie, L.; Li, Z.; Li, F. Ternary MgO/ZnO/In2O3 heterostructured photocatalysts derived from a layered precursor and visible-light-induced photocatalytic activity. Chem. Eng. J. 2013, 221, 7975. [Google Scholar] [CrossRef]
- Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alaizeri, Z.M.; Alhadlaq, H. Facile synthesis of Zn-doped Bi2O3nanoparticles and their selective cytotoxicity toward cancer cells. ACS Omega 2021, 6, 17353–17361. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Xue, Z.; Li, S.; Zhong, C. Comparison of cytotoxicity of Ag/ZnO and Ag@ZnO nanocomplexes to human umbilical vein endothelial cells in vitro. J. Appl. Toxicol. 2021, 41, 811–819. [Google Scholar] [CrossRef] [PubMed]

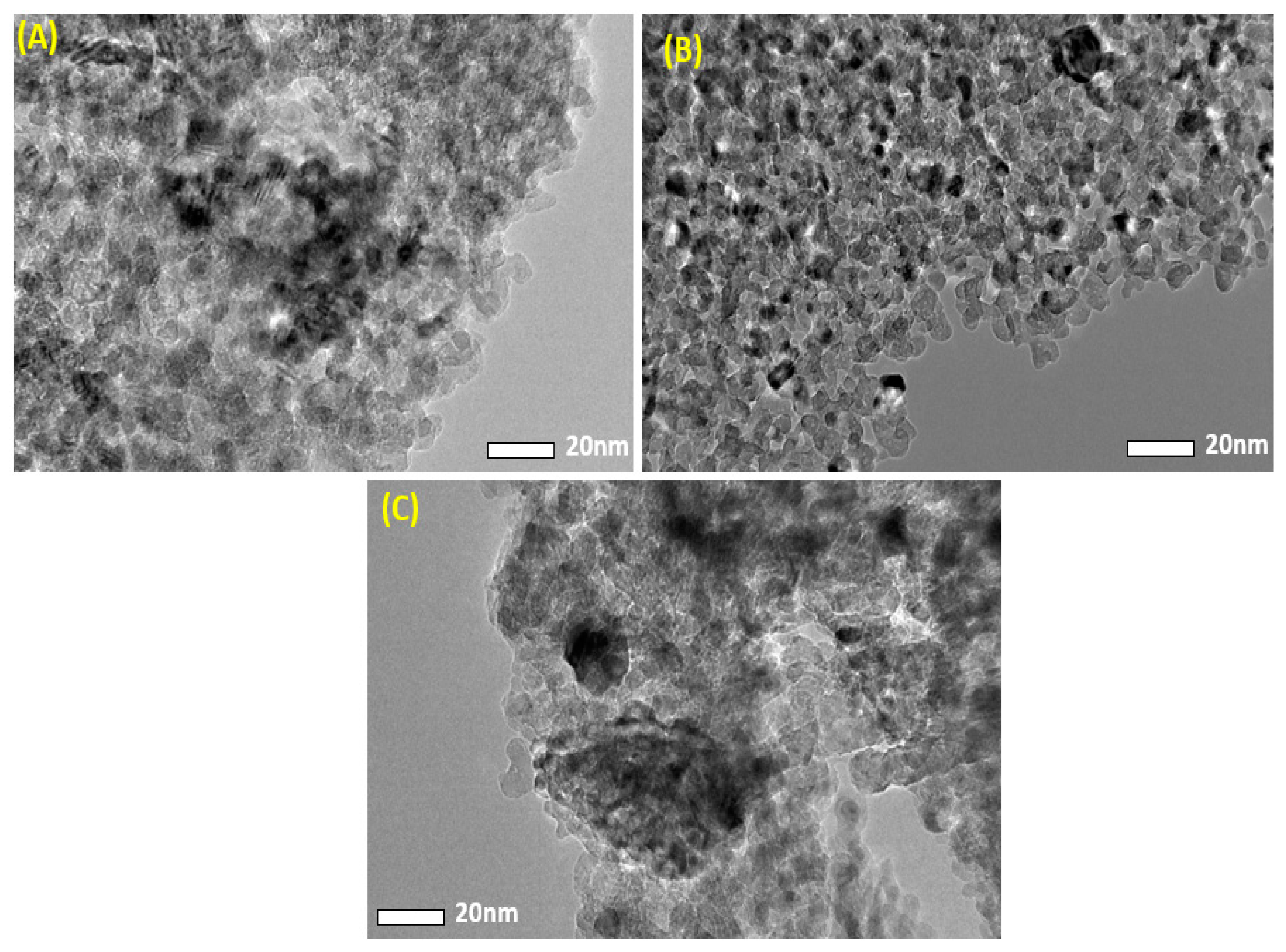

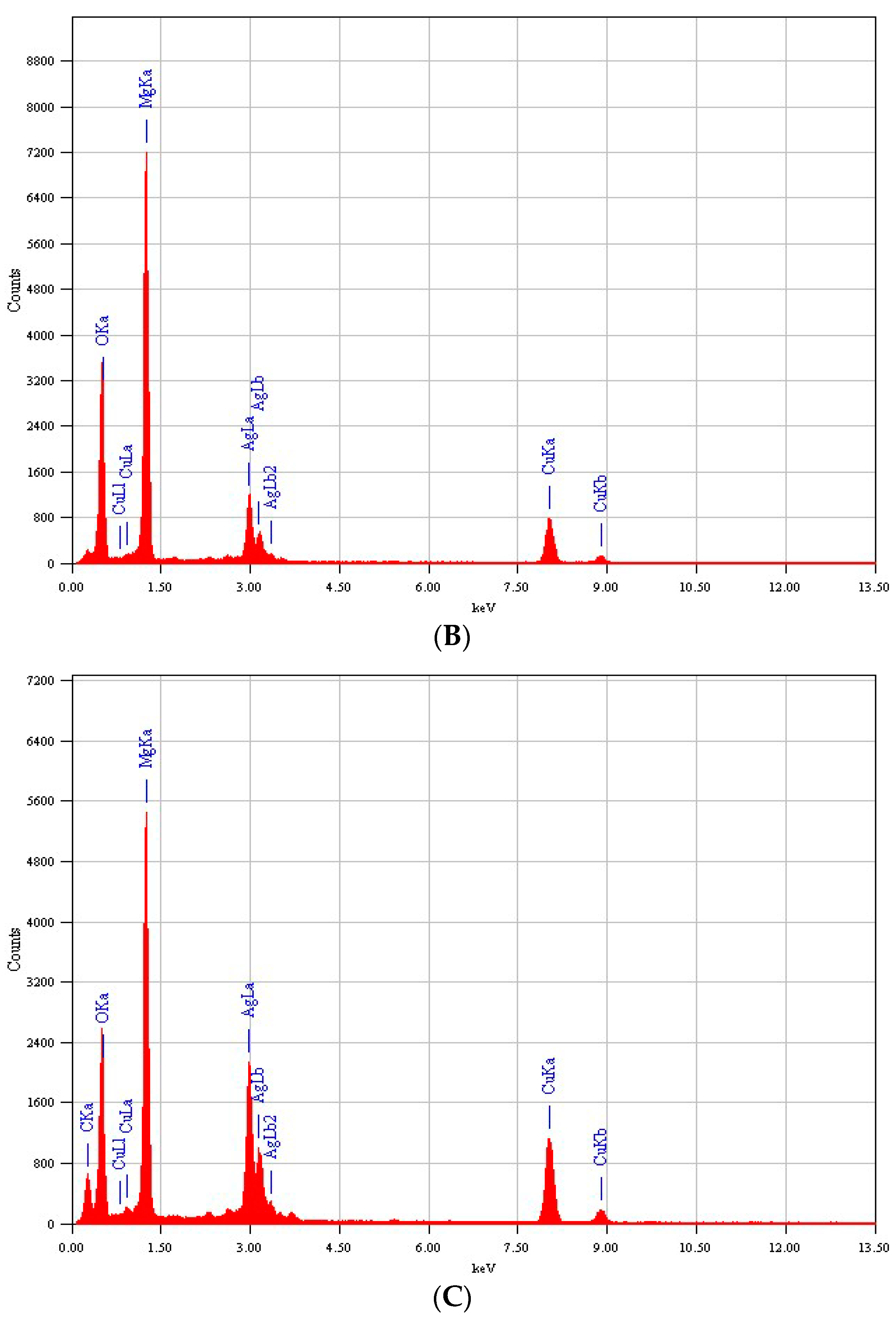


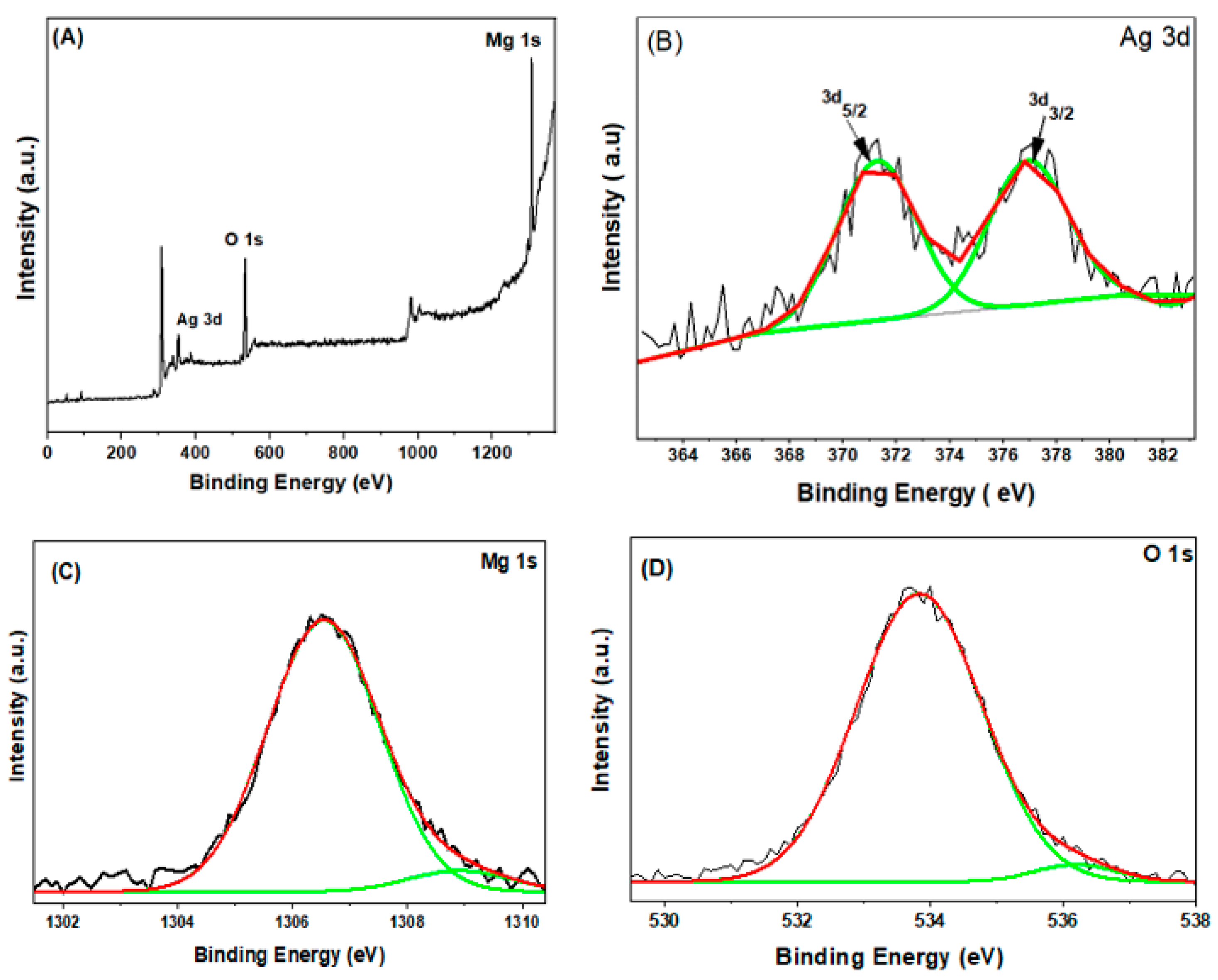
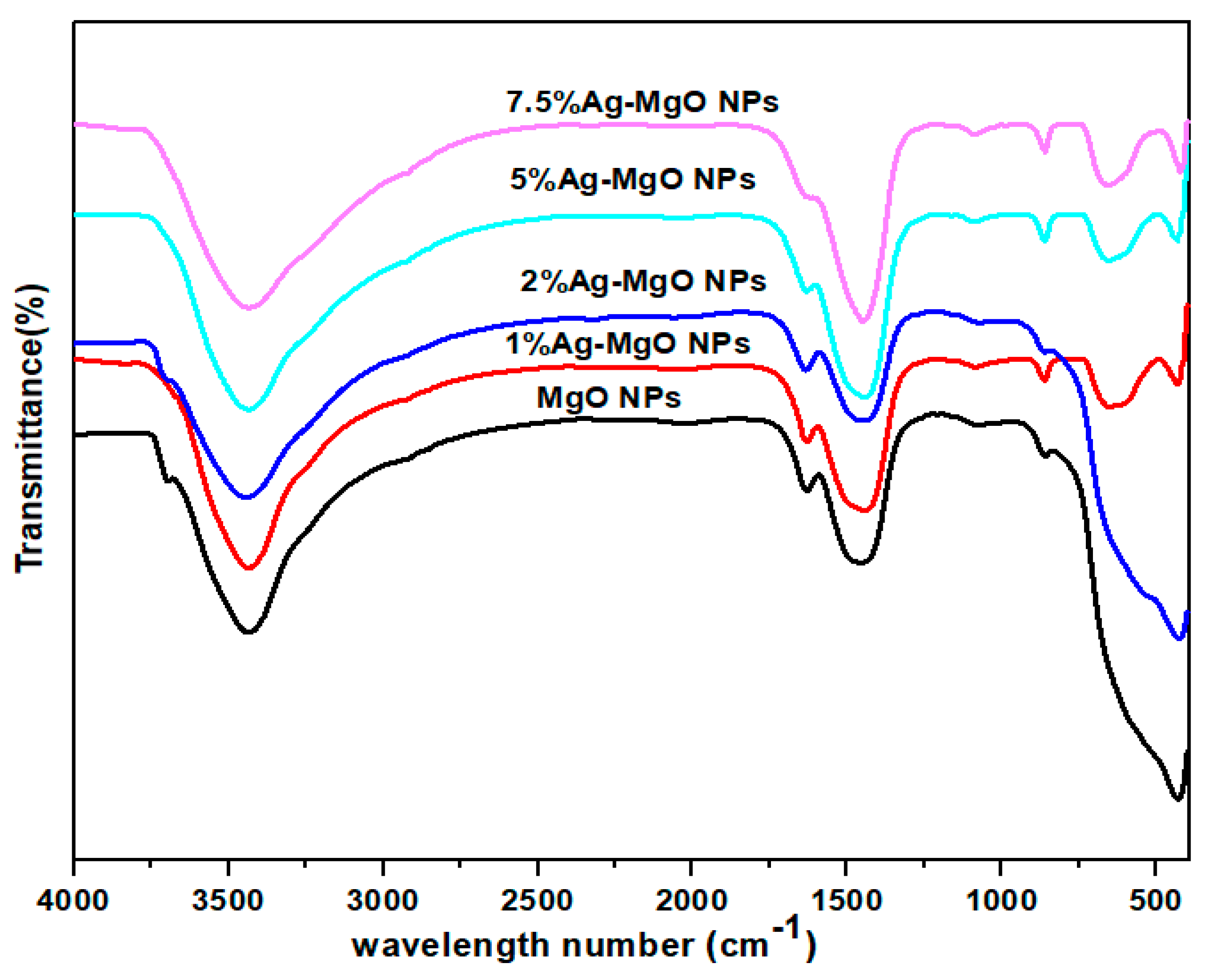

| Sample | XRD Size(nm) | TEM Size (nm) |
|---|---|---|
| MgO | 12 nm | 14.3 nm |
| 1% Ag-MgO | 11.5 nm | 13.3 nm |
| 2% Ag-MgO | 11 nm | 12 nm |
| 5% Ag-MgO | 9.5 nm | 10.5 nm |
| 7.5% Ag-MgO | 9 nm | 9.5 nm |
| Samples | Degradation Efficiency (%) | Source of Light | References |
|---|---|---|---|
| Ag/Ag2O NPs | 84.50% | UV–vis light irradiation | [55] |
| Fe/MgO NPs | 88% | UV–vis light irradiation | [18] |
| Ag/ZnO NPs | 96% | UV–vis light irradiation | [56] |
| ZnO/MgO nanocomposite | 91% | UV–vis light irradiation | [57] |
| Ag/TiO2/CNT NPs | 50% | Artificial light | [58] |
| Zn/In2O3 NPs | 81% | UV–vis light irradiation | [59] |
| MgO/ZnO/In2O3 nanocomposite | 80% | UV–vis light irradiation | [60] |
| Ag-MgO NPs | 75% | UV–vis light irradiation | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alaizeri, Z.M.; Alhadlaq, H.A.; Aldawood, S.; Akhtar, M.J.; Amer, M.S.; Ahamed, M. Facile Synthesis, Characterization, Photocatalytic Activity, and Cytotoxicity of Ag-Doped MgO Nanoparticles. Nanomaterials 2021, 11, 2915. https://doi.org/10.3390/nano11112915
Alaizeri ZM, Alhadlaq HA, Aldawood S, Akhtar MJ, Amer MS, Ahamed M. Facile Synthesis, Characterization, Photocatalytic Activity, and Cytotoxicity of Ag-Doped MgO Nanoparticles. Nanomaterials. 2021; 11(11):2915. https://doi.org/10.3390/nano11112915
Chicago/Turabian StyleAlaizeri, ZabnAllah M., Hisham A. Alhadlaq, Saad Aldawood, Mohd Javed Akhtar, Mabrook S. Amer, and Maqusood Ahamed. 2021. "Facile Synthesis, Characterization, Photocatalytic Activity, and Cytotoxicity of Ag-Doped MgO Nanoparticles" Nanomaterials 11, no. 11: 2915. https://doi.org/10.3390/nano11112915
APA StyleAlaizeri, Z. M., Alhadlaq, H. A., Aldawood, S., Akhtar, M. J., Amer, M. S., & Ahamed, M. (2021). Facile Synthesis, Characterization, Photocatalytic Activity, and Cytotoxicity of Ag-Doped MgO Nanoparticles. Nanomaterials, 11(11), 2915. https://doi.org/10.3390/nano11112915










