1. Introduction
Owing to their unique electrochemical and magnetic properties, magnetite (Fe
3O
4) nanoparticles have gained significant attention for application in various fields, including biomedical fields [
1], catalysis [
2], resistive switching memory [
3], energy storage [
4], and electromagnetic interference (EMI) shielding [
5]. However, they suffer from low chemical stability and agglomeration owing to their relatively high surface energy [
6,
7]. Therefore, to overcome these limitations of Fe
3O
4, complementary and related maintenance functions are implemented through the formation of composites with various functional groups [
8,
9,
10] and organic/inorganic materials [
7,
11,
12,
13,
14,
15].
Among the various materials that are used for forming composites with Fe
3O
4, tin dioxide (SnO
2), an n-type semiconductor with a wide bandgap (Eg = 3.6 eV at 300 K), has been extensively investigated [
16]. Owing to its unique characteristics, SnO
2 can stabilize the electronic, thermal, and chemical properties of Fe
3O
4 through the proximity effect and equilibration of potentials [
17,
18,
19], and various efforts have been made to realize the electrochemical applications of the composites of Fe
3O
4 and SnO
2. However, Fe
3O
4-SnO
2 composite materials show poor electrical conductivity, which limits their applications. Therefore, it is necessary to combine these composites with high-conductivity materials, such as precious metals and carbon materials, to enhance their conductivity [
20,
21].
In particular, numerous studies have been carried out on the preparation of composites of Fe
3O
4-SnO
2 particles with carbon-based materials to improve their conductivity and performance for application in various fields. Wang et al. [
22] prepared a composite obtained by combining core–shell Fe
3O
4@SnO
2 with reduced graphene oxide (rGO), a carbon-based oxide, for application in EMI shielding. Iron oxide with high magnetic permeability and low complex permittivity improved the dielectric properties of the composite, thus improving its EM wave absorption performance. Furthermore, the combination of rGO (with high electrical conductivity) with Fe
3O
4@SnO
2 resulted in electric dipolar polarization and interfacial polarization. Hence, the composite showed an enhanced electromagnetic wave absorption effect in the high-frequency region. Chen et al. [
23] fabricated Fe
3O
4/SnO
2/C composite particles for application as an anode for lithium-ion batteries. The complexation of Fe
3O
4 and SnO
2 improved the reversibility of the LiO
2 conversion reaction, thereby increasing the specific capacity and charge/discharge efficiency of the anode. In addition, the surface modification of the composite with carbon improved the conductivity of the active material and its affinity with the electrolyte. Thus, the Fe
3O
4/SnO
2/C composite showed a more stable rate performance than the Fe
3O
4/SnO
2 particles during cycling. Therefore, the preparation of Fe
3O
4-SnO
2-C composites has become a topic of great scientific interest.
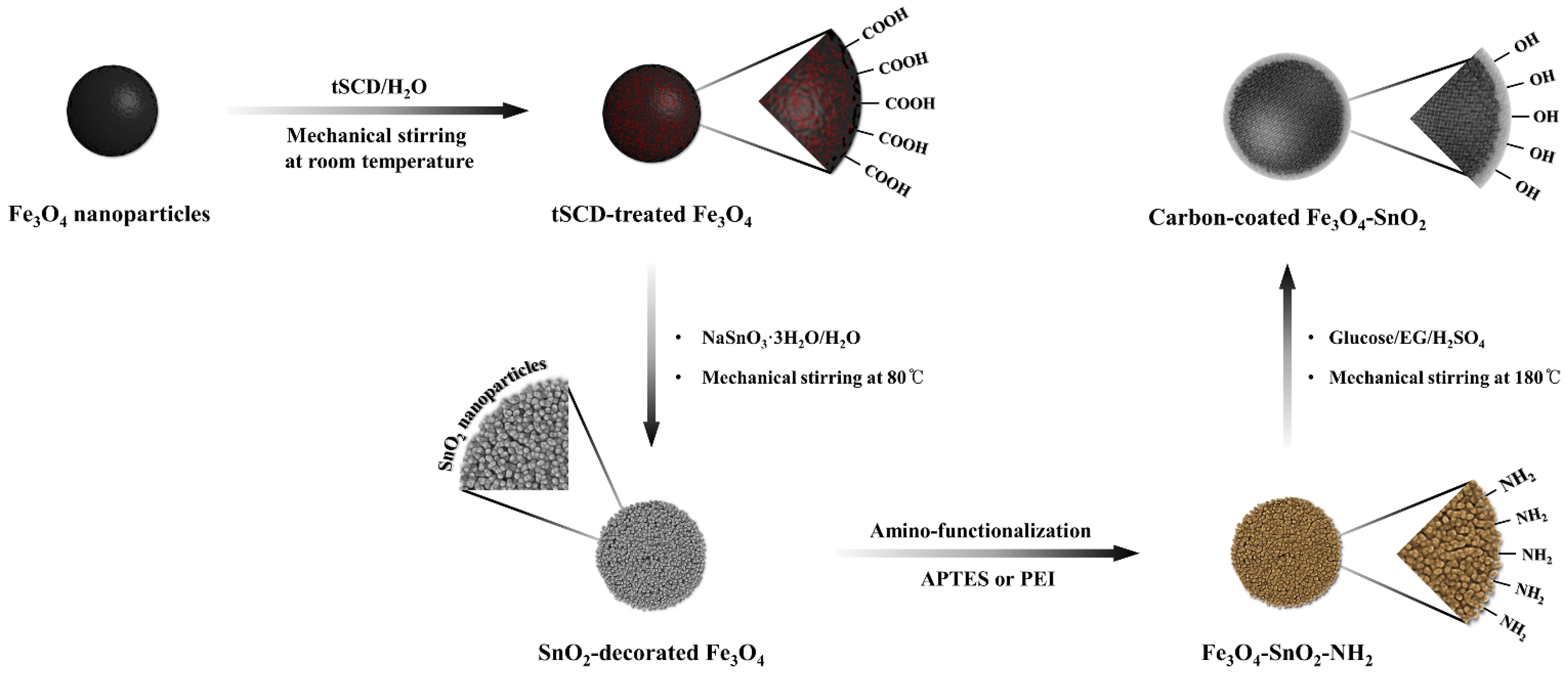
The fabrication of SnO
2-decorated Fe
3O
4 composites through facile surface modification has been reported [
24]. Fe
3O
4 nanoparticles were carboxylated using polyacrylic acid (PAA) or tri-sodium citrate dihydrate (tSCD), and the surface modification method using tSCD was more effective than that using PAA. The results demonstrated the feasibility of the preparation of spherical nanoparticles surrounded by ultrafine SnO
2 nanoparticles. Motivated by these results, in this study, we prepared Fe
3O
4-SnO
2-C composite particles with a three-layered core–shell structure through the dehydration of glucose under atmospheric pressure (
Figure 1). Fe
3O
4-SnO
2 nanoparticles were amino-functionalized using a polymeric precursor, PEI, and a silane precursor, APTES. Subsequently, the effects of the functionalizing amine groups on the structure of the multilayered particles were investigated. In addition, the mechanism underlying the formation of the core–shell structure by the reaction between the polymerized layer formed by combining glucose, a monosaccharide-based carbon precursor, and the aminated nanoparticles and sulfuric acid was investigated.
2. Materials and Methods
2.1. Materials
Ethylene glycol (EG, >99.5%, Samchun Pure Chemical, Pyeongtaek-si, Korea), ferric chloride hexahydrate (FeCl3·6H2O, >97%, Sigma Aldrich, USA), sodium acetate (NaOAc, >99.5%, Sigma Aldrich, St. Louis, MO, USA), ethanol (99.9%, Daejung Chemical, Siheung-si, Korea), tri-sodium citrate dihydrate (tSCD, HOC(COONa)(CH2COONa)2·2H2O, ≥99.0%, Sigma Aldrich, St. Louis, MO, USA), sodium hexahydroxostannate (Na2SnO3·3H2O, 95%, Junsei, Tokyo, Japan), (3-Aminopropyl)triethoxysilane (APTES, 98%, Sigma Aldrich, St. Louis, MO, USA), polyethyleneimine (PEI, branched, Mw ~25,000, Sigma Aldrich, St. Louis, MO, USA), D-(+)-glucose (>99.5%, Sigma Aldrich, St. Louis, MO, USA) and sulfuric acid (H2SO4, >98%, Daejung Chemical, Siheung-si, Korea) were used without further treatment.
2.2. Preparation of Core–Shell Structured Fe3O4-SnO2 Nanoparticles
Fe
3O
4-SnO
2 nanoparticles were prepared as described in our previous report [
24]. To prepare the Fe
3O
4 nanoparticles, 45 g of FeCl
3·6H
2O was added to distilled water under stirring at room temperature, and 0.5 M NaOAc was dissolved in 1000 mL of EG. These two solutions were then transferred to a three-necked round-bottom flask, and the resulting solution was heated to boiling with mechanical stirring and maintained at this temperature for 18 h. After the completion of the reaction, the solution was naturally cooled to room temperature, and the reactants were separated from the solution using a magnet. Subsequently, the reactants were rinsed with distilled water and ethanol several times to eliminate the organic and inorganic by-products.
The prepared Fe3O4 nanoparticles (1 g) were dispersed in 100 mL of distilled water. The resulting suspension was then transferred to a three-neck round-bottom flask, followed by the addition of 0.5 M tSCD. The mixed solution was mechanically stirred at 300 rpm for 24 h at room temperature. After the completion of the reaction, the surface-modified particles were separated from the solution and subsequently rinsed several times with distilled water.
The surface-modified Fe3O4 nanoparticles (0.5 g) were ultrasonically redispersed in 300 mL of distilled water for 30 min. Subsequently, 1 g of Na2SnO3·3H2O was added to the suspension under stirring at 300 rpm for 24 h at 80 °C. The reaction-terminated suspension was naturally cooled to room temperature, and the particles were separated using a magnet. The resultant particles were washed several times with distilled water.
2.3. Amino Functionalization of the Core–Shell Fe3O4-SnO2 Nanoparticles
The surface amino-functionalization process was carried out using two different methods. In the case of the inorganic-precursor-based amino functionalization, the Fe3O4-SnO2 nanoparticles (0.5 g) were ultrasonically dispersed for 15 min in a 100 mL solution of distilled water and ethanol (1:1 vol%). The dispersed Fe3O4-SnO2 suspension was then transferred to a three-neck round-bottom flask, followed by the injection of 0.09 M of APTES. The suspension was mechanically stirred at 350 rpm and 70 °C for 48 h. After the reaction, the solution was naturally cooled to room temperature and the reactants were separated from the solution using a magnet. The resultant particles were washed several times with ethanol and distilled water.
In the case of the polymeric-precursor-based amino functionalization, the Fe3O4-SnO2 nanoparticles (0.5 g) were ultrasonically redispersed for 20 min in 200 mL of distilled water with 5 wt% of PEI. Subsequently, the dispersed suspension was mechanically stirred at 300 rpm in a round-bottom flask and heat-treated at 80 °C for 18 h. The solution was cooled to room temperature, and the resulting particles were washed with distilled water several times to eliminate the by-products.
2.4. Carbonization of the Core–Shell Fe3O4-SnO2 Nanoparticles
The amino-functionalized Fe3O4–SnO2 nanoparticles (0.5 g) were redispersed in 400 mL of EG for 30 min via ultrasonication. The mixture solution was transferred to a three-neck round-bottom flask equipped with a reflux condenser, followed by the addition of 30 g of D-(+)-glucose. Subsequently, the solution was mechanically stirred at 300 rpm for 20 h at 180 °C under reflux conditions. Subsequently, the heated solution was slowly cooled to room temperature and immediately reheated to 60 °C. Then, 2 mL of 0.1 M H2SO4 was added to the solution, and the resulting solution was heated to 180 °C and maintained for 24 h. After the reaction, the solution was naturally cooled to room temperature, and the particles were separated from the solution using a magnet. The synthesized particles were washed several times with ethanol and distilled water.
2.5. Characterization
The surface characteristics of the surface-modified particles were investigated using Fourier transform infrared (FTIR) spectroscopy (Nicolet 5700, Thermo Electron, Waltham, MA, USA). The surface charges and dispersion properties of the particles were evaluated by carrying out zeta potential and particle size distribution analyses (Zetasizer Nano ZS, Malvern, UK). The crystal structures of the synthesized nanoparticles were analyzed using X-ray diffraction (XRD, UltimaIV, Rigaku, Japan) with Cu Kα radiation (λ = 1.5418 Å). The morphologies of the nanoparticles were investigated using high-resolution transmission electron microscopy (HRTEM, Tecnai G2 F30 S-Twin, FEI, Hillsboro, OR, USA). The degree of graphitization of the carbon layer on the particle surface was investigated using Raman spectroscopy (NRS-3100, Jasco, Easton, PN, USA), with an excitation wavelength of 532 nm. Moreover, the magnetic properties of the particles were evaluated using a vibrating sample magnetometer (Lake Shore 7400, Cryotronics Inc., Westerville, OH, USA) at the applied field of −10–10 kOe at room temperature.
3. Results and Discussion
To identify the surface functional groups of the as-prepared nanoparticles, their FTIR spectra were analyzed, as shown in
Figure 2. The Fe
3O
4 nanoparticles exhibited a peak at 582 cm
−1, corresponding to the stretching of the Fe-O bond in the tetrahedral sites [
8]. In contrast, the SnO
2 nanoparticles synthesized under the same experimental conditions exhibited peaks corresponding to the stretching of the Sn–OH, O–Sn–O, and Sn-O bonds at 550, 618, and 941 cm
−1, respectively [
25]. The peaks at 1639 and 3415 cm
−1 can be ascribed to the –OH stretching vibration of the particle surface of the hydroxyl group, which was generated by the absorption of H
2O from the ambient atmosphere [
25]. The tSCD-treated Fe
3O
4 nanoparticles exhibited –CH
2 and –CH
3 vibration peaks at 2857 and 2920 cm
−1, respectively. In addition, peaks corresponding to the vibration of the C–O and COO–Fe bonds were observed at 1393 and 1619 cm
−1, respectively [
26]. This confirms the presence of carboxyl groups on the particle surface.
The Fe
3O
4-SnO
2 nanoparticles exhibited peaks at 582 and 550 cm
−1, corresponding to the Fe–O and O–Sn–O bonds, respectively. Additionally, the peak at 941 cm
−1 can be ascribed to the stretching vibration of the Sn–O bond, which originated from the SnO
2 formed on the Fe
3O
4 surface. In the case of the APTES-treated Fe
3O
4-SnO
2 nanoparticles, an absorption peak was observed at approximately 3300 cm
−1, corresponding to the symmetric and asymmetric stretching modes of NH/NH
2 [
27,
28]. The peak at 1048 cm
−1 can be ascribed to the Si–O–Si bond, which originated from the silane group of APTES [
27]. On the other hand, the PEI-treated Fe
3O
4-SnO
2 nanoparticles exhibited –CH
2 and –CH
3 vibration peaks at 2843 and 2919 cm
−1, respectively [
29]. In addition, symmetric and asymmetric NH/NH
2 stretching modes were observed at 1639 and approximately 3300 cm
−1, respectively [
30]. Therefore, it can be stated that various organic functional groups were present on the surface of the PEI- and APTES-modified Fe
3O
4-SnO
2 nanoparticles with different structures.
The zeta potentials of the nanoparticles were measured to examine the changes in their surface properties after their surface modification under distilled water conditions (
Table 1). In the table, each sample displacement is the result of 10 measurements under the same conditions. The zeta potential value of the Fe
3O
4 nanoparticles was −5.4 mV, which indicates that these particles showed a small amount of surface charge. The Fe
3O
4-SnO
2 nanoparticles showed a zeta potential of −42.3 mV. In contrast, the zeta potentials of both the APTES- and PEI-treated particles were positive (31.8 and 32.9, respectively) because of the formation of amine groups on their surfaces. Although these modified nanoparticles showed similar zeta potential values, the average value and standard deviation of the PEI-treated particles were slightly larger. Therefore, the initial glucose conjugation amount could be set identically by minimizing the effect of electrostatic attraction that might have been caused by the surface charge on each particle during the carbon coating process.
Figure 3 shows the particle size distributions of the as-prepared Fe
3O
4, Fe
3O
4-SnO
2, APTES-treated Fe
3O
4-SnO
2, and PEI-treated Fe
3O
4-SnO
2 nanoparticles in distilled water. The distribution curve of the Fe
3O
4 nanoparticles showed a bimodal shape with a wide size distribution range of 200–2650 nm and a mean size of 821 nm. This can be attributed to the presence of particles aggregated because of the high surface energy generated at the nanoscale and the low surface charge of the Fe
3O
4 nanoparticles [
31]. In contrast, the surface-decorated/functionalized nanoparticles exhibited monomodal curves. The measured mean size distribution values of the Fe
3O
4-SnO
2, APTES-treated Fe
3O
4-SnO
2, PEI-treated Fe
3O
4-SnO
2 nanoparticles were 476, 551, and 578 nm, respectively. In the case of the Fe
3O
4-SnO
2 nanoparticles, the hydroxyl groups present on the SnO
2 surface generated a high surface charge, improving the dispersibility of the particles in the solvent. On the other hand, the APTES- and PEI-treated Fe
3O
4-SnO
2 nanoparticles showed relatively low surface charge, and thus showed slightly lower dispersibility than the unfunctionalized Fe
3O
4-SnO
2 nanoparticles.
The crystal structures of the Fe
3O
4, Fe
3O
4-SnO
2, PEI-treated Fe
3O
4-SnO
2, and APTES-treated Fe
3O
4-SnO
2 nanoparticles were investigated using XRD (
Figure 4). The Fe
3O
4 nanoparticles exhibited significant diffraction peaks at 2θ = 30.2, 35.5, 43.2, 53.5, 57.1, 62.7, and 74.3°, corresponding to the (220), (311), (400), (422), (511), (440), and (533) planes of the inverse-spinel structure (JCPDS No. 19-0629) [
32]. The Fe
3O
4-SnO
2 nanoparticles exhibited peaks corresponding to Fe
3O
4 in addition to those at 2θ = 26.1, 33.5, 37.3, 51.7, 65.4, and 71.7° corresponding to the (110), (101), (200), (211), (220), and (202) planes of the tetragonal rutile structure (JCPDS card no. 41-1445), respectively [
33]. The diffraction peaks corresponding to SnO
2 were broad because of its crystalline size of less than 5 nm [
34]. This was further confirmed by calculating the average crystallite sizes of the prepared nanoparticles using the Scherrer’s formula, D = Kλ/βcosθ, where D is the average crystallite size, K is the shape factor (K = approximately 0.94 for spherical crystallites), λ is the X-ray wavelength (λ = 1.5418 Å for Cu K
α radiation), β is the full width at half maximum of the high-intensity diffraction peak (in radians), and θ is the Bragg’s angle (in radians). The average crystallite sizes of Fe
3O
4 and SnO
2 were calculated to be 18.09 and 4.36 nm, respectively. Meanwhile, the XRD patterns of the APTES and PEI-treated Fe
3O
4-SnO
2 nanoparticles were the same as that of the Fe
3O
4-SnO
2 nanoparticles. This indicates that the Fe
3O
4-SnO
2 nanoparticles maintained their crystallinity even after the amino-functionalization treatment.
Figure 5 shows the TEM and HR-TEM images showing the morphologies and microstructures of the synthesized particles before carbon coating. As shown in the figure, all the synthesized particles were spherical, and the Fe
3O
4 particles, which acted as the core, had a diameter of approximately 300 nm (
Figure 5a). The magnified TEM (
Figure 5(b-1)) and HRTEM (
Figure 5(b-2)) images revealed that SnO
2 particles with a diameter of approximately 4.5 nm were formed on the surface of the Fe
3O
4 particles to a thickness of approximately 20 nm. This is almost consistent with the average crystallite sizes of the Fe
3O
4 and SnO
2 nanoparticles, as calculated from the XRD data (
Figure 4) according to Scherrer’s formula. In addition, lattice patterns with the interplanar spacings of 0.268, 0.334, and 0.233 nm corresponding to the (101), (110), and (200) planes, respectively, were observed on the particle surface. In
Figure 5(c-1), the outer layer of the PEI-treated Fe
3O
4-SnO
2 nanoparticles can be clearly distinguished from that of the untreated nanoparticles. This outer layer was confirmed to be grafted onto the particle surface with a thickness of approximately 6.5 nm because of the polymerization of the polymeric precursor. In contrast, no significant difference was observed in the images of the Fe
3O
4-SnO
2 nanoparticles before and after the APTES treatment (
Figure 5(d-1)).
Figure 5(c-2,d-2) show the HRTEM images of the PEI- and APTES-treated Fe
3O
4-SnO
2 nanoparticles. Both the amine-treated nanoparticles showed lattice patterns with the interplanar spacings of 0.334 and 0.233 nm corresponding to the (110) and (200) planes of SnO
2, respectively. This is consistent with the XRD results and confirms the successful amino functionalization of the Fe
3O
4-SnO
2 nanoparticles through the polymerization of PEI or the silane bonding of APTES. However, the surface microstructures of the amino-functionalized nanoparticles prepared using these functionalization methods were significantly different.
Figure 6 shows the carbon coating procedure of the amino-functionalized Fe
3O
4-SnO
2 nanoparticles using glucose. EG, which was used as the solvent, played an important role in forming a stable dispersion of the reaction medium and functionalized magnetic nanoparticles. First, owing to its negative charge, the hydroxyl group of glucose bonded strongly to the amine group (with a positive charge) on the surface of the Fe
3O
4-SnO
2 particles by electrostatic attraction. Glucose transformed into oligosaccharide chains by the condensation reaction and surrounded the particle surface. In addition, the rotational energy generated by stirring facilitated the deposition of a uniform layer of the oligosaccharide chains on the surface of the particles. Then, the added sulfuric acid dehydrated the oligosaccharide chains bonded to the surface, and a hydrophilic carbon layer with a hydroxyl group at the end was finally formed.
The TEM images of the amino-functionalized nanoparticles subjected to the carbon coating process are shown in
Figure 7. In the case of the PEI-treated nanoparticles, only naked nanoparticles with the polymer layer removed were observed after the carbon coating process (
Figure 7a). The polymer layer disappeared because of dissolution by high temperature or sulfuric acid during the reaction. Since PEI, as a binding polymeric material, has a melting point of approximately 75 °C, it is sensitive to heat and acidic environments [
35]. However, it seems that the SnO
2 particles formed on the surface of the Fe
3O
4 particles by electrostatic attraction were not removed by sulfuric acid (
Figure 7b). To analyze the structure of the nanoparticles in detail, their selected area electron diffraction (SAED) patterns were recorded, which confirmed the presence of the (101), (110), and (200) planes of SnO
2 in the composite nanoparticles (
Figure 7c) [
36].
In contrast, the particles treated with APTES formed a new coating layer on the surface with a thickness of approximately 17 nm (
Figure 7d,e). In addition, the lattice pattern corresponding to the (110) plane was observed with an interplanar spacing of 0.334 nm in the HRTEM image. Ring patterns corresponding to the (333) and (311) planes of Fe
3O
4 and the (101) plane of SnO
2 were observed, as shown in
Figure 7f [
29]. This can be attributed to the carbonization of the nanoparticles after their electrostatic bonding with glucose because the amino-functional group formed at the end of the particles by the silane bond of APTES was resistant to high temperatures and acidic environments. Therefore, it can be stated that the formation of the carbon layer was directly related to the thermal/chemical stability of the amine retention layer, and the surface treatment using APTES was more effective in carbon layer formation than that using the polymeric precursor, PEI. Finally, multilayered core–shell Fe
3O
4-SnO
2-C nanoparticles were synthesized by the amino functionalization of the Fe
3O
4-SnO
2 particles through APTES.
Figure 8a shows the XRD pattern of the Fe
3O
4-SnO
2-C composite nanoparticles. The nanoparticles showed peaks of Fe
3O
4 and SnO
2 corresponding to the (110), (101), (200), (211), (220), (202), (220), (311), (400), (422), (511), and (440) planes. In addition, the low-intensity peak at 2θ = 22.1° corresponds to the (002) plane of carbon, which originated from the carbon layer formed by the carbonization of glucose [
37].
As shown in
Figure 8b, the Raman spectrum of the Fe
3O
4-SnO
2-C composite nanoparticles confirmed the formation of carbon on their surface. Two strong characteristic carbon peaks were observed over the wavenumber range of 1000–2800 cm
−1. In general, the D band, which appears only when disordered or finite-sized graphite crystals are present, is not identified in well-crystallized graphite [
38]. The D band at 1371 cm
−1 indicates the presence of sp
3 defects in the carbon network, whereas the G band at 1584 cm
−1 corresponds to the E
2g stretching vibration of graphite with sp
2 electronic configuration.
The intensity ratio of the D- and G-bands (I
D/I
G) provides useful information about the crystallinity of carbon material. The closer the I
D/I
G value to zero, the higher is the degree of structural alignment of the formed carbon material [
39]. The I
D/I
G ratio of the Fe
3O
4-SnO
2-C composite nanoparticles was approximately 0.5706, as determined using Lorentz fitting. This indicates that the formed carbon layer had a fairly ordered structure. However, this carbon layer can be considered amorphous carbon because of its high I
D value. Thus, the layer formed on the surface of the Fe
3O
4-SnO
2 nanoparticles was confirmed to be amorphous graphitized carbon, which was formed when the sulfuric acid used in the coating process promoted the dehydration reaction of the oligosaccharide obtained from glucose. Thus, the TEM and Raman analysis results indicate that the Fe
3O
4-SnO
2-C nanoparticles were synthesized by the formation of a structurally aligned carbon layer in the reflux process under atmospheric pressure.
Figure 9a shows the FTIR spectra of the Fe
3O
4–SnO
2 and Fe
3O
4–SnO
2–C nanocomposites. Unlike Fe
3O
4-SnO
2, the Fe
3O
4-SnO
2-C nanocomposites exhibited a –OH stretching vibration peak over a broad spectral region of 3630–3200 cm
−1. The peaks at 2917 and 2871 cm
−1 correspond to the vibration of the –CH
2 and –CH
3 groups, respectively. The strong peak at 1636 cm
−1 can be ascribed to the C–C stretching vibration. In addition, the characteristic peaks at approximately 1387–1301 and 1042–1083 cm
−1 correspond to the stretching vibration of the C–O and C–OH bonds, respectively, which were probably formed because of the hydroxyl group formed on the carbon layer [
40]. As a result, the Fe
3O
4-SnO
2-C nanoparticles exhibited high hydrophilicity because of the large number of hydroxyl groups present in the carbon coating layer.
Figure 9b shows the size distribution curves of the Fe
3O
4-SnO
2-C nanoparticles. As can be observed, the estimated mean size of the particles was 669 nm, and their size distribution was in the range of 225–1700 nm. As compared with the Fe
3O
4-SnO
2 nanoparticles (mean size of 476 nm with a zeta potential of −42.3 mV), the Fe
3O
4-SnO
2-C nanoparticles (with a higher zeta potential of −51 mV) showed aggregation. This can be attributed to the carbonization of the Fe
3O
4-SnO
2 nanoparticles in a bonded state between the oligosaccharide coating layers during the carbon coating process. Nevertheless, the Fe
3O
4-SnO
2-C nanoparticles showed high dispersibility in aqueous solvents because of the hydroxyl groups present on the surface of the carbon layer.
As can be observed from the lower right inset of
Figure 10, the coercivity (Hc) and residual magnetization (Mr) of the Fe
3O
4, Fe
3O
4-SnO
2, and Fe
3O
4-SnO
2-C nanoparticles were almost zero. This suggests that all the prepared nanoparticles were superparamagnetic. As the prepared Fe
3O
4 particles gradually evolved into the Fe
3O
4-SnO
2-C particles, their saturation magnetization (M
S) value tended to decrease. The measured M
S values of the Fe
3O
4, Fe
3O
4-SnO
2, and Fe
3O
4-SnO
2-C nanoparticles were 121, 95, and 81 emu/g, respectively (
Figure 10). This decrease in the M
S of the Fe
3O
4-SnO
2-C nanoparticles can be attributed to the increase in the weight of the single particles because of the formation of non-magnetic SnO
2 and carbon in the Fe
3O
4 nanoparticles. As can be seen from the optical image inserted, the Fe
3O
4-SnO
2-C nanoparticles reacted strongly to the external magnetic fields despite their relatively lower magnetization than that of the Fe
3O
4-SnO
2 nanoparticles.