Aquatic Environment Exposure and Toxicity of Engineered Nanomaterials Released from Nano-Enabled Products: Current Status and Data Needs
Abstract
:1. Introduction
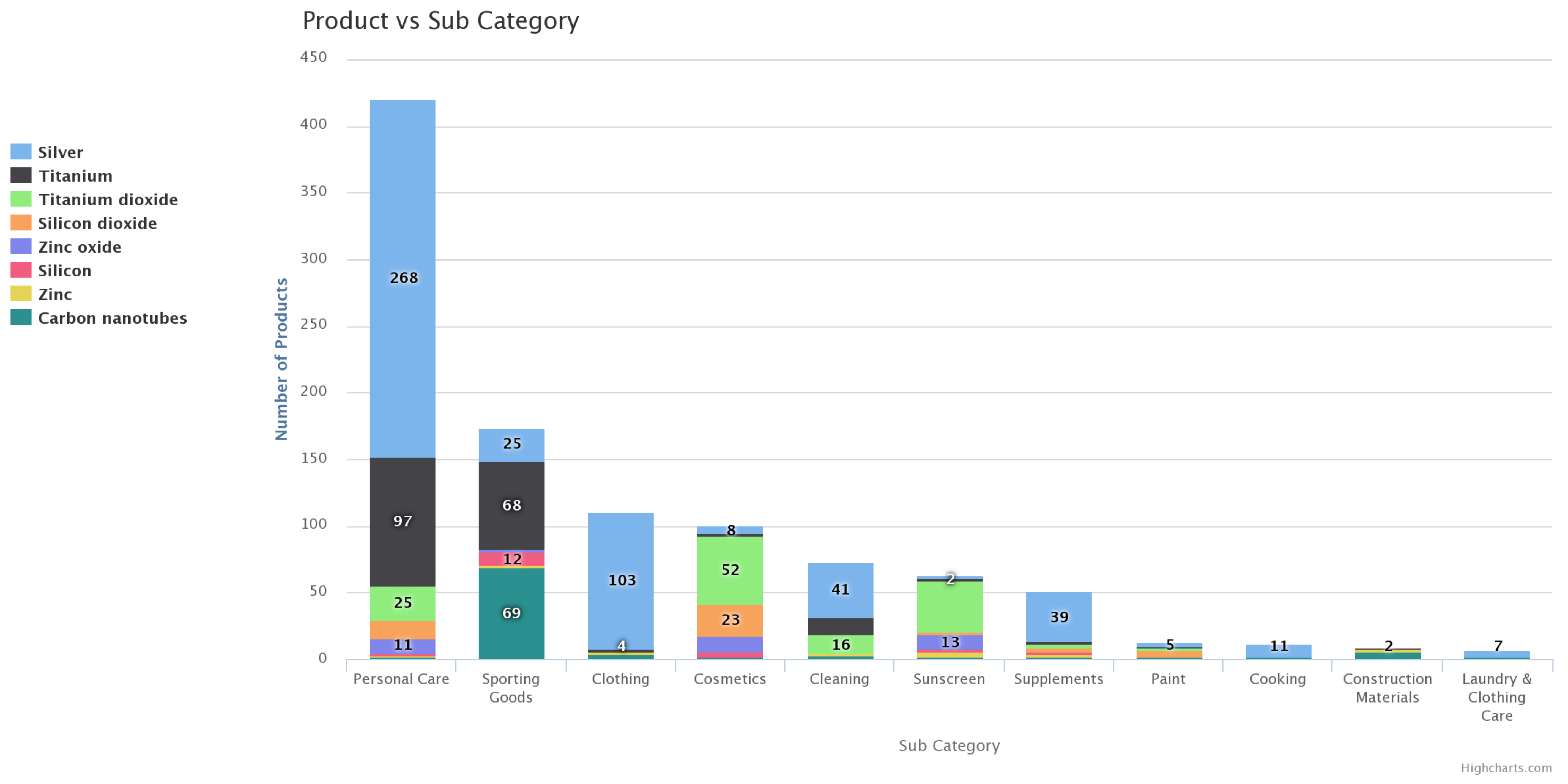
2. ENMs Identified to Be Commonly Used in NEPs That Exhibit Medium to High Environmental Exposure Potential
2.1. Titanium Dioxide Nanoparticles (nTiO2)
2.2. Zinc Oxide Nanoparticles (nZnO)
2.3. Silver Nanoparticles (nAg)
2.4. Silicon Dioxide Nanoparticles (nSiO2)
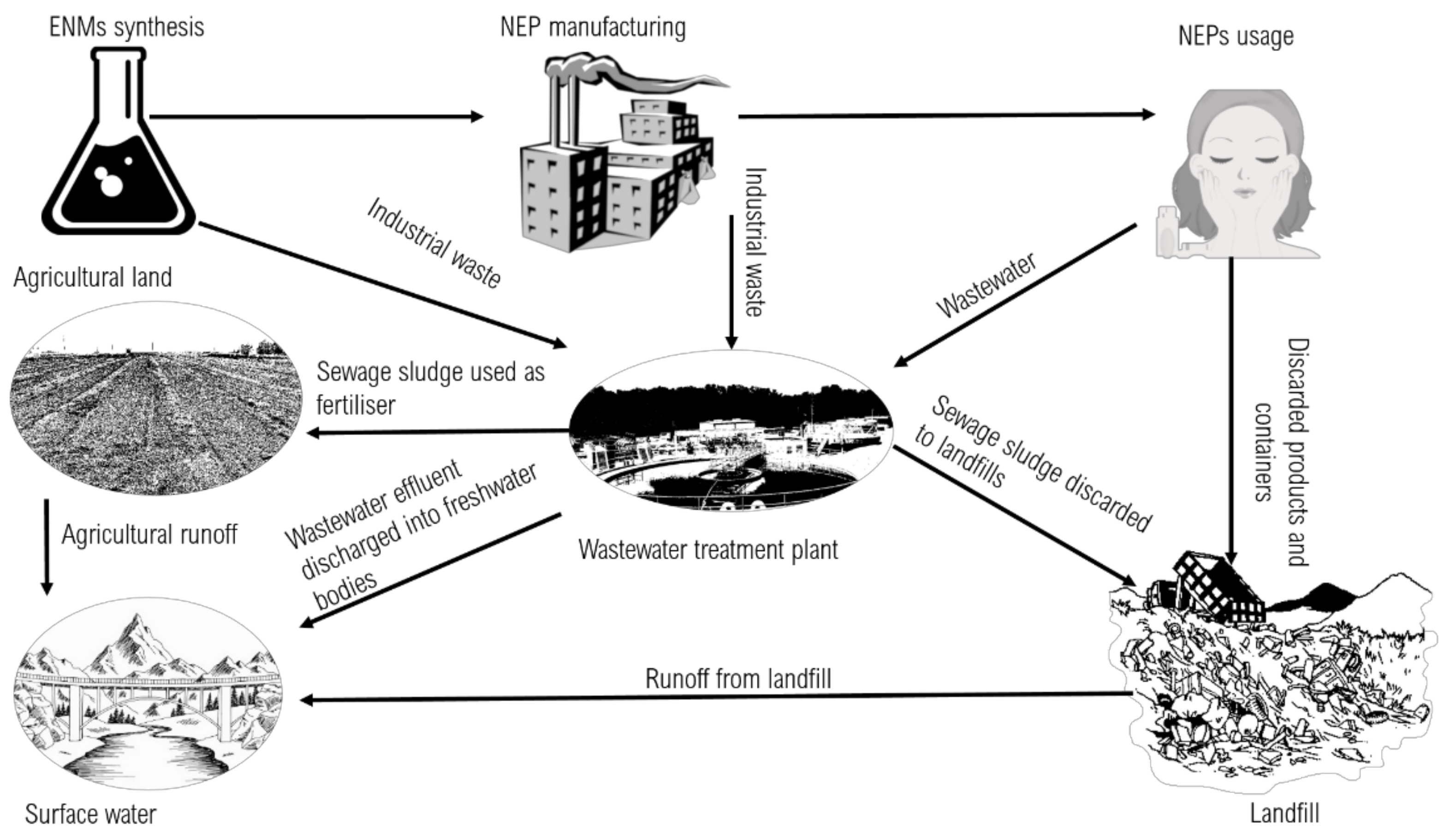
3. Release of ENMS from NEPs
3.1. Sunscreens
| NEP | ENMs Type | Concentration/Percentage Released | References |
|---|---|---|---|
| Sunscreen | nTiO2 | 7 × 10−4–0.00116 mg/L | [22] |
| 19–38% | [70] | ||
| nZnO | 0.58 mg/L | [100] | |
| nTiO2 + nZnO | 20–40% | [76] | |
| 0.4–8% | [140] | ||
| Paint | nAg | 0.5–20 mg/L | [23] |
| 3.5 × 107 particles/L | [13] | ||
| 1.7–15.7 µg/L | [121] | ||
| nTiO2 | 5 × 105 particles/mL | [127] | |
| 2 × 106–1.2 × 107 µg/m2 | [74] | ||
| 10–30 µg/m2 | [87] | ||
| Textiles | nTiO2 | 0.64–4.7 mg/L | [120] |
| 0.05 ± 0.02–3.13 ± 1.51 µg/g | [83] | ||
| nAg | 0.32–38.5 mg/L | [120] | |
| <1–100% | [14] | ||
| 0.3–377 µg/g | [15] | ||
| 18 ± 2–2925 ± 10 mg/kg | [138] | ||
| 3.4 ± 0.1–106 ± 10 µg/g | [124] | ||
| 15.8–34 µg | [141] | ||
| 1 × 10−3–5.969 mg/L | [24] | ||
| 5.3–6.4 mg/L | [126] | ||
| Washing machine | nAg | 8.1724 × 107 particles/mL | [123] |
| Baby products | nAg | 1–35% | [142] |
| Toothbrush | nAg | 3.6–6.6 × 107 particles/L (Baby) | [25] |
| 9.3–20.3 × 107 particles/L (Adult) | [25] |
3.2. Personal Care Products
3.3. Paints
3.4. Clothing/Textile
3.5. Washing Machine
4. Presence of PR–ENMs in Environmental Water Systems
5. Ecotoxicity of PR–ENMs in the Aquatic Environment
5.1. Sunscreen-Released ENMs
5.2. Household Detergent-Released ENMs
5.3. Textile-Released ENMs
5.4. Paint-Released ENMs
6. PR–ENMs Risk Characterisation Estimation
7. Conclusions, Recommendations and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Breznan, D.; Das, D.D.; Mackinnon-Roy, C.; Bernatchez, S.; Sayari, A.; Hill, M.; Vincent, R.; Kumarathasan, P. Physicochemical Properties Can Be Key Determinants of Mesoporous Silica Nanoparticle Potency in Vitro. ACS Nano 2018, 12, 12062–12079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adach, K.; Kroisova, D.; Fijalkowski, M. Biogenic silicon dioxide nanoparticles processed from natural sources. Part. Sci. Technol. 2021, 39, 481–489. [Google Scholar] [CrossRef]
- BCC Research Global Nanotechnology Market (by Component and Applications), Funding & Investment, Patent Analysis and 27 Companies Profile & Recent Developments—Forecast to 2024. Available online: https://www.researchandmarkets.com/reports/4520812/global-nanotechnology-market-by-component-and (accessed on 26 July 2021).
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F.; Hull, D.R. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [Green Version]
- Hansen, S.F.; Hansen, O.F.H.; Nielsen, M.B. Advances and challenges towards consumerization of nanomaterials. Nat. Nanotechnol. 2020, 15, 964–965. [Google Scholar] [CrossRef] [PubMed]
- Foss Hansen, S.; Heggelund, L.R.; Revilla Besora, P.; Mackevica, A.; Boldrin, A.; Baun, A. Nanoproducts—What is actually available to European consumers? Environ. Sci. Nano 2016, 3, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Moeta, P.J.; Wesley-Smith, J.; Maity, A.; Thwala, M. Nano-enabled products in South Africa and the assessment of environmental exposure potential for engineered nanomaterials. SN Appl. Sci. 2019, 1, 577. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Leu, Y.R.; Aitken, R.J.; Riediker, M. Inventory of engineered nanoparticle-containing consumer products available in the singapore retail market and likelihood of release into the aquatic environment. Int. J. Environ. Res. Public Health 2015, 12, 8717–8743. [Google Scholar] [CrossRef] [Green Version]
- Amorim, M.J.B.; Lin, S.; Schlich, K.; Navas, J.M.; Brunelli, A.; Neubauer, N.; Vilsmeier, K.; Costa, A.L.; Gondikas, A.; Xia, T.; et al. Environmental Impacts by Fragments Released from Nanoenabled Products: A Multiassay, Multimaterial Exploration by the SUN Approach. Environ. Sci. Technol. 2018, 52, 1514–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehutso, R.F.; Tancu, Y.; Maity, A.; Thwala, M. Characterisation of engineered nanomaterials in nano-enabled products exhibiting priority environmental exposure. Molecules 2021, 26, 1370. [Google Scholar] [CrossRef] [PubMed]
- Salieri, B.; Turner, D.A.; Nowack, B.; Hischier, R. Life cycle assessment of manufactured nanomaterials: Where are we? NanoImpact 2018, 10, 108–120. [Google Scholar] [CrossRef]
- Lehutso, R.F.; Tancu, Y.; Maity, A.; Thwala, M. Aquatic toxicity of transformed and product-released engineered nanomaterials: An overview of the current state of knowledge. Process Saf. Environ. Prot. 2020, 138, 39–56. [Google Scholar] [CrossRef]
- Kaegi, R.; Ulrich, A.; Sinnet, B.; Vonbank, R.; Wichser, A.; Zuleeg, S.; Simmler, H.; Brunner, S.; Vonmont, H.; Burkhardt, M.; et al. Synthetic TiO2 nanoparticle emission from exterior facades into the aquatic environment. Environ. Pollut. 2008, 156, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Benn, T.M.; Westerhoff, P. Nanoparticle Silver Released into Water from Commercially Available Sock Fabrics. Environ. Sci. Technol. 2008, 42, 4133–4139. [Google Scholar] [CrossRef] [PubMed]
- Geranio, L.; Heuberger, M.; Nowack, B. The Behavior of Silver Nanotextiles during Washing. Environ. Sci. Technol. 2009, 43, 8113–8118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Drug Administration Guidance for Industry: Safety of Nanomaterials in Cosmetic Products|FDA. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-safety-nanomaterials-cosmetic-products#IIIB1 (accessed on 19 April 2020).
- Food and Drug Administration Guidance for Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology FDA. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considering-whether-fda-regulated-product-involves-application-nanotechnology (accessed on 19 October 2021).
- European Commission Joint Research Centre. Technical Guidance Document on Risk Assessment in Support of Commission Directive 93/67/EEC on Risk Assessment for New Notified Substances. Commission Regulation (EC) No 1488/94 on Risk Assessment for Existing Substances Directive 98/8/EC of the European Parliament and of the Council Concerning the Placing of Biocidal Products on the Market. Part II. Available online: https://echa.europa.eu/documents/10162/987906/tgdpart2_2ed_en.pdf/138b7b71-a069-428e-9036-62f4300b752f (accessed on 21 October 2021).
- Oecd Physical-Chemical Decision Framework to Inform Decisions for Risk Assessment of Manufactured Nanomaterials. Available online: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2019)12&doclanguage=en (accessed on 28 July 2021).
- Koivisto, A.J.; Jensen, A.C.Ø.; Kling, K.I.; Nørgaard, A.; Brinch, A.; Christensen, F.; Jensen, K.A. Quantitative material releases from products and articles containing manufactured nanomaterials: Towards a release library. NanoImpact 2017, 5, 119–132. [Google Scholar] [CrossRef]
- Al-Kattan, A.; Wichser, A.; Vonbank, R.; Brunner, S.; Ulrich, A.; Zuin, S.; Nowack, B. Release of TiO2 from paints containing pigment-TiO2 or nano-TiO2 by weathering. Environ. Sci. Process. Impacts 2013, 15, 2186. [Google Scholar] [CrossRef] [Green Version]
- Nthwane, Y.B.; Tancu, Y.; Maity, A.; Thwala, M. Characterisation of titanium oxide nanomaterials in sunscreens obtained by extraction and release exposure scenarios. SN Appl. Sci. 2019, 1, 312. [Google Scholar] [CrossRef] [Green Version]
- Kaegi, R.; Sinnet, B.; Zuleeg, S.; Hagendorfer, H.; Mueller, E.; Vonbank, R.; Boller, M.; Burkhardt, M. Release of silver nanoparticles from outdoor facades. Environ. Pollut. 2010, 158, 2900–2905. [Google Scholar] [CrossRef]
- Limpiteeprakan, P.; Babel, S.; Lohwacharin, J.; Takizawa, S. Release of silver nanoparticles from fabrics during the course of sequential washing. Environ. Sci. Pollut. Res. 2016, 23, 22810–22818. [Google Scholar] [CrossRef] [PubMed]
- Mackevica, A.; Olsson, M.E.; Hansen, S.F. The release of silver nanoparticles from commercial toothbrushes. J. Hazard. Mater. 2017, 322, 270–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, B.F.; Pérez, S.; Gardinalli, P.; Singhal, R.K.; Mozeto, A.A.; Barceló, D. Analytical chemistry of metallic nanoparticles in natural environments. Trends Anal. Chem. 2011, 30, 528–540. [Google Scholar] [CrossRef]
- De la Calle, I.; Menta, M.; Séby, F. Current trends and challenges in sample preparation for metallic nanoparticles analysis in daily products and environmental samples: A review. Spectrochim. Acta Part B At. Spectrosc. 2016, 125, 66–96. [Google Scholar] [CrossRef]
- Laborda, F.; Bolea, E.; Cepriá, G.; Gómez, M.T.; Jiménez, M.S.; Pérez-Arantegui, J.; Castillo, J.R. Detection, characterization and quantification of inorganic engineered nanomaterials: A review of techniques and methodological approaches for the analysis of complex samples. Anal. Chim. Acta 2016, 904, 10–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amde, M.; Liu, J.; Tan, Z.-Q.; Bekana, D. Transformation and bioavailability of metal oxide nanoparticles in aquatic and terrestrial environments. A review. Environ. Pollut. 2017, 230, 250–267. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Advanced Analytical Techniques for the Measurement of Nanomaterials in Food and Agricultural Samples: A Review. Environ. Eng. Sci. 2013, 30, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, J.; Cai, Z.; Feng, Y.; Wang, Y.; Zhang, D.; Pan, X. Detection of engineered nanoparticles in aquatic environments: Current status and challenges in enrichment, separation, and analysis. Environ. Sci. Nano 2019, 6, 709–735. [Google Scholar] [CrossRef]
- Choi, S.; Johnston, M.; Wang, G.S.; Huang, C.P. A seasonal observation on the distribution of engineered nanoparticles in municipal wastewater treatment systems exemplified by TiO2 and ZnO. Sci. Total Environ. 2018, 625, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Maryam, B.; Büyükgüngör, H. Wastewater reclamation and reuse trends in Turkey: Opportunities and challenges. J. Water Process Eng. 2019, 30, 100501. [Google Scholar] [CrossRef]
- Musee, N. Simulated environmental risk estimation of engineered nanomaterials: A case of cosmetics in Johannesburg City. Hum. Exp. Toxicol. 2011, 30, 1181–1195. [Google Scholar] [CrossRef] [PubMed]
- Bathi, J.R.; Moazeni, F.; Upadhyayula, V.K.K.; Chowdhury, I.; Palchoudhury, S.; Potts, G.E.; Gadhamshetty, V. Behavior of engineered nanoparticles in aquatic environmental samples: Current status and challenges. Sci. Total Environ. 2021, 793, 148560. [Google Scholar] [CrossRef]
- Martínez, G.; Merinero, M.; Pérez-Aranda, M.; Pérez-Soriano, E.M.; Ortiz, T.; Begines, B.; Alcudia, A. Environmental impact of nanoparticles’ application as an emerging technology: A review. Materials 2021, 14, 166. [Google Scholar] [CrossRef]
- Heilgeist, S.; Sekine, R.; Sahin, O.; Stewart, R.A. Finding nano: Challenges involved in monitoring the presence and fate of engineered titanium dioxide nanoparticles in aquatic environments. Water 2021, 13, 734. [Google Scholar] [CrossRef]
- Ramachandran, G.; Ostraat, M.; Evans, D.E.; Methner, M.M.; O’Shaughnessy, P.; D’Arcy, J.; Geraci, C.L.; Stevenson, E.; Maynard, A.; Rickabaugh, K. A strategy for assessing workplace exposures to nanomaterials. J. Occup. Environ. Hyg. 2011, 8, 673–685. [Google Scholar] [CrossRef]
- Ma, H.; Williams, P.L.; Diamond, S.A. Ecotoxicity of manufactured ZnO nanoparticles—A review. Environ. Pollut. 2013, 172, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Labille, J.; Catalano, R.; Slomberg, D.; Motellier, S.; Pinsino, A.; Hennebert, P.; Santaella, C.; Bartolomei, V. Assessing Sunscreen Lifecycle to Minimize Environmental Risk Posed by Nanoparticulate UV-Filters—A Review for Safer-by-Design Products. Front. Environ. Sci. 2020, 8, 101. [Google Scholar] [CrossRef]
- Shevlin, D.; O’Brien, N.; Cummins, E. Silver engineered nanoparticles in freshwater systems–Likely fate and behaviour through natural attenuation processes. Sci. Total Environ. 2018, 621, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B.; Ranville, J.F.; Diamond, S.; Gallego-Urrea, J.A.; Metcalfe, C.; Rose, J.; Horne, N.; Koelmans, A.A.; Klaine, S.J. Potential scenarios for nanomaterial release and subsequent alteration in the environment. Environ. Toxicol. Chem. 2012, 31, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Mitrano, D.M.; Motellier, S.; Clavaguera, S.; Nowack, B. Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environ. Int. 2015, 77, 132–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parihar, V.; Raja, M.; Paulose, R. A brief review of structural, electrical and electrochemical properties of zinc oxide nanoparticles. Rev. Adv. Mater. Sci. 2018, 53, 119–130. [Google Scholar] [CrossRef]
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in cosmetics: Recent updates. Nanomaterials 2020, 10, 979. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Oranu, E.A.; Tao, M.; Keller, A.A. Release and detection of nanosized copper from a commercial antifouling paint. Water Res. 2016, 102, 374–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, J.; Li, T.; Miao, L.; You, G.; Xu, Y.; Liu, S. Effects of titanium dioxide nanoparticles on algal and bacterial communities in periphytic biofilms. Environ. Pollut. 2019, 251, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yang, S.; Du, W.; Yin, Y.; Zhang, J.; Guo, H. Effects of titanium dioxide nanoparticles on Microcystis aeruginosa and microcystins production and release. J. Hazard. Mater. 2019, 377, 1–7. [Google Scholar] [CrossRef]
- De Leersnyder, I.; Rijckaert, H.; De Gelder, L.; Van Driessche, I.; Vermeir, P. High Variability in Silver Particle Characteristics, Silver Concentrations, and Production Batches of Commercially Available Products Indicates the Need for a More Rigorous Approach. Nanomaterials 2020, 10, 1394. [Google Scholar] [CrossRef]
- Szymańska, R.; Kołodziej, K.; Ślesak, I.; Zimak-Piekarczyk, P.; Orzechowska, A.; Gabruk, M.; Zadło, A.; Habina, I.; Knap, W.; Burda, K.; et al. Titanium dioxide nanoparticles (100–1000 mg/L) can affect vitamin E response in Arabidopsis thaliana. Environ. Pollut. 2016, 213, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Pulit-Prociak, J.; Banach, M. Silver nanoparticles—A material of the future…? Open Chem. 2016, 14, 76–91. [Google Scholar] [CrossRef]
- Inshakova, E.; Inshakov, O. World market for nanomaterials: Structure and trends. MATEC Web Conf. 2017, 129, 02013. [Google Scholar] [CrossRef]
- Nanodatabase Consumer Products—The Nanodatabase. Available online: https://nanodb.dk/en/analysis/consumer-products/#chartHashsection (accessed on 26 July 2021).
- CPI Consumer Products Inventory: An Inventory of Nanotechnology-Based Consumer Products Introduced on the Market. Available online: https://processwire.com/sites/list/nanotechnology-consumer-products-inventory/ (accessed on 21 October 2021).
- Tan, C.; Fan, W.H.; Wang, W.X. Role of titanium dioxide nanoparticles in the elevated uptake and retention of cadmium and zinc in Daphnia magna. Environ. Sci. Technol. 2012, 46, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.W.; Song, I.H.; Um, S.H. Role of physicochemical properties in nanoparticle toxicity. Nanomaterials 2015, 5, 1351–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium dioxide nanoparticles: Prospects and applications in medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [Green Version]
- Dréno, B.; Alexis, A.; Chuberre, B.; Marinovich, M. Safety of titanium dioxide nanoparticles in cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 34–46. [Google Scholar] [CrossRef] [Green Version]
- Peters, R.J.B.; van Bemmel, G.; Herrera-Rivera, Z.; Helsper, H.P.F.G.; Marvin, H.J.P.; Weigel, S.; Tromp, P.C.; Oomen, A.G.; Rietveld, A.G.; Bouwmeester, H. Characterization of Titanium Dioxide Nanoparticles in Food Products: Analytical Methods To Define Nanoparticles. J. Agric. Food Chem. 2014, 62, 6285–6293. [Google Scholar] [CrossRef] [PubMed]
- Rompelberg, C.; Heringa, M.B.; van Donkersgoed, G.; Drijvers, J.; Roos, A.; Westenbrink, S.; Peters, R.; van Bemmel, G.; Brand, W.; Oomen, A.G. Oral intake of added titanium dioxide and its nanofraction from food products, food supplements and toothpaste by the Dutch population. Nanotoxicology 2016, 10, 1404–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, R.; Bautista, L.; de la Varga, M.; Botet, J.M.; Casals, E.; Puntes, V.; Marsal, F. Nano-cotton Fabrics with High Ultraviolet Protection. Text. Res. J. 2010, 80, 454–462. [Google Scholar] [CrossRef]
- Hincapié, I.; Caballero-Guzman, A.; Hiltbrunner, D.; Nowack, B. Use of engineered nanomaterials in the construction industry with specific emphasis on paints and their flows in construction and demolition waste in Switzerland. Waste Manag. 2015, 43, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Hischier, R.; Nowack, B.; Gottschalk, F.; Hincapie, I.; Steinfeldt, M.; Som, C. Life cycle assessment of façade coating systems containing manufactured nanomaterials. J. Nanopart. Res. 2015, 17, 68. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Huang, F.; Wang, F.; Duan, W.; Li, J.; Shen, Y.; Xie, A. Functionalization of cotton fabrics with rutile TiO2 nanoparticles: Applications for superhydrophobic, UV-shielding and self-cleaning properties. Russ. J. Phys. Chem. A 2012, 86, 413–417. [Google Scholar] [CrossRef]
- Shah, S.N.A.; Shah, Z.; Hussain, M.; Khan, M. Hazardous Effects of Titanium Dioxide Nanoparticles in Ecosystem. Bioinorg. Chem. Appl. 2017, 2017, 4101735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, A.; Daoud, W.A.; Liang, H.H.; Szeto, Y.S. Application of rutile and anatase onto cotton fabric and their effect on the NIR reflection/surface temperature of the fabric. Sol. Energy Mater. Sol. Cells 2015, 134, 425–437. [Google Scholar] [CrossRef]
- Iswarya, V.; Palanivel, A.; Chandrasekaran, N.; Mukherjee, A. Toxic effect of different types of titanium dioxide nanoparticles on Ceriodaphnia dubia in a freshwater system. Environ. Sci. Pollut. Res. 2019, 26, 11998–12013. [Google Scholar] [CrossRef] [PubMed]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.J.; Huang, S.C.; Chen, Y.P.; Chiueh, L.C.; Shih, D.Y.C. Analysis of titanium dioxide and zinc oxide nanoparticles in cosmetics. J. Food Drug Anal. 2015, 23, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Botta, C.; Labille, J.; Auffan, M.; Borschneck, D.; Miche, H.H.; Cabié, M.; Masion, A.; Rose, J.; Bottero, J.-Y.Y. TiO2-based nanoparticles released in water from commercialized sunscreens in a life-cycle perspective: Structures and quantities. Environ. Pollut. 2011, 159, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bairi, V.G.; Lim, J.-H.; Fong, A.; Linder, S.W. Size characterization of metal oxide nanoparticles in commercial sunscreen products. J. Nanopart. Res. 2017, 19, 256. [Google Scholar] [CrossRef]
- Shandilya, N.; Capron, I. Safer-by-design hybrid nanostructures: An alternative to conventional titanium dioxide UV filters in skin care products. RSC Adv. 2017, 7, 20430–20439. [Google Scholar] [CrossRef] [Green Version]
- Azimzada, A.; Farner, J.M.; Hadioui, M.; Liu-Kang, C.; Jreije, I.; Tufenkji, N.; Wilkinson, K.J. Release of TiO2 nanoparticles from painted surfaces in cold climates: Characterization using a high sensitivity single-particle ICP-MS. Environ. Sci. Nano 2020, 7, 139–148. [Google Scholar] [CrossRef]
- Virkutyte, J.; Al-Abed, S.R. Statistical evaluation of potential damage to the Al(OH)3 layer on nTiO2 particles in the presence of swimming pool and seawater. J. Nanopart. Res. 2012, 14, 787. [Google Scholar] [CrossRef]
- Jeon, S.-K.; Kim, E.-J.; Lee, J.; Lee, S. Potential risks of TiO2 and ZnO nanoparticles released from sunscreens into outdoor swimming pools. J. Hazard. Mater. 2016, 317, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Joo, S.H.; Blackwelder, P.; Toborek, M. Effects of coating materials on antibacterial properties of industrial and sunscreen-derived titanium-dioxide nanoparticles on Escherichia coli. Chemosphere 2018, 208, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Hanigan, D.; Truong, L.; Schoepf, J.; Nosaka, T.; Mulchandani, A.; Tanguay, R.L.; Westerhoff, P. Trade-offs in ecosystem impacts from nanomaterial versus organic chemical ultraviolet filters in sunscreens. Water Res. 2018, 139, 281–290. [Google Scholar] [CrossRef]
- Lorenz, C.; Tiede, K.; Tear, S.; Boxall, A.; Von Goetz, N.; Hungerbühler, K. Imaging and characterization of engineered nanoparticles in sunscreens by electron microscopy, under wet and dry conditions. Int. J. Occup. Environ. Health 2010, 16, 406–428. [Google Scholar] [CrossRef] [PubMed]
- Al-Kattan, A.; Wichser, A.; Zuin, S.; Arroyo, Y.; Golanski, L.; Ulrich, A.; Nowack, B. Behavior of TiO2 released from nano-TiO2-containing paint and comparison to pristine nano-TiO2. Environ. Sci. Technol. 2014, 48, 6710–6718. [Google Scholar] [CrossRef]
- Rand, L.N.; Bi, Y.; Poustie, A.; Bednar, A.J.; Hanigan, D.J.; Westerhoff, P.; Ranville, J.F. Quantifying temporal and geographic variation in sunscreen and mineralogic titanium-containing nanoparticles in three recreational rivers. Sci. Total Environ. 2020, 743, 140845. [Google Scholar] [CrossRef] [PubMed]
- Dedman, C.J.; King, A.; Christie-Oleza, J.; Davies, G.-L. Environmentally relevant concentrations of titanium dioxide nanoparticles pose negligible risk to marine microbes. Environ. Sci. Nano 2021, 8, 1236–1255. [Google Scholar] [CrossRef] [PubMed]
- Mackevica, A.; Olsson, M.E.; Hansen, S.F. Quantitative characterization of TiO2 nanoparticle release from textiles by conventional and single particle ICP-MS. J. Nanopart. Res. 2018, 20, 6. [Google Scholar] [CrossRef]
- Gottschalk, F.; Nowack, B. The release of engineered nanomaterials to the environment. J. Environ. Monit. 2011, 13, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Bottero, J.Y.; Rose, J.; De Garidel, C.; Masion, A.; Deutsch, T.; Brochard, G.; Carrière, M.; Gontard, N.; Wortham, H.; Rabilloud, T.; et al. SERENADE: Safer and ecodesign research and education applied to nanomaterial development, the new generation of materials safer by design. Environ. Sci. Nano 2017, 4, 526–538. [Google Scholar] [CrossRef]
- Truffier-Boutry, D.; Fiorentino, B.; Bartolomei, V.; Soulas, R.; Sicardy, O.; Benayad, A.; Damlencourt, J.F.; Pépin-Donat, B.; Lombard, C.; Gandolfo, A.; et al. Characterization of photocatalytic paints: A relationship between the photocatalytic properties-release of nanoparticles and volatile organic compounds. Environ. Sci. Nano 2017, 4, 1998–2009. [Google Scholar] [CrossRef]
- Azimzada, A.; Farner, J.M.; Jreije, I.; Hadioui, M.; Liu-Kang, C.; Tufenkji, N.; Shaw, P.; Wilkinson, K.J. Single- and Multi-Element Quantification and Characterization of TiO2 Nanoparticles Released From Outdoor Stains and Paints. Front. Environ. Sci. 2020, 8, 91. [Google Scholar] [CrossRef]
- Philippe, A.; Košík, J.; Welle, A.; Guigner, J.M.; Clemens, O.; Schaumann, G.E. Extraction and characterization methods for titanium dioxide nanoparticles from commercialized sunscreens. Environ. Sci. Nano 2018, 5, 191–202. [Google Scholar] [CrossRef]
- Rodríguez-Romero, A.; Ruiz-Gutiérrez, G.; Viguri, J.R.; Tovar-Sánchez, A. Sunscreens as a New Source of Metals and Nutrients to Coastal Waters. Environ. Sci. Technol. 2019, 53, 10177–10187. [Google Scholar] [CrossRef] [PubMed]
- Slomberg, D.L.; Catalano, R.; Ziarelli, F.; Viel, S.; Bartolomei, V.; Labille, J.; Masion, A. Aqueous aging of a silica coated TiO2 UV filter used in sunscreens: Investigations at the molecular scale with dynamic nuclear polarization NMR. RSC Adv. 2020, 10, 8266–8274. [Google Scholar] [CrossRef]
- Auffan, M.; Pedeutour, M.; Rose, J.; Masion, A.; Ziarelli, F.; Borschneck, D.; Chaneac, C.; Botta, C.; Chaurand, P.; Labille, J.; et al. Structural degradation at the surface of a TiO2-based nanomaterial used in cosmetics. Environ. Sci. Technol. 2010, 44, 2689–2694. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Watson, C.; Murdaugh, K.M.; Darrah, T.H.; Pyrgiotakis, G.; Elder, A.; Brain, J.D.; Demokritou, P. Engineering safer-by-design silica-coated ZnO nanorods with reduced DNA damage potential. Environ. Sci. Nano 2014, 1, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Semenzin, E.; Subramanian, V.; Pizzol, L.; Zabeo, A.; Fransman, W.; Oksel, C.; Hristozov, D.; Marcomini, A. Controlling the risks of nano-enabled products through the life cycle: The case of nano copper oxide paint for wood protection and nano-pigments used in the automotive industry. Environ. Int. 2019, 131, 104901. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Harper, B.J.; Harper, S.L. Comparative dissolution, uptake, and toxicity of zinc oxide particles in individual aquatic species and mixed populations. Environ. Toxicol. Chem. 2019, 38, 591–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djurišić, A.B.; Chen, X.; Leung, Y.H.; Man Ching Ng, A. ZnO nanostructures: Growth, properties and applications. J. Mater. Chem. 2012, 22, 6526–6535. [Google Scholar] [CrossRef]
- Osmond, M.J.; Mccall, M.J. Zinc oxide nanoparticles in modern sunscreens: An analysis of potential exposure and hazard. Nanotoxicology 2010, 4, 15–41. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Lewicka, Z.A.; Benedetto, A.F.; Benoit, D.N.; Yu, W.W.; Fortner, J.D.; Colvin, V.L. The structure, composition, and dimensions of TiO2 and ZnO nanomaterials in commercial sunscreens. J. Nanopart. Res. 2011, 13, 3607–3617. [Google Scholar] [CrossRef]
- Wong, S.W.Y.; Zhou, G.J.; Leung, P.T.Y.; Han, J.; Lee, J.S.; Kwok, K.W.H.; Leung, K.M.Y. Sunscreens containing zinc oxide nanoparticles can trigger oxidative stress and toxicity to the marine copepod Tigriopus japonicus. Mar. Pollut. Bull. 2020, 154, 111078. [Google Scholar] [CrossRef] [PubMed]
- Windler, L.; Lorenz, C.; Von Goetz, N.; Hungerbühler, K.; Amberg, M.; Heuberger, M.; Nowack, B. Release of titanium dioxide from textiles during washing. Environ. Sci. Technol. 2012, 46, 8181–8188. [Google Scholar] [CrossRef] [PubMed]
- Garza-Cervantes, J.A.; Chávez-Reyes, A.; Castillo, E.C.; García-Rivas, G.; Ortega-Rivera, O.A.; Salinas, E.; Ortiz-Martínez, M.; Gómez-Flores, S.L.; Peña-Martínez, J.A.; Pepi-Molina, A.; et al. Synergistic antimicrobial effects of silver/transition-metal combinatorial treatments. Sci. Rep. 2017, 7, 903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou El-Nour, K.M.M.; Eftaiha, A.; Al-Warthan, A.; Ammar, R.A.A. Synthesis and applications of silver nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.J.; Yin, Y.G.; Liu, J.F. Silver nanoparticles in the environment. Environ. Sci. Process. Impacts 2013, 15, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and differences between silver ions and silver in nanoforms as antibacterial agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Kheshen, A.A.; El-Rab, S.F.G. Effect of reducing and protecting agents on size of silver nanoparticles and their anti-bacterial activity. Der Pharma Chem. 2012, 4, 53–65. [Google Scholar]
- Pandiarajan, J.; Krishnan, M. Properties, synthesis and toxicity of silver nanoparticles. Environ. Chem. Lett. 2017, 15, 387–397. [Google Scholar] [CrossRef]
- Abbasi, E.; Milani, M.; Aval, S.F.; Kouhi, M.; Akbarzadeh, A.; Nasrabadi, H.T.; Nikasa, P.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; et al. Silver nanoparticles: Synthesis methods, bio-applications and properties. Crit. Rev. Microbiol. 2016, 42, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Kittler, S.; Greulich, C.; Diendorf, J.; Koller, M.; Epple, M. Toxicity of Silver Nanoparticles Increases during Storage Because of Slow Dissolution under Release of Silver Ions. Chem. Mater. 2010, 22, 4548–4554. [Google Scholar] [CrossRef]
- Jones, A.M.; Garg, S.; He, D.; Pham, A.N.; Waite, T.D. Superoxide-mediated formation and charging of silver nanoparticles. Environ. Sci. Technol. 2011, 45, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Levard, C.; Marinakos, S.M.; Cheng, Y.; Liu, J.; Michel, F.M.; Brown, G.E.; Lowry, G.V. Size-controlled dissolution of organic-coated silver nanoparticles. Environ. Sci. Technol. 2012, 46, 752–759. [Google Scholar] [CrossRef]
- Völker, C.; Kämpken, I.; Boedicker, C.; Oehlmann, J.; Oetken, M. Toxicity of silver nanoparticles and ionic silver: Comparison of adverse effects and potential toxicity mechanisms in the freshwater clam Sphaerium corneum. Nanotoxicology 2015, 9, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-Operation and Development. Environment, Health and Safety Publications Series on the Safety of Manufactured Nanomaterials No. 83 Silver Nanoparticles: Summary of the Dossier. Available online: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2017)31&doclanguage=en (accessed on 26 July 2021).
- Thwala, M.; Musee, N.; Sikhwivhilu, L.; Wepener, V. The oxidative toxicity of Ag and ZnO nanoparticles towards the aquatic plant Spirodela punctuta and the role of testing media parameters. Environ. Sci. Process. Impacts 2013, 15, 1830–1843. [Google Scholar] [CrossRef] [PubMed]
- Thwala, M.; Klaine, S.; Musee, N. Exposure Media and Nanoparticle Size Influence on the Fate, Bioaccumulation, and Toxicity of Silver Nanoparticles to Higher Plant Salvinia minima. Molecules 2021, 26, 2305. [Google Scholar] [CrossRef] [PubMed]
- Dallas, P.; Sharma, V.K.; Zboril, R. Silver polymeric nanocomposites as advanced antimicrobial agents: Classification, synthetic paths, applications, and perspectives. Adv. Colloid Interface Sci. 2011, 166, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Fabrega, J.; Luoma, S.N.; Tyler, C.R.; Galloway, T.S.; Lead, J.R. Silver nanoparticles: Behaviour and effects in the aquatic environment. Environ. Int. 2011, 37, 517–531. [Google Scholar] [CrossRef]
- García-Barrasa, J.; López-De-luzuriaga, J.M.; Monge, M. Silver nanoparticles: Synthesis through chemical methods in solution and biomedical applications. Cent. Eur. J. Chem. 2011, 9, 7–19. [Google Scholar] [CrossRef]
- Lorenz, C.; Windler, L.; von Goetz, N.; Lehmann, R.P.P.; Schuppler, M.; Hungerbühler, K.; Heuberger, M.; Nowack, B. Characterization of silver release from commercially available functional (nano)textiles. Chemosphere 2012, 89, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Künniger, T.; Gerecke, A.C.; Ulrich, A.; Huch, A.; Vonbank, R.; Heeb, M.; Wichser, A.; Haag, R.; Kunz, P.; Faller, M. Release and environmental impact of silver nanoparticles and conventional organic biocides from coated wooden façades. Environ. Pollut. 2014, 184, 464–471. [Google Scholar] [CrossRef]
- Echavarri-Bravo, V.; Paterson, L.; Aspray, T.J.; Porter, J.S.; Winson, M.K.; Hartl, M.G.J. Natural marine bacteria as model organisms for the hazard-assessment of consumer products containing silver nanoparticles. Mar. Environ. Res. 2017, 130, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Farkas, J.; Peter, H.; Christian, P.; Gallego Urrea, J.A.; Hassellöv, M.; Tuoriniemi, J.; Gustafsson, S.; Olsson, E.; Hylland, K.; Thomas, K.V. Characterization of the effluent from a nanosilver producing washing machine. Environ. Int. 2011, 37, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.B.; Zaikova, T.; Barber, A.; Simonich, M.; Lankone, R.; Marco, M.; Hristovski, K.; Herckes, P.; Passantino, L.; Fairbrother, D.H.; et al. Potential Environmental Impacts and Antimicrobial Efficacy of Silver- and Nanosilver-Containing Textiles. Environ. Sci. Technol. 2016, 50, 4018–4026. [Google Scholar] [CrossRef]
- Kim, J.B.; Kim, J.Y.; Yoon, T.H. Determination of silver nanoparticle species released from textiles into artificial sweat and laundry wash for a risk assessment. Hum. Ecol. Risk Assess. 2017, 23, 741–750. [Google Scholar] [CrossRef]
- Gagnon, V.; Button, M.; Boparai, H.K.; Nearing, M.; O’Carroll, D.M.; Weber, K.P. Influence of realistic wearing on the morphology and release of silver nanomaterials from textiles. Environ. Sci. Nano 2019, 6, 411–424. [Google Scholar] [CrossRef]
- Kaegi, R.; Englert, A.; Gondikas, A.; Sinnet, B.; von der Kammer, F.; Burkhardt, M. Release of TiO2–(Nano) particles from construction and demolition landfills. NanoImpact 2017, 8, 73–79. [Google Scholar] [CrossRef]
- Cascio, C.; Geiss, O.; Franchini, F.; Ojea-Jimenez, I.; Rossi, F.; Gilliland, D.; Calzolai, L. Detection, quantification and derivation of number size distribution of silver nanoparticles in antimicrobial consumer products †. J. Anal. At. Spectrom. 2015, 30, 1255–1265. [Google Scholar] [CrossRef] [Green Version]
- Aznar, R.; Barahona, F.; Geiss, O.; Ponti, J.; José Luis, T.; Barrero-Moreno, J. Quantification and size characterisation of silver nanoparticles in environmental aqueous samples and consumer products by single particle-ICPMS. Talanta 2017, 175, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Potter, P.M.; Navratilova, J.; Rogers, K.R.; Al-Abed, S.R. Transformation of silver nanoparticle consumer products during simulated usage and disposal. Environ. Sci. Nano 2019, 6, 592–598. [Google Scholar] [CrossRef]
- Liljenström, C.; Lazarevic, D.; Finnveden, G. Silicon-Based Nanomaterials in a Life-Cycle Perspective, Including a Case Study on Self-Cleaning Coatings; Royal Institute of Technology: Stockholm, Sweden, 2013; ISBN 9789175019420. [Google Scholar]
- Michel, K.; Scheel, J.; Karsten, S.; Stelter, N.; Wind, T. Risk assessment of amorphous silicon dioxide nanoparticles in a glass cleaner formulation. Nanotoxicology 2013, 7, 974–988. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Roblegg, E. Models for oral uptake of nanoparticles in consumer products. Toxicology 2012, 291, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuin, S.; Massari, A.; Ferrari, A.; Golanski, L. Formulation effects on the release of silica dioxide nanoparticles from paint debris to water. Sci. Total Environ. 2014, 476–477, 298–307. [Google Scholar] [CrossRef]
- Lajmanovich, R.C.; Peltzer, P.M.; Martinuzzi, C.S.; Attademo, A.M.; Colussi, C.L.; Bassó, A. Acute Toxicity of Colloidal Silicon Dioxide Nanoparticles on Amphibian Larvae: Emerging Environmental Concern. Int. J. Environ. Res. 2018, 12, 269–278. [Google Scholar] [CrossRef]
- Vidya, P.V.; Chitra, K.C. Irreversible Nanotoxicity of Silicon Dioxide Nanoparticles in the Freshwater Fish, Oreochromis mossambicus (Peters 1852). Asian Fish. Sci. 2018, 31, 146–160. [Google Scholar] [CrossRef]
- Sahu, D.; Kannan, G.M.; Tailang, M.; Vijayaraghavan, R. In Vitro Cytotoxicity of Nanoparticles: A Comparison between Particle Size and Cell Type. J. Nanosci. 2016, 2016, 4023852. [Google Scholar] [CrossRef] [Green Version]
- Lombi, E.; Donner, E.; Scheckel, K.G.; Sekine, R.; Lorenz, C.; Goetz, N.V.; Nowack, B. Silver speciation and release in commercial antimicrobial textiles as influenced by washing. Chemosphere 2014, 111, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Batchelor-McAuley, C.; Tschulik, K.; Neumann, C.C.M.; Laborda, E.; Compton, R.G. Why are silver nanoparticles more toxic than bulk silver? Towards understanding the dissolution and toxicity of silver nanoparticles. Int. J. Electrochem. Sci. 2014, 9, 1132–1138. [Google Scholar]
- Lehutso, R.F.; Thwala, M. Assessment of Nanopollution in Water Environments from Commercial Products. Nanomaterials 2021, 11, 2537. [Google Scholar] [CrossRef] [PubMed]
- Benn, T.; Cavanagh, B.; Hristovski, K.; Posner, J.D.; Westerhoff, P. The Release of Nanosilver from Consumer Products Used in the Home. Physiol. Behav. 2016, 176, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quadros, M.E.; Pierson, R.; Tulve, N.S.; Willis, R.; Rogers, K.; Thomas, T.A.; Marr, L.C. Release of silver from nanotechnology-based consumer products for children. Environ. Sci. Technol. 2013, 47, 8894–8901. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.B.; Martin, D.P.; Bednar, A.J.; Montaño, M.D.; Westerhoff, P.; Ranville, J.F. Multi-day diurnal measurements of Ti-containing nanoparticle and organic sunscreen chemical release during recreational use of a natural surface water. Environ. Sci. Nano 2017, 4, 69–77. [Google Scholar] [CrossRef]
- Markus, A.A.; Krystek, P.; Tromp, P.C.; Parsons, J.R.; Roex, E.W.M.; Voogt, P.D.; Laane, R.W.P.M. Determination of metal-based nanoparticles in the river Dommel in The Netherlands via ultrafiltration, HR-ICP-MS and SEM. Sci. Total Environ. 2018, 631–632, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Gondikas, A.; Von Der Kammer, F.; Kaegi, R.; Borovinskaya, O.; Neubauer, E.; Navratilova, J.; Praetorius, A.; Cornelis, G.; Hofmann, T. Where is the nano? Analytical approaches for the detection and quantification of TiO2 engineered nanoparticles in surface waters. Environ. Sci. Nano 2018, 5, 313–326. [Google Scholar] [CrossRef]
- Labille, J.; Slomberg, D.; Catalano, R.; Robert, S.; Apers-Tremelo, M.L.; Boudenne, J.L.; Manasfi, T.; Radakovitch, O. Assessing UV filter inputs into beach waters during recreational activity: A field study of three French Mediterranean beaches from consumer survey to water analysis. Sci. Total Environ. 2020, 706, 136010. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Sepúlveda, M.S.; Klinkhamer, C.; Wei, A.; Gao, Y.; Mahapatra, C.T. Nanosilver-coated socks and their toxicity to zebrafish (Danio rerio) embryos. Chemosphere 2015, 119, 948–952. [Google Scholar] [CrossRef]
- Fastelli, P.; Renzi, M. Exposure of key marine species to sunscreens: Changing ecotoxicity as a possible indirect effect of global warming. Mar. Pollut. Bull. 2019, 149, 110517. [Google Scholar] [CrossRef]
- Kalbassi, M.R.; Salari-joo, H.; Johari, A. Toxicity of Silver Nanoparticles in Aquatic Ecosystems: Salinity As the Main Cause in Reducing Toxicity. Iran. J. Toxicol. 2011, 5, 436–443. [Google Scholar]
- Bicherel, P.; Thomas, P.C. Aquatic Toxicity Calculation of Mixtures: A Chemical Activity Approach Incorporating a Bioavailability Reduction Concept. Environ. Sci. Technol. 2021, 55, 11183–11191. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, L.; Wang, C.; Zhang, S.; Liu, H.; Li, S.; Wang, X. Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J. Environ. Sci. 2019, 75, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, S.; Oliviero, M.; Philippe, A.; Manzo, S. Nanoparticles based sunscreens provoke adverse effects on marine microalgae Dunaliella tertiolecta. Environ. Sci. Nano 2018, 5, 3011–3022. [Google Scholar] [CrossRef]
- Mueller, N.C.; Nowack, B. Exposure Modeling of Engineered Nanoparticles in the Environment. EMPA Act. 2008, 42, 63. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Possibilities and limitations of modeling environmental exposure to engineered nanomaterials by probabilistic by probabilistic material flow analysis. Environ. Toxicol. Chem. 2010, 29, 1036–1048. [Google Scholar] [CrossRef] [PubMed]
| ENMs Type | Global Production (Tons/Year) | References |
|---|---|---|
| nTiO2 | 10,000–15,000 | [50,51] |
| nZnO | 1000–36,000 | [39,51] |
| nAg | 420 | [51] |
| nSiO2 | 1,400,000 | [51] |
| NEPs Type | ENMs Type | Organism | LC50 or EC50 | Observation | References |
|---|---|---|---|---|---|
| Sunscreen | nTiO2 + nZnO | P. lividus | 14–96 µL/L (standard salinity) | Growth inhibition | [148] |
| P. tricornutum | 9.9–82 µL/L (salinity stress) | ||||
| C. orientalis | |||||
| Textile | nAg | D. rerio | 0.26–0.4 mg/L | Hatching inhibition | [147] |
| * | Abnormal embryo development | ||||
| No effects | [124] | ||||
| Household detergent | nAg | P. aliena | * | Growth inhibition | [122] |
| C. fuciola | |||||
| A. agilis | |||||
| S. koyangensis | |||||
| Paint | nAg | P. subcapitata | * | No effects | [121] |
| V. fischeri | |||||
| D. magna |
| NEPs Type | Organisms Group | PR–ENMs | RQ (Least Case Scenarios) | RQ Interpretation | RQ (Worst Case Scenarios) | RQ Interpretation |
|---|---|---|---|---|---|---|
| Sunscreen | Algae | PR–nTiO2 | 0.073 | No significant risk | 34.1 | Potential adverse effects |
| PR–nZnO | 35.4 | Potential adverse effects | 35.4 | Potential adverse effects | ||
| Echinoderms | PR–nTiO2 | 0.007 | No significant risk | 0.083 | No significant risk | |
| PR–nZnO | 6.04 | Small adverse effects | 41.4 | Potential adverse effects | ||
| Crustacea | PR–nZnO | 25.9 | Potential adverse effects | 25.9 | Potential adverse effects | |
| Textile | Fish | PR–nAg | 800 | Significant adverse effects | 148,076.9 | Significant adverse effects |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moloi, M.S.; Lehutso, R.F.; Erasmus, M.; Oberholster, P.J.; Thwala, M. Aquatic Environment Exposure and Toxicity of Engineered Nanomaterials Released from Nano-Enabled Products: Current Status and Data Needs. Nanomaterials 2021, 11, 2868. https://doi.org/10.3390/nano11112868
Moloi MS, Lehutso RF, Erasmus M, Oberholster PJ, Thwala M. Aquatic Environment Exposure and Toxicity of Engineered Nanomaterials Released from Nano-Enabled Products: Current Status and Data Needs. Nanomaterials. 2021; 11(11):2868. https://doi.org/10.3390/nano11112868
Chicago/Turabian StyleMoloi, Mbuyiselwa Shadrack, Raisibe Florence Lehutso, Mariana Erasmus, Paul Johan Oberholster, and Melusi Thwala. 2021. "Aquatic Environment Exposure and Toxicity of Engineered Nanomaterials Released from Nano-Enabled Products: Current Status and Data Needs" Nanomaterials 11, no. 11: 2868. https://doi.org/10.3390/nano11112868
APA StyleMoloi, M. S., Lehutso, R. F., Erasmus, M., Oberholster, P. J., & Thwala, M. (2021). Aquatic Environment Exposure and Toxicity of Engineered Nanomaterials Released from Nano-Enabled Products: Current Status and Data Needs. Nanomaterials, 11(11), 2868. https://doi.org/10.3390/nano11112868








