A Review on Biphasic Calcium Phosphate Materials Derived from Fish Discards
Abstract
:1. Introduction
2. Biphasic Calcium Phosphate-Based Materials Derived from Fish Discards
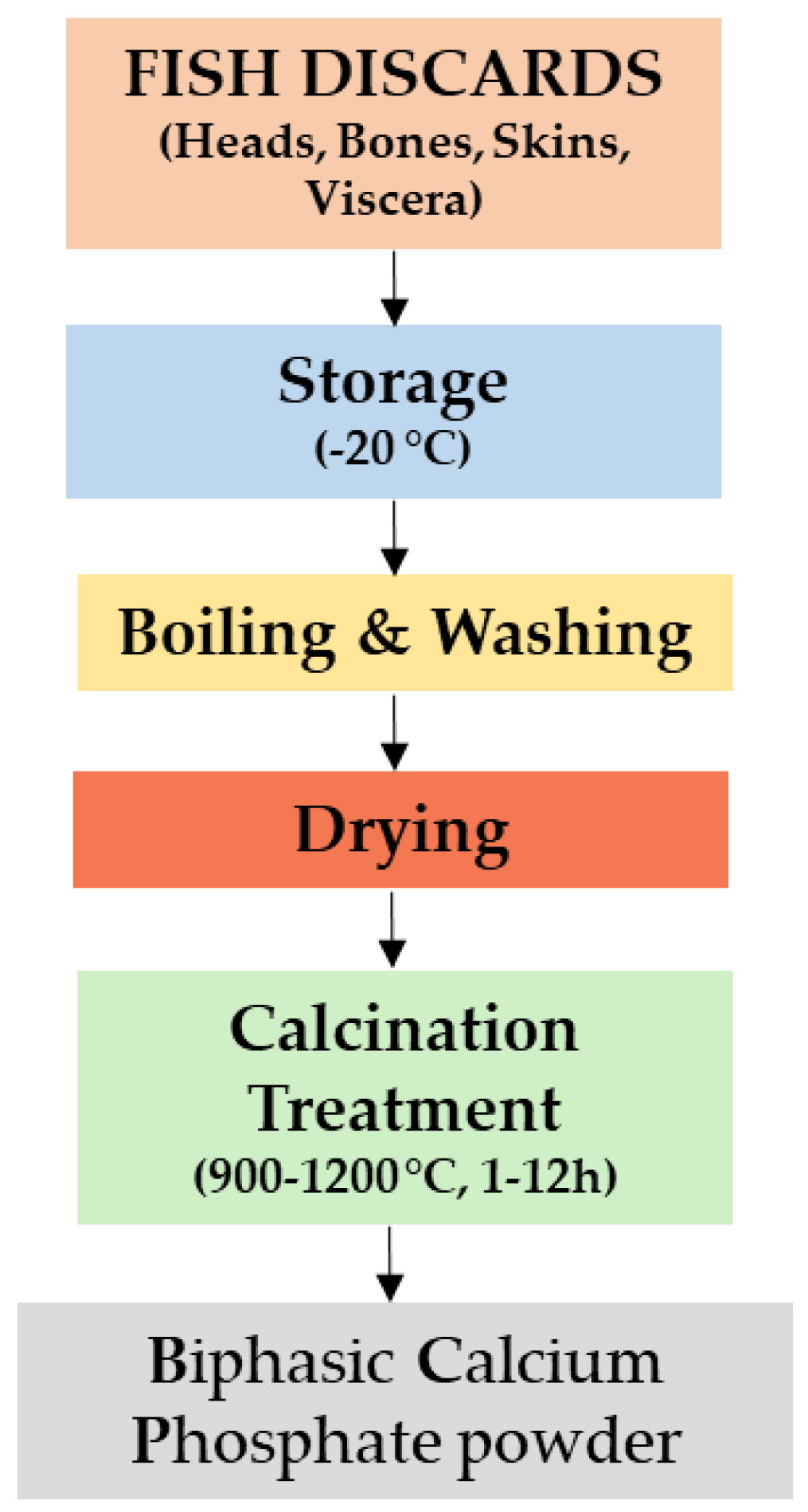
2.1. Preparation of Fish Discards to Produce Biphasic Calcium Phosphates
2.1.1. Pre-Treatment of Fish Discards
2.1.2. From Fish Discards to Powder Preparation
2.2. The Use of BCPs
2.2.1. BCP as Coatings
2.2.2. BCP as Scaffolds
2.2.3. BCP as Nanorods
3. Properties of Biphasic Calcium Phosphate Materials
3.1. BCP Powders
3.1.1. Morphological Characteristics
3.1.2. Elemental Composition
3.1.3. Structural Characteristics
3.1.4. Mechanical Properties
3.1.5. Biological Characteristics
- Interaction with the surroundings in terms of: allergic or toxicological effects, carcinogenic or mutagenic reactions, inflammatory processes, degree and quality of the biodegradation, and contact with human blood;
- Best period of the implant application: long- or short-term periods;
- Surface characteristics of the implants for the host tissue (chemical, biological, and morphological);
- Structural biocompatibility between the mechanical properties of the implant and of the host tissue;
- Function: optimal mechanical properties demanded by the application; and
- Proportion: optimal implant size and shape.
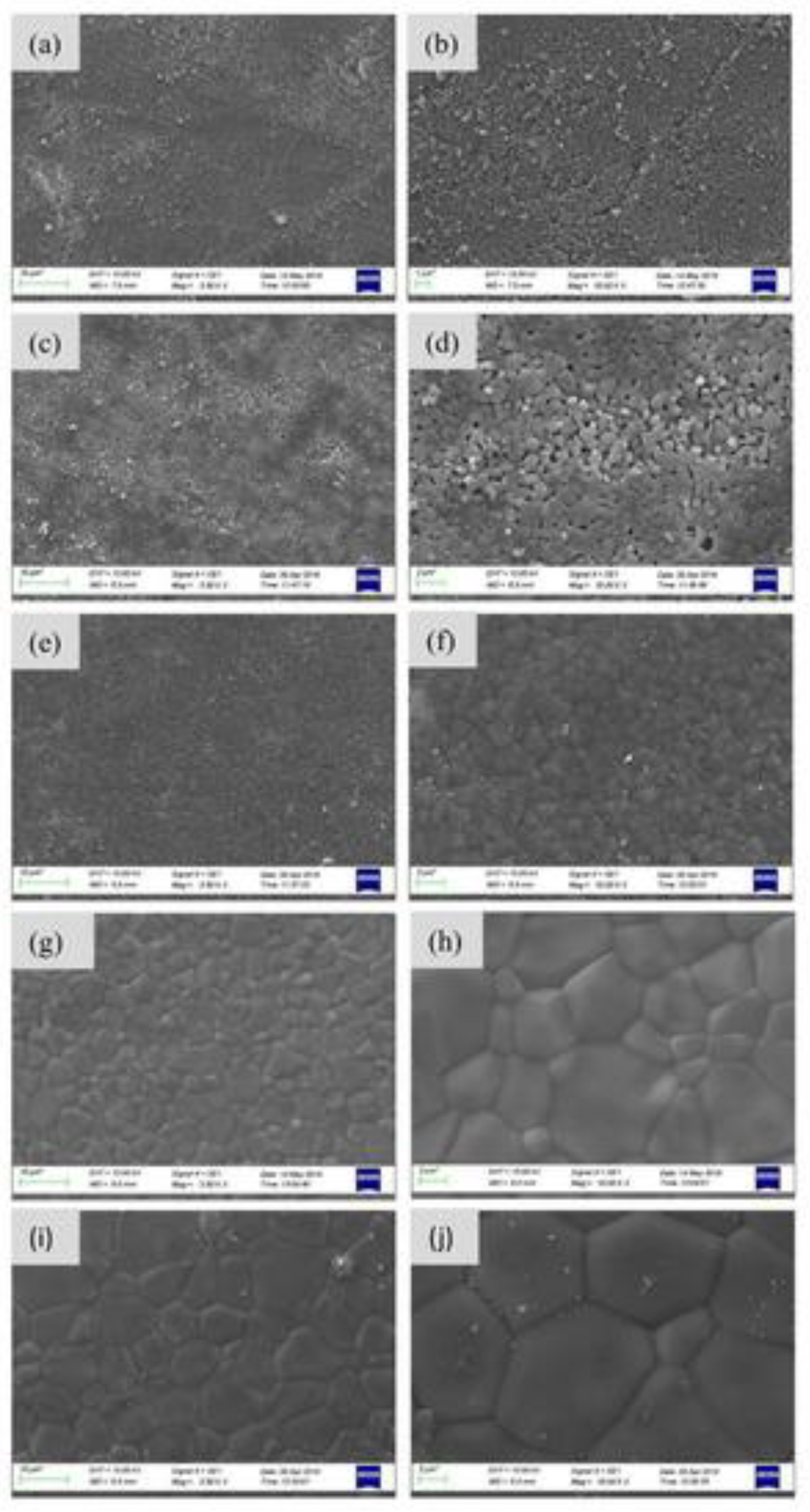
3.2. BCP Coatings
3.2.1. Morphological Characteristics
3.2.2. Elemental Composition
3.2.3. Structural Characteristics
3.2.4. Wettability Behavior
3.2.5. Mechanical Characteristics
3.2.6. Biological Characteristics
Cytocompatibility of Coatings
Antibacterial Activity
3.3. BCP Scaffolds
3.3.1. Morphological Characteristics
3.3.2. Elemental Composition
3.3.3. Structural Characteristics
3.3.4. Mechanical Properties
3.3.5. Biological Characteristics
3.4. BCP Nanorods
3.5. Applications of BCP Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Alliedmarketresearch. Available online: https://www.alliedmarketresearch.com/implantable-medical-devices-market (accessed on 23 June 2021).
- Oladele, I.O.; Agbabiaka, O.G.; Olasunkanmi, O.G.; Balogun, A.O.; Popoola, M.O. Non-synthetic sources for the development of hydroxyapatite. J. Appl. Biotechnol. Bioeng. 2018, 5, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Tite, T.; Popa, A.-C.; Balescu, L.M.; Bogdan, I.M.; Pasuk, I.; Ferreira, J.M.F.; Stan, G.E. Cationic Substitutions in Hydroxyapatite: Current Status of the Derived Biofunctional Effects and Their In Vitro Interrogation Methods. Materials 2018, 11, 2081. [Google Scholar] [CrossRef] [Green Version]
- Abidi, S.S.A.; Murtaza, Q. Synthesis and characterization of nanohydroxyapatite powder using wet chemical precipitation reaction. J. Mater. Sci. Technol. 2014, 30, 307–310. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S.; Bandyopadhyay, A. Hydroxyapatite coatings for metallic implants. In Hydroxyapatite (HAp) for Biomedical Applications, 1st ed.; Mucalo, M., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 143–157. [Google Scholar]
- Kang, Z.; Niu, J.Z.L. A one-step hydrothermal process to fabricate superhydrophobic hydroxyapatite coatings and determination of their properties. Surf. Coat. Tech. 2018, 334, 84–89. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Popa, C.L.; Predoi, D. Cerium-doped hydroxyapatite nanoparticles synthesized by the co-precipitation method. J. Serb. Chem. Soc. 2016, 81, 433–446. [Google Scholar] [CrossRef]
- Raucci, M.G.; Demitri, C.; Soriente, A.; Fasolino, I.; Sannino, A.; Ambrosio, L. Gelatin/nano-hydroxyapatite hydrogel scaffold prepared by sol-gel technology as filler to repair bone defects. J. Biomed. Mater. Res. Part A 2018, 106, 2007–2019. [Google Scholar] [CrossRef]
- Duta, L.; Mihailescu, N.; Popescu, A.C.; Luculescu, C.R.; Mihailescu, I.N.; Çetin, G.; Gunduz, O.; Oktar, F.N.; Popa, A.C.; Kuncser, A.; et al. Comparative physical, chemical and biological assessment of simple and titanium-doped ovine dentine-derived hydroxyapatite coatings fabricated by pulsed laser deposition. Appl. Surf. Sci. 2017, 413, 129–139. [Google Scholar] [CrossRef]
- Oktar, F.N. Microstructure and mechanical properties of sintered enamel hydroxyapatite. Ceram. Int. 2007, 33, 1309–1314. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Glimcher, M.J. Bone mineral: Update on chemical composition and structure. Osteoporos. Int. 2009, 20, 1013–1021. [Google Scholar] [CrossRef] [Green Version]
- Boutinguiza, M.; Pou, J.; Comesaña, R.; Lusquiños, F.; De Carlos, A.; León, B. Biological hydroxyapatite obtained from fish bones. Mater. Sci. Eng. C-Mater. Biol. Appl. 2012, 32, 478–486. [Google Scholar] [CrossRef]
- Venkatesan, J.; Lowe, B.; Manivasagan, P.; Kang, K.-H.; Chalisserry, E.P.; Anil, S.; Kim, D.G.; Kim, S.-K. Isolation and Characterization of Nano-Hydroxyapatite from Salmon Fish Bone. Materials 2015, 8, 5426–5439. [Google Scholar] [CrossRef]
- Coelho, T.M.; Nogueira, E.S.; Weinand, W.R.; Lima, W.M.; Steimacher, A.; Medina, A.N.; Baesso, M.L.; Bento, A.C. Thermal properties of natural nanostructured hydroxyapatite extracted from fish bone waste. J. Appl. Phys. 2007, 101, 084701. [Google Scholar] [CrossRef]
- Iriarte-Velasco, U.; Sierra, I.; Zudaire, L.; Ayastuy, J.L. Conversion of waste animal bones into porous hydroxyapatite by alkaline treatment: Effect of the impregnation ratio and investigation of the activation mechanism. J. Mater. Sci. 2015, 50, 7568–7582. [Google Scholar] [CrossRef]
- Trinkunaite-Felsen, J.; Birkedal, H.; Zarkov, A.; Tautkus, S.; Stankeviciute, Z.; Kareiva, A. Environmentally benign fabrication of calcium hydroxyapatite using seashells collected in Baltic Sea countries: A comparative study. Silicon Relat. Elem. 2016, 191, 919–925. [Google Scholar] [CrossRef]
- Terzioğlu, P.; Öğüt, H.; Kalemtaş, A. Natural calcium phosphates from fish bones and their potential biomedical applications. Mater. Sci. Eng. C 2018, 91, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Herliansyah, M.K.; Hamdi, M.; Ide-Ektessabi, A.; Wildan, M.W.; Toque, J.A. The influence of sintering temperature on the properties of compacted bovine hydroxyapatite. Mater. Sci. Eng. C 2009, 29, 1674–1680. [Google Scholar] [CrossRef]
- Iafisco, M.; Ruffini, A.; Adamiano, A.; Sprio, S.; Tampieri, A. Biomimetic magnesium–carbonate-apatite nanocrystals endowed with strontium ions as anti-osteoporotic trigger. Mater. Sci. Eng. C 2014, 35, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.; Shenoy, S.J.; Babu, S.S.; Nair, R.P.; Varma, H.K.; John, A. Strontium Hydroxyapatite scaffolds engineered with stem cells aid osteointegration and osteogenesis in osteoporotic sheep model. Colloids Surf. B Biointerfaces 2018, 163, 346–354. [Google Scholar] [CrossRef]
- Pietak, A.M.; Reid, J.W.; Stott, M.J.; Sayer, M. Silicon substitution in the calcium phosphate bioceramics. Biomaterials 2007, 28, 4023–4032. [Google Scholar] [CrossRef]
- Leventouri, T.H. Synthetic and biological hydroxyapatites: Crystal structure questions. Biomaterials 2006, 27, 3339–3342. [Google Scholar] [CrossRef] [PubMed]
- Ideia, P.; Pinto, J.; Ferreira, R.; Figueiredo, L.; Spínola, V.; Castilho, P.C. Fish processing industry residues: A review of valuable products extraction and characterization methods. Waste Biomass Valor. 2020, 11, 3223–3246. [Google Scholar] [CrossRef]
- Best, S.M.; Porter, A.E.; Thian, E.S.; Huang, J. Bioceramics: Past, present and for the future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327. [Google Scholar] [CrossRef]
- Hoyer, B.; Bernhardt, A.; Heinemann, S.; Stachel, I.; Meyer, M.; Gelinsky, M. Biomimetically Mineralized Salmon Collagen Scaffolds for Application in Bone Tissue Engineering. Biomacromolecules 2012, 13, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Rincón-López, J.A.; Hermann-Muñoz, J.A.; Giraldo-Betancur, A.L.; De Vizcaya-Ruiz, A.; Alvarado-Orozco, J.M.; Muñoz-Saldaña, J. Synthesis, Characterization and In Vitro Study of Synthetic and Bovine-Derived Hydroxyapatite Ceramics: A Comparison. Materials 2018, 11, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miculescu, F.; Stan, G.E.; Ciocan, L.T.; Miculescu, M.; Berbecaru, A.; Antoniac, I. Cortical bone as resource for producing biomimetic materials for clinical use. Dig. J. Nanomater. Biostruct. 2012, 7, 1667–1677. [Google Scholar]
- Niakan, A.; Singhbe, R.; Ganesan, P.; Tan, C.; Purbolaksono, J.; Chandran, H.; Teng, W. Sintering behaviour of natural porous hydroxyapatite derived from bovine bone. Ceram. Int. 2015, 41, 3024–3029. [Google Scholar] [CrossRef]
- Ramirez-Gutierrez, C.F.; Palechor-Ocampo, A.F.; Londoño-Restrepo, S.M.; Millán-Malo, B.M.; Rodriguez-García, M.E. Cooling rate effects on thermal, structural, and microstructural properties of bio-hydroxyapatite obtained from bovine bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 104, 339–344. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Zhang, R.; Jiang, D.; Zhu, Q.; Wang, S. Extraction and characterization of HA/b-TCP biphasic calcium phosphate from marine fish. Mater. Lett. 2019, 236, 680–682. [Google Scholar] [CrossRef]
- Nery, E.; Lynch, K.; Hirthe, W.; Mueller, K. Bioceramic implants in surgically produced infrabony defects. J. Periodontol. 1975, 46, 328–347. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphate materials in restorative dentistry: A review. Adv. Dent. Res. 1988, 2, 164–180. [Google Scholar] [CrossRef]
- Moore, D.; Chapman, M.; Manske, D. Evaluation of a new biphasic calcium phosphate ceramic for use in grafting long bone diaphyseal defects. Trans. Annu. Meet. Soc. Biomater. Conjunction Interna 1985, 160, 8. [Google Scholar]
- Ellinger, R.F.; Nery, E.B.; Lynch, K.L. Histological assessment of periodontal osseous defects following implantation of hydroxyapatite and biphasic calcium phosphate ceramics: A case report. Int. J. Periodontics Restor. Dent. 1986, 6, 22–33. [Google Scholar]
- LeGeros, R.Z. Variability of HAP/β-TCP ratios in sintered apatites. J. Dent. Res. 1986, 65, 292. [Google Scholar]
- Daculsi, G.; Legeros, R.Z.; Nery, E.; Lynch, K.; Kerebel, B. Transformation of biphasic calcium phosphate ceramics in vivo: Ultrastructural and physicochemical characterization. J. Biomed. Mater. Res. 1989, 23, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Suneelkumar, C.; Datta, K.; Srinivasan, M.R.; Kumar, S.T. Biphasic calcium phosphate in periapical surgery. J. Conserv. Dent. 2008, 11, 92–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behera, R.R.; Das, A.; Pamu, D.; Pandey, L.M.; Sankar, M.R. Mechano-tribological properties and in vitro bioactivity of biphasic calcium phosphate coating on Ti-6Al-4V. J. Mech. Behav. Biomed. Mater. 2018, 86, 143–157. [Google Scholar] [CrossRef]
- Kim, T.-W.; Lee, H.-S.; Kim, D.-H.; Jin, H.-H.; Hwang, K.-H.; Lee, J.K.; Park, H.-C.; Yoon, S.-Y. In Situ Synthesis of Magnesium-Substituted Biphasic Calcium Phosphate and in Vitro Biodegradation. Mater. Res. Bull. 2012, 47, 2506–2512. [Google Scholar] [CrossRef]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium Phosphate Ceramics in Bone Tissue Engineering: A Review of Properties and Their Influence on Cell Behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef] [PubMed]
- Bouler, J.M.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef]
- Goyenvalle, E.; Aguado, E.; Nguyen, J.M.; Passuit, N.; Le Guenennec, L.; Layrolle, P.; Daculsi, G. Osteointegration of femoral stem prostheses with a bilayered calcium phosphate coating. Biomaterials 2006, 27, 1119–1128. [Google Scholar] [CrossRef]
- Shim, K.; Kim, S.; Yun, Y.; Jeon, D.; Kim, H.; Park, K.; Song, H. Surface Immobilization of biphasic calcium phosphate nanoparticles on 3D printed poly(caprolactone) scaffolds enhances osteogenesis and bone tissue regeneration. J. Ind. Eng. Chem. 2017, 55, 101–109. [Google Scholar] [CrossRef]
- Marques, C.; Olhero, S.; Abrantes, J.; Marote, A.; Ferreira, S.; Vieira, S.; Ferreira, J. Biocompatibility and antimicrobial activity of biphasic calcium phosphate powders doped with metal ions for regenerative medicine. Ceram. Int. 2017, 43, 15719–15728. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, C.; Hong, Y.; Zhang, X. A review of protein adsorption on bioceramics. Interface Focus 2012, 2, 259–277. [Google Scholar] [CrossRef]
- Cheng, L.; Ye, F.; Yang, R.; Lu, X.; Shi, Y.; Li, L.; Fan, H.; Bu, H. Osteoinduction of hydroxyapatite/beta-tricalcium phosphate bioceramics in mice with a fractured fibula. Acta Biomater. 2010, 6, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Botelho, M.G.; Dorozhkin, S.V. Biphasic calcium phosphates bioceramics (HA/TCP): Concept, physicochemical properties and the impact of standardization of study protocols in biomaterials research. Mater. Sci. Eng. C 2017, 71, 1293–1312. [Google Scholar] [CrossRef]
- De Oliveira, R.S.; Brigato, R.; Madureira, J.F.G.; Cruz, A.A.V.; Filho, F.V.D.M.; Alonso, N.; Machado, H.R. Reconstruction of a large complex skull defect in a child: A case report and literature review. Child’s Nerv. Syst. 2007, 23, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, D.; Sola, A.; Gazzarri, M.; Chiellini, F.; Cannillo, V. A new hydroxyapatite-based biocomposite for bone replacement. Mater. Sci. Eng. C 2012, 33, 1091–1101. [Google Scholar] [CrossRef]
- Popescu-Pelin, G.; Ristoscu, C.; Duta, L.; Stan, G.E.; Pasuk, I.; Tite, T.; Stan, M.S.; Bleotu, C.; Popa, M.; Chifiriuc, M.C.; et al. Antimicrobial and Cytocompatible Bovine Hydroxyapatite-Alumina-Zeolite Composite Coatings Synthesized by Pulsed Laser Deposition from Low-Cost Sustainable Natural Resources. ACS Sustain. Chem. Eng. 2020, 8, 4026–4036. [Google Scholar] [CrossRef]
- Roda, M.A.P.; Gilman, E.; Huntington, T.; Kennelly, S.J.; Suuronen, P.; Chaloupka, M.; Medley, P.A. A Third Assessment of Global Marine Fisheries Discards. In FAO Fisheries and Aquaculture Technical Paper; No. 633; FAO: Rome, Italy, 2019; pp. 1–78. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2018-Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018; pp. 1–227. [Google Scholar]
- González, A.F.; Gracia, J.; Miniño, I.; Romón, J.; Larsson, C.; Maroto, J.; Requeira, M.; Pascual, S. Approach to reduce the zoonotic parasite load in fish stocks: When science meets technology. Fish. Res. 2018, 202, 140–148. [Google Scholar] [CrossRef]
- Rustad, T. Utilisation of marine by-products. Electron. J. Environ. Agric. Food Chem. 2003, 2, 458–463. [Google Scholar]
- Silva, T.; Moreira-Silva, J.; Marques, A.; Domingues, A.; Bayon, Y.; Reis, R.; Silva, T.H.; Moreira-Silva, J.; Marques, A.L.P.; Domingues, A.; et al. Marine origin collagens and its potential applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Alves, M.H.M.E.; Nascimento, G.A.; Cabrera, M.P.; Silvério, S.I.; Da, C.; Nobre, C.; Teixeira, J.A.; de Carvalho, L.B. Trypsin purification using magnetic particles of azocasein-iron composite. Food Chem. 2017, 226, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Lassoued, I.; Mora, L.; Nasri, R.; Jridi, M.; Toldrá, F.; Aristoy, M.-C.; Barkia, A.; Nasri, M. Characterization and comparative assessment of antioxidant and ACE inhibitory activities of thornback ray gelatin hydrolysates. J. Funct. Foods 2015, 13, 225–238. [Google Scholar] [CrossRef]
- Maccari, F.; Galeotti, F.; Volpi, N. Isolation and structural characterization of chondroitin sulfate from bony fishes. Carbohydr. Polym. 2015, 129, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M. Enhancing and improving the extraction of omega-3 from fish oil. Sustain. Chem. Pharm. 2017, 5, 54–59. [Google Scholar] [CrossRef]
- Ferraro, V.; Carvalho, A.P.; Piccirillo, C.; Santos, M.M.; Castro, P.M.; Pintado, M.E. Extraction of high added value biological compounds from sardine, sardine-type fish and mackerel canning residues—A review. Mater. Sci. Eng. C 2013, 33, 3111–3120. [Google Scholar] [CrossRef]
- Toppe, J.; Albrektsen, S.; Hope, B.; Aksnes, A. Chemical composition, mineral content and amino acid and lipid profiles in bones from various fish species. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 146, 395–401. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Dinesh kumar, B.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef]
- Zamora-Sillero, J.; Gharsallaoui, A.; Prentice, C. Peptides from fish by-product protein hydrolysates and its functional properties: An overview. Mar. Biotechnol. 2018, 20, 118–130. [Google Scholar] [CrossRef]
- Ishak, N.H.; Sarbon, N.M. A review of protein hydrolysates and bioactive peptides deriving from wastes generated by fish processing. Food Bioprocess Technol. 2018, 11, 2–16. [Google Scholar] [CrossRef]
- Rustad, T.; Storrø, I.; Slizyte, R. Possibilities for the utilisation of ma-rine by-products: Utilisation of marine by-products. Int. J. Food Sci. Technol. 2011, 46, 2001–2014. [Google Scholar] [CrossRef]
- Adeoti, I.A.; Hawboldt, K. A review of lipid extraction from fish processing by-product for use as a biofuel. Biomass Bioenergy 2014, 63, 330–340. [Google Scholar] [CrossRef]
- Olsen, R.L.; Toppe, J.; Karunasagar, I. Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends Food Sci. Technol. 2014, 36, 144–151. [Google Scholar] [CrossRef]
- Moriguchi, T.; Nakagawa, S.; Kaji, F. Reaction of Ca-deficient Hydroxyapatite with heavy metal ions along with metal substitution. Phosphorus Res. Bull. 2008, 22, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Annual Book of ASTM Standards, F 1581–1599. In Standard Specification for Composition of Anorganic Bone for Surgical Implants; USA, 1999; Volume 13, pp. 893–895. Available online: https://www.astm.org/Standard/alpha-lists/F.html (accessed on 21 October 2021).
- Griffith, M.; Islam, M.M.; Edin, J.; Papapavlou, G.; Buznyk, O.; Patra, H.K. The Quest for Anti-inflammatory and Anti-infective Biomaterials in Clinical Translation. Front. Bioeng. Biotechnol. 2016, 4, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, M.; Ingle, A.P.; Gaikwad, S.; Gupta, I.; Gade, A.; Silva, S.S. Nanotechnology based anti-infectives to fight microbial intrusions. J. Appl. Microbiol. 2016, 120, 527–542. [Google Scholar] [CrossRef]
- Létisse, M.; Comeau, L. Enrichment of eicosapentaenoic acid and docosahexaenoic acid from sardine by-products by supercritical fluid fractionation. J. Sep. Sci. 2008, 31, 1374–1380. [Google Scholar] [CrossRef]
- Murado, M.A.; Montemayor, M.I.; Cabo, M.L.; Vázquez, J.A.; González, M.P. Optimization of extraction and purification process of hyaluronic acid from fish eyeball. Food Bioprod. Process. 2012, 90, 491–498. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, A.; Shaw, N.B.; Murphy, S.C.; van de Vis, J.W.; van Pelt-Heerschap, H.; Kerry, J.P. Extraction of collagen from fish skins and its use in the manufacture of biopolymer films. J. Aquat. Food Prod. Technol. 2006, 15, 21–32. [Google Scholar] [CrossRef]
- Sousa, R.O.; Martins, E.; Carvalho, D.N.; Alves, A.L.; Oliveira, C.; Duarte, A.R.C.; Silva, T.H.; Reis, R.L. Collagen from Atlantic cod (Gadus morhua) skins extracted using CO2 acidified water with potential application in healthcare. J. Polym. Res. 2020, 27, 73. [Google Scholar] [CrossRef] [Green Version]
- Sousa, R.O.; Alves, A.L.; Carvalho, D.N.; Martins, E.; Oliveira, C.; Duarte, A.R.C.; Silva, T.H.; Reis, R.L. Acid and enzymatic extraction of collagen from Atlantic cod (Gadus Morhua) swim bladders envisaging health-related applications. Biomater. Sci. Polym. 2020, 31, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Seixas, M.J.; Martins, E.; Oliveira, C.; Duarte, A.R.C.; Silva, T.H.; Reis, R.L. Extraction and characterization of collagen from elasmobranch byproducts for potential biomaterial use. Mar. Drugs 2020, 18, 617. [Google Scholar] [CrossRef] [PubMed]
- Herpandi, H.; Huda, N.; Adzitey, F. Fish bone and scale as a potential source of halal gelatin. J. Fish. Aquat. Sci. 2011, 6, 379–389. [Google Scholar] [CrossRef] [Green Version]
- Lv, L.C.; Huang, Q.Y.; Ding, W.; Xiao, X.H.; Zhang, H.Y.; Xiong, L.X. Fish gelatin: The novel potential applications. J. Funct. Foods 2019, 63, 103581. [Google Scholar] [CrossRef]
- Rafael, M.Y.B.; Rafael, R.R.; Landingin, E.P.; Rafael, R.B.; Tayag, G.G.; Santos, J.P.E.; Rafael, M.J.R. Gelatin from Milkfish Scales for Food Application. CLSU Int. J. Sci. Technol. 2021, 5, 1. [Google Scholar] [CrossRef]
- Venkatesan, J.; Qian, Z.J.; Ryu, B.; Thomas, N.V.; Kim, S.K. A comparative study of thermal calcination and an alkaline hydrolysis method in the isolation of hydroxyapatite from Thunnus obesus bone. Biomed. Mater. 2011, 6, 035003. [Google Scholar] [CrossRef]
- Piccirillo, C.; Silva, M.; Pullar, R.; da Cruz, I.B.; Jorge, R.; Pintado, M.; Castro, P.M. Extraction and characterisation of apatite-and tricalcium phosphate-based materials from cod fish bones. Mater. Sci. Eng. C 2013, 33, 103–110. [Google Scholar] [CrossRef]
- Goto, T.; Sasaki, K. Effects of trace elements in fish bones on crystal characteristics of hydroxyapatite obtained by calcination. Ceram. Int. 2014, 40, 10777–10785. [Google Scholar] [CrossRef]
- Piccirillo, C.; Pullar, R.C.; Tobaldi, D.M.; Castro, P.M.L.; Pintado, M.M.E. Hydroxyapatite and chloroapatite derived from sardine by-products. Ceram. Int. 2014, 40, 13231–13240. [Google Scholar] [CrossRef]
- Sunil, B.R.; Jagannatham, M. Producing hydroxyapatite from fish bones by heat treatment. Mater. Lett. 2016, 185, 411–414. [Google Scholar] [CrossRef]
- Pal, A.; Paul, S.; Choudhury, A.R.; Balla, V.K.; Das, M.; Sinha, A. Synthesis of hydroxyapatite from Lates calcarifer fish bone for biomedical applications. Mater. Lett. 2017, 203, 89–92. [Google Scholar] [CrossRef]
- Boutinguiza, L.M.; Pou Saracho, J.M.; Comesana Pineiro, R.; Lusquinos Rodriguez, F.; Leon Fong, B.M. Biphasic Calcium Phosphate and Method for Obtaining Same from Fish Bones. European Patent Application EP 2,075,231 A1, 1 July 2009. [Google Scholar]
- Kongsri, S.; Janpradit, K.; Buapa, K.; Techawongstien, S.; Chanthai, S. Nanocrystalline hydroxyapatite from fish scale waste: Preparation, characterization and application for selenium adsorption in aqueous solution. Chem. Eng. J. 2013, 215–216, 522–532. [Google Scholar] [CrossRef]
- Montañez Supelano, N.D.; Estupiñan, H.A.; Garcia, S.J.; Peña Ballesteros, D.Y. Fabrication and characterization of novel biphasic calcium phosphate and nanosized hydroxyapatite derived from fish otoliths in different composition ratios. Chem. Eng. Trans. 2018, 64, 307–312. [Google Scholar] [CrossRef]
- Zhu, Q.; Ablikim, Z.; Chen, T.; Cai, Q.; Xia, J.; Jiang, D.; Wang, S. The Preparation and Characterization of HA/β-TCP Biphasic Ceramics from Fish Bones. Ceram. Int. 2017, 43, 12213–12220. [Google Scholar] [CrossRef]
- Dou, W.; Chen, H.; Chen, T.; Zhu, Q.; Jiang, D.; Xue, Z.; Wang, S.; Wang, S.; Tang, W. Design and construction of a microporous CO32−-containing HA/β- TCP biphasic ceramic as a novel bone graft material. Mater. Res. Express 2020, 7, 025401. [Google Scholar] [CrossRef]
- Kiyochi Junior, H.d.J.; Candido, A.G.; Bonadio, T.G.M.; Adauto da Cruz, J.; Baesso, M.L.; Weinand, W.R.; Hernandes, L. In vivo evaluation of interactions between biphasic calcium phosphate (BCP)-niobium pentoxide (Nb2O5) nanocomposite and tissues using a rat critical-size calvarial defect model. J. Mater. Sci. Mater. Med. 2020, 31, 71. [Google Scholar] [CrossRef]
- Piccirillo, C.; Dunnill, C.W.; Pullar, R.C.; Tobaldi, D.M.; Labrincha, J.A.; Parkin, I.P.; Pintado, M.M.; Castro, P.M.L. Calcium phosphate-based materials of natural origin showing photocatalytic activity. J. Mater. Chem. A 2013, 1, 6452–6461. [Google Scholar] [CrossRef]
- Soumia, B.; Ahmed, B.; Abdelmjid, A. Biphasic Calcium Phosphate Derived from a Sardine By-Product. Curr. Trends Biomed. Eng. Biosci. 2017, 6, 555690. [Google Scholar] [CrossRef]
- Bas, M.; Daglilar, S.; Kuskonmaz, N.; Kalkandelen, C.; Erdemir, G.; Kuruca, S.E.; Tulyaganov, D.; Yoshioka, T.; Gunduz, O.; Ficai, D.; et al. Mechanical and Biocompatibility Properties of Calcium Phosphate Bioceramics Derived from Salmon Fish Bone Wastes. Int. J. Mol. Sci. 2020, 21, 8082. [Google Scholar] [CrossRef]
- Santosh Kumar, B.Y.; Isloor, A.M.; Anil, S.; Venkatesan, J.; Kumar, G.C.M. Calcium phosphate bioceramics with polyvinyl alcohol hydrogels for biomedical applications. Mater. Res. Express 2019, 6, 125404. [Google Scholar] [CrossRef]
- Piccirillo, C.; Pullar, R.C.; Costa, E.; Santos-Silva, A.; Pintado, M.M.E.; Castro, P.M.L. Hydroxyapatite-based materials of marine origin: A bioactivity and sintering study. Mater. Sci. Eng. C 2015, 51, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Basu, B. Unravelling Doped Biphasic Calcium Phosphate: Synthesis to Application. ACS Appl. Bio. Mater. 2019, 2, 5263–5297. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.K. Effect of temperature on isolation and characterization of hydroxyapatite from tuna (Thunnus obesus) Bone. Materials 2010, 3, 4761–4772. [Google Scholar] [CrossRef]
- Piccirillo, C.; Adamiano, A.; Tobaldi, D.M.; Montalti, M.; Manzi, J.; Castro, P.M.L.; Panseri, S.; Montesi, M.; Sprio, S.; Tampieri, A.; et al. Luminescent calcium phosphate bioceramics doped with europium derived from fish industry byproducts. J. Am. Ceram. Soc. 2017, 100, 3402–3414. [Google Scholar] [CrossRef]
- Coelho, T.M.; Nogueira, E.S.; Steimacher, A.; Medina, A.N.; Weinand, W.R.; Lima, W.M.; Baesso, M.L.; Bento, A.C. Characterization of natural nanostructured hydroxyapatite obtained from the bones of Brazilian river fish. J. Appl. Phys. 2006, 100, 094312. [Google Scholar] [CrossRef]
- Silva, L.M.; Rosso, J.M.; Bonadio, T.G.M.; Silva, D.M.; Dias, G.S.; Weinand, W.R.; Tominaga, T.T.; Miyahara, R.Y.; Cótica, L.F.; Santos, I.A.; et al. On mechanical properties and bioactivity of PVDF-BCP composites. Cerâmica 2018, 64, 359–366. [Google Scholar] [CrossRef]
- Neto, A.S.; Fonseca, A.C.; Abrantes, J.C.C.; Coelho, J.F.J.; Ferreira, J.M.F. Surface functionalization of cuttlefish bone-derived biphasic calcium phosphate scaffolds with polymeric coatings. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110014. [Google Scholar] [CrossRef]
- Pon-On, W.; Suntornsaratoon, P.; Charoenphandhu, N.; Thongbunchoo, J.; Krishnamra, N.; Tang, I.M. Hydroxyapatite from fish scale for potential use as bone scaffold or regenerative material. Mater. Sci. Eng. C 2016, 62, 183–189. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Hsiao, P.-C.; Chai, H.-J. Hydroxyapatite extracted from fish scale: Effects on MG63 osteoblast-like cells. Ceram. Int. 2011, 37, 1825–1831. [Google Scholar] [CrossRef]
- Fernández-Arias, M.; Álvarez-Olcina, I.; Malvido-Fresnillo, P.; Vázquez, J.A.; Boutinguiza, M.; Comesaña, R.; Pou, J. Biogenic Calcium Phosphate from Fish Discards and By-Products. Appl. Sci. 2021, 11, 3387. [Google Scholar] [CrossRef]
- Bonadio, T.G.M.; Freitas, V.F.; Tominaga, T.T.; Miyahara, R.Y.; Rosso, J.M.; Cótica, L.F.; Baesso, M.L.; Weinand, W.R.; Santos, I.A.; Guo, R.; et al. Polyvinylidene fluoride/hydroxyapatite/β-tricalcium phosphate multifunctional biocomposite: Potentialities for bone tissue engineering. Curr. Appl. Phys. 2017, 17, 767–773. [Google Scholar] [CrossRef] [Green Version]
- Velten, D.; Eisenbarth, E.; Schanne, N.; Breme, J. Biocompatible Nb2O5 thin films prepared by means of the sol-gel process. J. Mater. Sci. Mater. Med. 2004, 15, 457–461. [Google Scholar] [CrossRef]
- Ribeiro, C.; Moreira, S.; Correia, V.; Sencadas, V.; Rocha, J.G.; Gama, F.M.; Ribelles, J.L.G.; Mendez, S.L. Enhanced proliferation of pre-osteoblastic cells by dynamic piezoelectric stimulation. RSC Adv. 2012, 2, 11504–11509. [Google Scholar] [CrossRef]
- Popescu-Pelin, G.; Ristoscu, C.; Duta, L.; Pasuk, I.; Stan, G.E.; Stan, M.S.; Popa, M.; Chifiriuc, M.C.; Hapenciuc, C.; Oktar, F.N.; et al. Fish Bone Derived Bi-Phasic Calcium Phosphate Coatings Fabricated by Pulsed Laser Deposition for Biomedical Applications. Mar. Drugs 2020, 18, 623. [Google Scholar] [CrossRef]
- Behera, R.R.; Das, A.; Hasan, A.; Pamu, D.; Pandey, L.M.; Sankar, M.R. Deposition of biphasic calcium phosphate film on laser surface textured Ti-6Al-4V and its effect on different biological properties for orthopedic applications. J. Alloy Compd. 2020, 842, 155683. [Google Scholar] [CrossRef]
- Chrisey, D.B.; Hubler, G.K. Pulsed Laser Deposition of Thin Films, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 1994; pp. 1–649. [Google Scholar]
- Eason, R. Pulsed Laser Deposition of Thin Films-Applications-Led Growth of Functional Materials; Wiley-Interscience: Hoboken, NJ, USA, 2006; pp. 1–682. [Google Scholar]
- Bao, Q.; Chen, C.; Wang, D.; Ji, Q.; Leia, T. Pulsed laser deposition and its current research status in preparing hydroxyapatite thin films. Appl. Surf. Sci. 2005, 252, 1538–1544. [Google Scholar] [CrossRef]
- Badea, M.; Braic, M.; Kiss, A.; Moga, M.; Pozna, E.; Pana, I.; Vladescu, A. Influence of Ag content on the antibacterial properties of SiC doped hydroxyapatite coatings. Ceram. Int. 2016, 42, 1801–1811. [Google Scholar] [CrossRef]
- Neto, A.S.; Brazete, D.; Ferreira, J.M.F. Cuttlefish Bone-Derived Biphasic Calcium Phosphate Scaffolds Coated with Sol-Gel Derived Bioactive Glass. Materials 2019, 12, 2711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesan, J.; Rekha, P.; Anil, S.; Bhatnagar, I.; Sudha, P.; Dechsakulwatana, C.; Kim, S.-K.; Shim, M.S. Hydroxyapatite from cuttlefish bone: Isolation, characterizations, and applications. Biotechnol. Bioprocess Eng. 2018, 23, 383–393. [Google Scholar] [CrossRef]
- Ideia, P.; Esposti, L.D.; Miguel, C.C.; Adamiano, A.; Iafisco, M.; Castilho, P.C. Extraction and characterization of hydroxyapatite-based materials from grey triggerfish skin and black scabbardfish bones. Int. J. Appl. Ceram. Technol. 2021, 18, 235–243. [Google Scholar] [CrossRef]
- Montañez-Supelano, N.D.; Sandoval-Amador, A.; Estupiñan-Durán, H.A.; Peña-Ballesteros, D.Y. A novel biphasic calcium phosphate derived from fish otoliths. J. Phys. Conf. Ser. 2017, 935, 012037. [Google Scholar] [CrossRef]
- Silva, C.C.; Pinheiro, A.G.; Figueiró, S.D.; Góes, J.C.; Sasaki, J.M.; Miranda, M.A.R.; Sombra, A.S.B. Piezoelectric properties of collagen-nanocrystalline hydroxyapatite composites. J. Mater. Sci. 2002, 37, 2061–2070. [Google Scholar] [CrossRef]
- Pollick, S.; Shors, E.C.; Holmes, R.E.; Kraut, R.A. Bone formation and implant degradation of coralline porous ceramics placed in bone and ectopic sites. J. Oral Maxillofac. Surg. 1995, 53, 915–922. [Google Scholar] [CrossRef]
- Ciobanu, G.; Ilisei, S.; Luca, C.; Carja, G.; Ciobanu, O. The effect of vitamins to hydroxyapatite growth on porous polyurethane substrate. Prog. Org. Coat. 2012, 74, 648–653. [Google Scholar] [CrossRef]
- Takeuchi, A.; Ohtsuki, C.; Miyazaki, T.; Kamitakahara, M.; Ogata, S.-I.; Yamazaki, M.; Furutani, Y.; Kinoshita, H.; Tanihara, M. Heterogeneous nucleation of hydroxyapatite on protein: Structural effect of silk sericin. J. R. Soc. Interface 2005, 2, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Heymann, D.; Bouler, J.M.; Daculsi, G. Osteoclastic resorption of calcium phosphate ceramics with different hydroxyapatite/β-tricalcium phosphate ratios. Biomaterials 1997, 18, 1037–1041. [Google Scholar] [CrossRef]
- Cooper, L.F.; Zhou, Y.; Takebe, J.; Guo, J.; Abron, A.; Holmén, A.; Ellingsen, J.E. Fluoride modification effects on osteoblast behavior and bone formation at TiO2 grit-blasted c.p. titanium endosseous implants. Biomaterials 2006, 27, 926–936. [Google Scholar] [CrossRef]
- Pazarçeviren, A.E.; Tezcaner, A.; Keskin, D.; Kolukısa, S.T.; Sürdem, S.; Evis, Z. Boron-doped Biphasic Hydroxyapatite/β-Tricalcium Phosphate for Bone Tissue Engineering. Biol. Trace Elem. Res. 2021, 199, 968–980. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Pripatnanont, P.; Monmaturapoj, N.; Suttapreyasri, S. Fabrication and characterization of novel nano hydroxyapatite/β-tricalcium phosphate scaffolds in three different composition ratios. J. Biomed. Mater. Res. A 2012, 100, 2260–2268. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; Lin, S.; Rohanizadeh, R.; Mijares, D.; LeGeros, J.P. Biphasic calcium phosphate bioceramics: Preparation, properties and applications. J. Mater. Sci. Mater. Med. 2003, 14, 201–209. [Google Scholar] [CrossRef]
- Stock, S.R. The Mineral–Collagen Interface in Bone. Calcif. Tissue Int. 2015, 97, 262–280. [Google Scholar] [CrossRef] [Green Version]
- Habelitz, S.; Pascual, L.; Durán, A. Transformation of tricalcium phosphate into apatite by ammonia treatment. J. Mater. Sci. 2001, 36, 4131–4135. [Google Scholar] [CrossRef]
- Smičiklas, I.; Onjia, A.; Raičević, S.; Mitrić, M. Factors influencing the removal of divalent cations by hydroxyapatite. J. Hazard. Mater. 2007, 152, 876–884. [Google Scholar] [CrossRef]
- Gianmar, D.E.; Xiu, L.; Pasteris, J.D. Immobilization of Lead with Nanocrystalline Carbonated Apatite Present in Fish Bone. Environ. Eng. Sci. 2008, 25, 725–735. [Google Scholar] [CrossRef]
- Ozawa, M.; Suzuki, M. Microstructural Development of Natural Hydroxyapatite Originated from Fish-Bone Waste through Heat Treatment. J. Am. Ceram. Soc. 2002, 85, 1315–1317. [Google Scholar] [CrossRef]
- Lim, J.J. Thermogravimetric analysis of human femur bone. J. Biol. Phys. 1975, 3, 111–129. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Yubao, L.; Wijn, J.D.; Klein, C.P.A.T.; Meer, S.V.; Groot, K.D. Preparation and characterization of nanograde osteoapatite-like rod crystals. J. Mater. Sci. Mater. Med. 1994, 5, 252–255. [Google Scholar] [CrossRef]
- Kannan, S.; Goetz-Neunhoeffer, F.; Neubauer, J.; Ferreira, J.M.F. Ionic substitutions in biphasic hydroxyapatite and β-tricalcium phosphate mixtures: Structural analysis by Rietveld refinement. J. Am. Ceram. Soc. 2008, 91, 1–12. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate-based bioceramics. Materials 2013, 6, 3840–3942. [Google Scholar] [CrossRef] [Green Version]
- Cesar, R.; Leivas, T.P.; Pereira, C.A.M.; Boffa, R.S.; Guarniero, R.; Reiff RBM Mandeli Netto, A.; Fortulan, C.A.; de Almeida Rollo, J.M.D. Axial compressive strength of human vertebrae trabecular bones classified as normal, osteopenic and osteoporotic by quantitative ultrasonometry of calcaneus. Res. Biomed. Eng. 2017, 33, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Havaldar, R.; Pilli, S.C.; Putti, B.B. Insights into the effects of tensile and compressive loadings on human femur bone. Adv. Biomed. Res. 2014, 3, 101. [Google Scholar] [CrossRef]
- Karpiński, R.; Jaworski, Ł.; Czuback, P. The structural and mechanical properties of the bone. J. Technol. Exploit Mech. Eng. 2017, 3, 43–50. [Google Scholar] [CrossRef]
- Parente, P.; Savoini, B.; Ferrari, B.; Monge, M.A.; Pareja, R.; Sanchez-Herencia, A.J. Effect of highly dispersed yttria addition on thermal stability of hydroxyapatite. Mater. Sci. Eng. C 2013, 33, 864–869. [Google Scholar] [CrossRef] [Green Version]
- Dorozhkin, S.V. Biocomposites and hybrid biomaterials based on calcium orthophosphates. Biomatter 2011, 1, 3–56. [Google Scholar] [CrossRef] [Green Version]
- Canillas, M.; De Lima, G.G.; Rodríguez, M.A.; Nugent, M.J.D.; Devine, D.M. Bioactive composites fabricated by freezing–thawing method for bone regeneration applications. J. Polym. Sci. Part B 2015, 54, 761–773. [Google Scholar] [CrossRef]
- Killion, A.; Geever, L.M.; Devine, D.M.; Farrell, H.; Higginbotham, C.L. Compressive strength and bioactivity properties of photopolymerizable hybrid composite hydrogels for bone tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 641–650. [Google Scholar] [CrossRef]
- Kenny, E.K.; Gately, N.M.; Killion, J.A.; Devine, D.M.; Higginbotham, C.L.; Geever, L.M. Melt extruded bioresorbable polymer composites for potential regenerative medicine applications. Polym. Plast. Technol. Eng. 2016, 55, 432–446. [Google Scholar] [CrossRef]
- Demirkol, N.; Oktar, F.N.; Kayali, E.S. Mechanical and microstructural properties of sheep hydroxyapatite (SHA)–niobium oxide composites. Acta Phys. Pol. A 2012, 121, 274–276. [Google Scholar] [CrossRef]
- Duta, L.; Oktar, F.; Stan, G.; Popescu-Pelin, G.; Serban, N.; Luculescu, C.; Mihailescu, I. Novel doped hydroxyapatite thin films obtained by pulsed laser deposition. Appl. Surf. Sci. 2013, 265, 41–49. [Google Scholar] [CrossRef]
- Nelea, V.; Jelinek, M.; Mihailescu, I.N. Biomaterials: New issues and breakthroughs for biomedical applications. In Pulsed Laser Deposition of Thin Films: Applications-Lead Growth of Functional Materials; Eason, R., Ed.; Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 421–459. [Google Scholar]
- Curcio, M.; De Stefanis, A.; De Bonis, A.; Teghil, R.; Rau, J.V. Pulsed laser deposited bioactive RKKP-Mn glass-ceramic coatings on titanium. Surf. Coat. Technol. 2019, 357, 122–128. [Google Scholar] [CrossRef]
- Von Euw, S.; Wang, Y.; Laurent, G.; Drouet, C.; Babonneau, F.; Nassif, N.; Azaïs, T. Bone mineral: New insights into its chemical composition. Sci. Rep. 2019, 9, 8456. [Google Scholar] [CrossRef] [Green Version]
- Sponer, P.; Strnadova, M.; Urban, K. In vivo behavior of low-temperature calcium-deficient hydroxyapatite: Comparison with deproteinised bovine bone. Int. Orthop. 2011, 35, 1553–1560. [Google Scholar] [CrossRef] [Green Version]
- Dulski, M.; Dudek, K.; Grelowski, M.; Kubacki, J.; Hertlein, J.; Wojtyniak, M.; Goryczka, T. Impact of annealing on features of BCP coating on NiTi shape memory alloy: Preparation and physicochemical characterization. Appl. Surf. Sci. 2018, 437, 28–40. [Google Scholar] [CrossRef]
- Xia, L.; Xie, Y.; Fang, B.; Wang, X.; Lin, K. In situ modulation of crystallinity and nano-structures to enhance the stability and osseointegration of hydroxyapatite coatings on Ti-6Al-4V implants. Chem. Eng. J. 2018, 347, 711–720. [Google Scholar] [CrossRef]
- Thian, E.S.; Huang, J.; Best, S.M.; Barber, Z.H.; Bonfield, W. Magnetron cosputtered silicon-containing hydroxyapatite thin films —An in vitro study. Biomaterials 2005, 26, 2947–2956. [Google Scholar] [CrossRef]
- Surmeneva, M.; Vladescu, A.; Surmenev, R.; Pantilimon, C.; Braic, M.; Cotrut, C. Study on a hydrophobic Ti-doped hydroxyapatite coating for corrosion protection of a titanium based alloy. RSC Adv. 2016, 6, 87665–87674. [Google Scholar] [CrossRef]
- Bohner, M.; Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Popescu, A.; Florian, P.; Stan, G.; Popescu-Pelin, G.; Zgura, I.; Enculescu, M.; Oktar, F.; Trusca, R.; Sima, L.E.; Roseanu, A.; et al. Physical-chemical characterization and biological assessment of simple and lithium-doped biological-derived hydroxyapatite thin films for a new generation of metallic implants. Appl. Surf. Sci. 2018, 439, 724–735. [Google Scholar] [CrossRef]
- Hasan, A.; Pattanayek, S.K.; Pandey, L.M. Effect of functional groups of selfassembled monolayers on protein adsorption and initial cell adhesion. ACS Biomater. Sci. Eng. 2018, 4, 3224–3233. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, S.L.; Predoi, D.; Stan, G.E.; Buton, N. Synthesis, Characterization, and Antimicrobial Activity of Magnesium-Doped Hydroxyapatite Suspensions. Nanomaterials 2019, 9, 1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutha, S.; Kavitha, K.; Karunakaran, G.; Rajendran, V. In-vitro bioactivity, biocorrosion and antibacterial activity of silicon integrated hydroxyapatite/chitosan composite coating on 316L stainless steel implants. Mater. Sci. Eng. C 2013, 33, 4046–4054. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Paralikar, P. Sulfur and sulfur nanoparticles as potential antimicrobials: From traditional medicine to nanomedicine. Expert Rev. Anti-Infect. Ther. 2016, 14, 969–978. [Google Scholar] [CrossRef]
- Rocha, J.H.G.; Lemos, A.F.; Agathopoulos, S.; Valério, P.; Kannan, S.; Oktar, F.N.; Ferreira, J.M.F. Scaffolds for bone restoration from cuttlefish. Bone 2005, 37, 850–857. [Google Scholar] [CrossRef]
- Cao, W.; Hench, L.L. Bioactive Materials. Ceram. Int. 1996, 22, 493–507. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Lowe, B.; Venkatesan, J.; Anil, S.; Shim, M.S.; Kim, S.-K. Preparation and characterization of chitosan-natural nano hydroxyapatitefucoidan nanocomposites for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1479–1487. [Google Scholar] [CrossRef]
- Nie, L.; Suo, J.; Zou, P.; Feng, S. Preparation and properties of biphasic calcium phosphate scaffolds multiply coated with HA/PLLA nanocomposites for bone tissue engineering applications. J. Nanomater. 2012, 2012, 213549. [Google Scholar] [CrossRef]
- Ou, K.; Dong, X.; Qin, C.; Ji, X.; He, J. Properties and toughening mechanisms of PVA/PAM double-network hydrogels prepared by freeze-thawing and anneal-swelling. Mater. Sci. Eng. C 2017, 77, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S. Calcium orthophosphate-containing biocomposites and hybrid biomaterials for biomedical applications. J. Funct. Biomater. 2015, 6, 708–832. [Google Scholar] [CrossRef] [Green Version]
- Rogina, A.; Ressler, A.; Matić, I.; Ferrer, G.G.; Marijanović, I.; Ivanković, M.; Ivanković, H. Cellular hydrogels based on pH-responsive chitosan-hydroxyapatite system. Carbohydr. Polym. 2017, 166, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, Z.C.; Lin, C.J. Preparation and characterization of nano-sized hydroxyapatite particles and hydroxyapatite/chitosan nano-composite for use in biomedical materials. Mater. Lett. 2002, 57, 858–861. [Google Scholar] [CrossRef]
- Damien, E.; Revell, P.A. Coralline hydroxyapatite bone graft substitute: A review of experimental studies and biomedical applications. J. Appl. Biomater. Biomech. 2004, 2, 65–73. [Google Scholar]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Peraire, C.; Arias, J.L.; Bernal, D.; Pou, J.; Leon, B.; Arano, A.; Roth, W. Biological stability and osteoconductivity in rabbit tibia of pulsed laser deposited hydroxylapatite coatings. J. Biomed. Mater. Res. A 2006, 77, 370–379. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Lusquiños, F.; Riveiro, A.; Comesaña, R.; Pou, J. Hydroxylapatite nanoparticles obtained by fiber laser-induced fracture. Appl. Surf. Sci. 2009, 255, 5382–5385. [Google Scholar] [CrossRef]
- Da Silva, E.A.B.; Costa, C.A.E.; Vilar, V.J.P.; Botelho, C.M.S.; Larosi, M.B.; Saracho, J.M.P.; Boaventura, R.A.R. Water remediation using calcium phosphate derived from marine residues. Water Air Soil Pollut. 2012, 223, 989–1003. [Google Scholar] [CrossRef]
- Nzihou, A.; Sharrock, P. Role of Phosphate in the Remediation and Reuse of Heavy Metal Polluted Wastes and Sites. Waste Biomass Valoriz. 2010, 1, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Piccirillo, C.; Fernández-Arias, M.; Boutinguiza, M.; Tobaldi, D.M.; Del Val, J.; Pintado, M.M.; Pou, J. Increased UV absorption properties of natural hydroxyapatite-based sunscreen through laser ablation in liquid. J. Amer. Ceram. Soc. 2019, 102, 3163–3174. [Google Scholar] [CrossRef]
- Atef, M.; Mahdi Ojagh, S. Health benefits and food applications of bioactive compounds from fish byproducts: A review. J. Funct. Foods 2017, 35, 673–681. [Google Scholar] [CrossRef]
- Halim, N.R.A.; Yusof, H.M.; Sarbon, N.M. Functional and bioactive properties of fish protein hydolysates and peptides: A comprehensive review. Trends Food Sci. Technol. 2016, 51, 24–33. [Google Scholar] [CrossRef]
- He, S.; Franco, C.; Zhang, W. Functions, applications and production of protein hydrolysates from fish processing co-products (FPCP). Food Res. Int. 2013, 50, 289–297. [Google Scholar] [CrossRef]
- Adamiano, A.; Fellet, G.; Vuerich, M.; Scarpin, D.; Carella, F.; Piccirillo, C.; Jeon, J.-R.; Pizzutti, A.; Marchiol, L.; Iafisco, M. Calcium Phosphate Particles Coated with Humic Substances: A Potential Plant Biostimulant from Circular Economy. Molecules 2021, 26, 2810. [Google Scholar] [CrossRef] [PubMed]

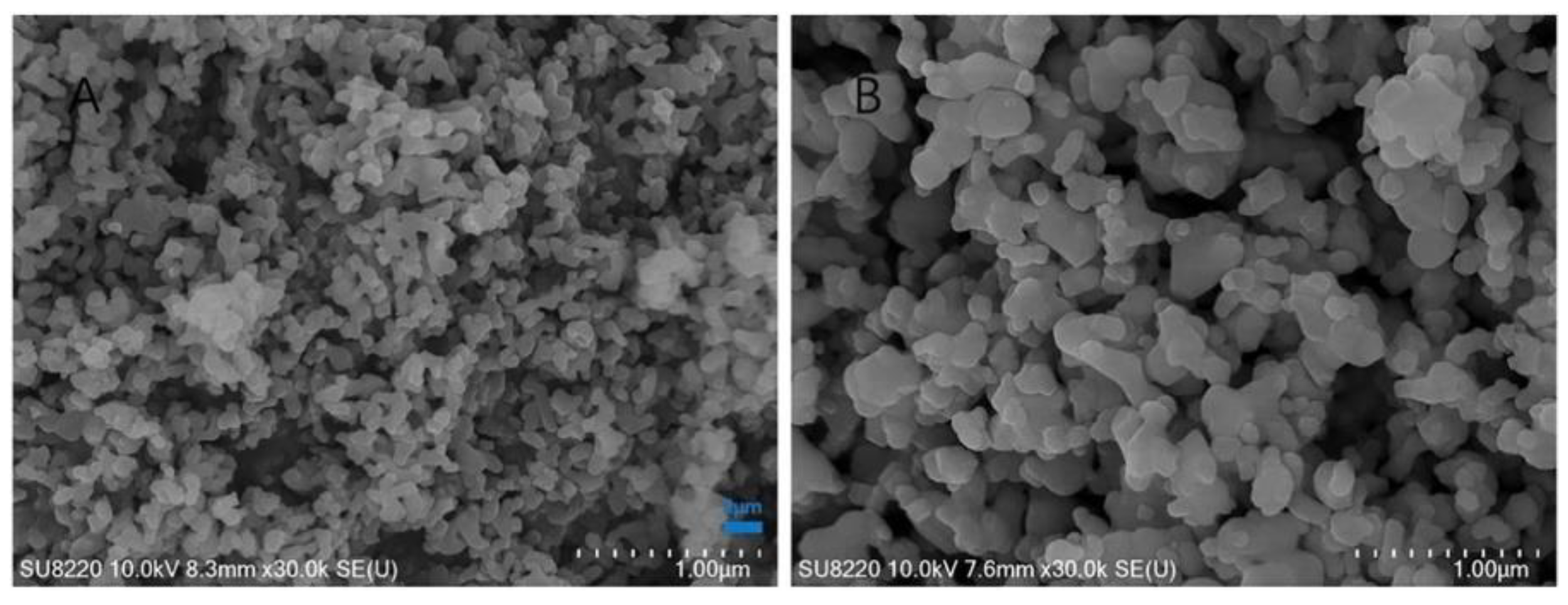


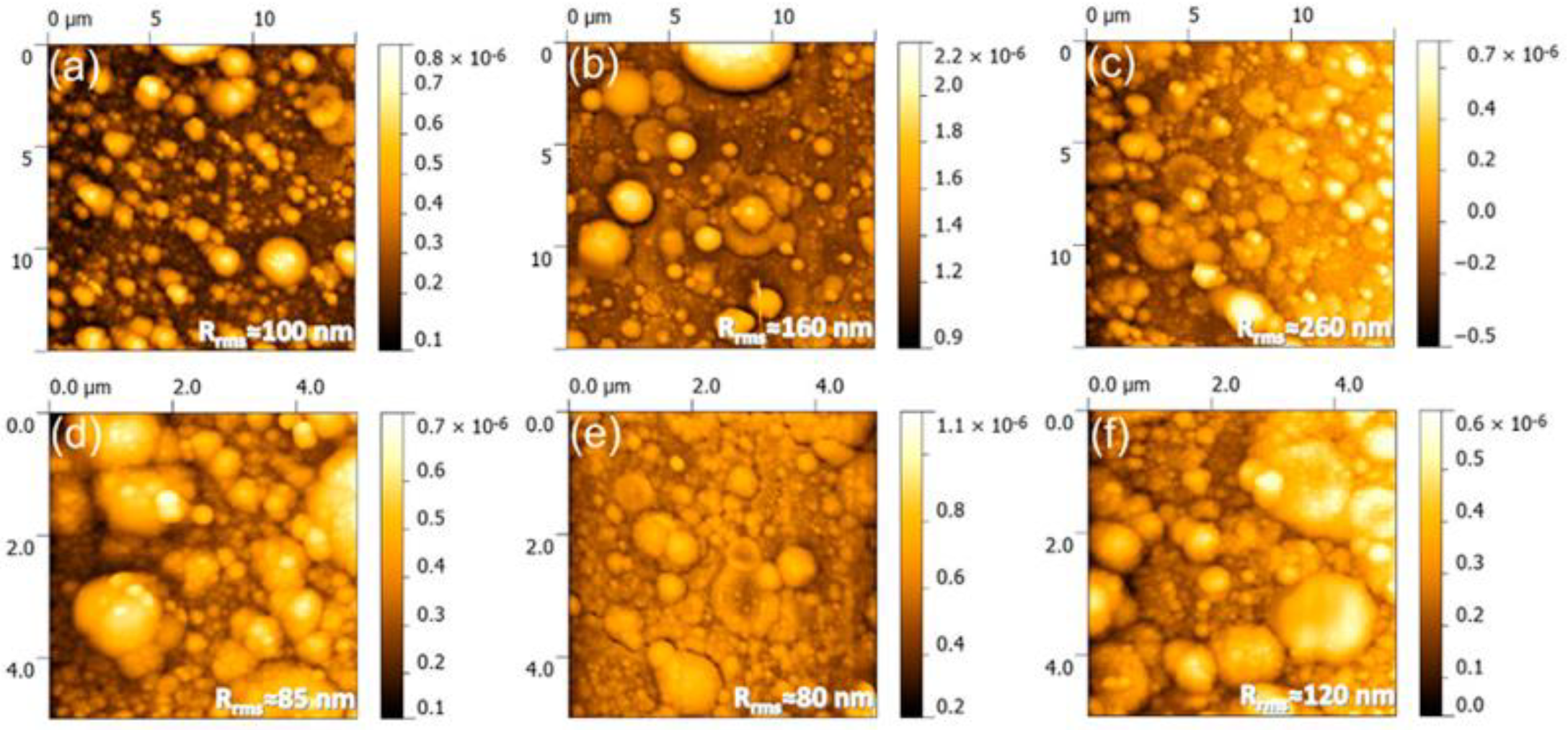
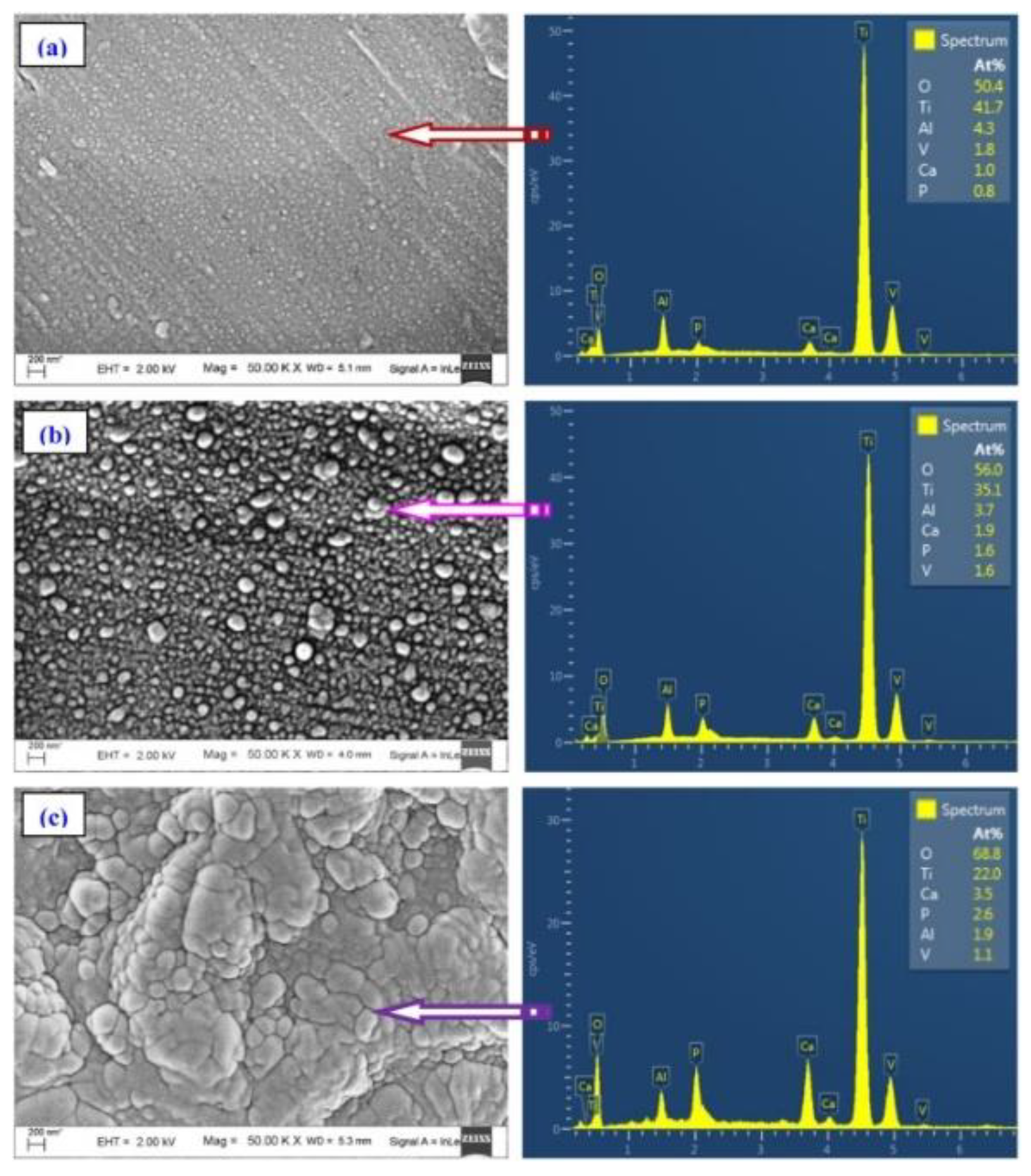
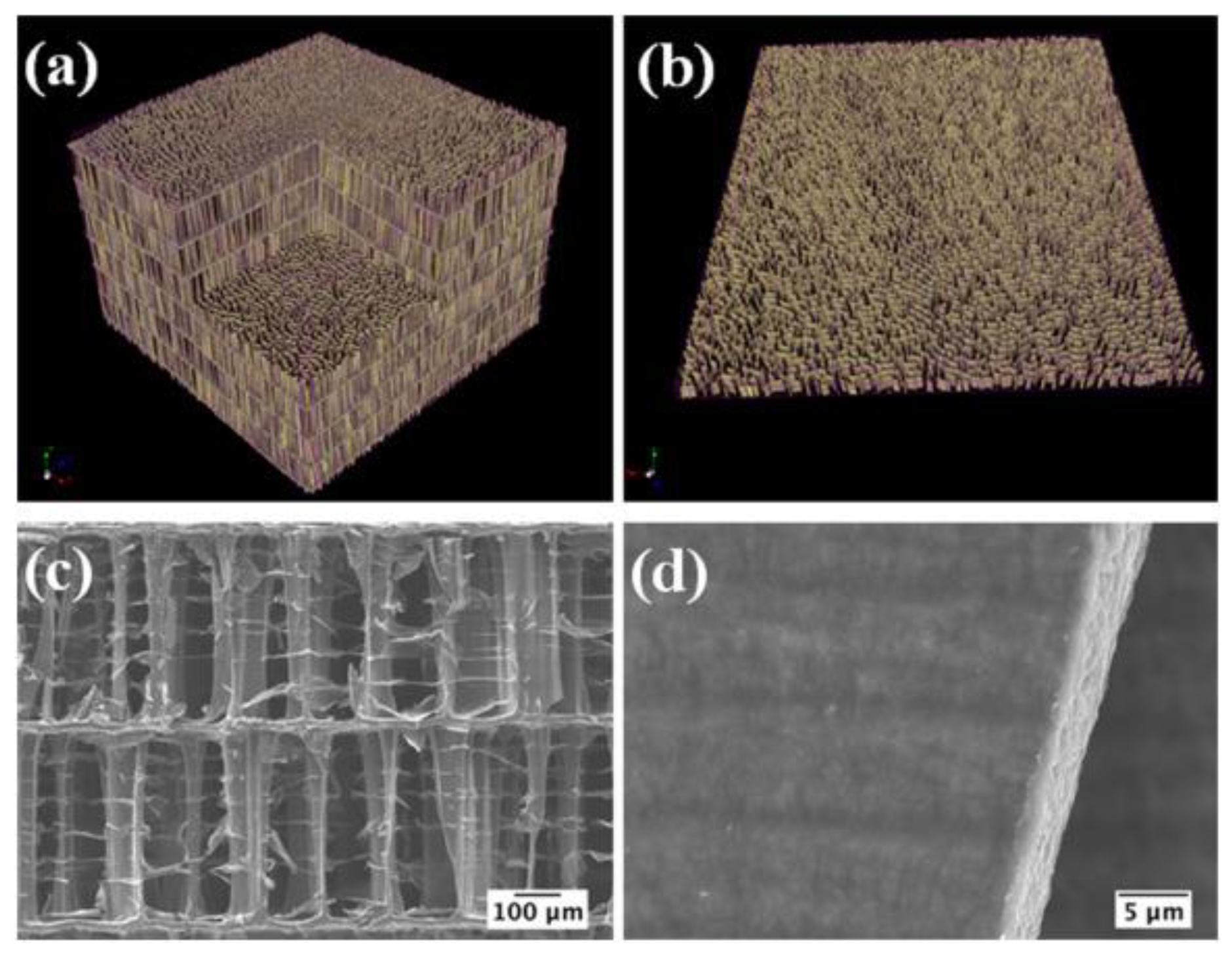
| Fish Discards | Prepared Form | Pre-Treatment | Calcination | Characterization Techniques | Ref. | |
|---|---|---|---|---|---|---|
| [°C] | Time [min] | |||||
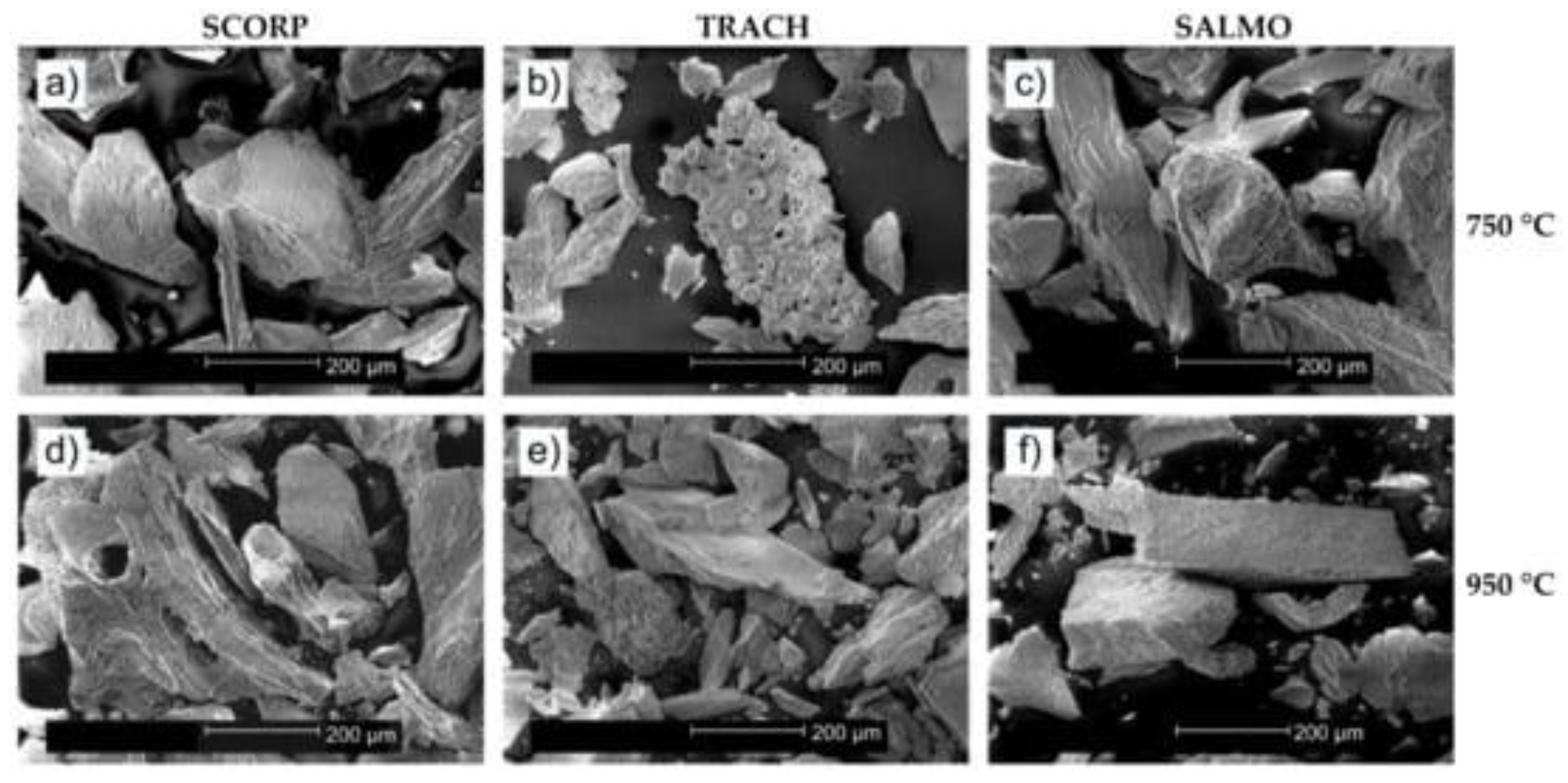
| Red scorpionfish (Scorpaena scrofa), Atlantic horse mackerel (Trachurus trachurus), salmon (Salmo salar) bones | Powders | Alkaline hydrolysis | 750, 900 | FEG/HR-SEM, EDS, XRD, FTIR | [109] | |
| Black scabbardfish (Aphanopus carbo) bones, grey triggerfish (Balistes capriscus) skin | Powders | Washed in hot water | 900, 950, 1000, 1100, 1200 | - | FAAS, XRD, FT-IR | [85] |
| Powders | Washed in hot water and dried at 60 °C | 800, 1000 | 60 | XRD, Raman | [96] | |
| Sea bream (Sparus aurata), salmon (Salmo salar) bones | Powders and thin films | NaOH solution | 850 | - | SEM, EDS, XRD, FT-IR, bonding strength, solubility/bioactivity, cytocompatibility, antibacterial activity tests | [113] |
| Black scabbardfish (Aphanopus carbo) bones, grey triggerfish (Balistes capriscus) skin | Powders | - | 400, 600, 800, 1000 | - | XRD, FT-IR, ICP-OES, SEM/FEG-SEM, SSA | [121] |
| Fish otoliths | Powders | Washed with ethanol and deionized water for 1 h | 1000 | - | XRD, FT-IR, Raman, SEM | [92,122] |
| Native Brazilian fish (Pseudoplatystoma corruscans) bones | Powders/discs | Cleaned with hot water, washed | 900 | XRD, Microhardness, Optical microscopy, | [95] | |
| Cuttlefish (Sepia officinalis) bones | Polymeric coating of scaffolds | Washed with water, dried | - | - | XRD, ICP-OES, FT-IR, SEM, µ-CT, compression tests, SBF | [106] |
| BG coating of scaffolds | - | 700 | 120 | XRD, FT-IR, SEM, µ-CT, compression tests, SBF | [119] | |
| Nanorods | Dipped in boiling water for 1 h | 900 | 240 | SEM, FT-IR, porosity, CA, mechanical, tribological tests, antimicrobial, cytotoxicity | [99] | |
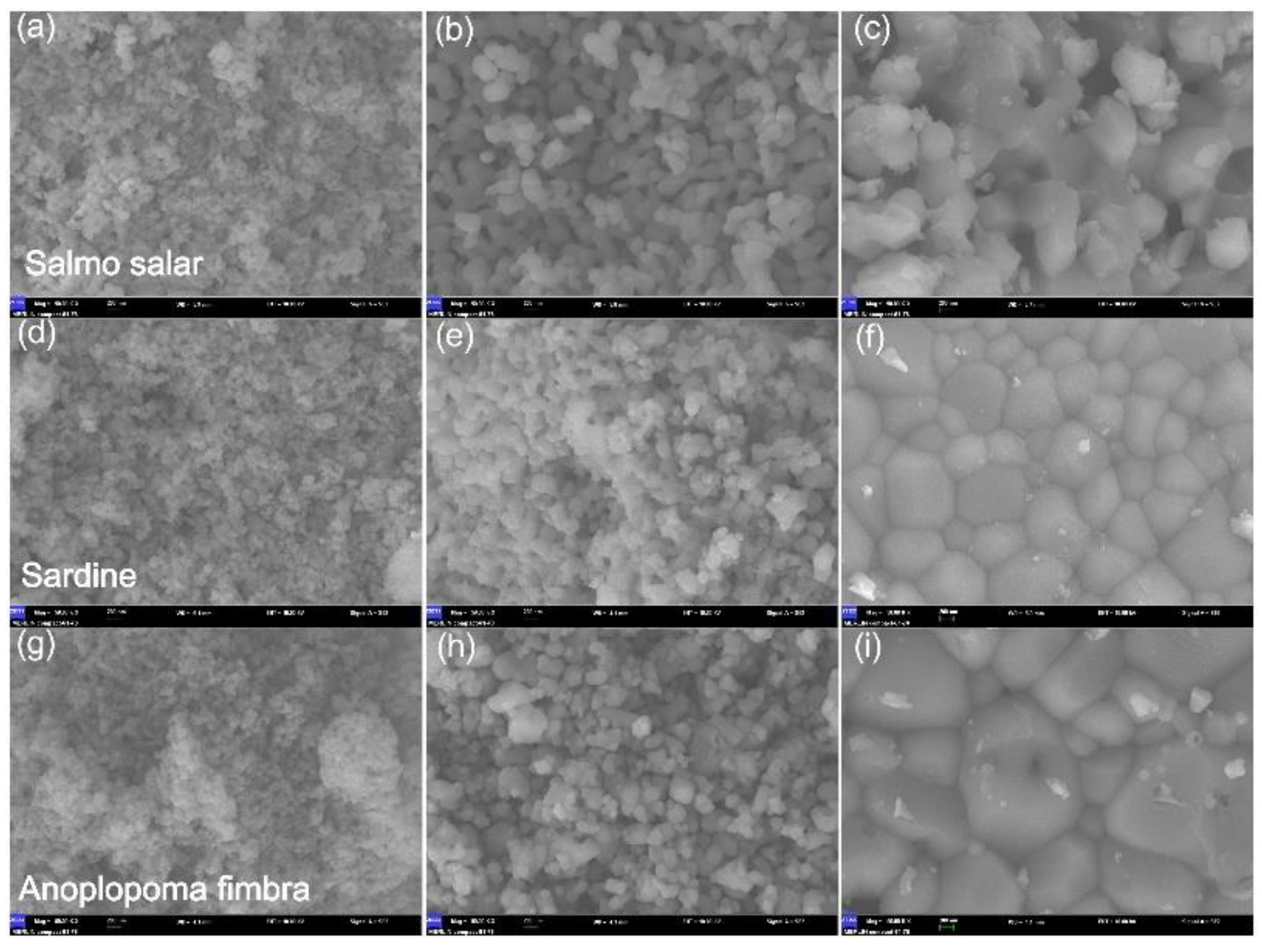
| Salmo salar, Anoplopoma fimbria, sardine bones | Powder | Boiled in deionized water for 2 h, washed with flowing water | 600–1100 | 60 | XRD, FT-IR, SEM, XRF, cell culture, cytotoxicity | [93] |
| Sardine scales | Powder | Washed with hot and distilled water | 1000 | – | XRD, IR | [97] |
| Salmon (Salmo salar) bones | Powder/cylindrical pellets | Boiled in water for 1 h, treated with 1% NaOH solution, washed with ultra-pure water | 800 | 180 | XRD, SEM, EDS, FT-IR, microhardness, in vitro biocompatibility tests | [98] |
| Powder | Boiled in deionized water for 2 h, washed with flowing water | 600 | 60 | XRD, FT-IR, SEM, Cytotoxicity, Western blotting analysis | [94] | |
| Fish (Tilapia nilotica) scales | Powder/films | Washed in distilled water, dried | 800 | – | XRD, SEM, Raman, profilometry, contact angle, mechano-tribological tests, in vitro bioactivity | [40] |
| Powder | Washed in distilled water | – | – | TEM, XRD, FT-IR, TGA | [91] | |
| Sword fish (Xiphias gladius), tuna (Thunnus thynnus) | Powder | Boiled in water for 1 h, washed in strong water jet | 600, 950 | 720 | FE-SEM, TEM/HRTEM, EDX, XRD, FT-IR, ICP-OES, Raman | [13] |
| Hairtails fish bones | Powder | Boiled in water for 3 h | 700, 800, 900 | 60 | XRD, FT-IR, XRF, SEM | [32] |
| Pintado (Pseudoplatystoma corruscans) bones | Powder | 900 | 480 | XRD, SEM, EDS hardness tests | [105] | |
| Pintado (Pseudoplatystoma corruscans), jaú (Paulicea lutkeni), cachara (Pseudoplatystoma fasciatum) bones | Powder | 900 | 480 | XRD, SEM, FT-IR | [110] | |
| BCP Coating Thickness (nm) | HA (wt.%) | β-TCP (wt.%) | Crystallite Sizes (nm) |
|---|---|---|---|
| 400 | 43.4 | 56.6 | 15 |
| 700 | 43.5 | 56.5 | 17 |
| 1000 | 44.9 | 55.1 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duta, L.; Dorcioman, G.; Grumezescu, V. A Review on Biphasic Calcium Phosphate Materials Derived from Fish Discards. Nanomaterials 2021, 11, 2856. https://doi.org/10.3390/nano11112856
Duta L, Dorcioman G, Grumezescu V. A Review on Biphasic Calcium Phosphate Materials Derived from Fish Discards. Nanomaterials. 2021; 11(11):2856. https://doi.org/10.3390/nano11112856
Chicago/Turabian StyleDuta, Liviu, Gabriela Dorcioman, and Valentina Grumezescu. 2021. "A Review on Biphasic Calcium Phosphate Materials Derived from Fish Discards" Nanomaterials 11, no. 11: 2856. https://doi.org/10.3390/nano11112856
APA StyleDuta, L., Dorcioman, G., & Grumezescu, V. (2021). A Review on Biphasic Calcium Phosphate Materials Derived from Fish Discards. Nanomaterials, 11(11), 2856. https://doi.org/10.3390/nano11112856







