CO2 Adsorption on Modified Mesoporous Silicas: The Role of the Adsorption Sites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of SBA-15 and MCM-48
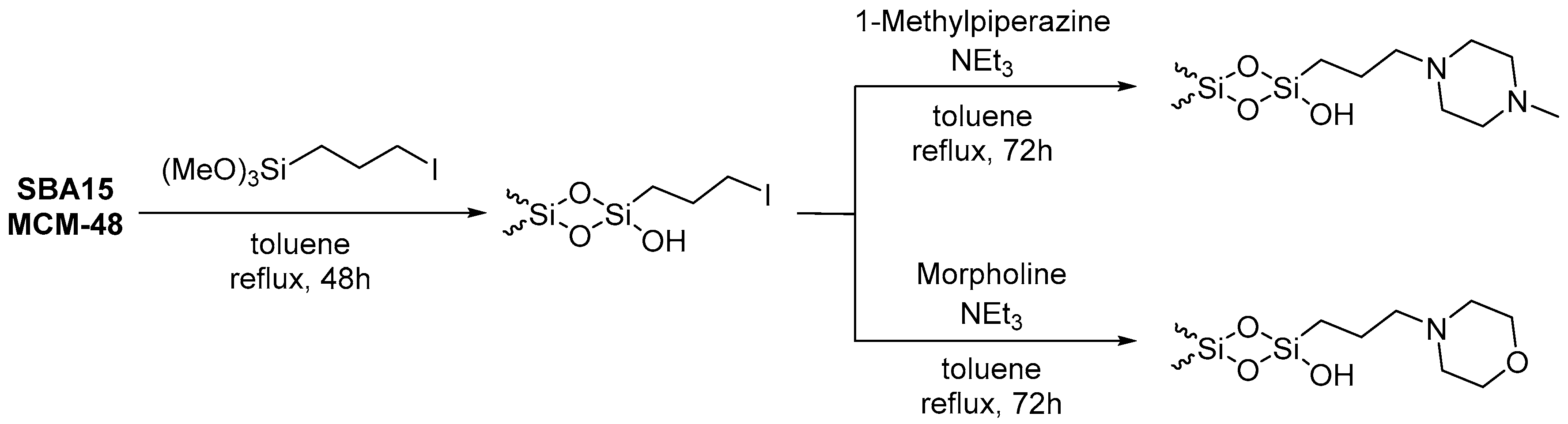
2.2. Preparation of Iodo-Functionalized Silica-Based Mesoporous Materials
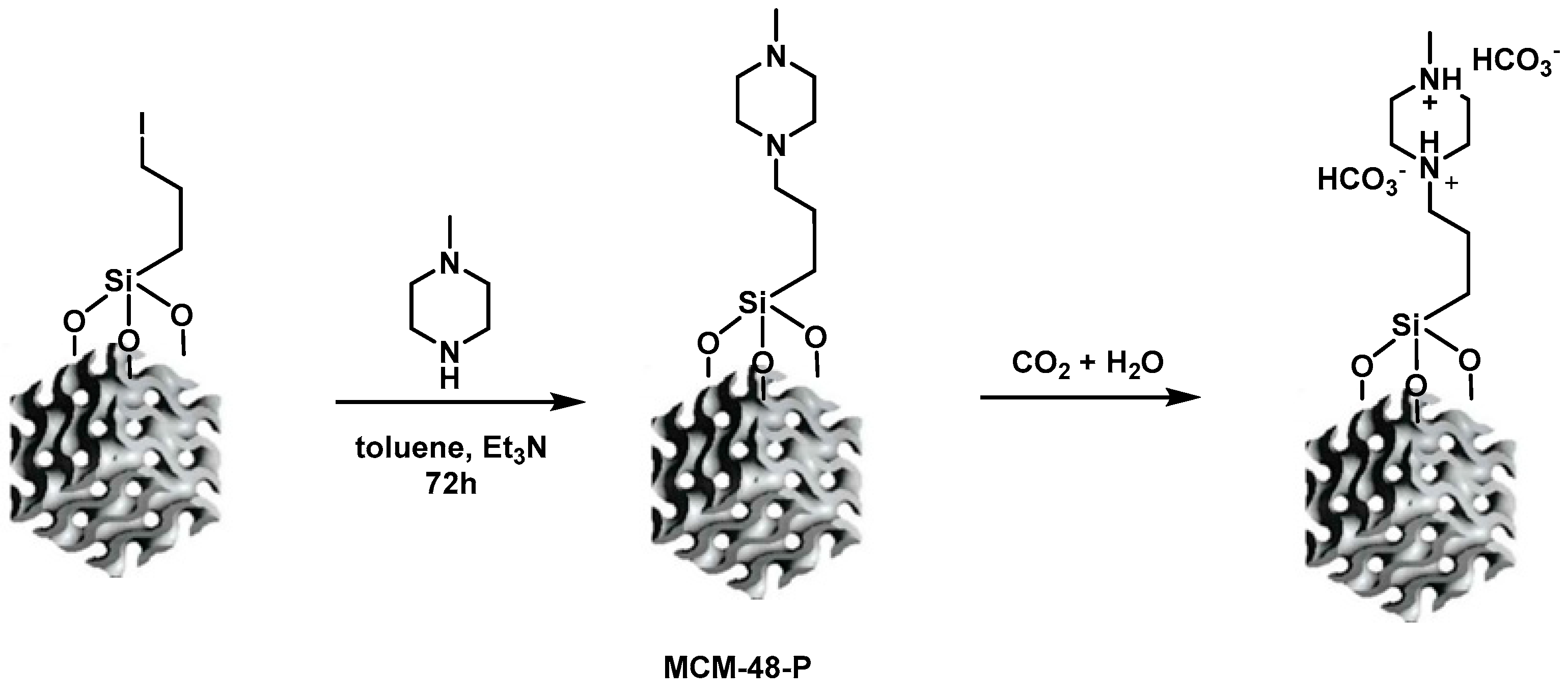
2.3. Preparation of Morpholine- and 1-Methylpiperazine-Functionalized Silica-Based Mesoporous Materials
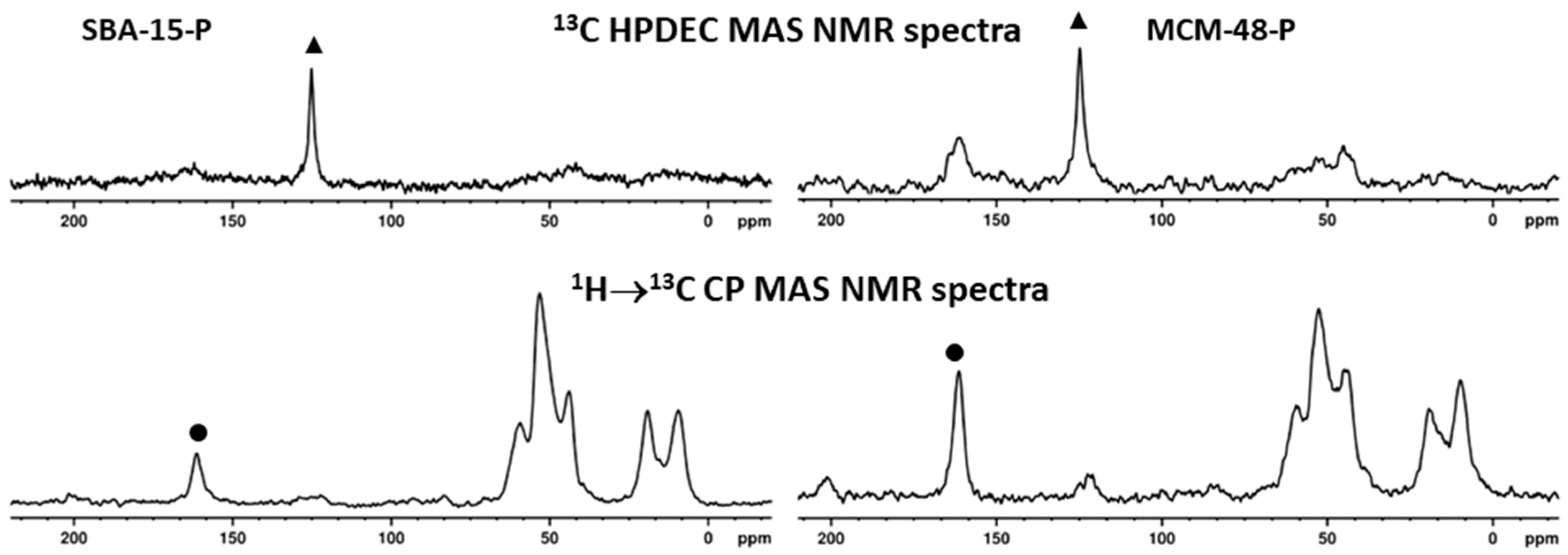
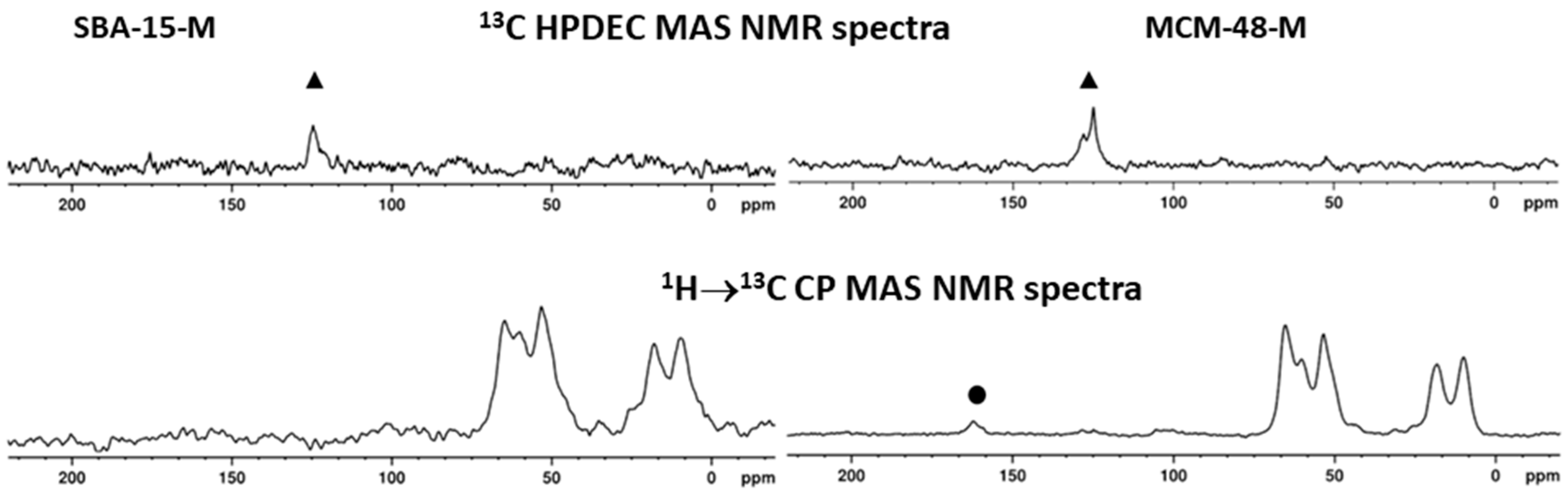
2.4. Characterization
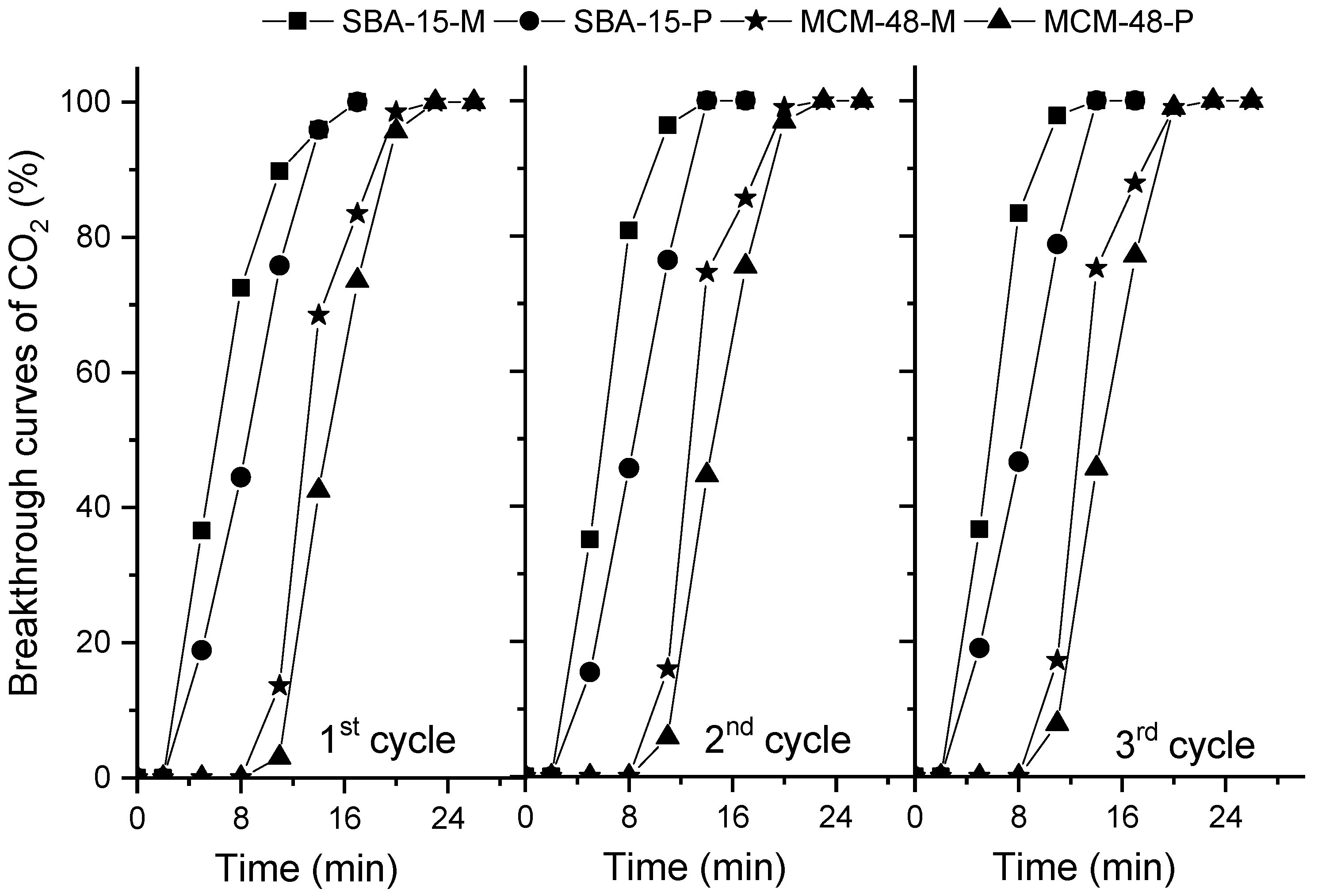
2.5. CO2 Adsorption Measurements in Dynamic Conditions
2.6. CO2 Adsorption Measurements in Static Conditions
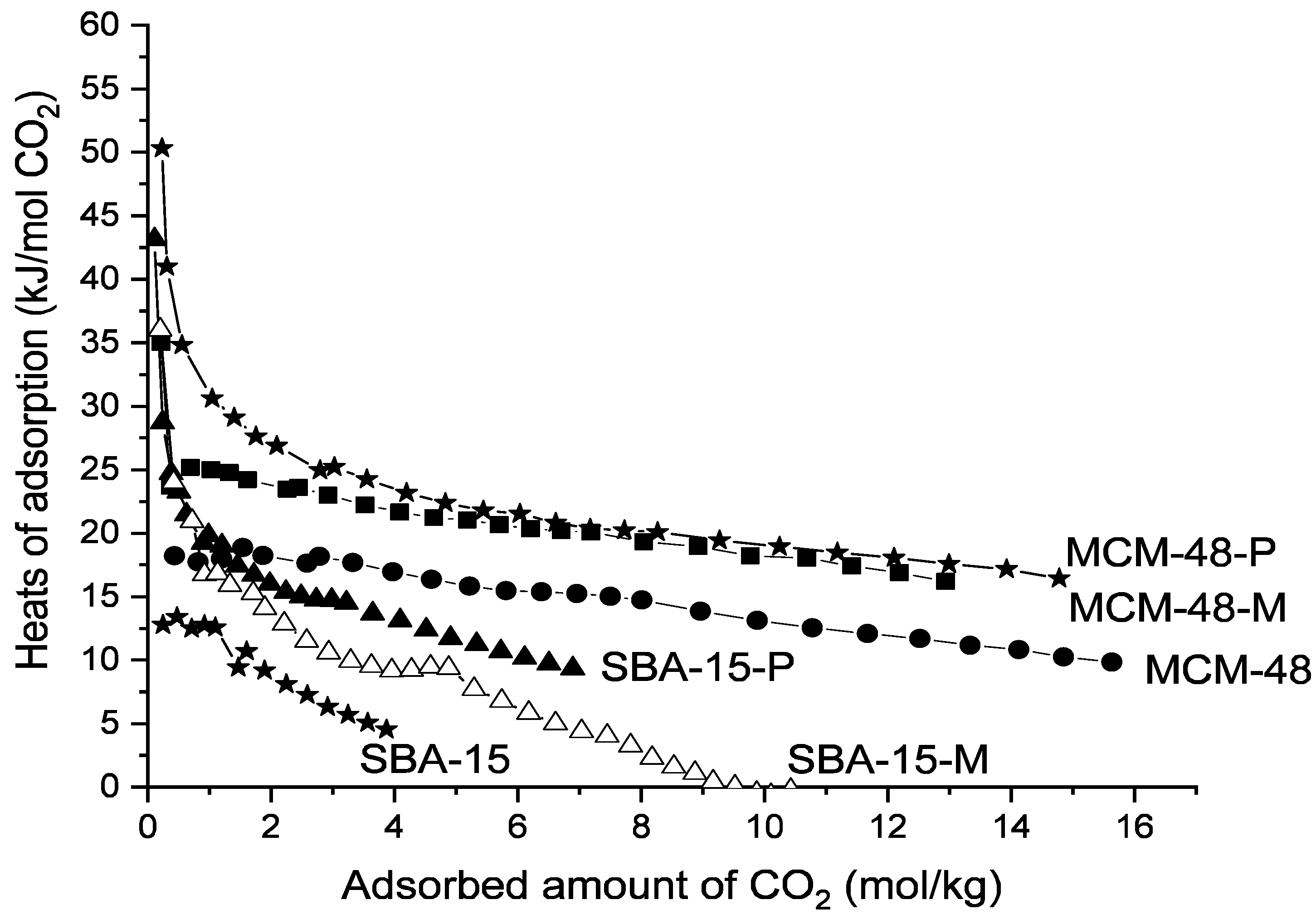
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon Dioxide Capture: Prospects for New Materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [Green Version]
- Rochelle, G.T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Harlick, P.J.E.; Sayari, A. Applications of pore-expanded mesoporous silicas. 3. Triamine silane grafting for enhanced CO2 adsorption. Ind. Eng. Chem. Res. 2006, 45, 3248–3255. [Google Scholar] [CrossRef]
- Harlick, P.J.E.; Sayari, A. Triamine grafted material with exceptional CO2 dynamic and equilibrium adsorption performance. Ind. Eng. Chem. Res. 2007, 46, 446–458. [Google Scholar] [CrossRef]
- Davarpanah, E.; Armandi, M.; Hernandez, S.; Fino, D.; Arletti, R.; Bensaid, S.; Piumetti, M. CO2 capture on natural zeolite clinoptilolite: Effect of temperature and role of the adsorption sites. J. Environ. Manag. 2020, 275, 111229. [Google Scholar] [CrossRef]
- Boukoussa, B.; Hakiki, A.; Bouazizi, N.; Beltrao-Nunes, A.-P.; Launay, F.; Pailleret, A.; Pillier, F.; Bengueddach, A.; Hamacha, R.; Azzouz, A. Mesoporous silica supported amine and amine-copper complex for CO2 adsorption: Detailed reaction mechanism of hydrophilic character and CO2 retention. J. Mol. Struct. 2019, 1191, 175–182. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources. ChemSusChem. 2009, 2, 796–854. [Google Scholar] [CrossRef] [PubMed]
- Busca, G. Acidity and basicity of zeolites: A fundamental approach. Microporous Mesoporous Mater. 2017, 254, 3–16. [Google Scholar] [CrossRef]
- IPCC. Special Report on Carbon Dioxide Capture and Storage: Working Group III of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2005; ISBN 92-9169-1190-4. [Google Scholar]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-Combustion CO2 Capture Using Solid Sorbents: A Review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Bonelli, B.; Armandi, M.; Garrone, E. Surface properties of alumino-silicate single walled nanotubes of the imogolite type. Phys. Chem. Chem. Phys. 2013, 15, 13381–13390. [Google Scholar] [CrossRef] [PubMed]
- Brandani, S.; Mangano, E.; Ahn, H.; Friedrich, D.; Hu, X. Diffusion mechanism of CO2 in 13X zeolite beads. Adsorption 2013, 20, 121–135. [Google Scholar]
- Sanz-Pérez, E.S.; Lobato, B.; Lopez-Anton, M.A.; Arencibia, A.; Sanz, R.; Martínez-Tarazona, M.R. Effectiveness of amino-functionalized sorbents for CO2 capture in the presence of Hg. Fuel 2020, 267, 117250. [Google Scholar] [CrossRef]
- Zhao, H.; Luo, X.; Zhang, H.; Sun, N.; Wei, W.; Sun, Y. Carbon-based adsorbents for postcombustion capture: A review. Greenh Gases Sci. Technol. 2018, 8, 11–36. [Google Scholar] [CrossRef]
- Xu, X.; Song, C.; Andrésen, J.M.; Miller, B.G.; Scaroni, A.W. Preparation and characterization of novel CO2 “molecular basket” adsorbents based on polymer-modified mesoporous molecular sieve MCM-41. Microporous Mesoporous Mater. 2003, 62, 29–45. [Google Scholar] [CrossRef]
- Liu, S.-H.; Lin, Y.-C.; Chien, Y.-C.; Hyu, H.-R. Adsorption of CO2 from Flue Gas Streams by a Highly Efficient and Stable Aminosilica Adsorbent. J. Air Waste Manag. Assoc. 2011, 61, 226–233. [Google Scholar] [CrossRef] [Green Version]
- Uehara, Y.; Karami, D.; Mahinpey, N. CO2 adsorption using amino acid ionic liquid-impregnated mesoporous silica sorbents with different textural properties. Microporous Mesoporous Mater. 2019, 278, 378–386. [Google Scholar] [CrossRef]
- Vinodh, R.; Babu, C.M.; Abidov, A.; Palanichamy, M.; Jang, H.T. Facile synthesis of amine modified silica/reduced graphene oxide composite sorbent for CO2 adsorption. Mater. Lett. 2019, 247, 44–47. [Google Scholar] [CrossRef]
- Belmabkhout, Y.; Serna-Guerrero, R.; Sayari, A. Adsorption of CO2-containing gas mixtures over amine-bearing pore-expanded MCM-41 silica: Application for CO2 separation. Adsorption 2011, 17, 395–401. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, L.; Zhang, Y.; Yang, G.; Yan, Z. Amine-modified SBA-15: Effect of pore structure on the performance for CO2 capture. Ind. Eng. Chem. Res. 2011, 50, 3220–3226. [Google Scholar] [CrossRef]
- Franchi, R.S.; Harlick, P.J.E.; Sayari, A. Applications of pore-expanded mesoporous silica. 2. Development of a high-capacity, water-tolerant adsorbent for CO2. Ind. Eng. Chem. Res. 2005, 44, 8007–8013. [Google Scholar] [CrossRef]
- Son, W.J.; Choi, J.S.; Ahn, W.S. Adsorptive removal of carbon dioxide using polyethyleneimine-loaded mesoporous silica materials. Microporous Mesoporous Mater. 2008, 113, 31–40. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, J.; Chen, J.; Ye, Q.; Pan, H.; Shao, Z.; Shi, Y. Dynamic performance of CO2 adsorption with tetraethylenepentamine-loaded KIT-6. Microporous Mesoporous Mater. 2010, 134, 16–21. [Google Scholar] [CrossRef]
- Lai, J.Y.; Ngu, L.H.; Hashim, S.S. A review of CO2 adsorbents performance for different carbon capture technology processes conditions. Greenhouse Gas Sci. Technol. 2021, 11, 1076–1117. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jena, H.S.; Leus, K.; Van Der Voort, P. Covalent triazine frameworks—A sustainable perspective. Green Chem. 2020, 22, 1038–1071. [Google Scholar] [CrossRef]
- Schmidt, J.; Leus, K.; Van Hecke, K.; Van Der Voort, P. Effect of Building Block Transformation in Covalent Triazine-Based Frameworks for Enhanced CO2 Uptake and Metal-Free Heterogeneous Catalysis. Chem. A Eur. J. 2020, 26, 1548–1557. [Google Scholar]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Shao, Y.; Zhang, J.; Anpo, M. Synthesis of MCM-48 mesoporous molecular sieve with thermal and hydrothermal stability with the aid of promoter anions. Microporous Mesoporous Mater. 2006, 95, 17–25. [Google Scholar] [CrossRef]
- Ariza, M.J.; Rodrıguez-Castellon, E.; Ric, R. X-Ray Photoelectron Spectroscopy Analysis of Di-(2-ethylhexyl)Phosphoric Acid Activated Membranes. J. Coll. Inter. Sci. 2000, 226, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Todea, M.; Muresan-Pop, M.; Simon, S.; Moisescu-Goia, C.; Simon, V.; Eniu, D. XPS investigation of new solid forms of 5-fluorouracil with piperazine. J. Mol. Struc. 2018, 1165. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, D.; Wang, J. Direct Synthesis of Amine-functionalized Mesoporous Silica for CO2 Adsorption. Chin. J. Chem. Eng. 2011, 19, 386–390. [Google Scholar] [CrossRef]
- Zeleňák, V.; Badaničová, M.; Halamova, D.; Čejka, J.; Zukal, A.; Murafa, N.; Goerigk, G. Amine-modified ordered mesoporous silica: Effect of pore size on carbon dioxide capture. Chem. Eng. J. 2008, 144, 336–342. [Google Scholar] [CrossRef]
- Mafra, L.; Cendak, T.; Schneider, S.; Wiper, P.V.; Pires, J.; Gomes, J.R.B.; Pinto, M.L. The structure of chemisorbed CO2 species in amine-functionalized mesoporous silicas studied by solid-state NMR and computer modeling. J. Am. Chem. Soc. 2017, 139, 389–408. [Google Scholar] [CrossRef]
- Pinto, M.L.; Mafra, L.; Guil, J.M.; Pires, J.; Rocha, J. Adsorption and Activation of CO2 by Amine-Modified Nanoporous Materials Studied by Solid-State NMR and 13CO2 Adsorption. Chem. Mater. 2011, 23, 1387–1395. [Google Scholar] [CrossRef]
- Chen, C.-H.; Shimon, D.; Lee, J.J.; Mentink-Vigier, F.; Hung, I.; Sievers, C.; Jones, C.W.; Hayes, S.E. The “missing” bicarbonate in CO2 chemisorption reactions on solid amine sorbents. J. Am. Chem. Soc. 2018, 140, 8648–8651. [Google Scholar] [CrossRef]
- Lee, J.J.; Yoo, C.-J.; Chen, C.-H.; Hayes, S.E.; Sievers, C.; Jones, C.W. Silica Supported Sterically Hindered Amines for CO2 Capture. Langmuir 2018, 34, 12279–12292. [Google Scholar] [CrossRef]
- Heydari-Gorji, A.; Belmabkhout, Y.; Sayari, A. Polyethylenimine-impregnated mesoporous silica: Effect of amine loading and surface alkyl chains on CO2 adsorption. Langmuir 2011, 27, 12411–12416. [Google Scholar] [CrossRef]
- Watabe, T.; Yogo, K. Isotherms and isosteric heats of adsorption for CO2 in amine-functionalized mesoporous silicas. Sep. Purif. Technol. 2013, 120, 20–23. [Google Scholar] [CrossRef]
- Chen, C.; Kim, J.; Ahn, W.-S. CO2 capture by amine-functionalized nanoporous materials: A review. Korean J. Chem. Eng. 2014, 31, 1919–1934. [Google Scholar] [CrossRef]

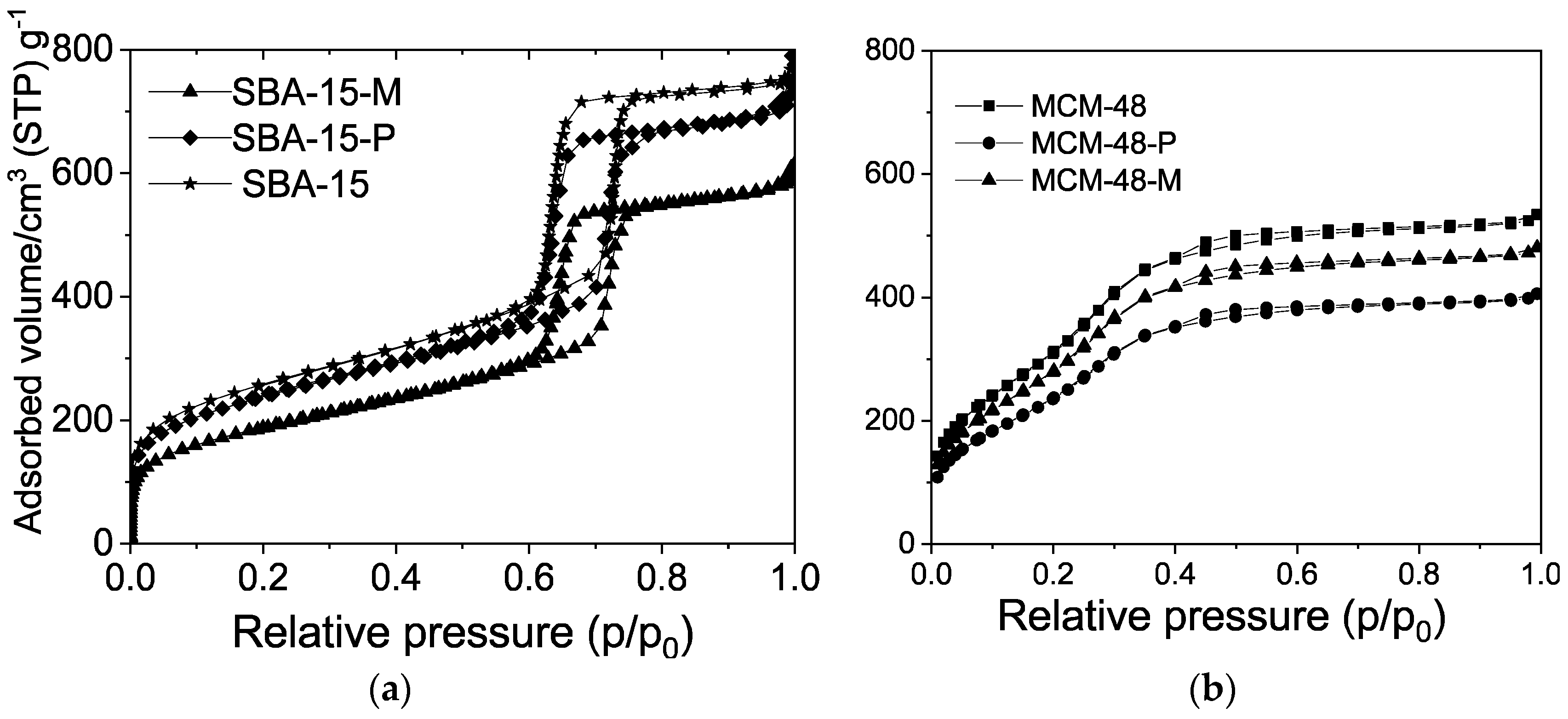
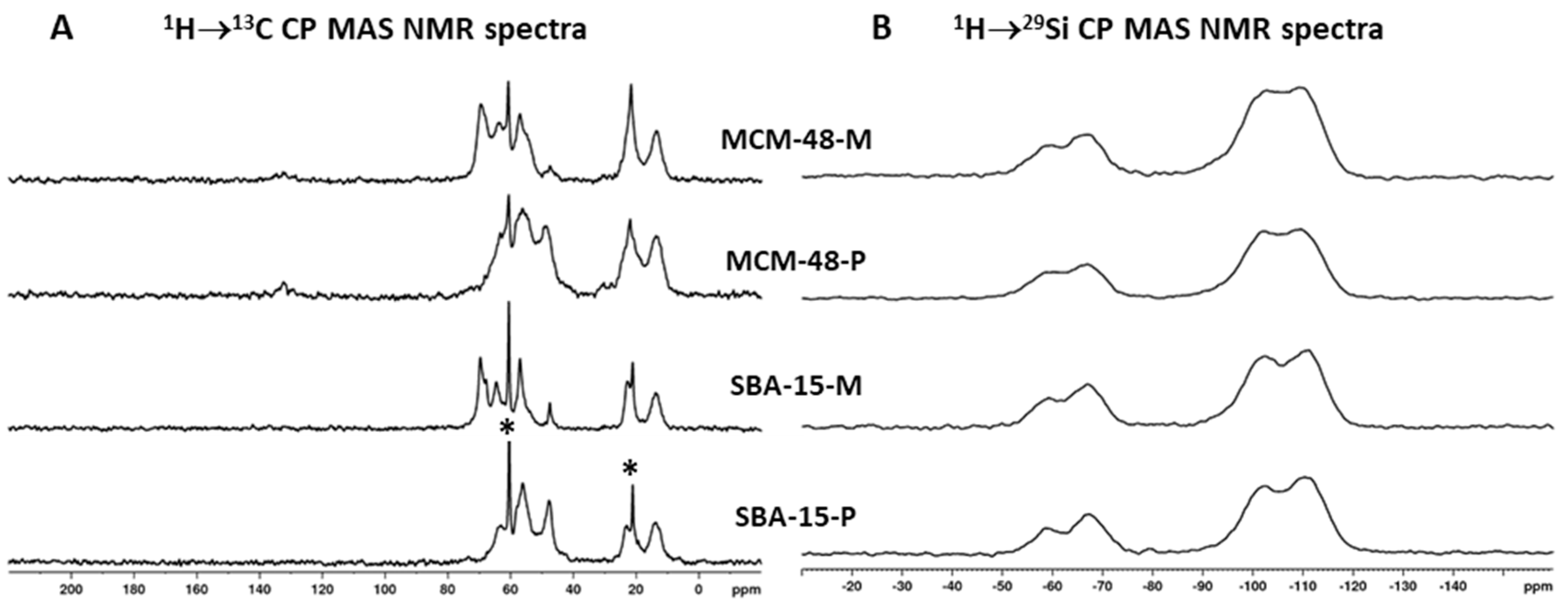
| Samples | BET (m2/g) | Pore Volume (cm3/g) | PDa (nm) | Content of Organic Functional Groups 1 (wt.%) | Content of Organic Functional Groups 1 (mmol/g) |
|---|---|---|---|---|---|
| SBA-15 | 770 | 0.90 | 6.0 | - | - |
| MCM-48 | 1235 | 0.83 | 2.4 | - | - |
| SBA-15-P | 680 | 0.76 | 5.8 | 26.0 | 2.13 |
| MCM-48-P | 945 | 0.63 | 2.3 | 28.7 | 2.35 |
| SBA-15-M | 650 | 0.70 | 5.8 | 27.2 | 2.23 |
| MCM-48-M | 885 | 0.58 | 2.3 | 27.5 | 2.25 |
| Entry | Samples | CO2 Adsorption from CO2/N2 1, mmol/g | Selectivity of CO2 over N2, Based on IAST Theory | Adsorption of CO2 from CO2/H2O/N2 1, mmol/g |
|---|---|---|---|---|
| 1 | SBA-15 | 1.5 | 56 | 1.4 |
| 2 | MCM-48 | 2.3 | 44 | 2.1 |
| 3 | SBA-15-P | 3.2 | 171 | 3.6 |
| 4 | MCM-48-P | 4.2 | 184 | 4.4 |
| 5 | SBA-15-M | 2.8 | 146 | 2.9 |
| 6 | MCM-48-M | 3.4 | 158 | 3.5 |
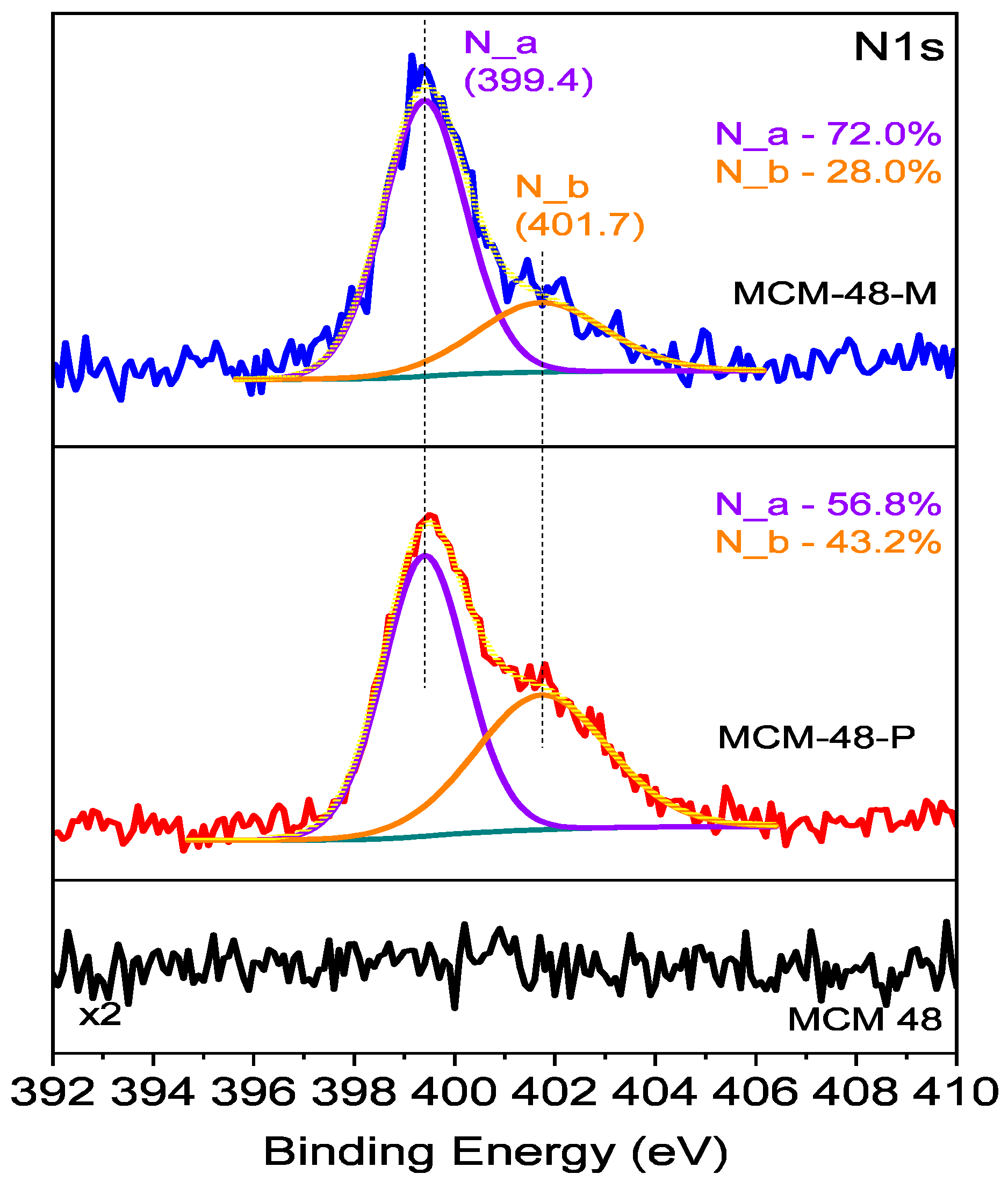
| Si (at%) | O (at%) | N (at%) | C (at%) | |
|---|---|---|---|---|
| MCM-48 | 28.1 | 71.9 | 0 | 0 |
| MCM-48-P | 17.8 | 43.7 | 7.4 | 31.1 |
| MCM-48-M | 19.7 | 52.5 | 3.6 | 24.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravutsov, M.; Mitrev, Y.; Shestakova, P.; Lazarova, H.; Simeonov, S.; Popova, M. CO2 Adsorption on Modified Mesoporous Silicas: The Role of the Adsorption Sites. Nanomaterials 2021, 11, 2831. https://doi.org/10.3390/nano11112831
Ravutsov M, Mitrev Y, Shestakova P, Lazarova H, Simeonov S, Popova M. CO2 Adsorption on Modified Mesoporous Silicas: The Role of the Adsorption Sites. Nanomaterials. 2021; 11(11):2831. https://doi.org/10.3390/nano11112831
Chicago/Turabian StyleRavutsov, Martin, Yavor Mitrev, Pavletta Shestakova, Hristina Lazarova, Svilen Simeonov, and Margarita Popova. 2021. "CO2 Adsorption on Modified Mesoporous Silicas: The Role of the Adsorption Sites" Nanomaterials 11, no. 11: 2831. https://doi.org/10.3390/nano11112831









