The Synthesis and Properties of TIPA-Dominated Porous Metal-Organic Frameworks
Abstract
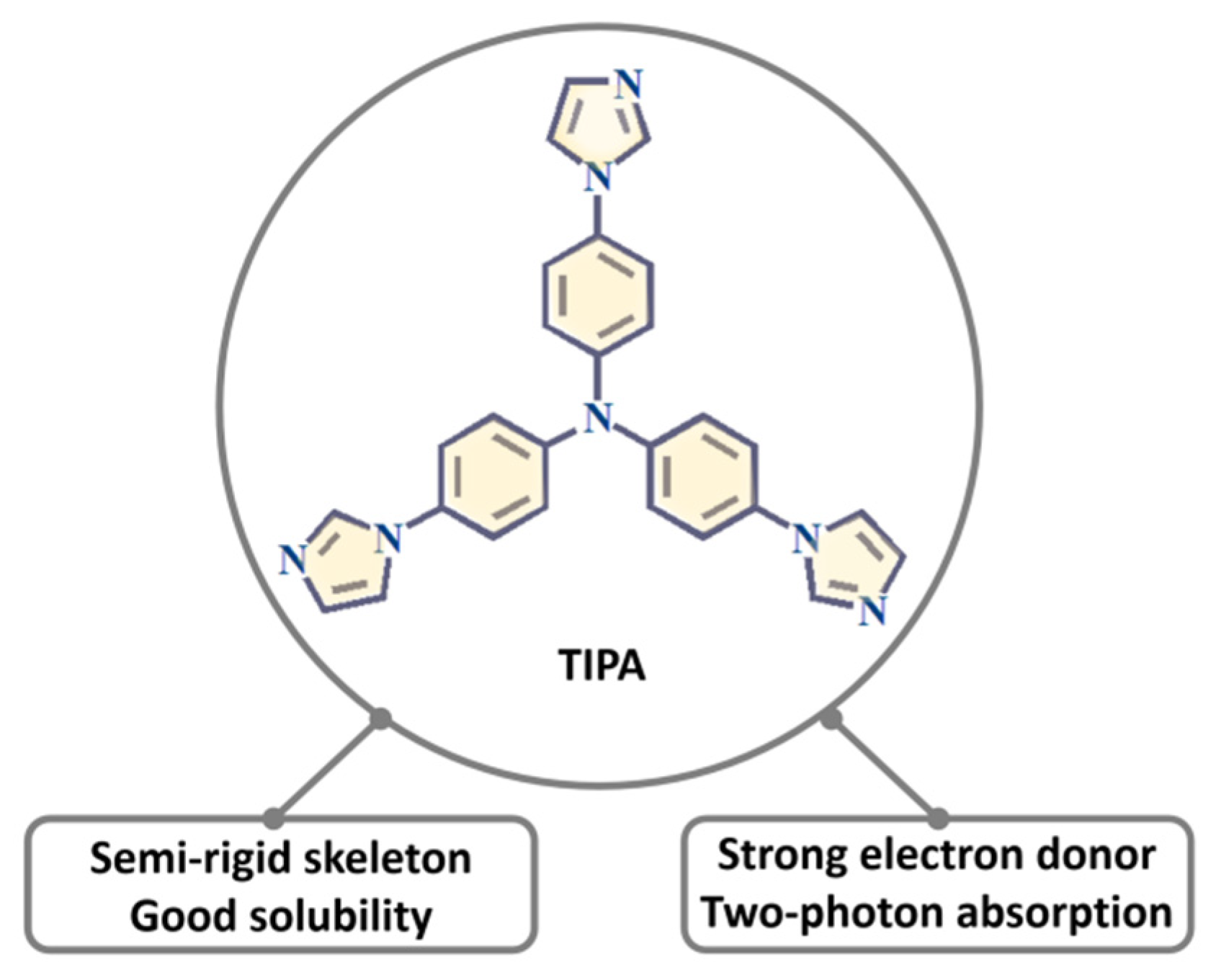
:1. Introduction
2. Construction Strategies for TIPA-Dominated Porous MOFs
2.1. Small Organic Molecule as Auxiliary
2.2. Inorganic Oxyanion as Auxiliary
2.3. Small Organic Molecule as Secondary Linker
2.4. Metal Clusters as Nodes
3. The Application of TIPA-Dominated Porous MOFs
3.1. The Capture of Toxic Oxoanions
3.2. Sensing
3.3. Gas Adsorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eddaoudi, M.; Moler, D.B.; Li, H.L.; Chen, B.L.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular Chemistry: Secondary Building Units as a Basis for the Design of Highly Porous and Robust Metal−Organic Carboxylate Frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.; Day, G.S.; Ryder, M.R.; Zhou, H. Destruction of Metal-Organic Frameworks: Positive and Negative Aspects of Stability and Lability. Chem. Rev. 2020, 120, 13087–13133. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zeng, H.; Zhu, X.; Lu, W.; Li, D. Metal-Organic Frameworks as Photoluminescent Biosensing Platforms: Mechanisms and Applications. Chem. Soc. Rev. 2021, 50, 4484–4513. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mukherjee, S.; Wang, F.; Fischer, R.A.; Zhang, J. Homochiral Metal-Organic Frameworks for Enantioseparation. Chem. Soc. Rev. 2021, 50, 5706–5745. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Flaig, R.W.; Jiang, H.; Yaghi, O.M. Carbon Capture and Conversion Using Metal-Organic Frameworks and Mof-based Materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, B.; Alsalmec, A.; Xiang, S.; Zhang, Z.; Chen, B. Design And Applications of Water-Stable Metal-Organic Frameworks: Status and Challenges. Coord. Chem. Rev. 2020, 423, 213507. [Google Scholar] [CrossRef]
- Tian, D.; Liu, X.; Feng, R.; Xu, J.; Chen, R.; Huang, L.; Bu, X. Microporous Luminescent Metal-Organic Framework for a Sensitive and Selective Fluorescence Sensing of Toxic Mycotoxin in Moldy Sugarcane. ACS Appl. Mater. Interfaces 2018, 10, 5618–5625. [Google Scholar] [CrossRef]
- Yao, Z.; Li, G.; Xu, J.; Hu, T.; Bu, X. A Water-Stable Luminescent ZnII Metal-Organic Framework as Chemosensor for High-Efficiency Detection of CrVI-Anions (Cr2O72− and CrO42−) in Aqueous Solution. Chem. Eur. J. 2018, 24, 3192–3198. [Google Scholar] [CrossRef]
- He, J.; Xu, J.; Yin, J.; Li, N.; Bu, X. Recent Advances in Luminescent Metalorganic Frameworks for Chemical Sensors. Sci. China Mater. 2019, 62, 1655–1678. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.P.; Tian, J.W.; Liu, S.; Li, B.; Zhao, J.; Ma, L.F.; Li, D.S.; Lan, Y.Q.; Bu, X. Microporous Metal-Organic Frameworks with Cubane [M4(OH)4] (M=Ni, Co) Clusters and Pore-Space Partition for Electrocatalytic Methanol Oxidation Reaction. Angew. Chem. Int. Ed. 2019, 58, 12185–12189. [Google Scholar] [CrossRef]
- Fu, H.R.; Wang, N.; Wu, X.; Li, F.; Zhao, Y.; Ma, L.; Du, M. Circularly Polarized Room-Temperature Phosphorescence and Encapsulation Engineering for MOF-based Fluorescent/Phosphorescent White Light-Emitting Devices. Adv. Optical Mater. 2020, 8, 2000330. [Google Scholar] [CrossRef]
- Rasheed, T.; Nabeel, F. Luminescent Metal-Organic Frameworks as Potential Sensory Materials for Various Environmental Toxic Agents. Coord. Chem. Rev. 2019, 401, 213065. [Google Scholar] [CrossRef]
- Li, H.; Zhao, S.; Zang, S.; Li, J. Functional Metal-Organic Frameworks as Effective Sensors of Gases and Volatile Compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef]
- Jiang, Y.Y.; Zhang, K.; Zhou, M.S.; Gao, P.F.; Fu, H.R. A Fluorescence/Phosphorescence Dual-Emitting Metal-Organic Framework Exhibiting Two Approaches for Single-Phase White-Light Emission. J. Solid State Chem. 2021, 304, 122563. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Dang, L.; Yang, G.; Ma, L.; Li, D.; Wang, Y. Highly Stable 3D Porous Hmof with Enhanced Catalysis and Fine Color Regulation by The Combination Of d- And p-Ions When Compared with Those of Its Monometallic MOFs. Chem. Commun. 2020, 56, 8758–8761. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, F.; Zhang, J. Design and Synthesis of Multifunctional Metal-Organic Zeolites. Chem. Soc. Rev. 2018, 47, 2130–2144. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Fu, H.R.; Han, M.; Zhou, Z.; Ma, L.F. Tetraphenylethylene Immobilized Metal−Organic Frameworks: Highly Sensitive Fluorescent Sensor for the Detection of Cr2O72− and Nitroaromatic Explosives. Cryst. Growth Des. 2017, 17, 6041–6048. [Google Scholar] [CrossRef]
- Liu, X.; Wang, K.; Chang, Z.; Zhang, Y.; Xu, J.; Zhao, Y.S.; Bu, X. Engineering Donor-Acceptor Heterostructure Metal-Organic Framework Crystals for Photonic Logic Computation. Angew. Chem. Int. Ed. 2019, 58, 13890–13896. [Google Scholar] [CrossRef]
- Leith, G.A.; Martin, C.R.; Mayers, J.M.; Kittikhunnatham, P.; Larsen, R.W.; Shustova, N.B. Confinement-Guided Photophysics in MOFs, COFs, and Cages. Chem. Soc. Rev. 2021, 50, 4382–4410. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, J.; He, H.; Qian, G. Photonic Functional Metal-Organic Frameworks. Chem. Soc. Rev. 2018, 47, 5740–5785. [Google Scholar] [CrossRef]
- Shu, Y.; Ye, Q.; Dai, T.; Xu, Q.; Hu, X. Encapsulation of Luminescent Guests to Construct Luminescent Metal-Organic Frameworks for Chemical Sensing. ACS Sens. 2021, 6, 641–658. [Google Scholar] [CrossRef]
- Xing, S.; Janiak, C. Design and Properties of Multiple-Emitter Luminescent Metal-Organic Frameworks. Chem. Commun. 2020, 56, 12290–12306. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yang, J.; Lu, B.; Li, C.; Ma, J. Water-Stable Metal-Organic Framework for Effective and Selective Cr2O72− Capture through Single-Crystal to Single-Crystal Anion Exchange. Inorg. Chem. 2018, 57, 11746–11752. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, F.; Zhang, J. Synthesis of Anionic Metal-Organic Zeolites for Selective Gas Adsorption and Ion Exchange. Inorg. Chem. 2019, 58, 4076–4079. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yu, X.; Wang, X.; Lai, S.; Sun, Y.; Yang, D. Recent Advances in Metal-Organic Frameworks for the Removal of Heavy Metal Oxoanions From Water. Chem. Eng. J. 2021, 407, 127221. [Google Scholar] [CrossRef]
- Kobielska, P.; Howarth, A.J.; Farha, O.K.; Nayak, S. Metal-Organic Frameworks for Heavy Metal Removal from Water. Coord. Chem. Rev. 2018, 358, 92–107. [Google Scholar] [CrossRef]
- Manna, B.; Desai, A.V.; Kumar, N.; Karmakar, A.; Ghosh, S.K. Single-Crystal-To-Single-Crystal Transformation of An Anion Exchangeable Dynamic Metal-Organic Framework. CrystEngComm 2015, 17, 8796–8800. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.X.; Shi, L.L.; Li, K.; Li, B.L.; Li, H.Y. An Unusual Porous Cationic Metal-Organic Framework Based On a Tetranuclear Hydroxyl-Copper(Ii) Cluster For Fast And Highly Efficient Dichromate Trapping Through a Single-Crystal To Single-Crystal Process. Chem. Commun. 2017, 53, 1860–1863. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, H.; Kong, F.; Wang, R. A Cationic Metal-Organic Framework Consisting of Nanoscale Cages: Capture, Separation, and Luminescent Probing of Cr2O72− through a Single-Crystal to Single-Crystal Process. Angew. Chem. Int. Ed. 2013, 52, 13769–1377326. [Google Scholar] [CrossRef]
- Ye, C.; Zhou, L.; Wang, X.; Liang, Z. Photon Upconversion: From Two-Photon Absorption (TPA) to Triplet–Triplet Annihilation (TTA). Phys. Chem. Chem. Phys. 2016, 18, 10818–10835. [Google Scholar] [CrossRef]
- He, Y.; Tan, Y.; Zhang, J. Functional Metal-Organic Frameworks Constructed from Triphenylamine-based Polycarboxylate Ligands. Coord. Chem. Rev. 2018, 420, 213354. [Google Scholar] [CrossRef]
- Summers, G.H.; Lefebvre, J.; Black, F.A.; Davies, E.S.; Gibson, E.A.; Pullerits, T.; Wood, C.J.; Zidek, K. Design And Characterisation of Bodipy Sensitizers for Dye-Sensitized NiO Solar Cells. Phys. Chem. Chem. Phys. 2016, 18, 1059–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Liao, Y.; Su, Z.; Zhang, H.; Wang, Y. Theoretical Study on Photophysical and Charge Transport Properties of 1,6-Bis(2-hydroxyphenol)pyridylboron Bis(4-n-butylphenyl)phenyleneamine Compound. J. Phys. Chem. A 2006, 110, 8758–8762. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Zhan, C.; Zhang, J.; Bu, X. Chiral Chemistry Of Metal–Camphorate Frameworks. Chem. Soc. Rev. 2016, 45, 3122–3144. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.; Rutledge, W.; Li, J.; Zhou, H. Zr-based Metal-Organic Frameworks: Design, Synthesis, Structure, and Applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Wang, N.; Zheng, P.; Fu, H.; Han, M.; Ma, L.; Wang, L. Tetraphenylethylene-Decorated Metal-Organic Frameworks as Energy-Transfer Platform for the Detection of Nitro-Antibiotics and White-Light Emission. Inorg. Chem. 2019, 58, 12700–12706. [Google Scholar] [CrossRef]
- Liu, J.; Yang, G.; Jin, J.; Wu, D.; Ma, L.; Wang, Y. A First New Porous d-p HMOF Material with Multiple Active Sites for Excellent CO2 Capture and Catalysis. Chem. Commun. 2020, 56, 2395–2398. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Sava, D.F.; Eubank, J.F.; Adil, K.; Guillerm, V. Zeolite-Like Metal-Organic Frameworks (ZMOFs): Design, Synthesis, and Properties. Chem. Soc. Rev. 2015, 44, 228–249. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, L.; Fan, N.; Han, M.; Yang, G.; Ma, L. Porous Zn(II)-Based Metal-Organic Frameworks Decorated with Carboxylate Groups Exhibiting High Gas Adsorption and Separation of Organic Dyes. Cryst. Growth Des. 2018, 18, 7114–7121. [Google Scholar] [CrossRef]
- Yao, X.; Cao, D.; Hu, J.; Li, Y.; Guo, Z.; Zheng, H. Chiral and Porous Coordination Polymers Based on an N-Centered Triangular Rigid Ligand. Cryst. Growth Des. 2011, 11, 231–239. [Google Scholar] [CrossRef]
- Wu, H.; Yang, J.; Liu, Y.; Ma, J. pH-Controlled Assembly of Two Unusual Entangled Motifs Based on a Tridentate Ligand and Octamolybdate Clusters: 1D + 1D → 3D Poly-Pseudorotaxane and 2D → 2D → 3D Polycatenation. Cryst. Growth Des. 2012, 12, 2272–2276. [Google Scholar] [CrossRef]
- Wu, H.; Yang, J.; Su, Z.; Batten, S.R.; Ma, J. An Exceptional 54-Fold Interpenetrated Coordination Polymer with 10(3)-Srs Network Topology. J. Am. Chem. Soc. 2011, 133, 11406–11409. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, H.; Yang, J.; Liu, B.; Ma, J.; Liu, Y.; Liu, Y. Series of Coordination Polymers based on Different Carboxylates and a Tri(4-imidazolylphenyl)amine Ligand: Entangled Structures and Photoluminescence. Cryst. Growth Des. 2011, 11, 2317–2324. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, M.; Hu, J.; Li, Y.; Guo, Z.; Zheng, H. Two Porous Zinc Coordination Polymers with (10,3) Topological Features Based on a N-Centered Tripodal Ligand and the Conversion of a (10,3)-d Subnet to a (10,3)-a Subnet. Chiral and Noncentrosymmetric Metal−Organic Frameworks Featuring A 2D → 3D Parallel/Parallel Inclined Subpolycatenation. Cryst. Growth Des. 2011, 11, 3039–3044. [Google Scholar]
- Yao, X.; Hu, J.; Zhang, M.; Qin, L.; Li, Y.; Guo, Z.; Zheng, H. Chiral and Noncentrosymmetric Metal−Organic Frameworks Featuring a 2D → 3D Parallel/Parallel Inclined Subpolycatenation. Cryst. Growth Des. 2013, 13, 3381–3388. [Google Scholar] [CrossRef]
- Zhang, M.; Di, C.; Qin, L.; Yao, X.; Li, Y.; Guo, Z.; Zheng, H. Diverse Structures of Metal−Organic Frameworks Based on a New Star-Like Tri(4-pyridylphenyl)amine Ligand. Cryst. Growth Des. 2012, 12, 3957–3963. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, H.; Lu, Z.; Yao, X. Application of W–Cu–S-based Secondary Building Units in Functional Metal-Organic Frameworks. CrystEngComm 2013, 15, 9265–9275. [Google Scholar] [CrossRef]
- Shen, K.; Zhang, M.; Zheng, H. Critical Factors Influencing the Structures and Properties of Metal-Organic Frameworks. CrystEngComm 2015, 17, 981–991. [Google Scholar] [CrossRef]
- Meng, F.; Qin, L.; Zhang, M.; Zheng, H. Two Pairs Of Isomorphism and Two 3D Metal-Organic Frameworks based on a Star-Like Ligand Tri(4-Pyridylphenyl)Amine. CrystEngComm 2014, 16, 698–706. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, M.; Shen, K.; Li, Y.; Zheng, H. A series of MOFs based on a Triangular Tri(4-Pyridylphenyl)Amine Ligand Combined With Carboxylate Or Nitrate Auxiliary Ligands. Dalton Trans. 2015, 44, 1412–1419. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, Z.; Chen, M.; Zheng, H. Chiral Crystallization and Optical Properties of Three Metal Complexes based on Two Non-Centrosymmetric Tripodal Ligands. Dalton Trans. 2015, 44, 5818–5825. [Google Scholar] [CrossRef]
- Shi, Z.; Pan, Z.; Zhang, C.; Zheng, H. Syntheses, Structures, and Properties of Six Cobalt(Ii) Complexes Based on a Tripodal Tris(4-(1H-1,2,4-Triazol-1-Yl)Phenyl)Amine Ligand. Dalton Trans. 2015, 44, 16854–16864. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F. Synthesis, Crystal Structure And Photoluminescence of a Copper(I)-Iodide Coordination Polymer Based On Polyimidazolate Ligand. Chinese J. Struct. Chem. 2019, 38, 1759–1763. [Google Scholar]
- Desai, A.V.; Sharma, S.; Roy, A.; Ghosh, S.K. Probing the Role of Anions in Influencing the Structure, Stability, and Properties in Neutral N-Donor Linker based Metal-Organic Frameworks. Cryst. Growth Des. 2019, 19, 7046–7054. [Google Scholar] [CrossRef]
- Yao, X.; Xiao, G.; Xie, H.; Qin, D.; Ma, H.; Liu, J.; Yan, P. Two Triphenylamine-Based Luminescent Metal-Organic Frameworks as a Dual-Functional Sensor for the Detection of Nitroaromatic Compounds and Ofloxacin Antibiotic. CrystEngComm 2019, 21, 2559–2570. [Google Scholar] [CrossRef]
- Yao, X.; Pan, Z.; Hu, J.; Li, Y.; Guo, Z.; Zheng, H. The rational synthesize of (10,3)-type MOFs based on tetraunclear [W(Mo)OS3Cu3]+ Secondary Building Units. Chem. Commun. 2011, 47, 10049–10051. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, C.; Sun, Y.; Wang, F.; Zhang, J. In Situ Encapsulation of Organic Sulfates in Layered Structures of Zinc and Tris(4-(1H-Imidazol-1-yl)phenyl)amine. Cryst. Growth Des. 2020, 20, 4228–4231. [Google Scholar] [CrossRef]
- Huo, J.; Li, H.; Yu, D.; Arulsamy, N. Three New Metal Complexes with Imidazole-Containing Tripodal Ligands as Fluorophores for Nitroaromatics- And Ion-Selective Sensing. Inorg. Chim. Acta 2020, 502, 119310. [Google Scholar] [CrossRef]
- Huo, J.; Yu, D.; Li, H.; Luo, B.; Arulsamy, N. Mechanistic Investigation Of Photocatalytic Degradation Of Organic Dyes by A Novel Zinc Coordination Polymer. RSC Adv. 2019, 9, 39323–39331. [Google Scholar] [CrossRef] [Green Version]
- Fu, H.R.; Kang, Y.; Zhang, J. Highly Selective Sorption of Small Hydrocarbons and Photocatalytic Properties of Three Metal-Organic Frameworks Based on Tris(4-(1H-imidazol-1-yl)phenyl)amine Ligand. Inorg. Chem. 2014, 53, 4209–4214. [Google Scholar] [CrossRef]
- Fu, H.R.; Xu, Z.; Zhang, J. Water-stable Metal-Organic Frameworks for Fast and High Dichromate Trapping via Single-Crystal-to-Single-Crystal Ion-exchange. Chem. Mater. 2015, 27, 205–210. [Google Scholar] [CrossRef]
- Fu, H.R.; Wang, N.; Qin, J.H.; Han, M.L.; Ma, L.F.; Wang, F. Spatial Confinement of A Cationic MOF: A SC-SC Approach for High Capacity Cr(VI)-oxyanion Capture in Aqueous Solution. Chem. Commun. 2018, 54, 11645–11648. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.R.; Zhao, Y.; Zhou, Z.; Yang, X.; Ma, L.F. Neutral ligand TIPA-based two 2D Metal-Organic frameworks: Ultrahigh selectivity of C2H2/CH4 and efficient sensing and sorption of Cr(VI). Dalton Trans. 2018, 47, 3725–3732. [Google Scholar] [CrossRef]
- Desai, A.V.; Manna, B.; Karmakar, A.; Sahu, A.; Ghosh, S.K. A Water-Stable Cationic Metal-Organic Framework as a Dual Adsorbent of Oxoanion Pollutants. Angew. Chem. Int. Ed. 2016, 55, 7811–7815. [Google Scholar] [CrossRef]
- Jiang, X.; Han, S.; Wang, A.; Pan, J.; Wang, G. The Tri(imidazole)-Derivative Moiety: A New Category of Electron Acceptors for the Design of Crystalline Hybrid Photochromic Materials. Chem. Eur. J. 2021, 27, 1410–1415. [Google Scholar] [CrossRef]
- Zhuo, C.; Wang, F.; Zhang, J. Mixed Short and Long Ligands toward the Construction of Metal-Organic Frameworks with Large Pore Openings. Cryst. Growth Des. 2019, 19, 3120–3123. [Google Scholar] [CrossRef]
- Yuan, S.; Deng, Y.; Xuan, W.; Wang, X.; Wang, S.; Dou, J.; Sun, D. Spontaneous Chiral Resolution of a 3D (3,12)-connected MOF with An Unprecedented ttt Topology Consisting of Cubic [Cd4(μ3-OH)4] Clusters and Propeller-like Ligands. CrystEngComm 2014, 16, 3829–3833. [Google Scholar] [CrossRef]
- He, Y.; Chen, G.; Yuan, L.; Zhang, L.; Zhang, J. Ti4(embonate)6 Cage-Ligand Strategy on the Construction of Metal-Organic Frameworks with High Stability and Gas Sorption Properties. Inorg. Chem. 2020, 59, 964–967. [Google Scholar] [CrossRef]
- Sharma, S.; Desai, A.V.; Joarder, B.; Ghosh, S.K. A Water-Stable Ionic MOF for the Selective Capture of Toxic Oxoanions of Se VI and As V and Crystallographic Insight into the Ion-Exchange Mechanism. Angew. Chem. Int. Ed. 2020, 59, 7788–7792. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Yang, Z.; Liu, S.; Dai, X.; Xiao, C.; Taylor-Pashow, K.; Li, D.; Yang, C.; Li, J.; Zhang, Y.; et al. 99TcO4− Removal from Legacy Defense Nuclear Waste by an Alkaline-Stable 2D CationicMetal organic Framework. Nat. Commun. 2020, 11, 5571. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.R.; Wu, X.; Ma, L.; Wang, F.; Zhang, J. Dual-Emission SG7@MOF Sensor via SC-SC Transformation: Enhancing the Formation of Excimer Emission and the Range and Sensitivity of Detection. ACS Appl. Mater. Interfaces 2018, 10, 18012–18020. [Google Scholar] [CrossRef]
- Yuan, S.; Deng, Y.; Sun, D. Unprecedented Second-Timescale Blue/Green Emissions and Iodine-Uptake-Induced Single-Crystal-to-Single-Crystal Transformation in ZnII/CdII Metal-Organic Frameworks. Chem. Eur. J. 2014, 20, 10093–10098. [Google Scholar] [CrossRef]
- Ge, R.; Li, X.X.; Zheng, S.T. Recent Advances in Polyoxometalate-Templated High-Nuclear Silver Clusters. Coord. Chem. Rev. 2020, 421, 213787. [Google Scholar]
- Jin, Y.; Zhang, C.; Dong, X.; Zang, S.; Mak, T.C.W. Shell Engineering to Achieve Modification and Assembly of Atomically-Precise Silver Clusters. Chem. Soc. Rev. 2021, 50, 2297–2319. [Google Scholar] [CrossRef]
- Guillerm, V.; Kim, D.; Eubank, J.F.; Luebke, R.; Liu, X.; Adil, K.; Lah, M.S.; Eddaoudi, M. A Supermolecular Building Approach For The Design and Construction Of Metal-Organic Frameworks. Chem. Soc. Rev. 2014, 43, 6141–6172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.; Wang, F.; Zhang, J.; Bu, X. Luminescent MTN-type Cluster-Organic Framework with 2.6 nm Cages. J. Am. Chem. Soc. 2012, 134, 17881–17884. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Su, C.; Fan, Y.; Shi, W.; Zhang, X. Assembly of Two Self-Interpenetrating Metal-Organic Frameworks Based on a Trigonal Ligand: Syntheses, Crystal Structures, and Properties. Inorg. Chem. 2020, 59, 7135–7142. [Google Scholar] [CrossRef]
- Winnik, F.M. Photophysics of Preassociated Pyrenes in Aqueous Polymer Solutions and In Other Organized Media. Chem. Rev. 1993, 93, 587–614. [Google Scholar] [CrossRef]
- Fu, H.R.; Yan, L.; Wu, N.; Ma, L.; Zang, S. Dual-emission MOF⊃dye Sensor For Ratiometric Fluorescence Recognition of RDX and Detection Of a Broad Class Of Nitro-Compounds. J. Mater. Chem. A 2018, 6, 9183–9191. [Google Scholar] [CrossRef]
- Zhai, Q.; Bu, X.; Zhao, X.; Li, D.; Feng, P. Pore Space Partition in Metal-Organic Frameworks. Acc. Chem. Res. 2017, 50, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Desaia, A.V.; Sharmaa, S.; Leta, S.; Ghosh, S.K. N-donor Linker Based Metal-Organic Frameworks (Mofs): Advancement and Prospects as Functional Materials. Coord. Chem. Rev. 2019, 395, 146–192. [Google Scholar] [CrossRef]
- Loukopoulos, E.; Kostakis, G.E. Recent Advances in The Coordination Chemistry of Benzotriazole-Based Ligands. Coord. Chem. Rev. 2019, 395, 193–229. [Google Scholar] [CrossRef]
- Jeoung, S.; Kim, S.; Kim, M.; Moon, H.R. Pore Engineering Of Metal-Organic Frameworks with Coordinating Functionalities. Coord. Chem. Rev. 2020, 420, 213377. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Z.; Su, Y.; Chen, P.; Jiang, Y.; Zhang, C.; Wang, C. Metal-Organic Layers with an Enhanced Two-Photon Absorption Cross-Section and Up-Converted Emission. Chem. Mater. 2021, 33, 1618–1624. [Google Scholar] [CrossRef]
- Hirata, S.; Totani, K.; Zhang, J.; Yamashita, T.; Kaji, H.; Marder, S.R.; Watanabe, T.; Adachi, C. Efficient Persistent Room Temperature Phosphorescence in Organic Amorphous Materials under Ambient Conditions. Adv. Funct. Mater. 2013, 23, 3386–3397. [Google Scholar] [CrossRef]
- Belyaev, A.; Cheng, Y.; Liu, Z.; Karttunen, A.J.; Chou, P.; Koshevoy, I.O. A Facile Molecular Machine: Optically Triggered Counterion Migration by Charge Transfer of Linear Donor-π-Acceptor Phosphonium Fluorophores. Angew. Chem. Int. Ed. 2019, 58, 13456–13465. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Qin, W.; Du, C.; Gao, H.; Zhu, F.; Liang, G. Long-Lived Room-Temperature Phosphorescence for Visual and Quantitative Detection of Oxygen. Angew. Chem. Int. Ed. 2019, 58, 12102–12106. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Dai, W.; Tian, Y.; Yang, J.; Li, P.; Shi, J.; Tong, B.; Cai, Z.; Dong, Y. Revealing Insight into Long-Lived Room-Temperature Phosphorescence of Host-Guest Systems. J. Phys. Chem. Lett. 2019, 10, 6019–6025. [Google Scholar] [CrossRef]
- Chen, K.C.; Liu, B. Enhancing the Performance of Pure Organic Room-Temperature Phosphorescent Luminophores. Nat. Commun. 2019, 10, 2111. [Google Scholar]





| MOF | Dimension | Porosity | Auxiliary Ligand | Ref. |
|---|---|---|---|---|
| [Cd (TIPA)·(NO3)2·5H2O] n | 2D | 28.7% | [40] | |
| [Co (TIPA)1/3·Cl3·2H2O] n | 2D | 52.8% | ||
| [Co (TIPA)·(5-OH-bdc)3·2H2O] n | 2D | none | ||
| [Cd2(TIPA)2·(5-OHbdc)2·5.5H2O] n | 3D | 34.4% | ||
| [Ag0.52Na0.48(β-Mo8O26) (H2O)] [Ag3(Tipa)2] | 3D | none | [41] | |
| [Ag6(Tipa)4(β-Mo8O26)] [H2(β-Mo8O26)] 5H2O | 2D | none | [42] | |
| [Ag3(OH)(H2O)2(Tipa)2.5] [Mo2O7]3·4.5H2O | 3D | none | ||
| [Cd (Tipa)(L1)2] H2O | 2D | none | HL1 = benzoic acid | [43] |
| [Cd (Tipa)(L2)] H2O | 2D | none | H2L2 = 5-NH2-1,3-benzenedicarboxylic acid | |
| [Cd (Tipa)(L2)] CH3OH·H2O | 3D | none | ||
| Cd (Tipa)(L3) (H2O) | 3D | none | H2L3 = 2-(4-carboxybenzylamino) benzoic acid | |
| [Mn (Tipa)(L2)] H2O | 2D | none | ||
| [Ni2(Tipa)2(L4) (H2O)2]2·Cl4·4H2O | 2D | none | H2L4 = 1,4-benzenedicarboxylic acid | |
| [Ni2(Tipa)2(L5) (H2O)4] (H4L5) 0.5H2O | 2D | none | H4L5 = 1,2,4,5-benzenetetracarboxylic acid | |
| {[Zn (TIPA)(mal)1/2] (NO3)·3H2O}n | 3D | 41.9% | mal = malonic acid | [44] |
| {[Zn (TIPA)(glu)1/2] (NO3)·H2O}n | 3D | 27.2% | glu = glutaric acid | |
| {[Co (TIPA)(trans-chdc) (H2O)]·H2O}n | 2D | none | trans-chdc = trans-1,4-cyclohexanedicarboxylic acid | [45] |
| {[Ni (TIPA)(trans-chdc) (H2O)]·H2O}n | 2D | none | ||
| {[Co (TIPA)(seb)1/2] (NO3)·H2O}n | 2D | none | H2seb = sebacic acid | |
| {[Ni (TIPA)(seb)1/27](NO3)·H2O}n | 2D | none | ||
| {[Zn2 (TIPA)(btc)(μ2-OH)]·4H2O}n | 2D | none | H3btc = 1,3,5-benzenetricarboxylic acid | |
| {[Cd (TPPA)(trans-chdc)]} n | 3D | none | trans-H2chdc = trans-1,4-cyclohexanedicarboxylic acid | [47] |
| {[Co (TPPA)2(D-ca)2]·(H2O)}n | 2D | none | d-H2ca = d-camphor acid | |
| {[Ni (TPPA)(bdc)(H2O)]·(H2O)4}n | 3D | none | H2bdc = benzene-p-dicarboxylic acid | |
| {[Ni (TPPA)(trans-chdc)(H2O)]·(H2O)4}n | 3D | none | ||
| [Co2(TPPA)2(1,3-bdc)2(H2O)] n | 2D | none | 1,3-H2bdc = 1,3-benzenedicarboxylic acid | [50] |
| [Zn (TPPA)(1,3-bdc)] n | 2D | none | ||
| [Zn6(TPPA)2(betc)(Hbetc)2(H2betc) (H2O)6·7H2O·2DMA]n | 3D | 24.2% | betc = 1,2,4,5-benzenetetracarboxylic dianhydride | |
| [Cu (TPPA)(NO3)2(H2O)]·2H2O]n | 2D | none | ||
| {[Cd (DIMPPA)(5-OH-bdc)](H2O)}n | 2D | none | 5-OH-H2bdc = 5-hydroxyisophthalic acid | [51] |
| {[Co (DIMPPA)(5-OH-bdc)](H2O)}n | 2D | none | ||
| {[Cd2(MIDPPA)2(D-ca)2(H2O)2] (H2O)5} n | 3D | none | D-H2ca = D-camphoric acid | |
| {[Co1.5(TTPA)(BTC) (H2O)]2·13H2O} n | 3D | 51% | H3BTC = 1,3,5-benzenetricarboxylic acid | [52] |
| [Co (TTPA)(PA)] n | 3D | none | H2PA = phthalic acid | |
| {[Co (TTPA)(BDA)0.5(NO3)]·3H2O}n | 3D | 27.2% | H2BDA = (1,1′-biphenyl)-4,4′-dicarboxylic acid | |
| {[Co2(TTPA)3(OBA)2(H2O)3]·2CH3CN·4H2O} n | 2D | none | H2OBA = 4,4′-oxydibenzoic acid | |
| {[Co (TTPA)(AIP) (H2O)]·2H2O} n | 2D | none | H2AIP = 5-aminoisophthalic acid | |
| {[Co (TTPA)(MIP) (H2O)]·2H2O} n | 2D | none | H2MIP = 5-methylisophthalic acid | |
| {[Cd(tipa)2]·(ClO4)2} n | 2D | 41.9% | [54] | |
| {[Cd(tipa)(NO3)2]} n | 1D | 33.2% | ||
| {[Cd2(SO4)2(tipa)2]} n | 2D | 28.3% | ||
| {[Cd(tipa)(NO3) (SA)]·(DMF)} n | 2D | none | sulfanilic acid | |
| {[Cd(tipa)(HCOO)2]·xG} n | 3D | 25.3% | ||
| {[Zn (TIPA)pim0.5]2H2O·NO3} n | 3D | 26.6% | H2pim = pimelic acid | [55] |
| {[Zn (TIPA)(pim)]3H2O} n | 2D | 38.4% | ||
| {[WOS3Cu3Br (TIPA)]-(H2O)(DMF)} n | 3D | 29.8% | [56] | |
| [Zn4 (Tipa)4Cl4]·4(G1) | 3D | none | [57] | |
| [Zn4(Tipa)4Cl4]·4(G2) | 3D | none | ||
| [Zn2(Tipa)2Cl2]·(G3) | 3D | none | ||
| [Zn2(Tipa)2(OH)]·(G4) | 3D | none | ||
| [Zn3(Tipa)2(OH)3]·(G5) | 3D | none | ||
| [Zn3(Tipa)2F2(H2O)4]·2(G6) | 3D | none | ||
| [Cd (TIPA)(suc)0.5(NO3)·½H2O]n | 3D | none | succinic acid | [59] |
| [Ni (TIPA)(tda)0.5(H2O)·¼H2O]n | 3D | none | 2,5-thiophenedicarboxylic acid | |
| [Cd (TIPA)(tda)0.5·½H2O] | 3D | none | ||
| {[Zn (TIPA)(seb)0.5](NO3)·3.5H2O}n | 3D | none | seb = sebacic acid | |
| [Zn2(Tipa)(4,4′-bpdc)1.5(H2O)(NO3)]·2(DMF)·H2O | 3D | none | [60] | |
| [Cd (Tipa)Cl2]·2(DMF)·H2O | 3D | 33.0% | ||
| [Co (Tipa)Cl2(H2O)]·DMF·H2O | 2D | none | ||
| [Zn2(Tipa)2(OH)]·3NO3·12H2O | 2D | 41.6% | [61] | |
| [Zn2(Tipa)2(OH)]·NO3·Cr2O7·5H2O | 3D | none | ||
| [Zn(Tipa)]·2NO3·DMF·4H2O) | 3D | 41.8% | ||
| [Zn2(TIPA)2(OH)(NO3)3]·5H2O | 3D | 49.0% | [62] | |
| [Zn2(TIPA)2(OH)(Cr2O7)1.5]·3H2O | 3D | none | ||
| [Ni (TIPA)(COO−)2(H2O)]·2(DMF)2 | 2D | 39.1% | [63] | |
| [Cd(TIPA)2(ClO4−)2]·(DMF)3(H2O) | 2D | 50.5% | ||
| [{Ni2(TIPA)3(SO4)(H2O)3}·(SO4)·x(G)]n | 3D | 24.6% | [64] | |
| [Zn2(HPO3)2(TIPA)]·2H2O | 2D | none | [65] | |
| [Zn3(HPO3)3(TIPA)]·6H2O | 1D | none | ||
| [Zn3(Tipa)2(Im)3]·3NO3·solvent | 3D | 28.8% | Im = imidazole | [66] |
| [Zn2(Tipa)2(Tz)][Zn(Tipa)(NO3)(H2O)]·4NO3·3DOA | 2D | 32.6% | Tz = tetrazole, DOA = 1,4-dioxane | |
| [Cd(tipa)(μ3-OH)·NO3·EtOH·DMF]n | 3D | none | [67] | |
| (Me2NH2)3[(Ti4L6)Zn3(OH)(tipa)2]·Guests | 3D | 36.2% | L = embonate | [68] |
| {[Ni2(L)3(SO4)-(H2O)3]·(HAsO4)·xG}n | 3D | none | [69] | |
| [Ni(tipa)2](NO3)2 | 2D | none | [70] | |
| [Zn2(tipa)2(OH−)](NO3−)(SG7)2/3·5H2O | 3D | none | SG7 = solvent green 7 | [71] |
| {[Zn(tipa)Cl]·NO3·2DMF}n | 3D | 37.4 % | [72] | |
| {[Cd2(tipa)2Cl4]·6DMF}n | 3D | none | ||
| {[Zn2(tipa)2Cl2]·2I3·2 DMF}n | 3D | none | ||
| {[Cd2(tipa)2Cl2(dmf)2]·2I3·4 DMF}n | 3D | none |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, H.; Jiang, Y.; Wang, F.; Zhang, J. The Synthesis and Properties of TIPA-Dominated Porous Metal-Organic Frameworks. Nanomaterials 2021, 11, 2791. https://doi.org/10.3390/nano11112791
Fu H, Jiang Y, Wang F, Zhang J. The Synthesis and Properties of TIPA-Dominated Porous Metal-Organic Frameworks. Nanomaterials. 2021; 11(11):2791. https://doi.org/10.3390/nano11112791
Chicago/Turabian StyleFu, Hongru, Yuying Jiang, Fei Wang, and Jian Zhang. 2021. "The Synthesis and Properties of TIPA-Dominated Porous Metal-Organic Frameworks" Nanomaterials 11, no. 11: 2791. https://doi.org/10.3390/nano11112791
APA StyleFu, H., Jiang, Y., Wang, F., & Zhang, J. (2021). The Synthesis and Properties of TIPA-Dominated Porous Metal-Organic Frameworks. Nanomaterials, 11(11), 2791. https://doi.org/10.3390/nano11112791






