Graphene-Based Cathode Materials for Lithium-Ion Capacitors: A Review
Abstract
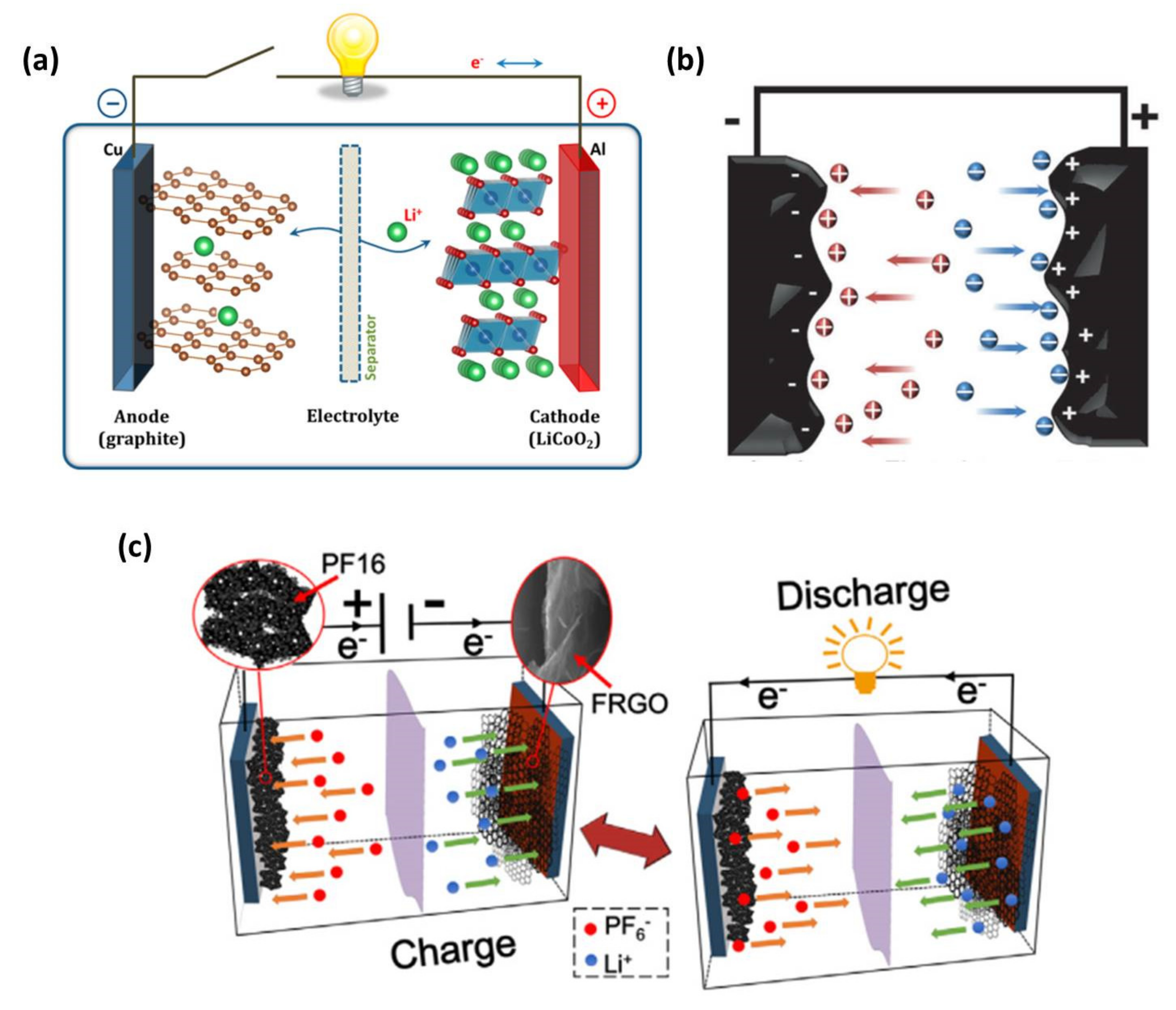
:1. Introduction
2. Reduced Graphene Oxide as a Cathode Material
2.1. Reduced Graphene Oxide
2.2. Three-Dimensional Reduced Graphene Oxide
3. Pure Porous Graphene as a Cathode Material
3.1. Porous Graphene Prepared with Template
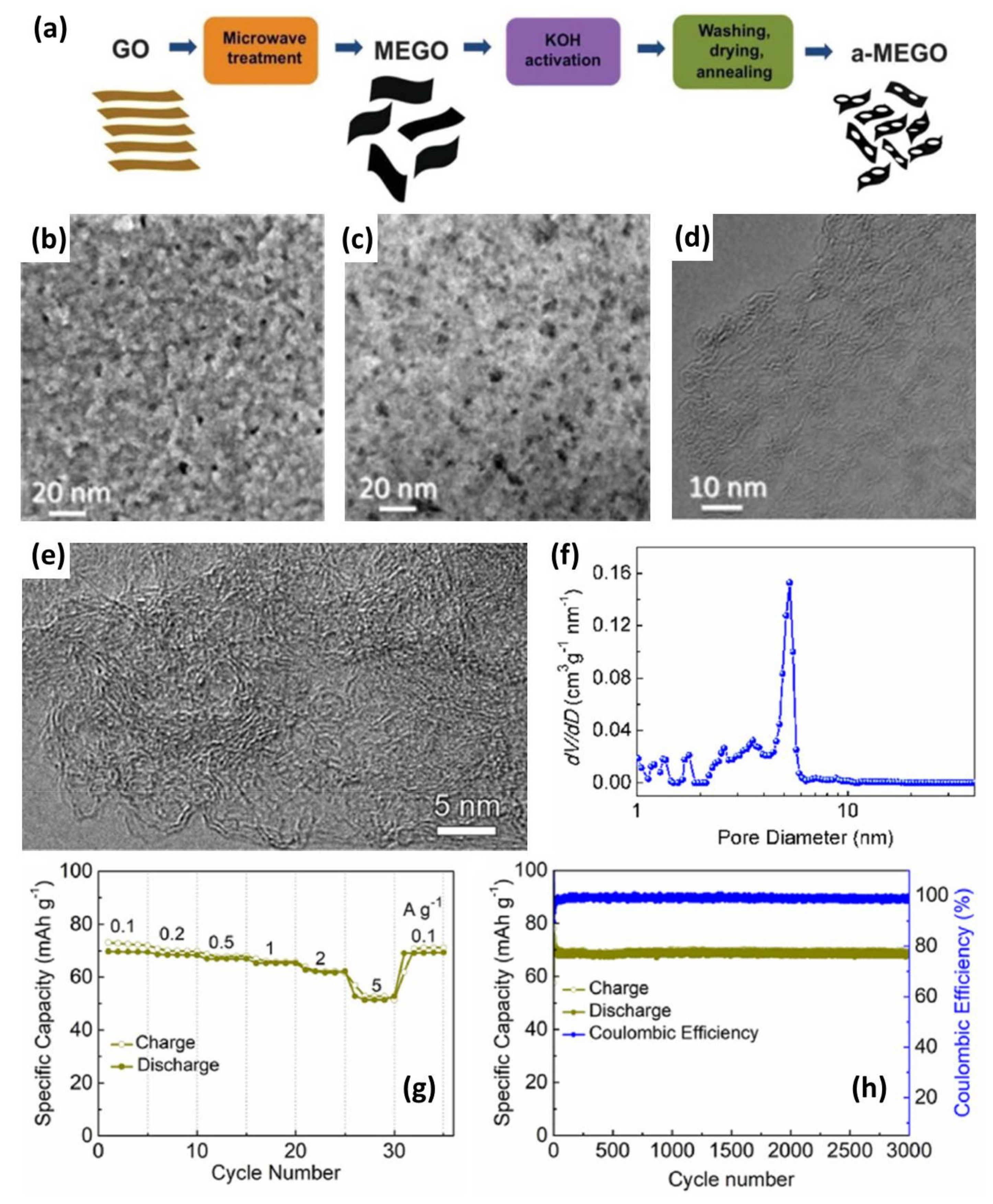
3.2. Porous Graphene Prepared by Chemical Activation
3.3. Porous Graphene Prepared by Other Methods
4. Graphene-Based 3D Composites as Cathode Materials
4.1. Grahene@Porous Carbon-Based 3D Composites
4.1.1. Grahene@Non-Doped Porous Carbon 3D Composites
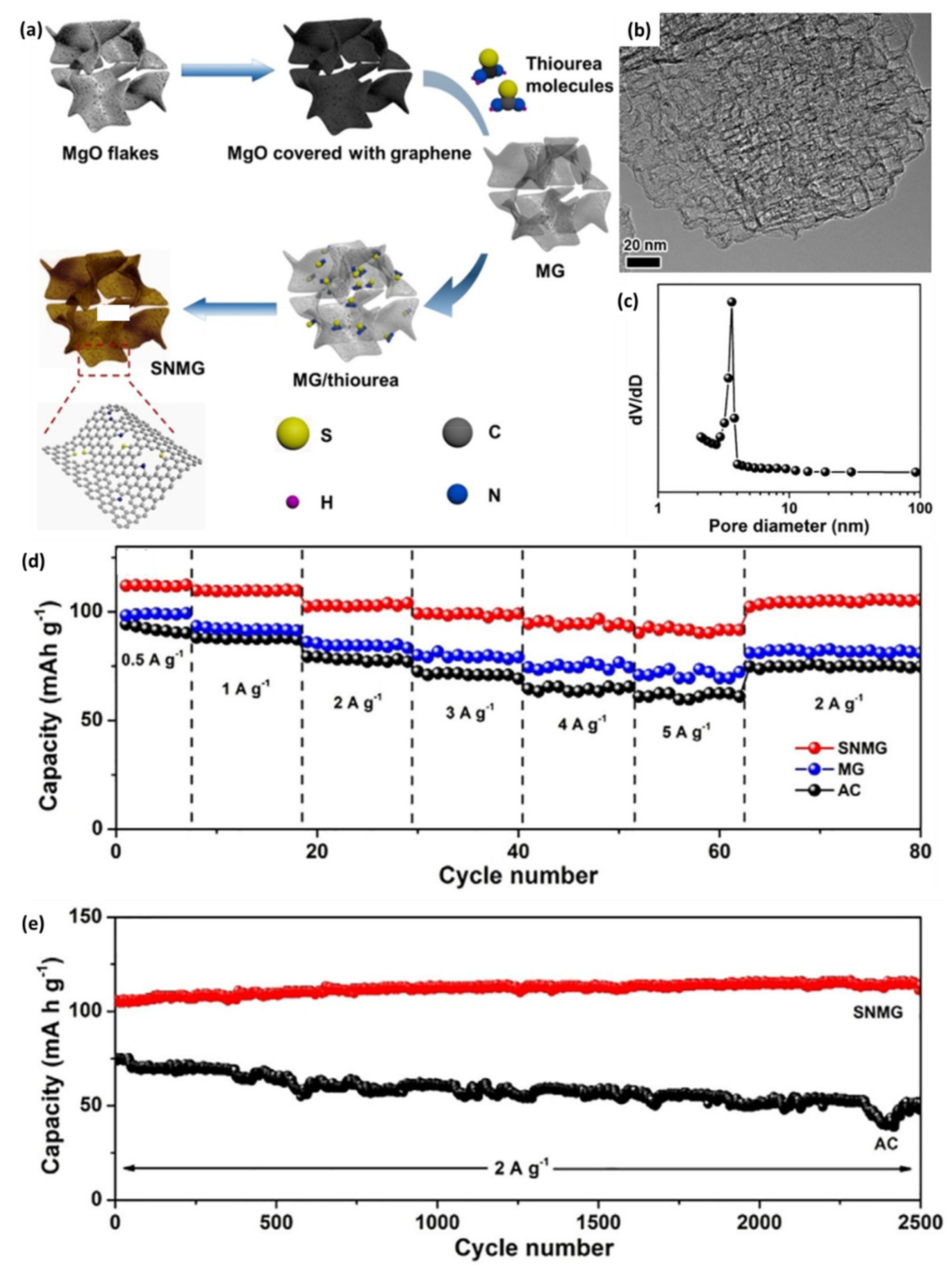
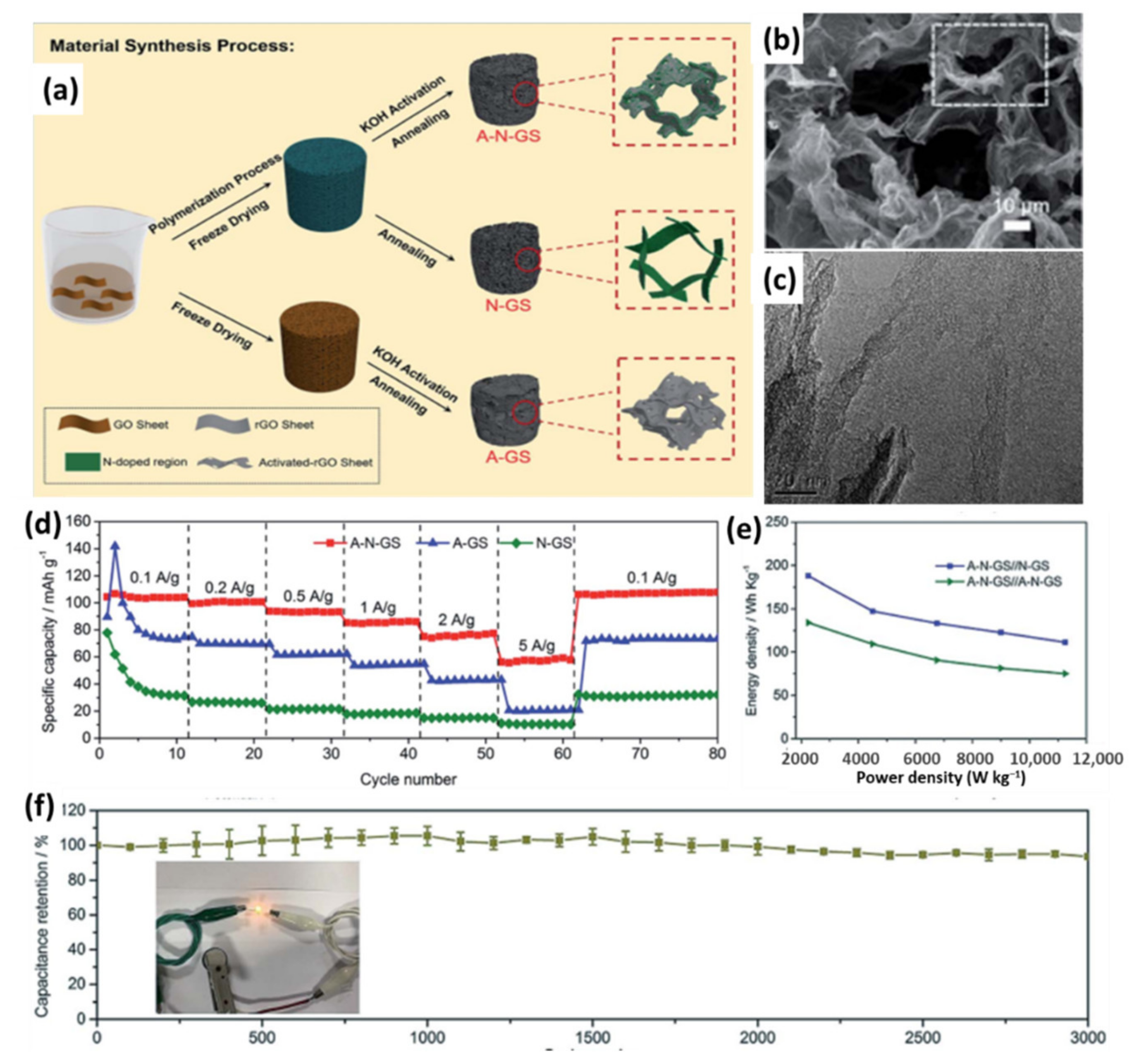
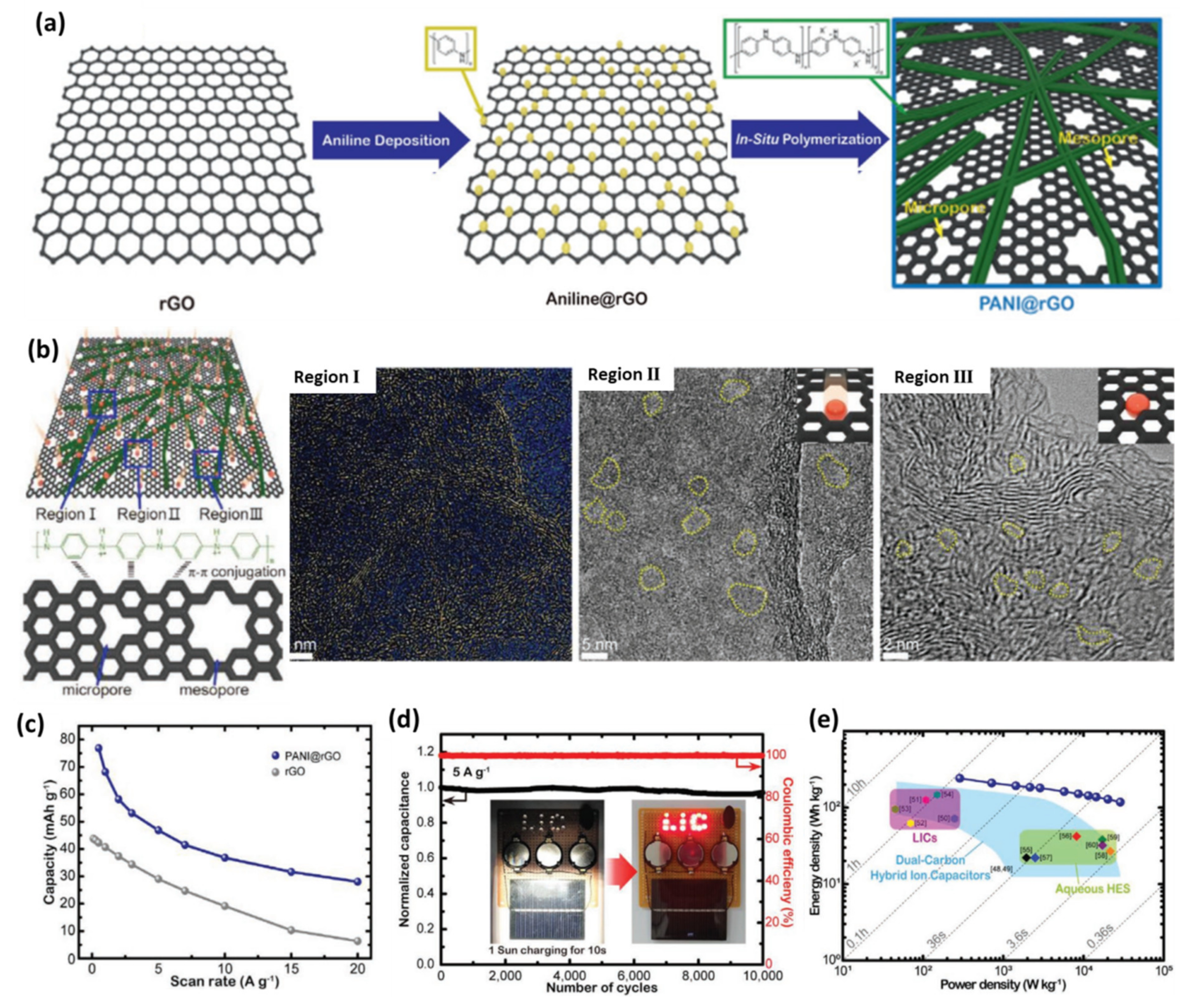
4.1.2. Graphene@Doped Porous Carbon 3D Composites
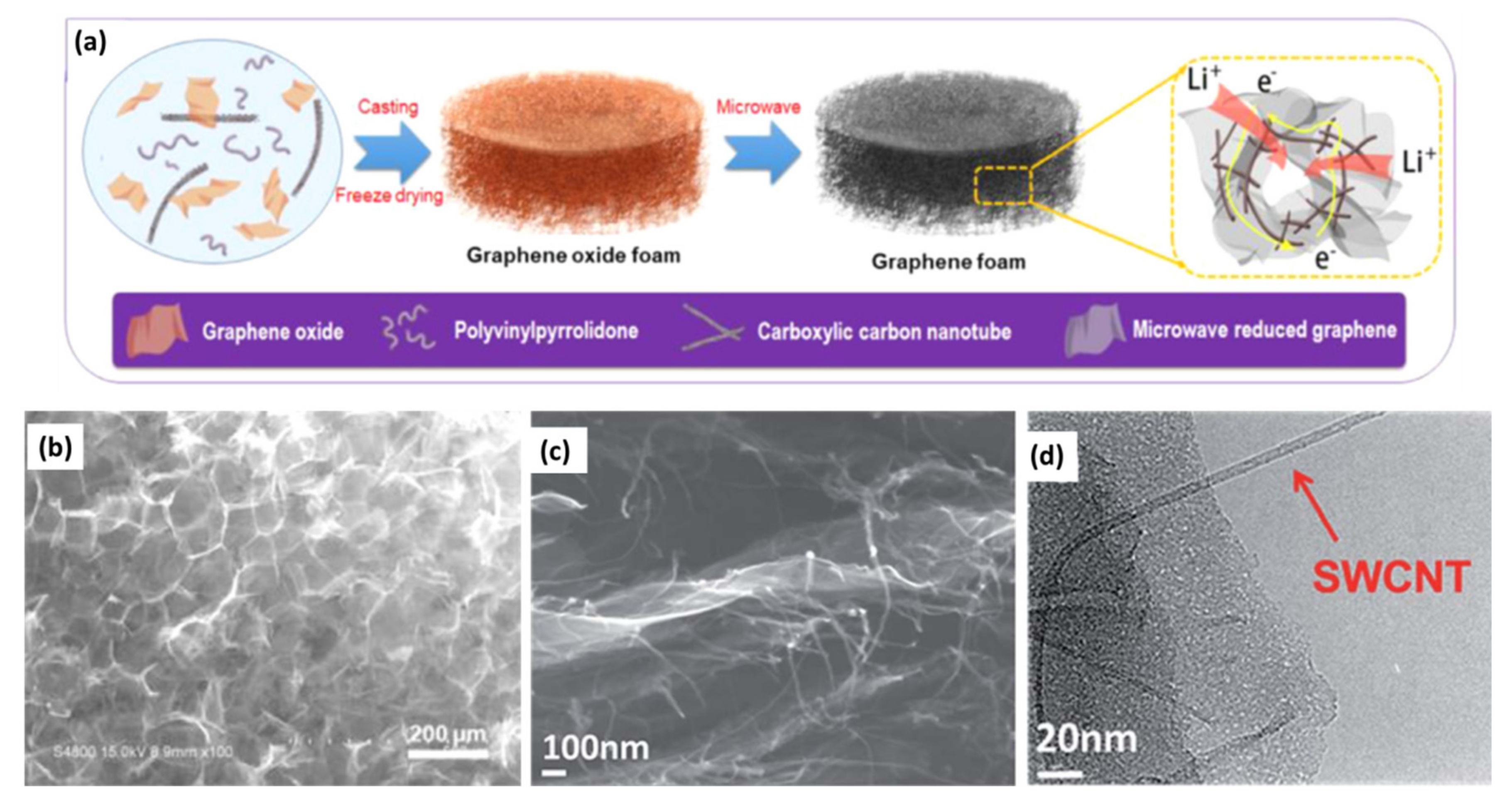
4.2. Graphene/Nanostructured Material 3D Composites
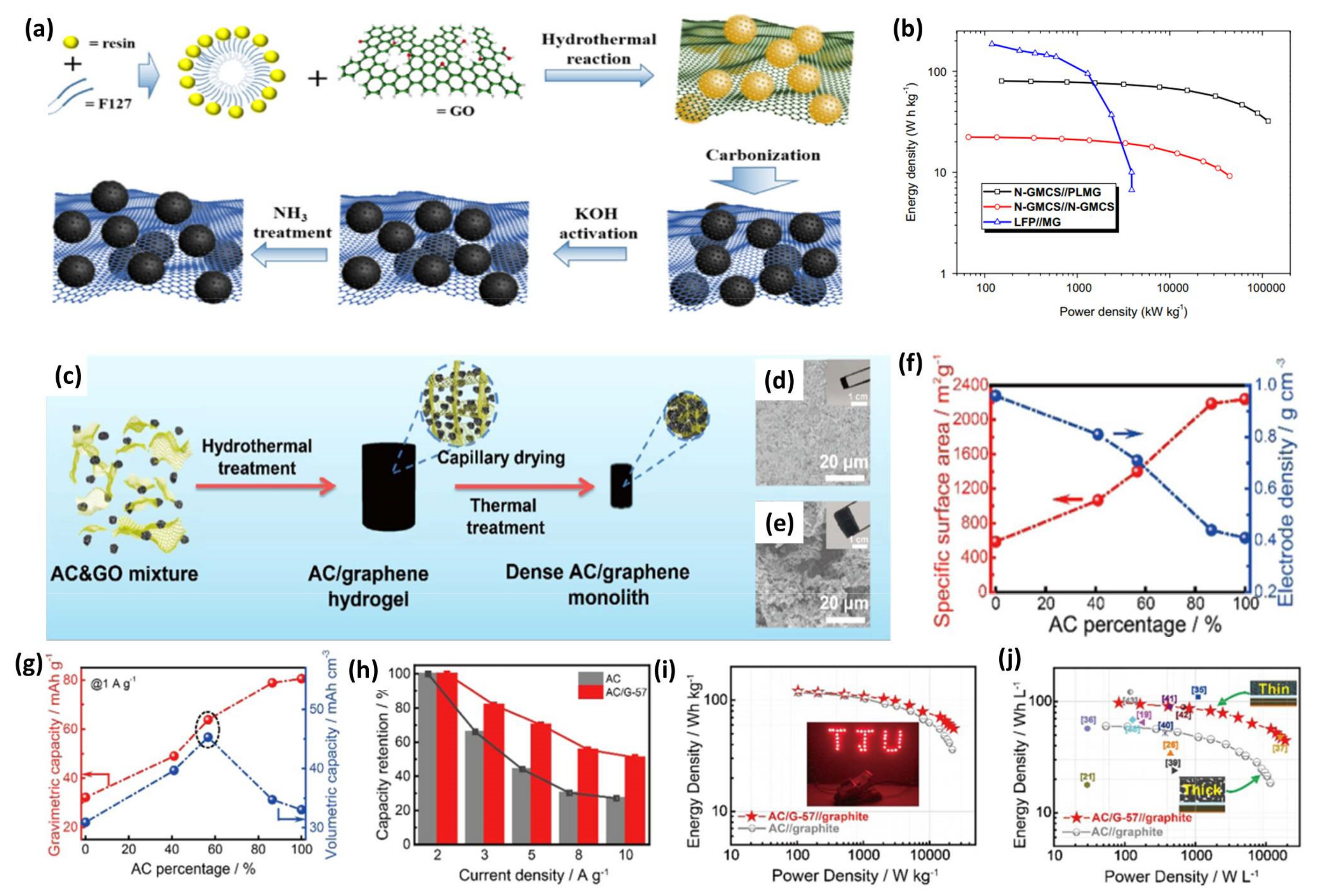
4.3. High-Density Graphene-Based 3D Composites
5. Summary and Outlook
- (1)
- High-capacity capacitor-type materials are urgently needed. To match the high capacity of battery-type anodes, developing cathode materials with improved capacity is the top priority. The cathode stores energy through a physical adsorption/desorption process at the electrolyte/electrode interface, which leads to a fast charge/discharge rate. On the other hand, the non-Faradaic energy storage mechanism also results in low capacity because it is critically influenced by the SSA. A high SSA could provide more active sites for ion adsorption and, to a certain extent, the capacity is raised with the increase in the SSA. However, it should be pointed out that not all the surface can be accessible by the electrolyte ions [134]. Therefore, the morphology, pore size and surface chemistry of graphene-based cathode materials should be carefully regulated to increase the effective surface area and in turn enhance their capacity. In addition, heteroatom doping and porosity engineering should also receive enough attention to obtain high-capacity and high-rate capacitor-type cathodes. Doping can not only provide extra capacity by fast redox reactions but enhance the electrical conductivity, while rational and tunable porosity is beneficial for the electrolyte ions’ diffusion, together resulting in excellent rate capability.
- (2)
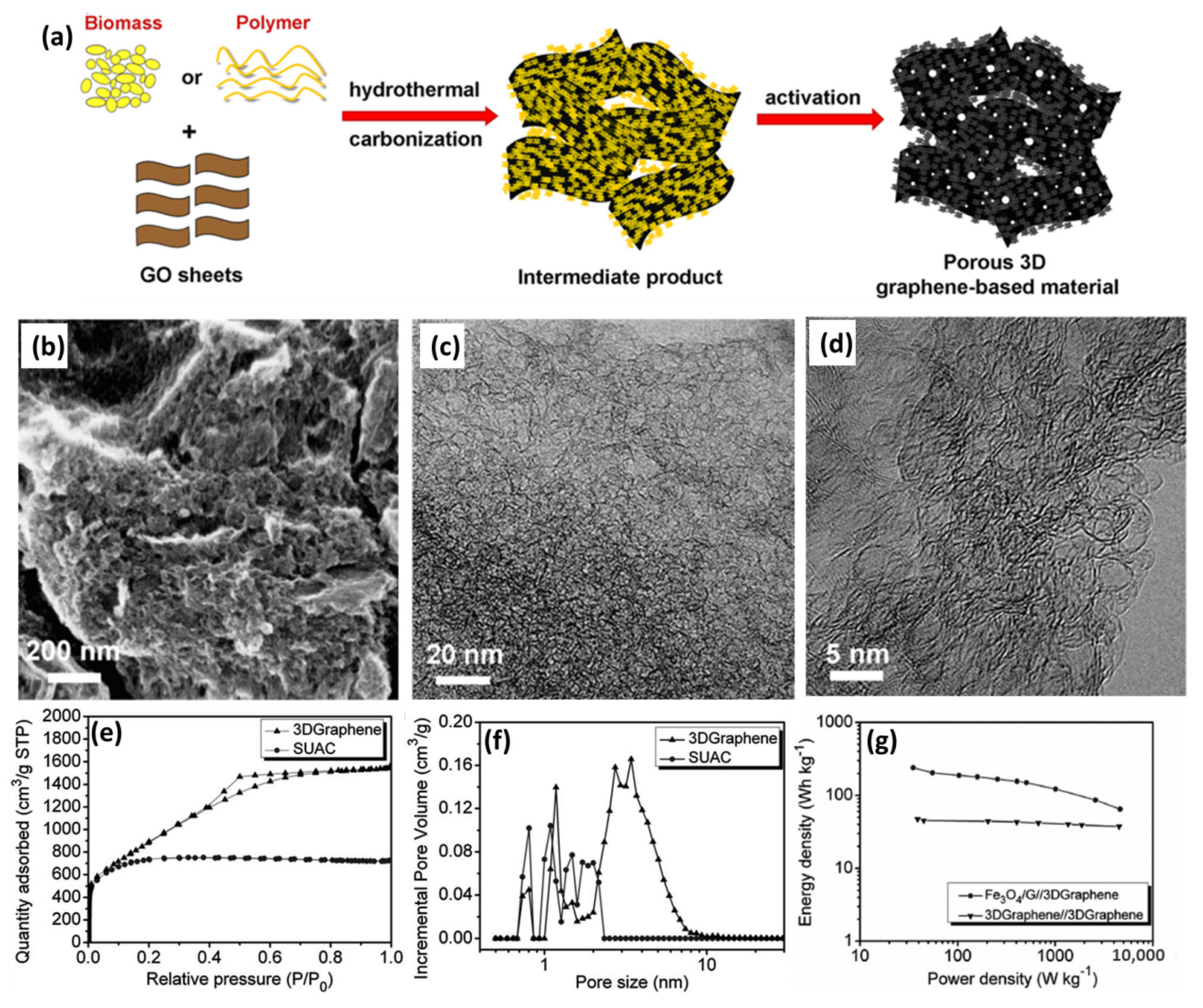
- The preparation of graphene-based cathode materials at low cost is one of the critical factors for large-scale applications. Although pure graphene-based porous materials can serve as outstanding capacitor-type electrodes due to their high electrical conductivity and large SSA, the high cost impedes their further commercial utilization. Forming composites with other materials is a facile but feasible method to solve this problem, such as biomass or polymer/graphene hydrogel-derived graphene-based 3D porous materials initially proposed by Chen’s group [24]. In such cases, the overall cost of the cathode materials can be largely reduced but the high conductivity and porous structure can be kept.
- (3)
- Developing battery-type materials with a high rate and long-term stability is another big challenge but imperative issue. High-capacity anodes ensure the high energy of the full cell. However, the high energy density of state-of-the-art LICs could only be realized at the cost of low power output because of the sluggish redox reaction and/or inferior electrical conductivity of battery-type anodes. Hence, designing nanostructured materials with tunable porosity and compositing with highly conductive materials are always applied to achieve high-rate anodes. These strategies could help to reduce the capacity and kinetics imbalances between the cathode and anode, leading to improved energy and power densities [12]. With the booming of lithium metal batteries, Li metal anodes have also been applied as the battery-type electrode to develop high-energy LICs [131,135]. In this circumstance, elaborate surface coating or electrolyte regulation is needed to suppress lithium dendrite growth to avoid safety accidents [136,137].
- (4)
- The volumetric performance of graphene-based materials should receive more attention for commercial applications. Graphene-based cathode materials have shown enhanced gravimetric capacity compared with commercial AC. However, their large SSA and highly porous structure result in a low taping density and consequently low volumetric energy density, which is a big obstacle for practical utilization. In addition, more binders and solvents are needed in the electrode fabrication process because of the highly porous nanostructured carbon materials, increasing the manufacturing cost and decreasing the energy density. Thus, the porosity and taping density should be well balanced. To achieve high volumetric energy density at a low cost, future research can focus on forming graphene-based 3D composites by using capillary drying or rapid drying processes [131,132].
- (5)
- Advanced electrolytes with a wide working voltage and high safety are also needed. Currently, typical LICs commonly adopt organic electrolytes to achieve a high operation voltage. However, they suffer from safety issues associated with volatility, flammability and toxicity. Hence, other novel electrolytes have been explored. For example, ionic liquids are regarded as a promising alternative to the organic electrolyte owing to their large working voltage, high conductivity and excellent thermal stability without risk of catching fire [134,138]. Recently, “Water-in-Salt” electrolytes have drawn tremendous interest as they inherit the safety advantage of aqueous electrolytes while keeping the high working voltage of organic electrolytes [139,140,141,142].
- (6)
- The decomposition of electrolytes should not be ignored. Generally, electrolyte decomposition on the cathode and anode takes place during the charge/discharge process, especially at a high working voltage, resulting in gas emission, impedance increase and low energy conversion efficiency. At the anode side, this phenomenon can be largely restrained by forming a stable solid–electrolyte interface (SEI) during the first several cycles. However, an effective strategy to suppress the electrolyte decomposition at the cathode side is still absent [143]. Fortunately, Li et al. proposed a preliminary electrochemical coating process to form a well-formed protective layer on a graphene-based cathode [144]. The protective layer could block the electron flow from the cathode to the electrolyte and thus terminate the decomposition. This may be a possible and promising solution to obtain high-voltage and long-durability LICs. Furthermore, some in situ and ex situ characterization technologies could be applied to investigate the decomposition mechanism of electrolytes, the degradation and/or evolution of the electrode surface and electrode/electrolyte interface and the composition of decomposed products [145,146,147]. It is expected that these in situ and ex situ tools will help us to understand the beneath decomposition mechanism and find an effective protection method.
- (7)
- Besides the above discussion, other critical issues should also be solved before industrial-level production and widespread applications. First of all, feasible pre-lithiation technology should be developed because the anode, especially nanostructured materials with a large SSA and rich porosity, would consume a large amount of lithium ions when forming a stable SEI [148,149]. Well-controlled pre-lithiation could largely improve the structural stability of the electrode, enhance the reversible capacity and working voltage of the full cell and reduce the resistance, which thus increases the energy and power densities and cycling life. However, current pre-lithiation technologies such as internal or external short circuit [150,151] or using lithium-containing compounds [152,153,154] are either unsafe, time-consuming or inefficient. Hence, high-efficiency pre-lithiation methods are highly needed. Benefiting from well-developed LIBs and SCs, the design strategies, assembly technology and components (conductive additives, binders and shell) could be easily transferred to LICs [133]. More importantly, special attention should be given to thermal management, which is exceptionally critical for the safe and efficient operation of LICs, especially at high and low temperatures [155].
- (8)
- Additionally, considering the limited and uneven distribution of lithium resources, other metal-ion capacitors are drawing increasing attention and have recently become the research hotspot. In particular, monovalent ion systems (i.e., sodium-ion capacitors and potassium-ion capacitors) are the most promising alternatives to LICs because they have a similar cell configuration and energy storage mechanism as well as abundant resources [156,157]. However, like LICs, Na/K-ion capacitors also suffer from safety problems derived from the use of organic electrolytes and the formation of highly reactive metal dendrites. Recently, multivalent ion systems are drawing more interest due to the merits of providing twice or triple the amount of electrons per unit of active materials as well as being less sensitive to air and water, rendering them low-cost, high-energy and safe devices [158,159]. These novel systems are still in the early stage and more efforts should be devoted to preparing advanced electrode materials, developing suitable electrolytes and investigating the energy storage mechanism.
Author Contributions
Funding
Conflicts of Interest
References
- Mahmoudzadeh Andwari, A.; Pesiridis, A.; Rajoo, S.; Martinez-Botas, R.; Esfahanian, V. A review of Battery Electric Vehicle technology and readiness levels. Renew. Sustain. Energy Rev. 2017, 78, 414–430. [Google Scholar] [CrossRef]
- Sui, D.; Chang, M.; Wang, H.; Qian, H.; Yang, Y.; Li, S.; Zhang, Y.; Song, Y. A Brief Review of Catalytic Cathode Materials for Na-CO2 Batteries. Catalysts 2021, 11, 603. [Google Scholar] [CrossRef]
- Chen, H.; Liu, P.; Liu, J.; Feng, X.; Zhou, S. Mechanochemical in-situ incorporation of Ni on MgO/MgH2 surface for the selective O-/C-terminal catalytic hydrogenation of CO2 to CH4. J. Catal. 2021, 394, 397–405. [Google Scholar] [CrossRef]
- Poizot, P.; Dolhem, F. Clean energy new deal for a sustainable world: From non-CO2 generating energy sources to greener electrochemical storage devices. Energy Environ. Sci. 2011, 4, 2003–2019. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 2019, 4, 540–550. [Google Scholar] [CrossRef]
- Chang, M.; Zhang, Y.; Lu, B.-S.; Sui, D.; Wang, F.; Wang, J.; Yang, Y.; Kan, B. The design of quinoxaline based unfused non-fullerene acceptors for high performance and stable organic solar cells. Chem. Eng. J. 2022, 427, 131473. [Google Scholar] [CrossRef]
- Hadjipaschalis, I.; Poullikkas, A.; Efthimiou, V. Overview of current and future energy storage technologies for electric power applications. Renew. Sustain. Energy Rev. 2009, 13, 1513–1522. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Xu, Z.; Zhou, Z.; Xi, S.; Xia, Y.; Zhang, Q.; Huang, L.; Mei, L.; Jiang, Y.; Gao, J.; et al. A Safe Flexible Self-Powered Wristband System by Integrating Defective MnO2–x Nanosheet-Based Zinc-Ion Batteries with Perovskite Solar Cells. ACS Nano 2021, 15, 10597–10608. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Wei, H.-J.; Li, T.-F.; Xiong, X.-H.; Wei, S.-Z.; Ren, F.-Z.; Volinsky, A.A. Recent advances and perspective in metal coordination materials-based electrode materials for potassium-ion batteries. Rare Met. 2021, 40, 448–470. [Google Scholar] [CrossRef]
- Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest advances in supercapacitors: From new electrode materials to novel device designs. Chem. Soc. Rev. 2017, 46, 6816–6854. [Google Scholar] [CrossRef] [PubMed]
- Tie, D.; Huang, S.; Wang, J.; Ma, J.; Zhang, J.; Zhao, Y. Hybrid energy storage devices: Advanced electrode materials and matching principles. Energy Storage Mater. 2019, 21, 22–40. [Google Scholar] [CrossRef]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Berckmans, G.; Messagie, M.; Smekens, J.; Omar, N.; Vanhaverbeke, L.; Van Mierlo, J. Cost Projection of State of the Art Lithium-Ion Batteries for Electric Vehicles Up to 2030. Energies 2017, 10, 1314. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.; Wu, H.; Kim, J.-K.; Liu, X.; Zhang, Y. Restoration of Degraded Nickel-Rich Cathode Materials for Long-Life Lithium-Ion Batteries. ChemElectroChem 2018, 5, 78–83. [Google Scholar] [CrossRef] [Green Version]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief History of Early Lithium-Battery Development. Materials 2020, 13, 1884. [Google Scholar] [CrossRef] [Green Version]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Ying, D.; Ding, R.; Huang, Y.; Shi, W.; Xu, Q.; Tan, C.; Sun, X.; Gao, P.; Liu, E. Conversion pseudocapacitance-contributing and robust hetero-nanostructural perovskite KCo0.54Mn0.46F3 nanocrystals anchored on graphene nanosheet anodes for advanced lithium-ion capacitors, batteries and their hybrids. J. Mater. Chem. A 2019, 7, 18257–18266. [Google Scholar] [CrossRef]
- Liu, G.; Li, M.; Wu, N.; Cui, L.; Huang, X.; Liu, X.; Zhao, Y.; Chen, H.; Yuan, W.; Bai, Y. Single-Crystalline Particles: An Effective Way to Ameliorate the Intragranular Cracking, Thermal Stability, and Capacity Fading of the LiNi0.6Co0.2Mn0.2O2 Electrodes. J. Electrochem. Soc. 2018, 165, A3040–A3047. [Google Scholar] [CrossRef]
- Kim, H.; Park, K.-Y.; Cho, M.-Y.; Kim, M.-H.; Hong, J.; Jung, S.-K.; Roh, K.C.; Kang, K. High-Performance Hybrid Supercapacitor Based on Graphene-Wrapped Li4Ti5O12 and Activated Carbon. ChemElectroChem 2014, 1, 125–130. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Yang, X.; Long, G.; Wu, Y.; Zhang, T.; Leng, K.; Huang, Y.; Ma, Y.; Yu, A.; et al. Porous 3D graphene-based bulk materials with exceptional high surface area and excellent conductivity for supercapacitors. Sci. Rep. 2013, 3, 1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable batteries. J. Power Sources 2011, 196, 6688–6694. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Brousse, T.; Bélanger, D.; Long, J.W. To Be or Not to Be Pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185–A5189. [Google Scholar] [CrossRef] [Green Version]
- Zuo, W.; Li, R.; Zhou, C.; Li, Y.; Xia, J.; Liu, J. Battery-Supercapacitor Hybrid Devices: Recent Progress and Future Prospects. Adv. Sci. 2017, 4, 1600539. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, H.; Xing, C.; Wang, Y.; Zhang, J.; Zhang, X.; Zhang, S. Intensified Energy Storage in High-Voltage Nanohybrid Supercapacitors via the Efficient Coupling between TiNb2O7/Holey-rGO Nanoarchitectures and Ionic Liquid-Based Electrolytes. Acs Appl. Mater. Interfaces 2021, 13, 21349–21361. [Google Scholar] [CrossRef]
- Wu, N.; Qiao, X.; Shen, J.; Liu, G.; Sun, T.; Wu, H.; Hou, H.; Liu, X.; Zhang, Y.; Ji, X. Anatase inverse opal TiO2-x@N-doped C induced the dominant pseudocapacitive effect for durable and fast lithium/sodium storage. Electrochim. Acta 2019, 299, 540–548. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Kong, S.; Zhu, K.; Yan, J.; Ye, K.; Wang, G.; Cheng, K.; Zhou, L.; Cao, D. A novel calendula-like MnNb2O6 anchored on graphene sheet as high-performance intercalation pseudocapacitive anode for lithium-ion capacitors. J. Mater. Chem. A 2019, 7, 2855–2863. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Yang, X.; Leng, K.; Huang, Y.; Chen, Y. High-Performance Supercapacitor Electrode Materials Prepared from Various Pollens. Small 2013, 9, 1342–1347. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, S.; Zhang, B.; Shao, Y.; Wu, Y.; Zhao, H.; Lei, Y.; Hao, X. High performance lithium-ion capacitors based on LiNbO3-arched 3D graphene aerogel anode and BCNNT cathode with enhanced kinetics match. Chem. Eng. J. 2020, 396, 125207. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Niu, Z. Carbon-based materials for lithium-ion capacitors. Mater. Chem. Front. 2019, 3, 1265–1279. [Google Scholar] [CrossRef]
- Zheng, S.; Wu, Z.-S.; Wang, S.; Xiao, H.; Zhou, F.; Sun, C.; Bao, X.; Cheng, H.-M. Graphene-based materials for high-voltage and high-energy asymmetric supercapacitors. Energy Storage Mater. 2017, 6, 70–97. [Google Scholar] [CrossRef]
- Ma, Y.; Chang, H.; Zhang, M.; Chen, Y. Graphene-Based Materials for Lithium-Ion Hybrid Supercapacitors. Adv. Mater. 2015, 27, 5296–5308. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, Z.; Yin, Z.; Guo, H.; Wang, Z.; Yan, G.; Liu, Y.; Li, L.; Wang, J. Non-aqueous dual-carbon lithium-ion capacitors: A review. J. Mater. Chem. A 2019, 7, 15541–15563. [Google Scholar] [CrossRef]
- Ding, J.; Hu, W.; Paek, E.; Mitlin, D. Review of Hybrid Ion Capacitors: From Aqueous to Lithium to Sodium. Chem. Rev. 2018, 118, 6457–6498. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, J.; Liu, B.; Ding, Y.; Tang, Y.; Yan, X. One dimensional graphene nanoscroll-wrapped MnO nanoparticles for high-performance lithium ion hybrid capacitors. J. Mater. Chem. A 2021, 9, 6352–6360. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, F.; Zhang, L.; Lu, Y.; Zhang, Y.; Yang, X.; Ma, Y.; Huang, Y. High energy density Li-ion capacitor assembled with all graphene-based electrodes. Carbon 2015, 92, 106–118. [Google Scholar] [CrossRef]
- Lang, J.; Zhang, X.; Liu, B.; Wang, R.; Chen, J.; Yan, X. The roles of graphene in advanced Li-ion hybrid supercapacitors. J. Energy Chem. 2018, 27, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Amatucci, G.G.; Badway, F.; Du Pasquier, A.; Zheng, T. An Asymmetric Hybrid Nonaqueous Energy Storage Cell. J. Electrochem. Soc. 2001, 148, A930. [Google Scholar] [CrossRef]
- Li, B.; Zheng, J.; Zhang, H.; Jin, L.; Yang, D.; Lv, H.; Shen, C.; Shellikeri, A.; Zheng, Y.; Gong, R.; et al. Electrode Materials, Electrolytes, and Challenges in Nonaqueous Lithium-Ion Capacitors. Adv. Mater. 2018, 30, 1705670. [Google Scholar] [CrossRef]
- Han, P.; Xu, G.; Han, X.; Zhao, J.; Zhou, X.; Cui, G. Lithium Ion Capacitors in Organic Electrolyte System: Scientific Problems, Material Development, and Key Technologies. Adv. Energy Mater. 2018, 8, 1801243. [Google Scholar] [CrossRef]
- Jagadale, A.; Zhou, X.; Xiong, R.; Dubal, D.P.; Xu, J.; Yang, S. Lithium ion capacitors (LICs): Development of the materials. Energy Storage Mater. 2019, 19, 314–329. [Google Scholar] [CrossRef]
- Soltani, M.; Beheshti, S.H. A comprehensive review of lithium ion capacitor: Development, modelling, thermal management and applications. J. Energy Storage 2021, 34, 102019. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Sudhakaran, M.S.P.; Kim, S.J. Fabrication of High-Performance Aqueous Li-Ion Hybrid Capacitor with LiMn2O4 and Graphene. ChemElectroChem 2017, 4, 396–403. [Google Scholar] [CrossRef]
- Aswathy, R.; Kesavan, T.; Kumaran, K.T.; Ragupathy, P. Octahedral high voltage LiNi0.5Mn1.5O4 spinel cathode: Enhanced capacity retention of hybrid aqueous capacitors with nitrogen doped graphene. J. Mater. Chem. A 2015, 3, 12386–12395. [Google Scholar] [CrossRef]
- Shao, H.; Wu, Y.-C.; Lin, Z.; Taberna, P.-L.; Simon, P. Nanoporous carbon for electrochemical capacitive energy storage. Chem. Soc. Rev. 2020, 49, 3005–3039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Yan, Z.; Yan, G.; Guo, H.; Li, X.; Wang, Z.; Wang, X.; Yang, Z. Spiral Graphene Coupling Hierarchically Porous Carbon Advances Dual-Carbon Lithium Ion Capacitor. Energy Storage Mater. 2021, 38, 528–534. [Google Scholar] [CrossRef]
- Han, C.; Li, H.; Shi, R.; Xu, L.; Li, J.; Kang, F.; Li, B. Nanostructured Anode Materials for Non-aqueous Lithium Ion Hybrid Capacitors. Energy Environ. Mater. 2018, 1, 75–87. [Google Scholar] [CrossRef]
- Park, G.D.; Park, J.-S.; Kim, J.K.; Kang, Y.C. Recent Advances in Heterostructured Anode Materials with Multiple Anions for Advanced Alkali-Ion Batteries. Adv. Energy Mater. 2021, 11, 2003058. [Google Scholar] [CrossRef]
- Du, H.; Yang, H.; Huang, C.; He, J.; Liu, H.; Li, Y. Graphdiyne applied for lithium-ion capacitors displaying high power and energy densities. Nano Energy 2016, 22, 615–622. [Google Scholar] [CrossRef]
- Li, Z.; Cao, L.; Chen, W.; Huang, Z.; Liu, H. Mesh-Like Carbon Nanosheets with High-Level Nitrogen Doping for High-Energy Dual-Carbon Lithium-Ion Capacitors. Small 2019, 15, 1805173. [Google Scholar] [CrossRef] [PubMed]
- Arnaiz, M.; Nair, V.; Mitra, S.; Ajuria, J. Furfuryl alcohol derived high-end carbons for ultrafast dual carbon lithium ion capacitors. Electrochim. Acta 2019, 304, 437–446. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Huang, Y.; Wei, Z.; Li, G.; Ng, D.H.L.; Lian, J.; Qiu, J.; Zhao, Y.; Zhang, X.; et al. Porous Nb4N5/rGO Nanocomposite for Ultrahigh-Energy-Density Lithium-Ion Hybrid Capacitor. ACS Appl. Mater. Interfaces 2019, 11, 24114–24121. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Hao, Q.; Xia, X.; Yao, D.; Ouyang, Y.; Lei, W. Boosting long-cycle-life energy storage with holey graphene supported TiNb2O7 network nanostructure for lithium ion hybrid supercapacitors. J. Power Sources 2018, 403, 66–75. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Li, H.; Gao, Y.; Yan, J.; Zhu, K.; Ye, K.; Cheng, K.; Wang, G.; Cao, D. Copper niobate nanowires immobilized on reduced graphene oxide nanosheets as rate capability anode for lithium ion capacitor. J. Colloid Interface Sci. 2021, 583, 652–660. [Google Scholar] [CrossRef]
- Yang, C.; Sun, M.; Zhang, L.; Liu, P.; Wang, P.; Lu, H. ZnFe2O4@Carbon Core–Shell Nanoparticles Encapsulated in Reduced Graphene Oxide for High-Performance Li-Ion Hybrid Supercapacitors. ACS Appl. Mater. Interfaces 2019, 11, 14713–14721. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, S.; Shao, Y.; Wu, Y.; Miao, S. High-Energy Density Li-Ion Capacitor with Layered SnS2/Reduced Graphene Oxide Anode and BCN Nanosheet Cathode. Adv. Energy Mater. 2020, 10, 1902836. [Google Scholar] [CrossRef]
- Li, F.-F.; Gao, J.-F.; He, Z.-H.; Kong, L.-B. Design and Synthesis of CoP/r-GO Hierarchical Architecture: Dominated Pseudocapacitance, Fasted Kinetics Features, and Li-Ion Capacitor Applications. ACS Appl. Energy Mater. 2020, 3, 5448–5461. [Google Scholar] [CrossRef]
- Ulaganathan, M.; Aravindan, V.; Ling, W.C.; Yan, Q.; Madhavi, S. High energy Li-ion capacitors with conversion type Mn3O4 particulates anchored to few layer graphene as the negative electrode. J. Mater. Chem. A 2016, 4, 15134–15139. [Google Scholar] [CrossRef]
- Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V.R. Metal Oxides and Oxysalts as Anode Materials for Li Ion Batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.G.; Arulraj, A.; Khalid, M.; Reddy, M.V.; Jose, R. Energy storage in metal cobaltite electrodes: Opportunities & challenges in magnesium cobalt oxide. Renew. Sustain. Energy Rev. 2021, 141, 110798. [Google Scholar]
- Kulkarni, P.; Nataraj, S.K.; Balakrishna, R.G.; Nagaraju, D.H.; Reddy, M.V. Nanostructured binary and ternary metal sulfides: Synthesis methods and their application in energy conversion and storage devices. J. Mater. Chem. A 2017, 5, 22040–22094. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, S.; Shi, D.; Chen, F.; Shao, Y.; Wu, Y.; Hao, X. Lithium-ion capacitor with improved energy density via perfect matching silicon@3D graphene aerogel anode and BCNNTs cathode. J. Mater. Chem. A 2021, 9, 1134–1142. [Google Scholar] [CrossRef]
- Ahn, S.; Haniu, Y.; Nara, H.; Momma, T.; Sugimoto, W.; Osaka, T. Synthesis of Stacked Graphene-Sn Composite as a High-Performance Anode for Lithium-Ion Capacitors. J. Electrochem. Soc. 2020, 167, 040519. [Google Scholar] [CrossRef]
- Sun, X.; Geng, L.; Yi, S.; Li, C.; An, Y.; Zhang, X.; Zhang, X.; Wang, K.; Ma, Y. Effects of carbon black on the electrochemical performances of SiOx anode for lithium-ion capacitors. J. Power Sources 2021, 499, 229936. [Google Scholar] [CrossRef]
- Sun, F.; Gao, J.; Zhu, Y.; Pi, X.; Wang, L.; Liu, X.; Qin, Y. A high performance lithium ion capacitor achieved by the integration of a Sn-C anode and a biomass-derived microporous activated carbon cathode. Sci. Rep. 2017, 7, 40990. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Yu, Z.; Ayub, M.; Li, S.; Ma, X.; Xu, C. High energy and power lithium-ion capacitor based on MnO-encased graphene spheres anode and hollow carbon nano-rods cathode. Chem. Eng. Sci. 2021, 245, 116968. [Google Scholar] [CrossRef]
- Petnikota, S.; Rotte, N.K.; Reddy, M.V.; Srikanth, V.V.S.S.; Chowdari, B.V.R. MgO-Decorated Few-Layered Graphene as an Anode for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Dubey, P.; Shrivastav, V.; Maheshwari, P.H.; Sundriyal, S. Recent advances in biomass derived activated carbon electrodes for hybrid electrochemical capacitor applications: Challenges and opportunities. Carbon 2020, 170, 1–29. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, T.; Yang, X.; Zhang, L.; Leng, K.; Huang, Y.; Chen, Y. A high-performance supercapacitor-battery hybrid energy storage device based on graphene-enhanced electrode materials with ultrahigh energy density. Energy Environ. Sci. 2013, 6, 1623–1632. [Google Scholar] [CrossRef]
- Mahmood, N.; Zhang, C.; Yin, H.; Hou, Y. Graphene-based nanocomposites for energy storage and conversion in lithium batteries, supercapacitors and fuel cells. J. Mater. Chem. A 2014, 2, 15–32. [Google Scholar] [CrossRef] [Green Version]
- Petnikota, S.; Rotte, N.K.; Srikanth, V.V.S.S.; Kota, B.S.R.; Reddy, M.V.; Loh, K.P.; Chowdari, B.V.R. Electrochemical studies of few-layered graphene as an anode material for Li ion batteries. J. Solid State Electrochem. 2014, 18, 941–949. [Google Scholar] [CrossRef]
- Goh, B.-M.; Wang, Y.; Reddy, M.V.; Ding, Y.L.; Lu, L.; Bunker, C.; Loh, K.P. Filling the Voids of Graphene Foam with Graphene “Eggshell” for Improved Lithium-Ion Storage. ACS Appl. Mater. Interfaces 2014, 6, 9835–9841. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, Z.; Zhang, T.; Qin, B.; Sui, D.; Xie, Y.; Ma, Y.; Chen, Y. Porous asphalt/graphene composite for supercapacitors with high energy density at superior power density without added conducting materials. J. Mater. Chem. A 2017, 5, 21757–21764. [Google Scholar] [CrossRef]
- Su, F.; Hou, X.; Qin, J.; Wu, Z.-S. Recent Advances and Challenges of Two-Dimensional Materials for High-Energy and High-Power Lithium-Ion Capacitors. Batter. Supercaps 2020, 3, 10–29. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wu, Y.; Huang, Y.; Zhang, F.; Yang, X.; Ma, Y.; Chen, Y. Preventing Graphene Sheets from Restacking for High-Capacitance Performance. J. Phys. Chem. C 2011, 115, 23192–23197. [Google Scholar] [CrossRef]
- Han, D.; Zhang, J.; Weng, Z.; Kong, D.; Tao, Y.; Ding, F.; Ruan, D.; Yang, Q.-H. Two-dimensional materials for lithium/sodium-ion capacitors. Mater. Today Energy 2019, 11, 30–45. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Sun, C.; Wang, K.; Sun, X.; Ma, Y. Recent progress of graphene-based materials in lithium-ion capacitors. J. Phys. D Appl. Phys. 2019, 52, 143001. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, J.; Huang, Y.; Ma, Y.; Wang, Y.; Chen, Y. Size-controlled synthesis of graphene oxide sheets on a large scale using chemical exfoliation. Carbon 2009, 47, 3365–3368. [Google Scholar] [CrossRef]
- Tu, F.; Liu, S.; Wu, T.; Jin, G.; Pan, C. Porous graphene as cathode material for lithium ion capacitor with high electrochemical performance. Powder Technol. 2014, 253, 580–583. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, W.H.; Ryou, M.-H.; Jin, J.K.; Kim, J.; Choi, J.W. Functionalized Graphene for High Performance Lithium Ion Capacitors. ChemSusChem 2012, 5, 2328–2333. [Google Scholar] [CrossRef]
- Sui, D.; Xu, L.; Zhang, H.; Sun, Z.; Kan, B.; Ma, Y.; Chen, Y. A 3D cross-linked graphene-based honeycomb carbon composite with excellent confinement effect of organic cathode material for lithium-ion batteries. Carbon 2020, 157, 656–662. [Google Scholar] [CrossRef]
- Wu, M.; Zhao, Y.; Sun, B.; Sun, Z.; Li, C.; Han, Y.; Xu, L.; Ge, Z.; Ren, Y.; Zhang, M.; et al. A 2D covalent organic framework as a high-performance cathode material for lithium-ion batteries. Nano Energy 2020, 70, 104498. [Google Scholar] [CrossRef]
- Aravindan, V.; Mhamane, D.; Ling, W.C.; Ogale, S.; Madhavi, S. Nonaqueous Lithium-Ion Capacitors with High Energy Densities using Trigol-Reduced Graphene Oxide Nanosheets as Cathode-Active Material. ChemSusChem 2013, 6, 2240–2244. [Google Scholar] [CrossRef]
- Dubal, D.P.; Gomez-Romero, P. All nanocarbon Li-Ion capacitor with high energy and high power density. Mater. Today Energy 2018, 8, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Guan, C.; Wang, X.; Fan, H.J. A High Energy and Power Li-Ion Capacitor Based on a TiO2 Nanobelt Array Anode and a Graphene Hydrogel Cathode. Small 2015, 11, 1470–1477. [Google Scholar] [CrossRef]
- Ye, L.; Liang, Q.; Lei, Y.; Yu, X.; Han, C.; Shen, W.; Huang, Z.-H.; Kang, F.; Yang, Q.-H. A high performance Li-ion capacitor constructed with Li4Ti5O12/C hybrid and porous graphene macroform. J. Power Sources 2015, 282, 174–178. [Google Scholar] [CrossRef]
- Li, H.; Shen, L.; Wang, J.; Fang, S.; Zhang, Y.; Dou, H.; Zhang, X. Three-dimensionally ordered porous TiNb2O7 nanotubes: A superior anode material for next generation hybrid supercapacitors. J. Mater. Chem. A 2015, 3, 16785–16790. [Google Scholar] [CrossRef]
- Wu, Y.; Yi, N.; Huang, L.; Zhang, T.; Fang, S.; Chang, H.; Li, N.; Oh, J.; Lee, J.A.; Kozlov, M.; et al. Three-dimensionally bonded spongy graphene material with super compressive elasticity and near-zero Poisson’s ratio. Nat. Commun. 2015, 6, 6141. [Google Scholar] [CrossRef] [Green Version]
- Sha, J.; Chu, X.; Xu, T.; Li, Y.; Tang, Y.; Ma, L.; Shi, C.; Liu, E.; Zhao, D.; He, C.; et al. Bi-functional modular graphene network with high rate and cycling performance. J. Power Sources 2021, 504, 230075. [Google Scholar] [CrossRef]
- Jin, L.; Guo, X.; Gong, R.; Zheng, J.; Xiang, Z.; Zhang, C.; Zheng, J.P. Target-oriented electrode constructions toward ultra-fast and ultra-stable all-graphene lithium ion capacitors. Energy Storage Mater. 2019, 23, 409–417. [Google Scholar] [CrossRef]
- Ma, X.; Gao, D. High Capacitive Storage Performance of Sulfur and Nitrogen Codoped Mesoporous Graphene. ChemSusChem 2018, 11, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, L.; Song, X.; Yu, Z.; Zhao, L.; Yu, Y.; Xiao, Z.; Ning, G.; Gao, J. Superior capacitive behaviors of the micron-sized porous graphene belts with high ratio of length to diameter. Carbon 2018, 140, 314–323. [Google Scholar] [CrossRef]
- Fan, Q.; Yang, M.; Meng, Q.; Cao, B.; Yu, Y. Activated-Nitrogen-Doped Graphene-Based Aerogel Composites as Cathode Materials for High Energy Density Lithium-Ion Supercapacitor. J. Electrochem. Soc. 2016, 163, A1736–A1742. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [Green Version]
- Stoller, M.D.; Murali, S.; Quarles, N.; Zhu, Y.; Potts, J.R.; Zhu, X.; Ha, H.-W.; Ruoff, R.S. Activated graphene as a cathode material for Li-ion hybrid supercapacitors. Phys. Chem. Chem. Phys. 2012, 14, 3388–3391. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Zheng, W.; Zhao, Q.; Sun, S.; Ji, G.; Li, S.; Fan, X.; Xu, C. Design of Nb2O5/graphene hybrid aerogel as polymer binder-free electrodes for lithium-ion capacitors. Mater. Technol. 2020, 35, 625–634. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Q.; Ding, B.; Wang, J.; Malgras, V.; Jiang, J.; Li, Z.; Chen, S.; Dou, H.; Alshehri, S.M.; et al. Lithium-ion capacitor based on nanoarchitectured polydopamine/graphene composite anode and porous graphene cathode. Carbon 2020, 167, 627–633. [Google Scholar] [CrossRef]
- Jeong, J.H.; Lee, G.-W.; Kim, Y.H.; Choi, Y.J.; Roh, K.C.; Kim, K.-B. A holey graphene-based hybrid supercapacitor. Chem. Eng. J. 2019, 378, 122126. [Google Scholar] [CrossRef]
- Kim, E.; Kim, H.; Park, B.-J.; Han, Y.-H.; Park, J.H.; Cho, J.; Lee, S.-S.; Son, J.G. Etching-Assisted Crumpled Graphene Wrapped Spiky Iron Oxide Particles for High-Performance Li-Ion Hybrid Supercapacitor. Small 2018, 14, 1704209. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Wang, K.; Sun, X.; Xu, Y.; Su, F.; Chen, C.-M.; Liu, F.; Wu, Z.-S.; Ma, Y. Nitrogen-enriched graphene framework from a large-scale magnesiothermic conversion of CO2 with synergistic kinetics for high-power lithium-ion capacitors. NPG Asia Mater. 2021, 13, 59. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Y. Three-dimensional graphene networks: Synthesis, properties and applications. Natl. Sci. Rev. 2014, 2, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Bu, F.; Shakir, I.; Xu, Y. 3D Graphene Composites for Efficient Electrochemical Energy Storage. Adv. Mater. Interfaces 2018, 5, 1800468. [Google Scholar] [CrossRef]
- Mao, S.; Lu, G.; Chen, J. Three-dimensional graphene-based composites for energy applications. Nanoscale 2015, 7, 6924–6943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Fernández, G.; Granados-Moreno, M.; Gómez-Urbano, J.L.; Carriazo, D. Phosphorus-Functionalized Graphene for Lithium-Ion Capacitors with Improved Power and Cyclability. Batter. Supercaps 2021, 4, 469–478. [Google Scholar] [CrossRef]
- Leng, K.; Zhang, F.; Zhang, L.; Zhang, T.; Wu, Y.; Lu, Y.; Huang, Y.; Chen, Y. Graphene-based Li-ion hybrid supercapacitors with ultrahigh performance. Nano Res. 2013, 6, 581–592. [Google Scholar] [CrossRef]
- Sui, D.; Wu, M.; Liu, Y.; Yang, Y.; Zhang, H.; Ma, Y.; Zhang, L.; Chen, Y. High performance Li-ion capacitor fabricated with dual graphene-based materials. Nanotechnology 2020, 32, 015403. [Google Scholar] [CrossRef]
- Dai, X.; Lei, S.; Liu, J.; Shang, Z.; Zhong, S.; Li, X. Promoting the energy density of lithium-ion capacitor by coupling the pore-size and nitrogen content in capacitive carbon cathode. J. Power Sources 2021, 498, 229912. [Google Scholar] [CrossRef]
- Yang, J.; Xu, D.; Hou, R.; Lang, J.; Wang, Z.; Dong, Z.; Ma, J. Nitrogen-doped carbon nanotubes by multistep pyrolysis process as a promising anode material for lithium ion hybrid capacitors. Chin. Chem. Lett. 2020, 31, 2239–2244. [Google Scholar] [CrossRef]
- Salvatierra, R.V.; Zakhidov, D.; Sha, J.; Kim, N.D.; Lee, S.-K.; Raji, A.-R.O.; Zhao, N.; Tour, J.M. Graphene Carbon Nanotube Carpets Grown Using Binary Catalysts for High-Performance Lithium-Ion Capacitors. ACS Nano 2017, 11, 2724–2733. [Google Scholar] [CrossRef] [PubMed]
- Li, N.-W.; Du, X.; Shi, J.-L.; Zhang, X.; Fan, W.; Wang, J.; Zhao, S.; Liu, Y.; Xu, W.; Li, M.; et al. Graphene@hierarchical meso-/microporous carbon for ultrahigh energy density lithium-ion capacitors. Electrochim. Acta 2018, 281, 459–465. [Google Scholar] [CrossRef]
- Yan, D.; Li, S.-H.; Guo, L.-P.; Dong, X.-L.; Chen, Z.-Y.; Li, W.-C. Hard@Soft Integrated Morning Glory Like Porous Carbon as a Cathode for a High-Energy Lithium Ion Capacitor. ACS Appl. Mater. Interfaces 2018, 10, 43946–43952. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, F.; Zhang, T.; Leng, K.; Zhang, L.; Yang, X.; Ma, Y.; Huang, Y.; Zhang, M.; Chen, Y. Synthesis and supercapacitor performance studies of N-doped graphene materials using o-phenylenediamine as the double-N precursor. Carbon 2013, 63, 508–516. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Feng, K.; Sy, A.; Liu, Y.; Lim, L.; Lui, G.; Tjandra, R.; Rasenthiram, L.; Chiu, G.; et al. Advanced Li-Ion Hybrid Supercapacitors Based on 3D Graphene–Foam Composites. ACS Appl. Mater. Interfaces 2016, 8, 25941–25953. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, Q.; Zheng, W.; Ren, Z.; Hu, X.; Li, J.; Lu, L.; Hu, N.; Molenda, J.; Liu, X.; et al. Achieving high energy density in a 4.5 V all nitrogen-doped graphene based lithium-ion capacitor. J. Mater. Chem. A 2019, 7, 19909–19921. [Google Scholar] [CrossRef]
- Li, P.; Li, H.; Han, D.; Shang, T.; Deng, Y.; Tao, Y.; Lv, W.; Yang, Q.-H. Packing Activated Carbons into Dense Graphene Network by Capillarity for High Volumetric Performance Supercapacitors. Adv. Sci. 2019, 6, 1802355. [Google Scholar] [CrossRef]
- Wang, J.-A.; Li, S.-M.; Wang, Y.-S.; Lan, P.-Y.; Liao, W.-H.; Hsiao, S.-T.; Lin, S.-C.; Lin, C.-W.; Ma, C.-C.M.; Hu, C.-C. Preparation and Properties of NrGO-CNT Composite for Lithium-Ion Capacitors. J. Electrochem. Soc. 2017, 164, A3657–A3665. [Google Scholar] [CrossRef]
- Adelowo, E.; Baboukani, A.R.; Chen, C.; Wang, C. Electrostatically Sprayed Reduced Graphene Oxide-Carbon Nanotubes Electrodes for Lithium-Ion Capacitors. C 2018, 4, 31. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Zhang, H.; Zhang, C. Agricultural waste-derived activated carbon/graphene composites for high performance lithium-ion capacitors. RSC Adv. 2019, 9, 29190–29194. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, Z.; Zhang, X.; Peng, H.; Xin, G.; Lu, C.; Zhong, Y.; Wang, G.; Zhang, Y. Nitrogen-Doped Defective Graphene Aerogel as Anode for all Graphene-Based Lithium Ion Capacitor. ChemistrySelect 2017, 2, 8436–8445. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Chen, J.; Li, X.; Cheng, Q.; Wang, G. Fabrication of porous lithium titanate self-supporting anode for high performance lithium-ion capacitor. J. Energy Chem. 2020, 50, 344–350. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, J.; Qin, F.; Yuan, J.; Zhang, K.; Li, J.; Zhu, D.-M.; Qin, L.-C. Hybrid lithium-ion capacitors with asymmetric graphene electrodes. J. Mater. Chem. A 2017, 5, 13601–13609. [Google Scholar] [CrossRef]
- Ock, I.W.; Lee, J.; Kang, J.K. Metal–Organic Framework-Derived Anode and Polyaniline Chain Networked Cathode with Mesoporous and Conductive Pathways for High Energy Density, Ultrafast Rechargeable, and Long-Life Hybrid Capacitors. Adv. Energy Mater. 2020, 10, 2001851. [Google Scholar] [CrossRef]
- Yang, H.; Kannappan, S.; Pandian, A.S.; Jang, J.-H.; Lee, Y.S.; Lu, W. Graphene supercapacitor with both high power and energy density. Nanotechnology 2017, 28, 445401. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhan, C.; Lv, R.; Bai, Y.; Lin, Y.; Huang, Z.-H.; Shen, W.; Qiu, X.; Kang, F. Ultrahigh-rate and high-density lithium-ion capacitors through hybriding nitrogen-enriched hierarchical porous carbon cathode with prelithiated microcrystalline graphite anode. Nano Energy 2015, 15, 43–53. [Google Scholar] [CrossRef]
- Han, D.; Weng, Z.; Li, P.; Tao, Y.; Cui, C.; Zhang, L.; Lin, W.; Gao, Y.; Kong, D.; Yang, Q.-H. Electrode thickness matching for achieving high-volumetric-performance lithium-ion capacitors. Energy Storage Mater. 2019, 18, 133–138. [Google Scholar] [CrossRef]
- Zong, J.; Ni, W.; Xu, H.; Ding, F.; Wang, T.; Feng, W.; Liu, X. High tap-density graphene cathode material for lithium-ion capacitors via a mass-scalable synthesis method. Chem. Eng. J. 2019, 360, 1233–1240. [Google Scholar] [CrossRef]
- An, Y.; Liu, T.; Li, C.; Zhang, X.; Hu, T.; Sun, X.; Wang, K.; Wang, C.; Ma, Y. A general route for the mass production of graphene-enhanced carbon composites toward practical pouch lithium-ion capacitors. J. Mater. Chem. A 2021, 9, 15654–15664. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Zhang, F.; Long, G.; Zhang, T.; Leng, K.; Zhang, Y.; Huang, Y.; Ma, Y.; Zhang, M.; et al. Controlling the Effective Surface Area and Pore Size Distribution of sp2 Carbon Materials and Their Impact on the Capacitance Performance of These Materials. J. Am. Chem. Soc. 2013, 135, 5921–5929. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, J.; Yang, B.; Liu, L.; Sun, Y.; Hou, R.; Lin, Z.; Yan, X. Boosting the performance of lithium metal capacitors with a Li composite anode. J. Mater. Chem. A 2021, 9, 10722–10730. [Google Scholar] [CrossRef]
- Guo, F.; Wu, C.; Chen, H.; Zhong, F.; Ai, X.; Yang, H.; Qian, J. Dendrite-free lithium deposition by coating a lithiophilic heterogeneous metal layer on lithium metal anode. Energy Storage Mater. 2020, 24, 635–643. [Google Scholar] [CrossRef]
- Luo, D.; Li, M.; Zheng, Y.; Ma, Q.; Gao, R.; Zhang, Z.; Dou, H.; Wen, G.; Shui, L.; Yu, A.; et al. Electrolyte Design for Lithium Metal Anode-Based Batteries Toward Extreme Temperature Application. Adv. Sci. 2021, 8, 2101051. [Google Scholar] [CrossRef] [PubMed]
- Hirota, N.; Okuno, K.; Majima, M.; Hosoe, A.; Uchida, S.; Ishikawa, M. High-performance lithium-ion capacitor composed of electrodes with porous three-dimensional current collector and bis(fluorosulfonyl)imide-based ionic liquid electrolyte. Electrochim. Acta 2018, 276, 125–133. [Google Scholar] [CrossRef]
- Dou, Q.; Wang, Y.; Wang, A.; Ye, M.; Hou, R.; Lu, Y.; Su, L.; Shi, S.; Zhang, H.; Yan, X. “Water in salt/ionic liquid” electrolyte for 2.8 V aqueous lithium-ion capacitor. Sci. Bull. 2020, 65, 1812–1822. [Google Scholar] [CrossRef]
- Tian, X.; Zhu, Q.; Xu, B. “Water-in-Salt” Electrolytes for Supercapacitors: A Review. ChemSusChem 2021, 14, 2501–2515. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, B.; Liu, X.; Liu, J.; Ding, J.; Zhong, C.; Hu, W. Water-in-salt electrolyte for safe and high-energy aqueous battery. Energy Storage Mater. 2021, 34, 461–474. [Google Scholar] [CrossRef]
- Dong, S.; Wang, Y.; Chen, C.; Shen, L.; Zhang, X. Niobium Tungsten Oxide in a Green Water-in-Salt Electrolyte Enables Ultra-Stable Aqueous Lithium-Ion Capacitors. Nano-Micro Lett. 2020, 12, 168. [Google Scholar] [CrossRef]
- Qin, H.; Chao, H.; Zhang, M.; Huang, Y.; Liu, H.; Cheng, J.; Cao, L.; Xu, Q.; Guan, L.; Teng, X.; et al. Precious potential regulation of carbon cathode enabling high-performance lithium-ion capacitors. Carbon 2021, 180, 110–117. [Google Scholar] [CrossRef]
- Shan, X.-Y.; Wang, Y.; Wang, D.-W.; Li, F.; Cheng, H.-M. Armoring Graphene Cathodes for High-Rate and Long-Life Lithium Ion Supercapacitors. Adv. Energy Mater. 2016, 6, 1502064. [Google Scholar] [CrossRef]
- Yousaf, M.; Naseer, U.; Li, Y.; Ali, Z.; Mahmood, N.; Wang, L.; Gao, P.; Guo, S. A mechanistic study of electrode materials for rechargeable batteries beyond lithium ions by in situ transmission electron microscopy. Energy Environ. Sci. 2021, 14, 2670–2707. [Google Scholar] [CrossRef]
- Gbadamasi, S.; Mohiuddin, M.; Krishnamurthi, V.; Verma, R.; Khan, M.W.; Pathak, S.; Kalantar-Zadeh, K.; Mahmood, N. Interface chemistry of two-dimensional heterostructures – fundamentals to applications. Chem. Soc. Rev. 2021, 50, 4684–4729. [Google Scholar] [CrossRef] [PubMed]
- Jian, X.; Wang, H.; Rao, G.; Jiang, L.; Wang, H.; Subramaniyam, C.M.; Mahmood, A.; Zhang, W.; Xiang, Y.; Dou, S.X.; et al. Self-tunable ultrathin carbon nanocups as the electrode material of sodium-ion batteries with unprecedented capacity and stability. Chem. Eng. J. 2019, 364, 578–588. [Google Scholar] [CrossRef]
- Jin, L.; Shen, C.; Shellikeri, A.; Wu, Q.; Zheng, J.; Andrei, P.; Zhang, J.-G.; Zheng, J.P. Progress and perspectives on pre-lithiation technologies for lithium ion capacitors. Energy Environ. Sci. 2020, 13, 2341–2362. [Google Scholar] [CrossRef]
- Rao, Z.; Wu, J.; He, B.; Chen, W.; Wang, H.; Fu, Q.; Huang, Y. A Prelithiation Separator for Compensating the Initial Capacity Loss of Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 38194–38201. [Google Scholar] [CrossRef]
- Sivakkumar, S.R.; Pandolfo, A.G. Evaluation of lithium-ion capacitors assembled with pre-lithiated graphite anode and activated carbon cathode. Electrochim. Acta 2012, 65, 280–287. [Google Scholar] [CrossRef]
- Kim, M.; Xu, F.; Lee, J.H.; Jung, C.; Hong, S.M.; Zhang, Q.M.; Koo, C.M. A fast and efficient pre-doping approach to high energy density lithium-ion hybrid capacitors. J. Mater. Chem. A 2014, 2, 10029–10033. [Google Scholar] [CrossRef]
- Jeżowski, P.; Crosnier, O.; Deunf, E.; Poizot, P.; Béguin, F.; Brousse, T. Safe and recyclable lithium-ion capacitors using sacrificial organic lithium salt. Nat. Mater. 2018, 17, 167–173. [Google Scholar] [CrossRef]
- Park, M.-S.; Lim, Y.-G.; Hwang, S.M.; Kim, J.H.; Kim, J.-S.; Dou, S.X.; Cho, J.; Kim, Y.-J. Scalable Integration of Li5FeO4 towards Robust, High-Performance Lithium-Ion Hybrid Capacitors. ChemSusChem 2014, 7, 3138–3144. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, T.; Zhang, H.; Song, Z.; Qu, C.; Hou, G.; Zhang, H.; Ni, C.; Li, X. DMF stabilized Li3N slurry for manufacturing self-prelithiatable lithium-ion capacitors. Sci. Bull. 2020, 65, 434–442. [Google Scholar] [CrossRef] [Green Version]
- Karimi, D.; Khaleghi, S.; Behi, H.; Beheshti, H.; Hosen, M.S.; Akbarzadeh, M.; Van Mierlo, J.; Berecibar, M. Lithium-Ion Capacitor Lifetime Extension through an Optimal Thermal Management System for Smart Grid Applications. Energies 2021, 14, 2907. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, J.; An, Y.; Wu, L.; Dou, H.; Zhang, J.; Zhang, Y.; Wu, S.; Dong, M.; Zhang, X.; et al. Sodium-ion capacitors: Materials, Mechanism, and Challenges. ChemSusChem 2020, 13, 2522–2539. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chang, L.; Le, Z.; Jiang, J.; Li, J.; Wang, H.; Zhao, C.; Xu, T.; Nie, P.; Wang, L. Emerging Potassium-ion Hybrid Capacitors. ChemSusChem 2020, 13, 5837–5862. [Google Scholar] [CrossRef]
- Ma, X.; Cheng, J.; Dong, L.; Liu, W.; Mou, J.; Zhao, L.; Wang, J.; Ren, D.; Wu, J.; Xu, C.; et al. Multivalent ion storage towards high-performance aqueous zinc-ion hybrid supercapacitors. Energy Storage Mater. 2019, 20, 335–342. [Google Scholar] [CrossRef]
- Sui, D.; Wu, M.; Shi, K.; Li, C.; Lang, J.; Yang, Y.; Zhang, X.; Yan, X.; Chen, Y. Recent progress of cathode materials for aqueous zinc-ion capacitors: Carbon-based materials and beyond. Carbon 2021, 185, 126–151. [Google Scholar] [CrossRef]




| Energy Storage Systems | Anode//Cathode | Electrolyte | Voltage (V) a | Energy Density (Wh kg−1) b | Power Density (W kg−1) c | Cycling Life |
|---|---|---|---|---|---|---|
| LABs | Pb//PbO2 | H2SO4 aqueous solution | 2 | 30–50 | <1000 | <800 |
| NiMHBs | Metal hydride//Ni(OH)2 | KOH aqueous solution | 1.2 | 40–60 | ~1000 | <1000 |
| LIBs | Graphite//Lithium-based compounds d | LiPF6 in organic solution | 3.6–4.35 | 150–300 | <1000 | <5000 |
| EDLCs | AC//AC | (CH3CH2)4NBF4 in acetonitrile | 2.7–3.0 | 5–10 | >10,000 | >100,000 |
| LICs | Battery-type anode//Capacitor-type cathode | Lithium salts in organic solution | 3.0–4.5 | 20–100 | 1000–10,000 | >10,000 |
| Cathode//Anode | Electrode Preparation | Capacity of Cathode (mAh g−1) | Electrolyte | Cell Voltage (V) | Maximum Energy Density (Wh kg−1) | Maximum Power Density (kW kg−1) | Cycling Stability | Ref. |
|---|---|---|---|---|---|---|---|---|
| URGO//graphite | Reduced by urea | 35 | 1 M LiPF6 in EC/DEC | 2.2–3.8 | 106 | 4.2 | ~100% at 1000 | [85] |
| TRGO//LTO | Reduced by trigol | 58 | 1 M LiPF6 in EC/DEC | 1–3 | 45 | 3.3 | ~100% at 5000 | [88] |
| EG-GO//Li | Hydrothermal reduction | 172 | 1 M LiPF6 in EC/DEC/DMC | 2–4.5 | 240 | 53.5 | ~100% at 3000 | [84] |
| Graphene grass//TNO | Hydrothermal reduction | 63.2 | 1 M LiPF6 in EC/DMC | 0–3 | 74 | 7.5 | 81.2% at 3000 | [92] |
| PRGO//N-CNPipes | Thermal annealing | 171 | 1 M LiPF6 in EC/PC | 0.01–4 | 262 | 9.0 | 91% at 4000 | [89] |
| Graphene hydrogel//TiO2 NBA | Hydrothermal reduction | 52 | 1 M LiPF6 in EC/DMC | 0–3.8 | 82 | 19 | 73% at 600 | [90] |
| PGM//LTO/C | Hydrothermal reduction | 66 | LiPF6 | 1–3 | 72 | 8.3 | 65% at 1000 | [91] |
| Cathode//Anode | Electrode Preparation | Capacity of Cathode (mAh g−1) | Electrolyte | Cell Voltage (V) | Maximum Energy Density (Wh kg−1) | Maximum Power Density (kW kg−1) | Cycling Stability | Ref. |
|---|---|---|---|---|---|---|---|---|
| SNMG//LTO | CVD | 112 | 1 M LiPF6 in EC/DEC | 0–4 | 86.2 | 7.4 | 87% at 2000 | [96] |
| PGBs//LTO | CVD | 92 | 1 M LiPF6 in EC/DEC | 0–4 | 120 | 8.04 | 83.7% at 2000 | [97] |
| CG | Template-guided | 212.3 F g−1 | 1 M LiPF6 in EC/DEC/DMC | 1–4 | 121 | 18 | 87% at 2000 | [105] |
| HG | Catalytic carbon gasification | 97.2 | 1 M LiPF6 in EC/DEC | 1.5–3 | 117.3 | 19.7 | 81.7% at 2000 | [104] |
| a-MEGO//graphite | Chemical activation | 125 | 1 M LiPF6 in EC/DEC | 2–4 | 147.8 | / | / | [101] |
| a-NGA//LTO | Chemical activation | 76 | 1 M LiPF6 in EC/DEC/DMC | 1–3 | 70 | 8.0 | 64% at 10,000 | [98] |
| AGF | Chemical activation | 93 F g−1 | 1 M LiPF6 in EC/DEC | 0–3 | 53 | 2.09 | 89% at 3000 | [102] |
| PG | Chemical activation | 69 | 1 M LiPF6 in EC/DEC/DMC | 0.01–4.2 | 135.6 | 21 | 65% at 3000 | [103] |
| NGF-0//NGF-2 | Magnesiothermic combustion synthesis | 82 | 1 M LiPF6 in EC/DEC/DMC | 1–4 | 151 | 49 | 87% at 10,000 | [106] |
| Cathode//Anode | Electrode Preparation | Capacity of Cathode (mAh g−1) | Electrolyte | Cell Voltage (V) | Maximum Energy Density (Wh kg−1) | Maximum Power Density (kW kg−1) | Cycling Stability | Ref. |
|---|---|---|---|---|---|---|---|---|
| PC-75//MnO@C | Chemical activation | 50 | 1 M LiPF6 in EC/EMC/DMC | 0.1–4 | 117.6 | 10.25 | 76% at 3000 | [117] |
| N-GMCS//graphite | Chemical activation | / | 1 M LiPF6 in EC/DEC | 2.2–4.2 | 66.7 Wh L−1 | 292 kW L−1 | 93.1 at 3000 | [130] |
| 3DGraphene// Fe3O4/G | Chemical activation | / | 1 M LiPF6 in EC/DEC/DMC | 1–4 | 204 | 4.6 | 70% at 1000 | [74] |
| 3D PANI/GNSs//3D MoO3/GNSs | Chemical activation | 67.8 | 1 M LiPF6 in EC/DEC | 0–3.8 | 128.3 | 13.5 | 90% at 3000 | [119] |
| GF//CNT@pLTO | Microwave oven irradiation | 151.9 F g−1 | 1 M LiPF6 in EC/DMC | 0–3.5 | 108.1 | 12.3 | 84.8% at 5000 | [126] |
| rGO-CNT//lithiated rGO-CNT | Electrostaticspray deposition | 72 | 1 M LiPF6 in EC/EMC | 0.01–4.3 | 114.5 | 2.57 | 68.5% at 2000 | [123] |
| SG//Li-SG | Reduced by hydrazine | 137 F g−1 | 1 M LiPF6 in EC/DMC | 0–4 | 222 | / | 58% at 5000 | [127] |
| OAC/rGO//Si/C | Ball milling | 140 | 1 M LiPF6 in DMC/FEC | 2–4.5 | 141 | 10.3 | 78.9% at 1000 | [124] |
| PANI@rGO// MoO2@rGO | In situ polymerization | / | 1 M LiPF6 in EC/DEC | 1.25–4.5 | 241.7 | 28.75 | 96% at 10000 | [128] |
| G@HMMC//graphite | Chemical activation | 112 | 1 M LiPF6 in EC/DEC/DMC | 2–4.5 | 233.3 | 15.6 | 90.6% at 3000 | [116] |
| AC/G//graphite | Hydrothermal Process and thermal treatment | 45 mAh cm−3 | 1 M LiPF6 in EC/EMC/DMC | 2–4.5 | 98 Wh L−1 | 19 kW L−1 | 98.9% at 3000 | [131] |
| G/AC//G/SC | Self-propagating high-temperature synthesis | 113.7F g−1 | 1 M LiPF6 in EC/DEC/DMC | 1–4 | 151 | 18.9 | 93.8% at 10,000 | [133] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, D.; Chang, M.; Peng, Z.; Li, C.; He, X.; Yang, Y.; Liu, Y.; Lu, Y. Graphene-Based Cathode Materials for Lithium-Ion Capacitors: A Review. Nanomaterials 2021, 11, 2771. https://doi.org/10.3390/nano11102771
Sui D, Chang M, Peng Z, Li C, He X, Yang Y, Liu Y, Lu Y. Graphene-Based Cathode Materials for Lithium-Ion Capacitors: A Review. Nanomaterials. 2021; 11(10):2771. https://doi.org/10.3390/nano11102771
Chicago/Turabian StyleSui, Dong, Meijia Chang, Zexin Peng, Changle Li, Xiaotong He, Yanliang Yang, Yong Liu, and Yanhong Lu. 2021. "Graphene-Based Cathode Materials for Lithium-Ion Capacitors: A Review" Nanomaterials 11, no. 10: 2771. https://doi.org/10.3390/nano11102771
APA StyleSui, D., Chang, M., Peng, Z., Li, C., He, X., Yang, Y., Liu, Y., & Lu, Y. (2021). Graphene-Based Cathode Materials for Lithium-Ion Capacitors: A Review. Nanomaterials, 11(10), 2771. https://doi.org/10.3390/nano11102771





