Physical–Chemical Exfoliation of n-Alkylamine Derivatives of Layered Perovskite-like Oxide H2K0.5Bi2.5Ti4O13 into Nanosheets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Characterization
2.1.1. Initial Substances
Preparation of Inorganic Hosts
Preparation of Inorganic-Organic Hybrids
2.1.2. Precursors Characterization
2.1.3. Exfoliation
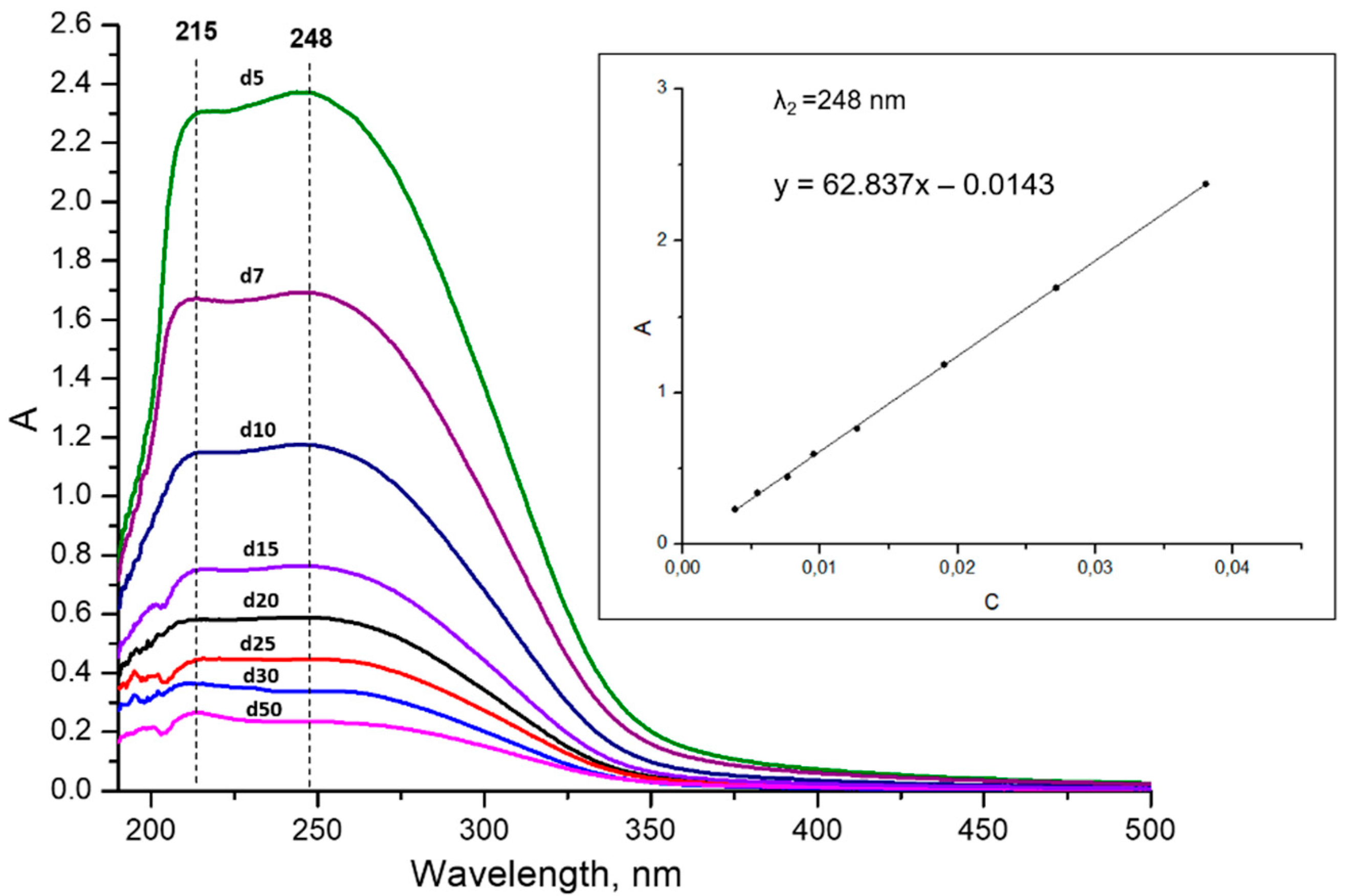
2.1.4. Concentration Determination
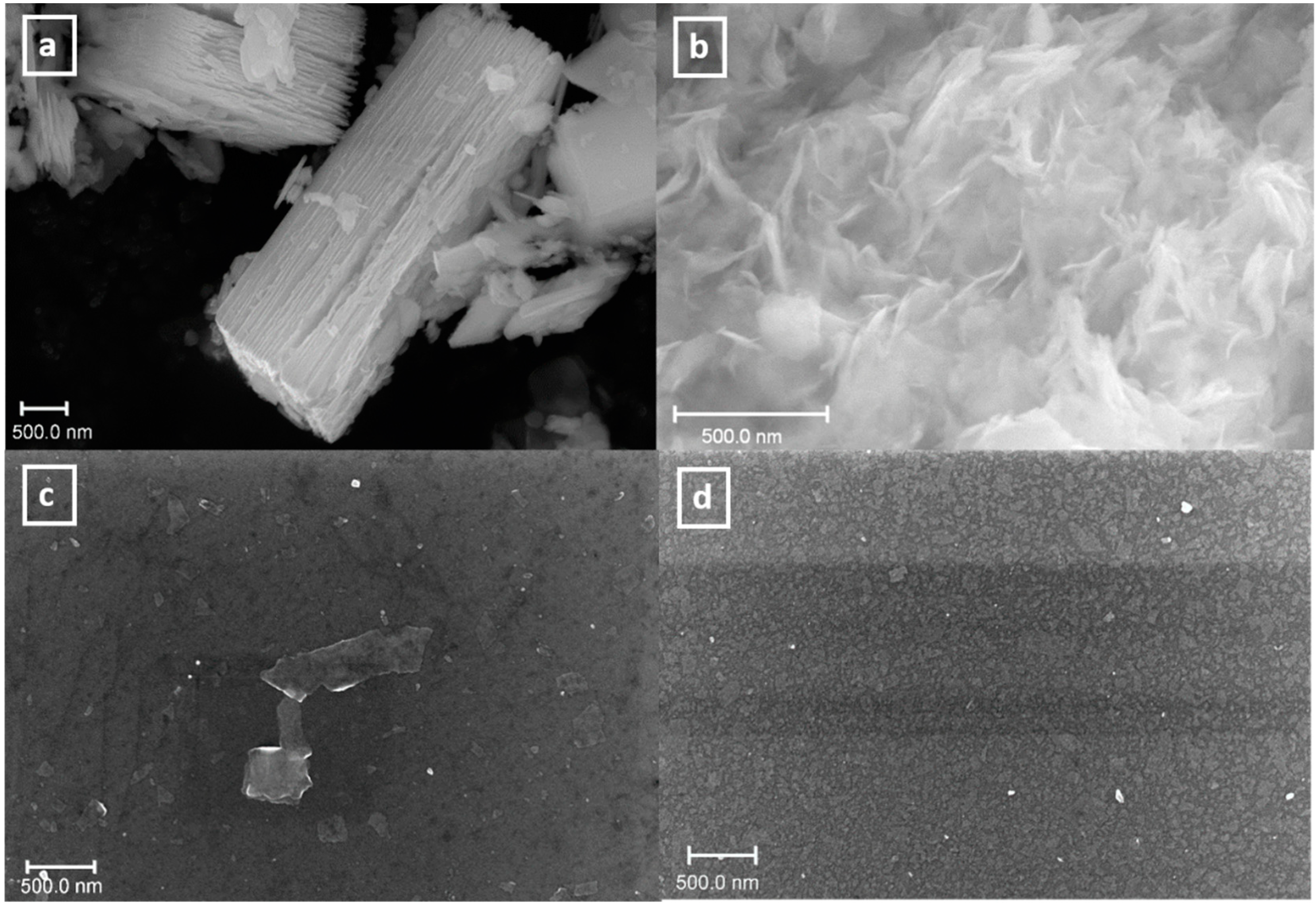
2.1.5. Sample Preparation for SEM and AFM Measurements
2.2. Instrumentation
3. Results and Discussion
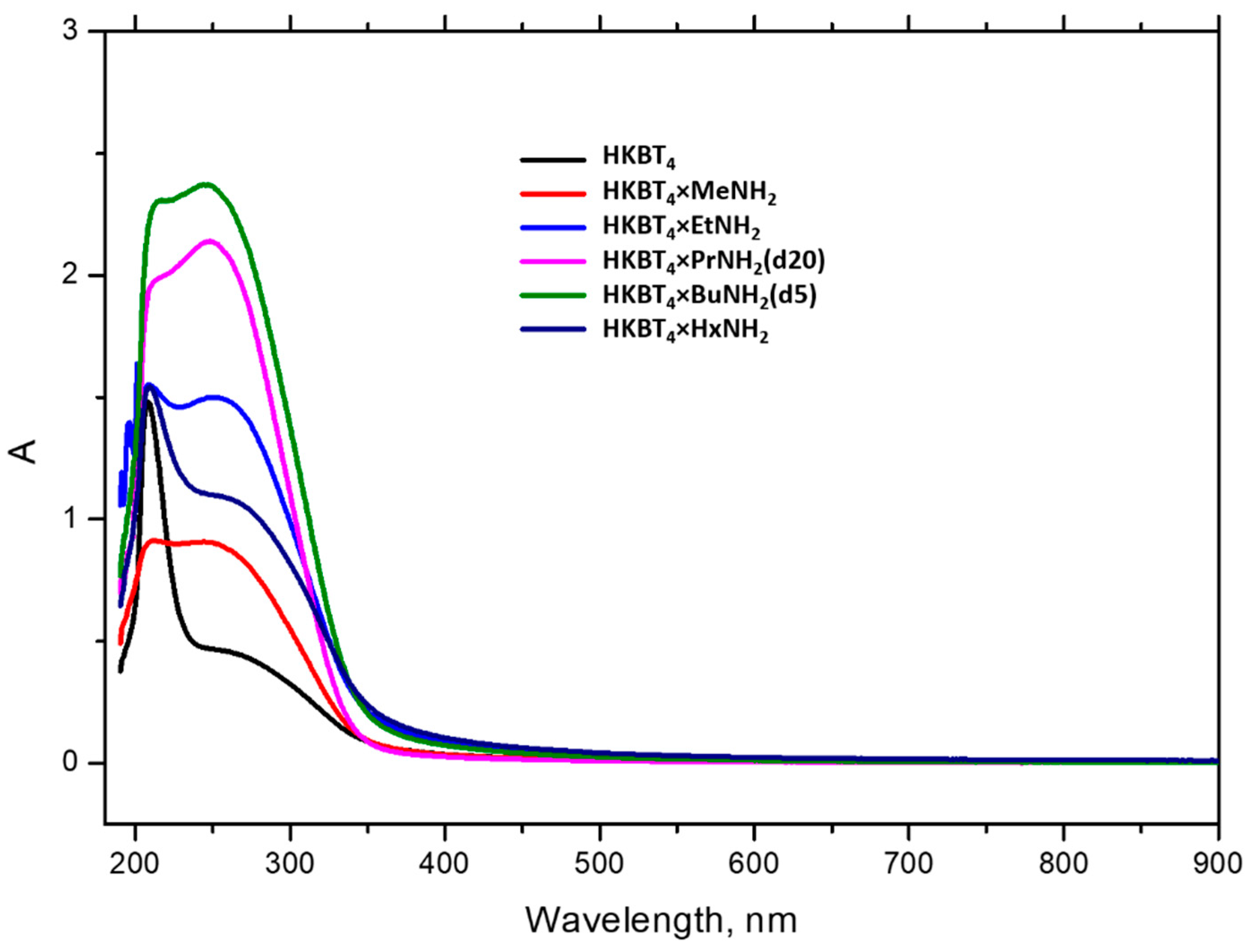
3.1. Optimization of Suspensions Preparations
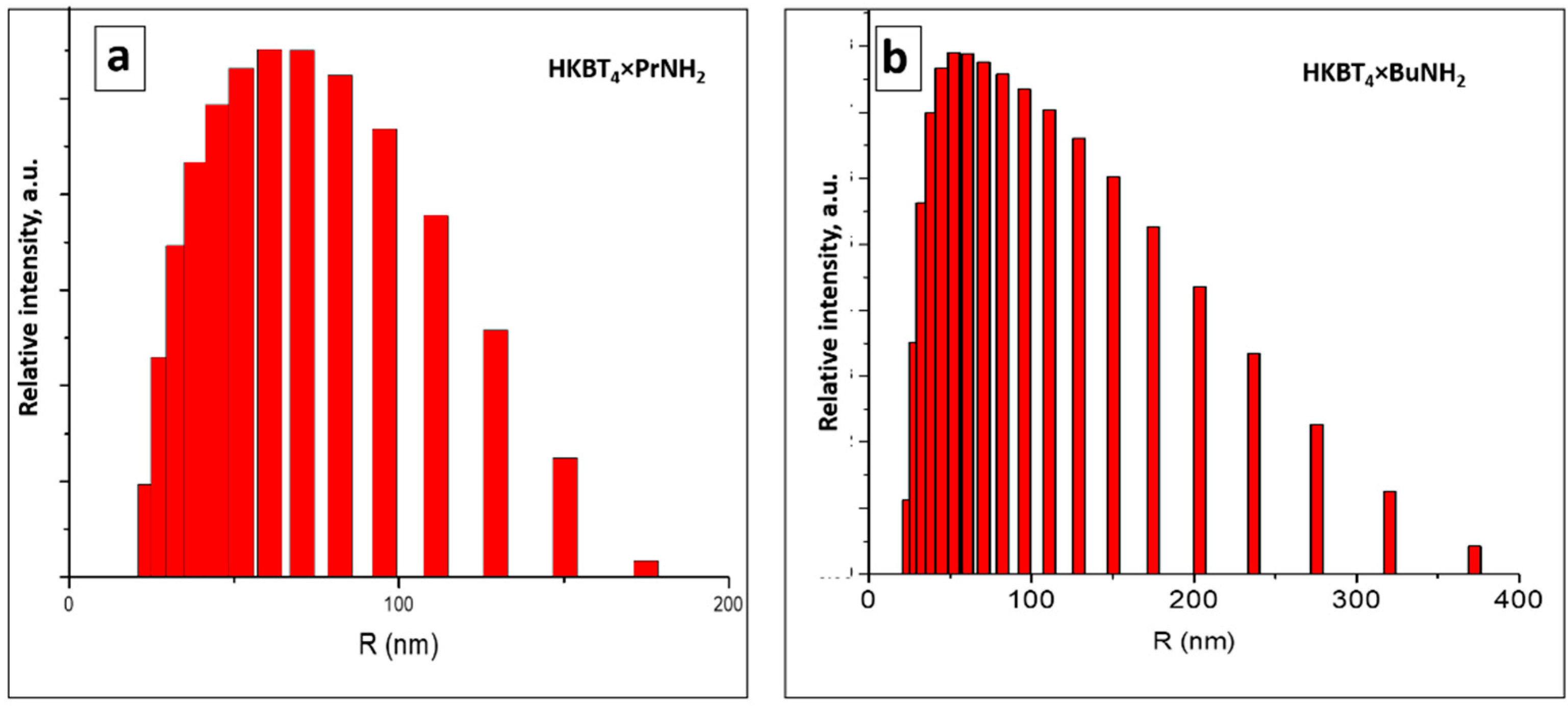
3.2. DLS Measurements
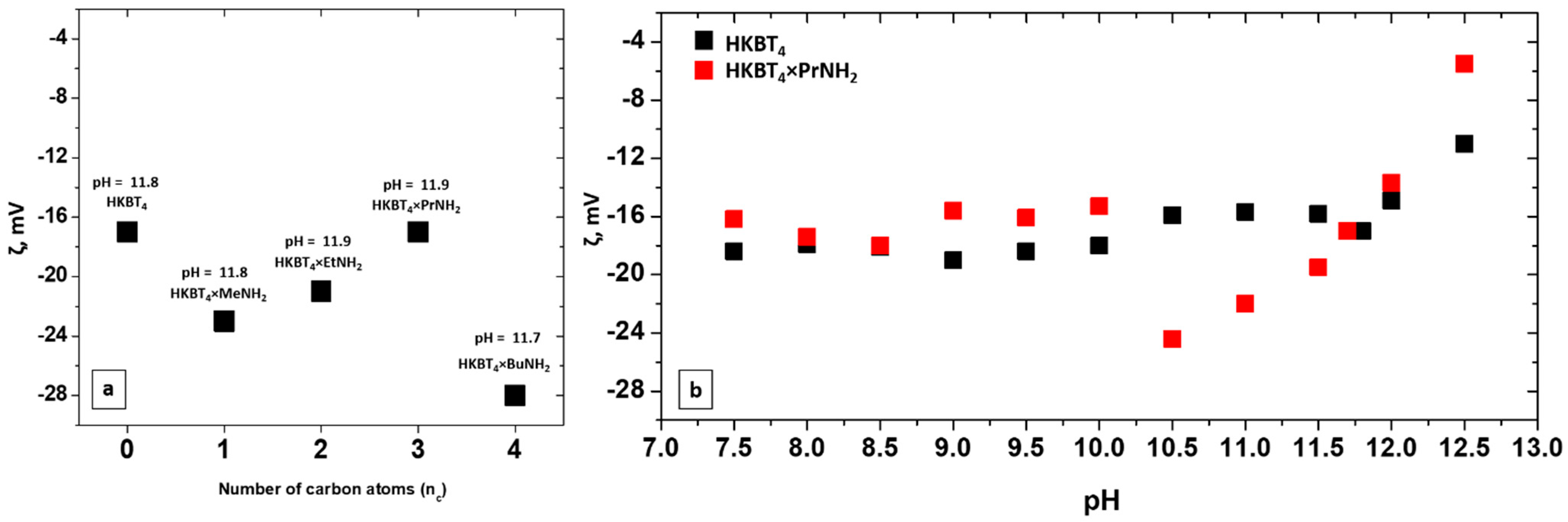
3.3. Stability of Nanoparticles Suspensions
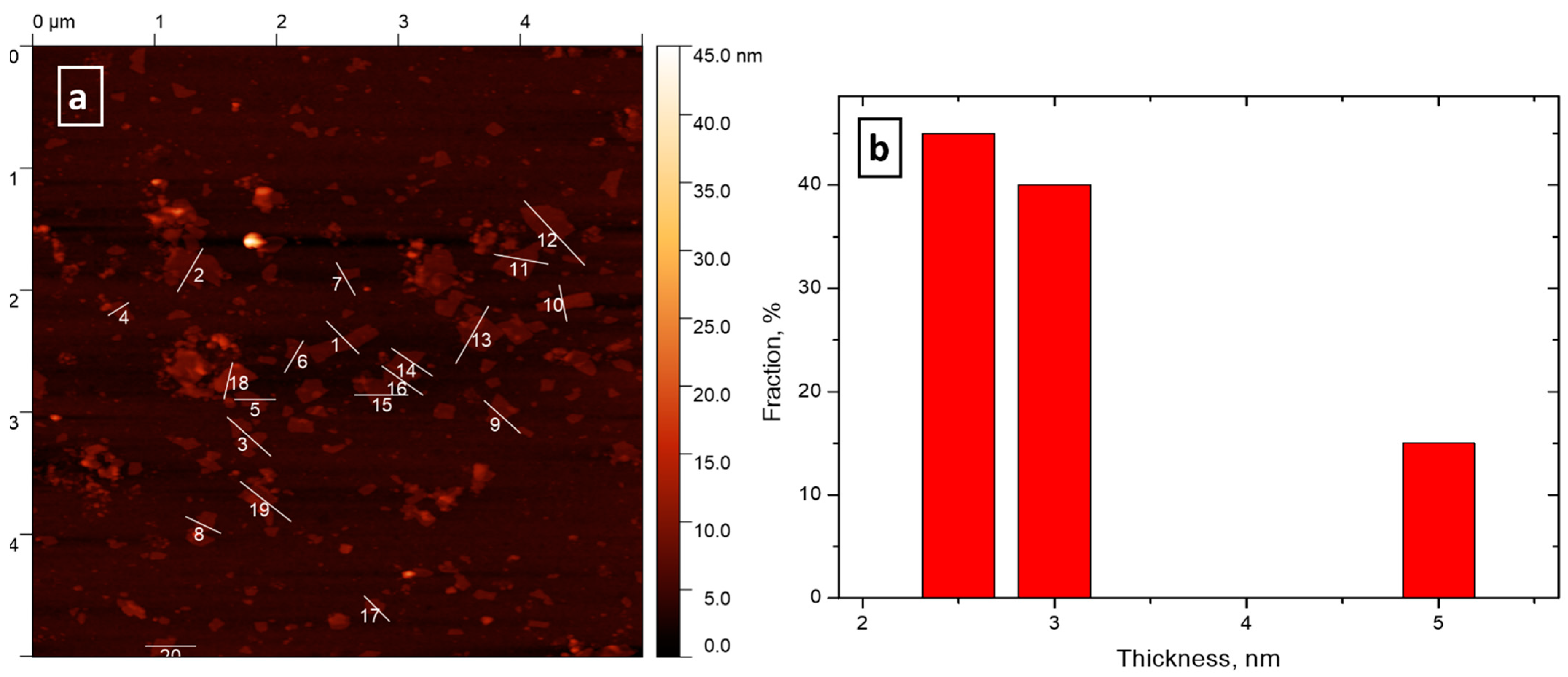
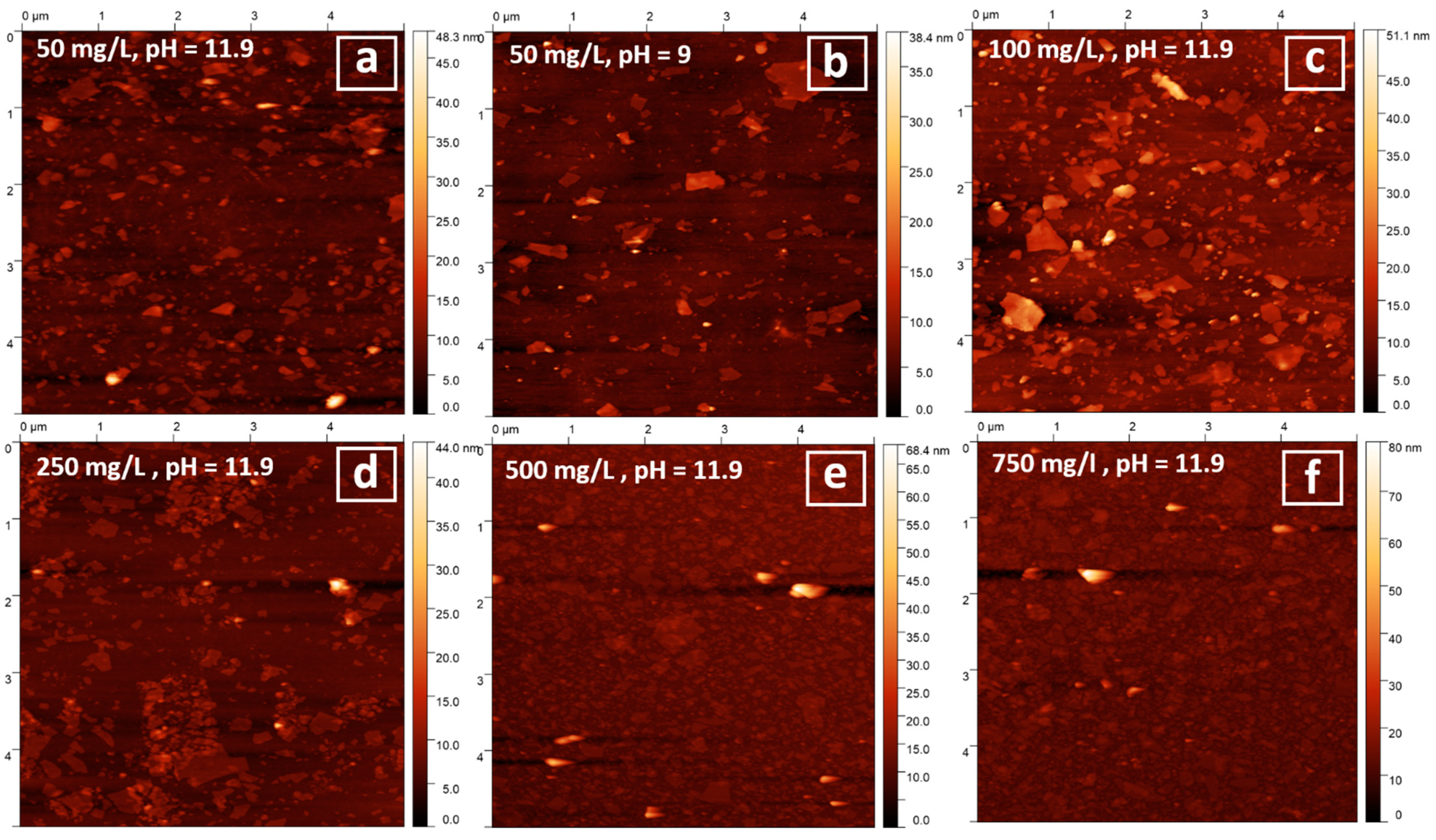
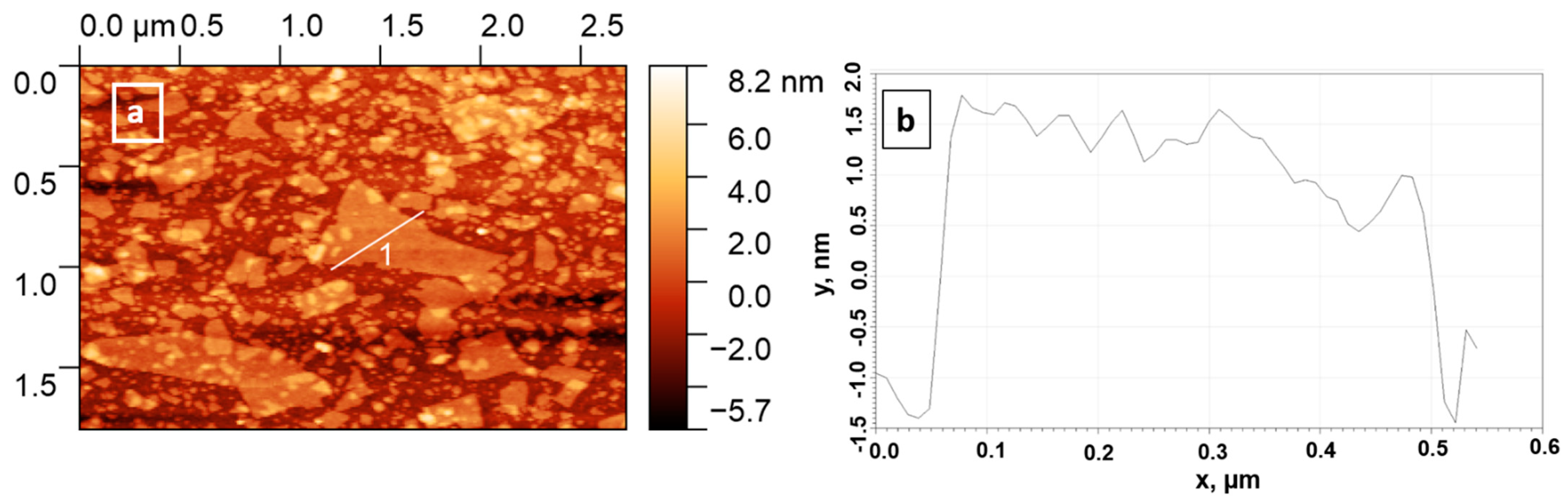
3.4. Particles Deposition on Silicon Wafers
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Dion, M.; Ganne, M.; Tournoux, M. Nouvelles familles de phases MIMII2Nb3O10 a feuillets “perovskites”. Mater. Res. Bull. 1981, 16, 1429–1435. [Google Scholar] [CrossRef]
- Jacobson, A.J.; Johnson, J.W.; Lewandowski, J.T. Interlayer Chemistry between Thick Transition-Metal Oxide Layers: Synthesis and Intercalation Reactions of K[Ca2Nan−3NbnO3n+1] (3 ≤ n ≤ 7). Inorg. Chem. 1985, 24, 3727–3729. [Google Scholar] [CrossRef]
- Kato, M.; Kajita, T.; Hanakago, R.; Koike, Y. Search for new superconductors by the Li-intercalation into layered perovskites. Phys. C Supercond. 2006, 445–448, 26–30. [Google Scholar] [CrossRef]
- Toda, K.; Teranishi, T.; Sato, M. Possibility of superconductivity in new reduced tantalate and titanate with the layered perovskite structure. J. Eur. Ceram. Soc. 1999, 19, 1525–1529. [Google Scholar] [CrossRef]
- Chen, C.; Ning, H.; Lepadatu, S.; Cain, M.; Yan, H.; Reece, M.J. Ferroelectricity in Dion–Jacobson ABiNb2O7 (A = Rb, Cs) compounds. J. Mater. Chem. C 2015, 3, 19–22. [Google Scholar] [CrossRef] [Green Version]
- Benedek, N.A. Origin of ferroelectricity in a family of polar oxides: The dion-jacobson phases. Inorg. Chem. 2014, 53, 3769–3777. [Google Scholar] [CrossRef]
- Fennie, C.J.; Rabe, K.M. Ferroelectricity in the Dion-Jacobson CsBiNb2O7 from first principles. Appl. Phys. Lett. 2006, 88, 262902. [Google Scholar] [CrossRef] [Green Version]
- Li, B.-W.; Osada, M.; Ebina, Y.; Ozawa, T.C.; Ma, R.; Sasaki, T. Impact of perovskite layer stacking on dielectric responses in KCa2Nan−3NbnO3n+1 (n = 3–6) Dion–Jacobson homologous series. Appl. Phys. Lett. 2010, 96, 182903. [Google Scholar] [CrossRef]
- Gou, G.; Shi, J. Piezoelectricity enhancement in Dion-Jacobson RbBiNb2O7 via negative pressure. EPL (Europhys. Lett.) 2014, 108, 67006. [Google Scholar] [CrossRef]
- Zhang, N.; Guo, C.; Jing, H. Photoluminescence and cathode-luminescence of Eu3+-doped NaLnTiO4 (Ln = Gd and Y) phosphors. RSC Adv. 2013, 3, 7495. [Google Scholar] [CrossRef]
- Gu, H.; Hu, Z.; Hu, Y.; Yuan, Y.; You, J.; Zou, W. The structure and photoluminescence of Bi4Ti3O12 nanoplates synthesized by hydrothermal method. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 294–298. [Google Scholar] [CrossRef]
- Ida, S.; Ogata, C.; Eguchi, M.; Youngblood, W.J.; Mallouk, T.E.; Matsumoto, Y. Photoluminescence of perovskite nanosheets prepared by exfoliation of layered oxides, K2Ln2Ti3O10, KLnNb2O7, and RbLnTa2O7 (Ln: Lanthanide ion). J. Am. Chem. Soc. 2008, 130, 7052–7059. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Silyukov, O.I.; Zvereva, I.A. Study of photocatalytic activity of layered oxides: NaNdTiO4, LiNdTiO4, and HNdTiO4 titanates. Russ. J. Gen. Chem. 2012, 82, 635–638. [Google Scholar] [CrossRef]
- Domen, K.; Yoshimura, J.; Sekine, T.; Kondo, J.; Tanaka, A.; Maruya, K.; Onishi, T. A Novel Series of Photocatalysts with an Ion-Exchangeable Layered Structure of Niobate. Stud. Surf. Sci. Catal. 1993, 2159–2162. [Google Scholar] [CrossRef]
- Hu, Y.; Mao, L.; Guan, X.; Tucker, K.A.; Xie, H.; Wu, X.; Shi, J. Layered perovskite oxides and their derivative nanosheets adopting different modification strategies towards better photocatalytic performance of water splitting. Renew. Sustain. Energy Rev. 2020, 119, 109527. [Google Scholar] [CrossRef]
- Rodionov, I.A.; Silyukov, O.I.; Utkina, T.D.; Chislov, M.V.; Sokolova, Y.P.; Zvereva, I.A. Photocatalytic properties and hydration of perovskite-type layered titanates A2Ln2Ti3O10 (A = Li, Na, K.; Ln = La, Nd). Russ. J. Gen. Chem. 2012, 82, 1191–1196. [Google Scholar] [CrossRef]
- Lichtenberg, F.; Herrnberger, A.; Wiedenmann, K. Synthesis, structural, magnetic and transport properties of layered perovskite-related titanates, niobates and tantalates of the type AnBnO3n+2, A′Ak−1BkO3k+1 and AmBm−1O3m. Prog. Solid State Chem. 2008, 36, 253–387. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Q.; Yin, Z.; Li, J. Study on the photocatalytic activity of K2La2Ti3O10 doped with vanadium (V). J. Alloys Compd. 2009, 488, 364–369. [Google Scholar] [CrossRef]
- Ozawa, T.C.; Fukuda, K.; Akatsuka, K.; Ebina, Y.; Sasaki, T. Preparation and Characterization of the Eu3+ Doped Perovskite Nanosheet Phosphor: La0.90Eu0.05Nb2O7. Chem. Mater. 2007, 19, 6575–6580. [Google Scholar] [CrossRef]
- Wei, T.; Li, C.P.; Zhou, Q.J.; Zou, Y.L.; Zhang, L.S. Upconversion luminescence and ferroelectric properties of Er3+ doped Bi4Ti3O12–SrBi4Ti4O15. Mater. Lett. 2014, 118, 92–95. [Google Scholar] [CrossRef]
- Ya-Hui, Y.; Qi-Yuan, C.; Zhou-Lan, Y.; Jie, L. Study on the photocatalytic activity of K2La2Ti3O10 doped with zinc(Zn). Appl. Surf. Sci. 2009, 255, 8419–8424. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, Y.; Fan, L.; Li, Y.; Wei, Y.; Lin, J.; Wu, J. Synthesis and photochemical properties of La-doped HCa2Nb3O10. Int. J. Hydrog. Energy 2008, 33, 6432–6438. [Google Scholar] [CrossRef]
- Gopalakrishnan, J. Chimie Douce Approaches to the Synthesis of Metastable Oxide Materials. Am. Chem. Soc. 1995, 7, 1265–1275. [Google Scholar] [CrossRef]
- Schaak, R.E.; Mallouk, T.E. Perovskites by Design: A Toolbox of Solid-State Reactions. Chem. Mater. 2002, 14, 1455–1471. [Google Scholar] [CrossRef]
- Yafarova, L.V.; Silyukov, O.I.; Myshkovskaya, T.D.; Minich, I.A.; Zvereva, I.A. New data on protonation and hydration of perovskite-type layered oxide KCa2Nb3O10. J. Therm. Anal. Calorim. 2021, 143, 87–93. [Google Scholar] [CrossRef]
- Jacobson, A.J.; Johnson, J.W.; Lewandowski, J. Intercalation of the layered solid acid HCa2Nb3O10 by organic amines. Mater. Res. Bull. 1987, 22, 45–51. [Google Scholar] [CrossRef]
- Hong, Y.; Kim, S.-J. Intercalation of Primary Diamines in the Layered Perovskite Oxides, HSr2Nb3O10. Bull. Korean Chem. Soc. 1996, 17, 730–735. [Google Scholar]
- Kurnosenko, S.A.; Silyukov, O.I.; Mazur, A.S.; Zvereva, I.A. Synthesis and thermal stability of new inorganic-organic perovskite-like hybrids based on layered titanates HLnTiO4 (Ln = La, Nd). Ceram. Int. 2020, 46, 5058–5068. [Google Scholar] [CrossRef]
- Silyukov, O.I.; Khramova, A.D.; Zvereva, I.A. Synthesis of Organic-Inorganic Derivatives of Perovskite-Like Layered HCa2Nb3O10 Oxide with Monoethanolamine and Glycine. Glas. Phys. Chem. 2020, 46, 256–259. [Google Scholar] [CrossRef]
- Wang, T.H.; Henderson, C.N.; Draskovic, T.I.; Mallouk, T.E. Synthesis, exfoliation, and electronic/protonic conductivity of the dion-jacobson phase layer perovskite HLa2TiTa2O10. Chem. Mater. 2014, 26, 898–906. [Google Scholar] [CrossRef]
- Nicolosi, V.; Chhowalla, M.; Kanatzidis, M.G.; Strano, M.S.; Coleman, J.N. Liquid Exfoliation of Layered Materials. Science 2013, 340, 1226419. [Google Scholar] [CrossRef] [Green Version]
- Osada, M.; Sasaki, T. Exfoliated oxide nanosheets: New solution to nanoelectronics. J. Mater. Chem. 2009, 19, 2503–2511. [Google Scholar] [CrossRef]
- Lee, W.-J.; Yeo, H.J.; Kim, D.-Y.; Paek, S.-M.; Kim, Y.-I. Exfoliation of Dion-Jacobson Layered Perovskite into Macromolecular Nanoplatelet. Bull. Korean Chem. Soc. 2013, 34, 2041–2043. [Google Scholar] [CrossRef] [Green Version]
- Li, B.-W.; Osada, M.; Akatsuka, K.; Ebina, Y.; Ozawa, T.C.; Sasaki, T. Solution-Based Fabrication of Perovskite Multilayers and Superlattices Using Nanosheet Process. Jpn. J. Appl. Phys. 2011, 50, 09NA10. [Google Scholar] [CrossRef]
- Pulyalina, A.; Rostovtseva, V.; Minich, I.; Silyukov, O.; Toikka, M.; Saprykina, N.; Polotskaya, G. Specific Structure and Properties of Composite Membranes Based on the Torlon® (Polyamide-imide)/Layered Perovskite Oxide. Symmetry 2020, 12, 1142. [Google Scholar] [CrossRef]
- Sasaki, T.; Ebina, Y.; Tanaka, T.; Harada, M.; Watanabe, M.; Decher, G. Layer-by-layer assembly of titania nanosheet/polycation composite films. Chem. Mater. 2001, 13, 4661–4667. [Google Scholar] [CrossRef]
- Hong, Y.-S.; Kim, S.-J. Synthesis and Characterization of Molecular Composite Prepared from Layered Perovskite Oxide, HLa2Ti2NbO10. Bull. Korean Chem. Soc. 1997, 18, 623–628. [Google Scholar] [CrossRef]
- Ida, S.; Ogata, C.; Unal, U.; Izawa, K.; Inoue, T.; Altuntasoglu, O.; Matsumoto, Y. Preparation of a blue luminescent nanosheet derived from layered perovskite Bi2SrTa2O9. J. Am. Chem. Soc. 2007, 129, 8956–8957. [Google Scholar] [CrossRef]
- Tetsuka, H.; Takashima, H.; Ikegami, K.; Nanjo, H.; Ebina, T.; Mizukami, F. Nanosheet seed-layer assists oriented growth of highly luminescent perovskite films. Chem. Mater. 2009, 21, 21–26. [Google Scholar] [CrossRef]
- Takagaki, A.; Sugisawa, M.; Lu, D.; Kondo, J.N.; Hara, M.; Domen, K.; Hayashi, S. Exfoliated nanosheets as a new strong solid acid catalyst. J. Am. Chem. Soc. 2003, 125, 5479–5485. [Google Scholar] [CrossRef]
- Zhou, Y.; Wen, T.; Zhang, X.; Chang, B.; Kong, W.; Guo, Y.; Yang, B.; Wang, Y. A Multiple Structure-Design Strategy towards Ultrathin Niobate Perovskite Nanosheets with Thickness-Dependent Photocatalytic Hydrogen-Evolution Performance. Chem.-Asian J. 2017, 12, 2727–2733. [Google Scholar] [CrossRef]
- Hu, S.; Chi, B.; Pu, J.; Jian, L. Novel heterojunction photocatalysts based on lanthanum titanate nanosheets and indium oxide nanoparticles with enhanced photocatalytic hydrogen production activity. J. Mater. Chem. A 2014, 2, 19260–19267. [Google Scholar] [CrossRef]
- Osada, M.; Akatsuka, K.; Ebina, Y.; Kotani, Y.; Ono, K.; Funakubo, H.; Ueda, S.; Kobayashi, K.; Takada, K.; Sasaki, T. Langmuir–Blodgett Fabrication of Nanosheet-Based Dielectric Films without an Interfacial Dead Layer. Jpn. J. Appl. Phys. 2008, 47, 7556–7560. [Google Scholar] [CrossRef]
- Khan, M.S.; Kim, H.; Kim, Y.; Ebina, Y.; Sugimoto, W.; Sasaki, T.; Osada, M. Scalable Design of Two-Dimensional Oxide Nanosheets for Construction of Ultrathin Multilayer Nanocapacitor. Small 2020, 16, 2003485. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Avdeev, M.; Liu, Y.; Johnson, M.R.; Ling, C.D. A New n = 4 Layered Ruddlesden–Popper Phase K2.5Bi2.5Ti4O13 Showing Stoichiometric Hydration. Inorg. Chem. 2016, 55, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Silyukov, O.I.; Minich, I.A.; Zvereva, I.A. Synthesis of protonated derivatives of layered perovskite-like bismuth titanates. Glas. Phys. Chem. 2018, 44, 115–119. [Google Scholar] [CrossRef]
- Minich, I.A.; Silyukov, O.I.; Kulish, L.D.; Zvereva, I.A. Study on thermolysis process of a new hydrated and protonated perovskite-like oxides H2K0.5Bi2.5Ti4O13·yH2O. Ceram. Int. 2019, 45, 2704–2709. [Google Scholar] [CrossRef]
- Minich, I.A.; Silyukov, O.I.; Gak, V.V.; Borisov, E.V.; Zvereva, I.A. Synthesis of Organic-Inorganic Hybrids Based on Perovskite-like Bismuth Titanate H2K0.5Bi2.5Ti4O13·H2O and n-Alkylamines. ACS Omega 2020, 5, 8158–8168. [Google Scholar] [CrossRef]
- Minich, I.A.; Silyukov, O.I.; Mazur, A.S.; Zvereva, I.A. Grafting reactions of perovskite-like bismuth titanate H2K0.5Bi2.5Ti4O13·H2O with n-alcohols. Ceram. Int. 2020, 46, 29373–29381. [Google Scholar] [CrossRef]
- Ebina, Y.; Sasaki, T.; Watanabe, M. Study on exfoliation of layered perovskite-type niobates. Solid State Ion. 2002, 151, 177–182. [Google Scholar] [CrossRef]
- Gao, H.; Shori, S.; Chen, X.; zur Loye, H.C.; Ploehn, H.J. Quantitative analysis of exfoliation and aspect ratio of calcium niobate platelets. J. Colloid Interface Sci. 2013, 392, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Ebina, Y.; Akatsuka, K.; Fukuda, K.; Sasaki, T. Synthesis and in situ X-ray diffraction characterization of two-dimensional perovskite-type oxide colloids with a controlled molecular thickness. Chem. Mater. 2012, 24, 4201–4208. [Google Scholar] [CrossRef]
- Kurnosenko, S.A.; Silyukov, O.I.; Minich, I.A.Z. Exfoliation of Methylamine and n-Butylamine Derivatives of Layered Perovskite-Like Oxides HLnTiO4 and H2Ln2Ti3O10 (Ln = La, Nd) into Nanolayers. Glas. Phys. Chem. 2021, 47, 372–381. [Google Scholar] [CrossRef]
- Li, S.; Leroy, P.; Heberling, F.; Devau, N.; Jougnot, D.; Chiaberge, C. Influence of surface conductivity on the apparent zeta potential of calcite. J. Colloid Interface Sci. 2016, 468, 262–275. [Google Scholar] [CrossRef] [Green Version]
- Tantra, R.; Schulze, P.; Quincey, P. Effect of nanoparticle concentration on zeta-potential measurement results and reproducibility. Particuology 2010, 8, 279–285. [Google Scholar] [CrossRef]
- Suh, J.; Hwang, B.K. Ionization of PEI and PAA at various pH’s. Bioorg. Chem. 1994, 22, 318–327. [Google Scholar] [CrossRef]
| Sample | Concentration (24 h), mg/L | Concentration (1 w), mg/L |
|---|---|---|
| HKBT4 × MeNH2 | 18 | 13 |
| HKBT4 × EtNH2 | 26 | 23 |
| HKBT4 × PrNH2 | 730 | 770 |
| HKBT4 × BuNH2 | 156 | 245 |
| Sample | Average Size, nm |
|---|---|
| HKBT4 (aggregated) | ~2000 |
| HKBT4 (resonicated) | 96 |
| HKBT4 × MeNH2 | 77 |
| HKBT4 × EtNH2 | 83 |
| HKBT4 × PrNH2 | 65 |
| HKBT4 × BuNH2 | 88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minich, I.A.; Silyukov, O.I.; Kurnosenko, S.A.; Gak, V.V.; Kalganov, V.D.; Kolonitskiy, P.D.; Zvereva, I.A. Physical–Chemical Exfoliation of n-Alkylamine Derivatives of Layered Perovskite-like Oxide H2K0.5Bi2.5Ti4O13 into Nanosheets. Nanomaterials 2021, 11, 2708. https://doi.org/10.3390/nano11102708
Minich IA, Silyukov OI, Kurnosenko SA, Gak VV, Kalganov VD, Kolonitskiy PD, Zvereva IA. Physical–Chemical Exfoliation of n-Alkylamine Derivatives of Layered Perovskite-like Oxide H2K0.5Bi2.5Ti4O13 into Nanosheets. Nanomaterials. 2021; 11(10):2708. https://doi.org/10.3390/nano11102708
Chicago/Turabian StyleMinich, Iana A., Oleg I. Silyukov, Sergei A. Kurnosenko, Veronika V. Gak, Vladimir D. Kalganov, Petr D. Kolonitskiy, and Irina A. Zvereva. 2021. "Physical–Chemical Exfoliation of n-Alkylamine Derivatives of Layered Perovskite-like Oxide H2K0.5Bi2.5Ti4O13 into Nanosheets" Nanomaterials 11, no. 10: 2708. https://doi.org/10.3390/nano11102708
APA StyleMinich, I. A., Silyukov, O. I., Kurnosenko, S. A., Gak, V. V., Kalganov, V. D., Kolonitskiy, P. D., & Zvereva, I. A. (2021). Physical–Chemical Exfoliation of n-Alkylamine Derivatives of Layered Perovskite-like Oxide H2K0.5Bi2.5Ti4O13 into Nanosheets. Nanomaterials, 11(10), 2708. https://doi.org/10.3390/nano11102708







