Recent Advances in Fabrication of Flexible, Thermochromic Vanadium Dioxide Films for Smart Windows
Abstract
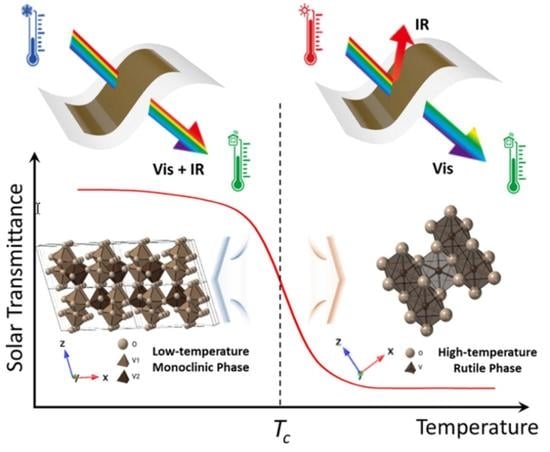
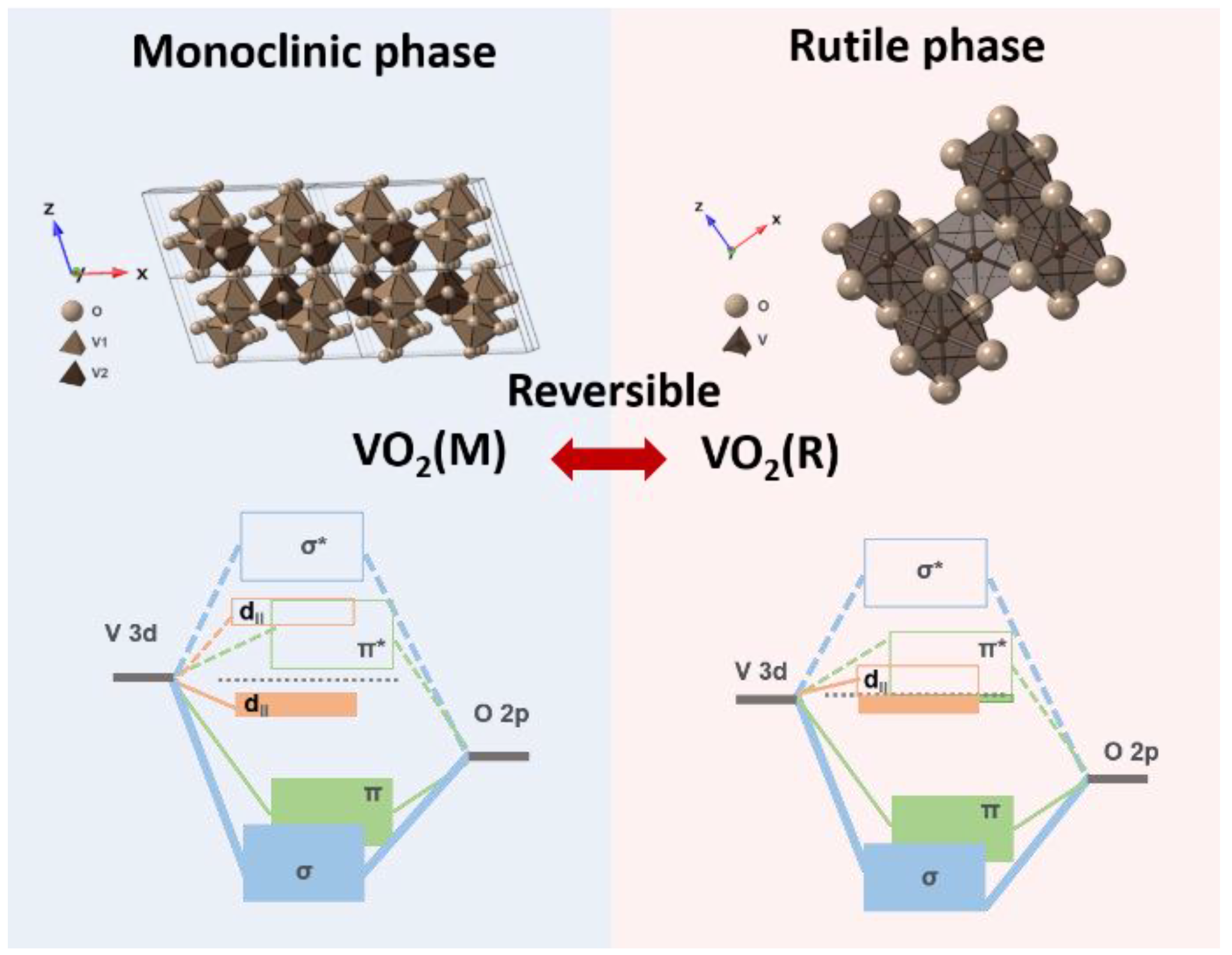
:1. Introduction
2. Fabrication Methods
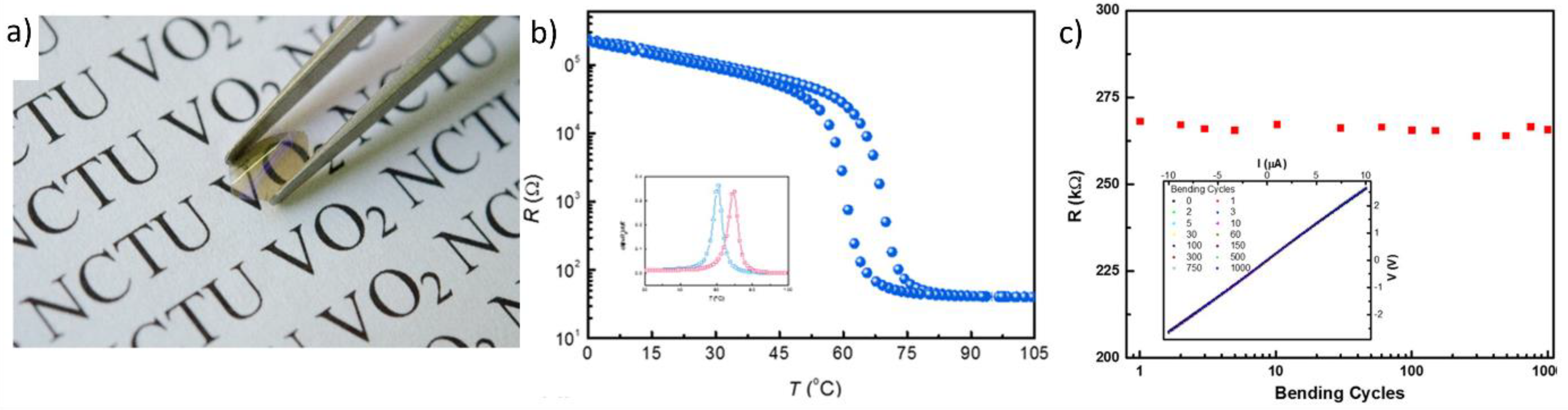
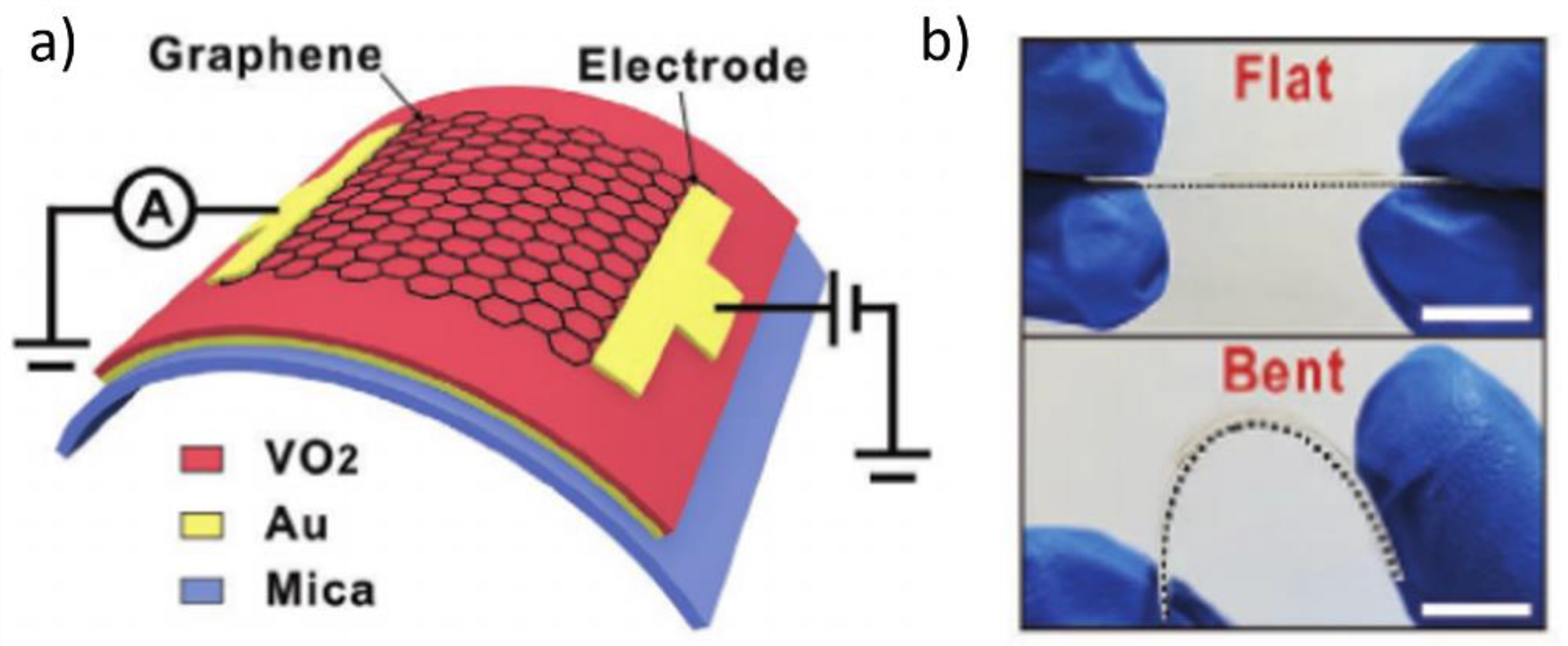
2.1. Fabrication of Flexible Monoclinic-Phase VO2 (VO2(M)) Films via Chamber-Based Deposition
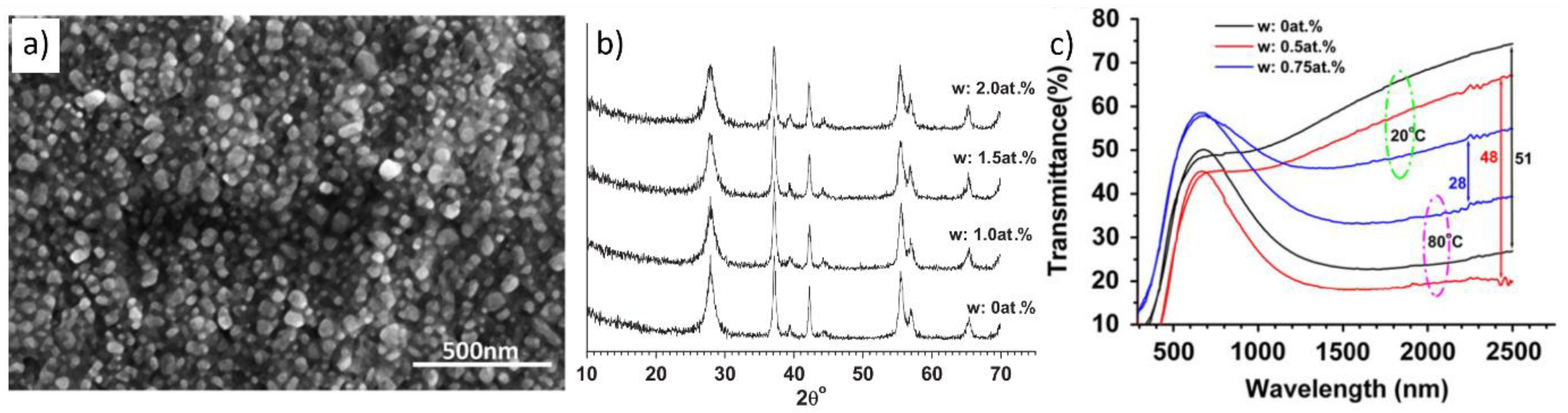
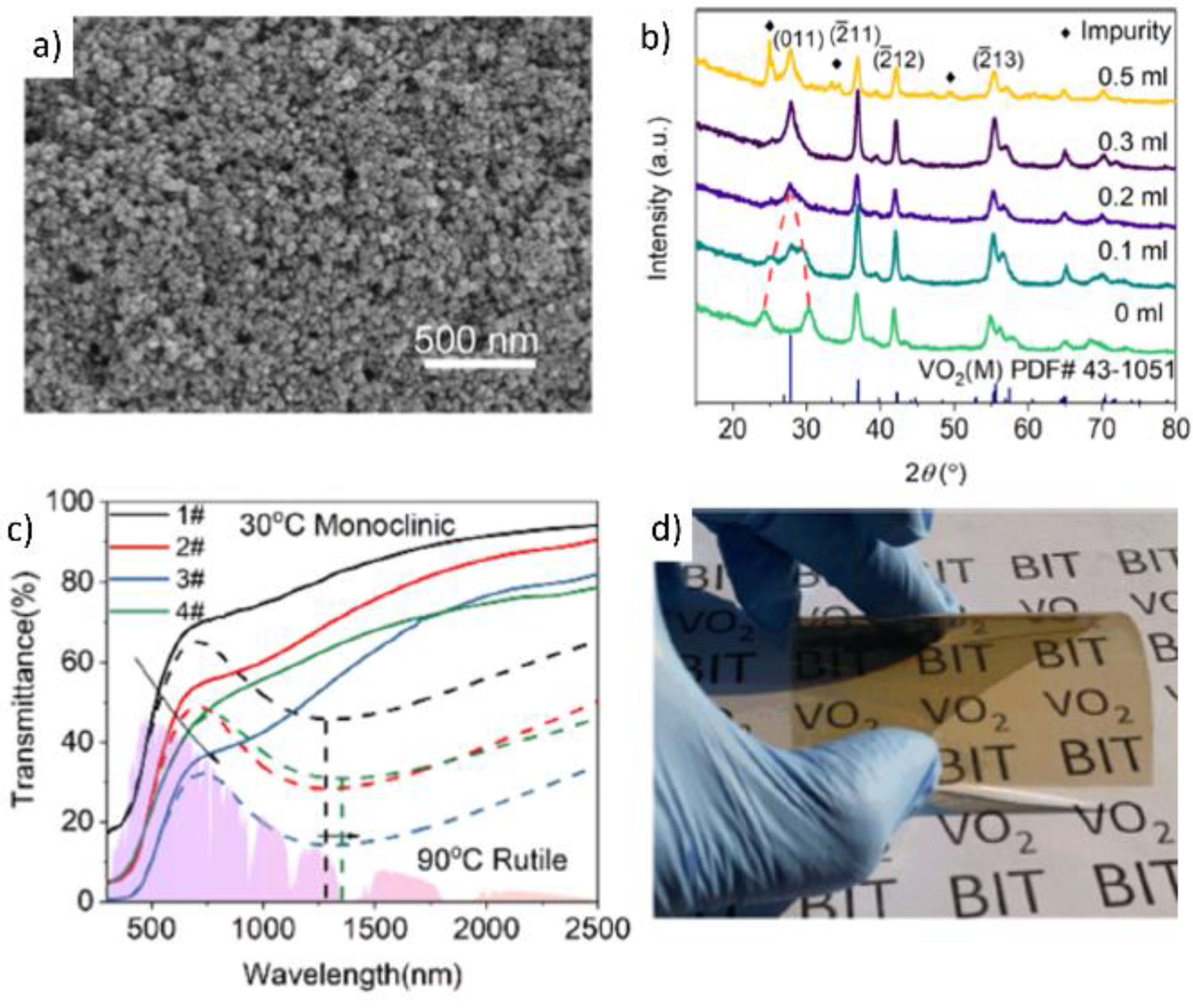
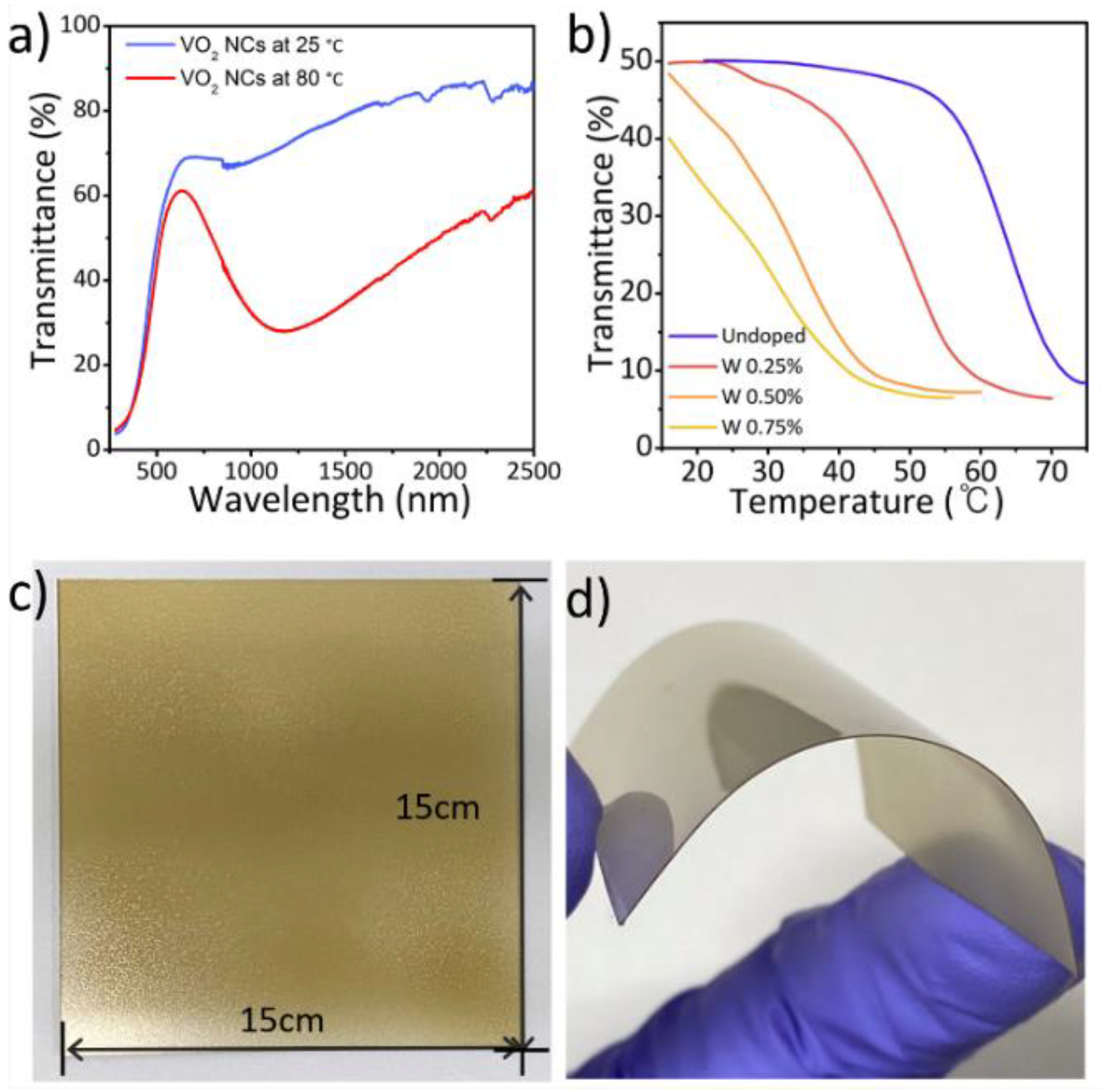
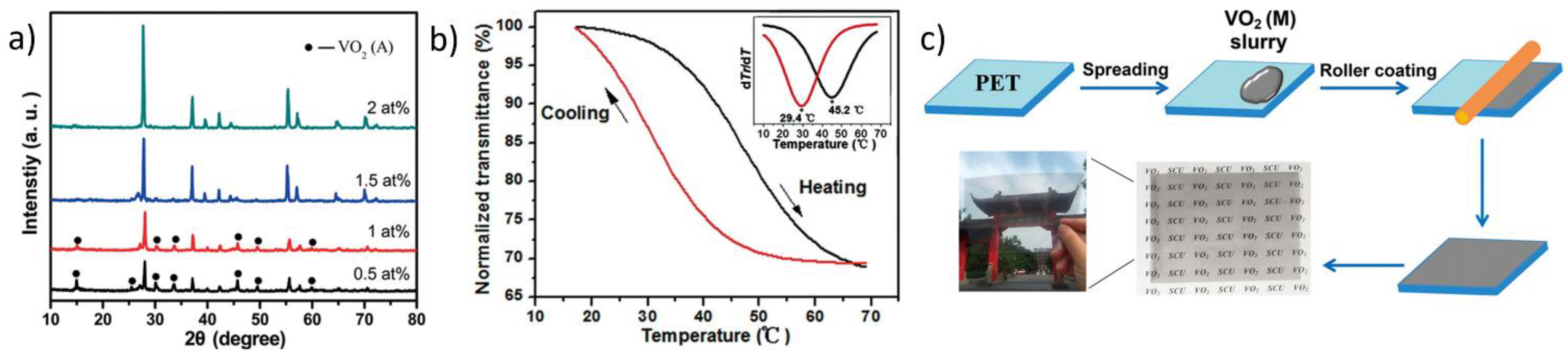
2.2. Fabrication of Flexible VO2(M) Films through Solution-Based Deposition Process
3. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, Y.; Luo, H.; Zhang, Z.; Kang, L.; Chen, Z.; Du, J.; Kanehira, M.; Cao, C. Nanoceramic VO2 thermochromic smart glass: A review on progress in solution processing. Nano Energy 2012, 1, 221–246. [Google Scholar] [CrossRef]
- Granqvist, C.G. Transparent conductors as solar energy materials: A panoramic review. Sol. Energy Mater. Sol. Cells 2007, 91, 1529–1598. [Google Scholar] [CrossRef]
- Ke, Y.; Zhou, C.; Zhou, Y.; Wang, S.; Chan, S.H.; Long, Y. Emerging thermal-responsive materials and integrated techniques targeting the energy-efficient smart window application. Adv. Funct. Mater. 2018, 28, 1800113. [Google Scholar] [CrossRef]
- Chao, D.; Zhu, C.; Xia, X.; Liu, J.; Zhang, X.; Wang, J.; Liang, P.; Lin, J.; Zhang, H.; Shen, Z.X. Graphene quantum dots coated VO2 arrays for highly durable electrodes for Li and Na ion batteries. Nano Lett. 2015, 15, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Morin, F. Oxides which show a metal-to-insulator transition at the Neel temperature. Phys. Rev. Lett. 1959, 3, 34. [Google Scholar] [CrossRef]
- Tripathi, A.; John, J.; Kruk, S.; Zhang, Z.; Nguyen, H.S.; Berguiga, L.; Romeo, P.R.; Orobtchouk, R.; Ramanathan, S.; Kivshar, Y. Tunable Mie-Resonant Dielectric Metasurfaces Based on VO2 Phase-Transition Materials. ACS Photonics 2021, 8, 1206–1213. [Google Scholar] [CrossRef]
- Jorgenson, G.; Lee, J. Doped vanadium oxide for optical switching films. Sol. Energy Mater. 1986, 14, 205–214. [Google Scholar] [CrossRef]
- Budai, J.D.; Hong, J.; Manley, M.E.; Specht, E.D.; Li, C.W.; Tischler, J.Z.; Abernathy, D.L.; Said, A.H.; Leu, B.M.; Boatner, L.A. Metallization of vanadium dioxide driven by large phonon entropy. Nature 2014, 515, 535–539. [Google Scholar] [CrossRef]
- Aetukuri, N.B.; Gray, A.X.; Drouard, M.; Cossale, M.; Gao, L.; Reid, A.H.; Kukreja, R.; Ohldag, H.; Jenkins, C.A.; Arenholz, E. Control of the metal–insulator transition in vanadium dioxide by modifying orbital occupancy. Nat. Phys. 2013, 9, 661–666. [Google Scholar] [CrossRef]
- Wu, C.; Feng, F.; Xie, Y. Design of vanadium oxide structures with controllable electrical properties for energy applications. Chem Soc Rev 2013, 42, 5157–5183. [Google Scholar] [CrossRef]
- Li, Y.; Ji, S.; Gao, Y.; Luo, H.; Kanehira, M. Core-shell VO2@TiO2 nanorods that combine thermochromic and photocatalytic properties for application as energy-saving smart coatings. Sci. Rep. 2013, 3, 1–13. [Google Scholar] [CrossRef]
- Goodenough, J.B. The two components of the crystallographic transition in VO2. J. Solid State Chem. 1971, 3, 490–500. [Google Scholar] [CrossRef]
- Whittaker, L.; Patridge, C.J.; Banerjee, S. Microscopic and nanoscale perspective of the metal− insulator phase transitions of VO2: Some new twists to an old tale. J. Phys. Chem. Lett. 2011, 2, 745–758. [Google Scholar] [CrossRef]
- Wu, B.; Zimmers, A.; Aubin, H.; Ghosh, R.; Liu, Y.; Lopez, R. Electric-field-driven phase transition in vanadium dioxide. Phys. Rev. B 2011, 84, 241410. [Google Scholar] [CrossRef] [Green Version]
- Kikuzuki, T.; Lippmaa, M. Characterizing a strain-driven phase transition in VO2. Appl. Phys. Lett. 2010, 96, 132107. [Google Scholar] [CrossRef]
- Gea, L.A.; Boatner, L. Optical switching of coherent VO2 precipitates formed in sapphire by ion implantation and annealing. Appl. Phys. Lett. 1996, 68, 3081–3083. [Google Scholar] [CrossRef]
- Wu, C.; Feng, F.; Feng, J.; Dai, J.; Peng, L.; Zhao, J.; Yang, J.; Si, C.; Wu, Z.; Xie, Y. Hydrogen-incorporation stabilization of metallic VO2 (R) phase to room temperature, displaying promising low-temperature thermoelectric effect. J. Am. Chem. Soc. 2011, 133, 13798–13801. [Google Scholar] [CrossRef]
- Xie, J.; Wu, C.; Hu, S.; Dai, J.; Zhang, N.; Feng, J.; Yang, J.; Xie, Y. Ambient rutile VO2(R) hollow hierarchitectures with rich grain boundaries from new-state nsutite-type VO2, displaying enhanced hydrogen adsorption behavior. Phys. Chem. Chem. Phys. 2012, 14, 4810–4816. [Google Scholar] [CrossRef]
- Yoon, H.; Choi, M.; Lim, T.-W.; Kwon, H.; Ihm, K.; Kim, J.K.; Choi, S.-Y.; Son, J. Reversible phase modulation and hydrogen storage in multivalent VO2 epitaxial thin films. Nat. Mater. 2016, 15, 1113–1119. [Google Scholar] [CrossRef]
- Hu, B.; Ding, Y.; Chen, W.; Kulkarni, D.; Shen, Y.; Tsukruk, V.V.; Wang, Z.L. External-strain induced insulating phase transition in VO2 nanobeam and its application as flexible strain sensor. Adv. Mater. 2010, 22, 5134–5139. [Google Scholar] [CrossRef]
- Liu, N.; Mesch, M.; Weiss, T.; Hentschel, M.; Giessen, H. Infrared perfect absorber and its application as plasmonic sensor. Nano Lett. 2010, 10, 2342–2348. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Wang, K.; Liu, K.; Bhat, A.K.; Dhara, S.; Wu, J.; Deshmukh, M.M. Field-effect modulation of conductance in VO2 nanobeam transistors with HfO2 as the gate dielectric. Appl. Phys. Lett. 2011, 99, 062114. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-T.; Chae, B.-G.; Youn, D.-H.; Maeng, S.-L.; Kim, G.; Kang, K.-Y.; Lim, Y.-S. Mechanism and observation of Mott transition in VO2-based two-and three-terminal devices. N. J. Phys. 2004, 6, 52. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Ramanathan, S. Relaxation dynamics of ionic liquid—VO2 interfaces and influence in electric double-layer transistors. J. Appl. Phys 2012, 111, 084508. [Google Scholar] [CrossRef]
- Liu, M.; Hwang, H.Y.; Tao, H.; Strikwerda, A.C.; Fan, K.; Keiser, G.R.; Sternbach, A.J.; West, K.G.; Kittiwatanakul, S.; Lu, J. Terahertz-field-induced insulator-to-metal transition in vanadium dioxide metamaterial. Nature 2012, 487, 345–348. [Google Scholar] [CrossRef]
- Schurig, D.; Mock, J.J.; Justice, B.; Cummer, S.A.; Pendry, J.B.; Starr, A.F.; Smith, D.R. Metamaterial electromagnetic cloak at microwave frequencies. Science 2006, 314, 977–980. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-W.; Choi, H.J.; Jeong, H. Tunable metasurfaces for visible and SWIR applications. Nano Converg. 2020, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Liu, H.; Deng, S.; Zheng, M.; Wang, Y.; van Kan, J.A.; Tang, S.H.; Zhang, X.; Sow, C.H.; Mhaisalkar, S.G. Highly sensitive and multispectral responsive phototransistor using tungsten-doped VO2 nanowires. Nanoscale 2014, 6, 7619–7627. [Google Scholar] [CrossRef]
- Babulanam, S.; Eriksson, T.; Niklasson, G.; Granqvist, C. Thermochromic VO2 films for energy-efficient windows. Sol. Energy Mater. 1987, 16, 347–363. [Google Scholar] [CrossRef]
- Kamalisarvestani, M.; Saidur, R.; Mekhilef, S.; Javadi, F. Performance, materials and coating technologies of thermochromic thin films on smart windows. Renew. Sust. Energ. Rev. 2013, 26, 353–364. [Google Scholar] [CrossRef]
- Lampert, C.M. Large-area smart glass and integrated photovoltaics. Sol. Energy Mater. Sol. Cells 2003, 76, 489–499. [Google Scholar] [CrossRef]
- Lin, S.; Bai, X.; Wang, H.; Wang, H.; Song, J.; Huang, K.; Wang, C.; Wang, N.; Li, B.; Lei, M. Roll-to-roll production of transparent silver-nanofiber-network electrodes for flexible electrochromic smart Windows. Adv. Mater. 2017, 29, 1703238. [Google Scholar] [CrossRef]
- Kim, M.-J.; Sung, G.; Sun, J.-Y. Stretchable and reflective displays: Materials, technologies and strategies. Nano Converg. 2019, 6, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Ai, B.; Wong, Z.J. Soft optical metamaterials. Nano Converg. 2020, 7, 1–17. [Google Scholar] [CrossRef]
- Hoffmann, S.; Lee, E.S.; Clavero, C. Examination of the technical potential of near-infrared switching thermochromic windows for commercial building applications. Sol. Energy Mater. Sol. Cells 2014, 123, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.; Cao, X.; Long, Y.; Luo, H.; Jin, P. How to properly evaluate and compare the thermochromic performance of VO2-based smart coatings. J. Mater. Chem. A 2019, 7, 24164–24172. [Google Scholar] [CrossRef]
- Li, M.; Magdassi, S.; Gao, Y.; Long, Y. Hydrothermal synthesis of VO2 polymorphs: Advantages, challenges and prospects for the application of energy efficient smart windows. Small 2017, 13, 1701147. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Niklasson, G.A.; Granqvist, C.-G. Thermochromic fenestration with VO2-based materials: Three challenges and how they can be met. Thin Solid Films 2012, 520, 3823–3828. [Google Scholar] [CrossRef]
- Saeli, M.; Piccirillo, C.; Parkin, I.P.; Binions, R.; Ridley, I. Energy modelling studies of thermochromic glazing. Energy Build. 2010, 42, 1666–1673. [Google Scholar] [CrossRef]
- Majid, S.; Sahu, S.; Ahad, A.; Dey, K.; Gautam, K.; Rahman, F.; Behera, P.; Deshpande, U.; Sathe, V.; Shukla, D. Role of VV dimerization in the insulator-metal transition and optical transmittance of pure and doped VO2 thin films. Phys. Rev. B 2020, 101, 014108. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Miao, L.; Liu, C.; Li, C.; Asaka, T.; Kang, Y.; Iwamoto, Y.; Tanemura, S.; Gu, H.; Su, H. Solution-processed VO2-SiO2 composite films with simultaneously enhanced luminous transmittance, solar modulation ability and anti-oxidation property. Sci. Rep. 2014, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Whittaker, L.; Wu, T.-L.; Patridge, C.J.; Sambandamurthy, G.; Banerjee, S. Distinctive finite size effects on the phase diagram and metal–insulator transitions of tungsten-doped vanadium (iv) oxide. J. Mater. Chem. 2011, 21, 5580–5592. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, W.; Shi, Q.; Zhang, Y.; Song, L.; Zhang, Y. Synthesis and properties of Mo and W ions co-doped porous nano-structured VO2 films by sol–gel process. J. Solgel Sci. Technol. 2012, 64, 493–499. [Google Scholar] [CrossRef]
- Gao, Y.; Cao, C.; Dai, L.; Luo, H.; Kanehira, M.; Ding, Y.; Wang, Z.L. Phase and shape controlled VO2 nanostructures by antimony doping. Energy Environ. Sci. 2012, 5, 8708–8715. [Google Scholar] [CrossRef]
- Griffiths, C.; Eastwood, H. Influence of stoichiometry on the metal-semiconductor transition in vanadium dioxide. J. Appl. Phys 1974, 45, 2201–2206. [Google Scholar] [CrossRef]
- Muraoka, Y.; Hiroi, Z. Metal–insulator transition of VO2 thin films grown on TiO2 (001) and (110) substrates. Appl. Phys. Lett. 2002, 80, 583–585. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Cao, C.; Gao, Y.; Luo, H. Synthesis and phase transition behavior of undoped VO2 with a strong nano-size effect. Sol. Energy Mater. Sol. Cells 2011, 95, 712–715. [Google Scholar] [CrossRef]
- Liang, S.; Shi, Q.; Zhu, H.; Peng, B.; Huang, W. One-step hydrothermal synthesis of W-doped VO2 (M) nanorods with a tunable phase-transition temperature for infrared smart windows. ACS Omega 2016, 1, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Strelcov, E.; Tselev, A.; Ivanov, I.; Budai, J.D.; Zhang, J.; Tischler, J.Z.; Kravchenko, I.; Kalinin, S.V.; Kolmakov, A. Doping-based stabilization of the M2 phase in free-standing VO2 nanostructures at room temperature. Nano Lett. 2012, 12, 6198–6205. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulou, M.; Gagaoudakis, E.; Boukos, N.; Aperathitis, E.; Kiriakidis, G.; Tsoukalas, D.; Raptis, Y. Thermochromic performance of Mg-doped VO2 thin films on functional substrates for glazing applications. Sol. Energy Mater. Sol. Cells 2016, 157, 1004–1010. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Y.; Wang, D.; Ling, C.; Chang, Q.; Li, J.; Zhao, Y.; Jin, H. Sn dopants improve the visible transmittance of VO2 films achieving excellent thermochromic performance for smart window. Sol. Energy Mater. Sol. Cells 2020, 209, 110443. [Google Scholar] [CrossRef]
- Patridge, C.J.; Whittaker, L.; Ravel, B.; Banerjee, S. Elucidating the Influence of Local Structure Perturbations on the Metal–Insulator Transitions of V1–xMoxO2 Nanowires: Mechanistic Insights from an X-ray Absorption Spectroscopy Study. J. Phys. Chem. C 2012, 116, 3728–3736. [Google Scholar] [CrossRef]
- Li, D.; Li, M.; Pan, J.; Luo, Y.; Wu, H.; Zhang, Y.; Li, G. Hydrothermal synthesis of Mo-doped VO2/TiO2 composite nanocrystals with enhanced thermochromic performance. ACS Appl. Mater. Interfaces 2014, 6, 6555–6561. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Chen, S.; Liu, J.; Gao, Y.; Zhou, J.; Chen, Z.; Cao, C.; Luo, H.; Kanehira, M. F-doped VO2 nanoparticles for thermochromic energy-saving foils with modified color and enhanced solar-heat shielding ability. Phys. Chem. Chem. Phys. 2013, 15, 11723–11729. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, S.; Luo, H.; Dai, L.; Cao, C.; Liu, Y.; Chen, Z.; Kanehira, M. Enhanced chemical stability of VO2 nanoparticles by the formation of SiO2/VO2 core/shell structures and the application to transparent and flexible VO2-based composite foils with excellent thermochromic properties for solar heat control. Energy Environ. Sci. 2012, 5, 6104–6110. [Google Scholar] [CrossRef]
- Cui, Y.; Ke, Y.; Liu, C.; Chen, Z.; Wang, N.; Zhang, L.; Zhou, Y.; Wang, S.; Gao, Y.; Long, Y. Thermochromic VO2 for energy-efficient smart windows. Joule 2018, 2, 1707–1746. [Google Scholar] [CrossRef] [Green Version]
- Li, S.-Y.; Niklasson, G.A.; Granqvist, C.-G. Nanothermochromics: Calculations for VO2 nanoparticles in dielectric hosts show much improved luminous transmittance and solar energy transmittance modulation. J. Appl. Phys 2010, 108, 063525. [Google Scholar] [CrossRef]
- Ke, Y.; Balin, I.; Wang, N.; Lu, Q.; Tok, A.I.Y.; White, T.J.; Magdassi, S.; Abdulhalim, I.; Long, Y. Two-dimensional SiO2/VO2 photonic crystals with statically visible and dynamically infrared modulated for smart window deployment. ACS Appl. Mater. Interfaces 2016, 8, 33112–33120. [Google Scholar] [CrossRef]
- Kang, L.; Gao, Y.; Luo, H.; Chen, Z.; Du, J.; Zhang, Z. Nanoporous thermochromic VO2 films with low optical constants, enhanced luminous transmittance and thermochromic properties. ACS Appl. Mater. Interfaces 2011, 3, 135–138. [Google Scholar] [CrossRef]
- Mlyuka, N.; Niklasson, G.A.; Granqvist, C.-G. Thermochromic multilayer films of VO2 and TiO2 with enhanced transmittance. Sol. Energy Mater. Sol. Cells 2009, 93, 1685–1687. [Google Scholar] [CrossRef]
- Ke, Y.; Chen, J.; Lin, G.; Wang, S.; Zhou, Y.; Yin, J.; Lee, P.S.; Long, Y. Smart windows: Electro-, thermo-, mechano-, photochromics, and beyond. Adv. Energy Mater. 2019, 9, 1902066. [Google Scholar] [CrossRef]
- Cao, X.; Chang, T.; Shao, Z.; Xu, F.; Luo, H.; Jin, P. Challenges and opportunities toward real application of VO2-based smart glazing. Matter 2020, 2, 862–881. [Google Scholar] [CrossRef]
- Chang, Q.; Wang, D.; Zhao, Z.; Ling, C.; Wang, C.; Jin, H.; Li, J. Size-Controllable M-Phase VO2 Nanocrystals for Flexible Thermochromic Energy-Saving Windows. ACS Appl. Nano Mater. 2021, 4, 6778–6785. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, S.; Kang, L.; Chen, Z.; Du, J.; Liu, X.; Luo, H.; Kanehira, M. VO2–Sb: SnO2 composite thermochromic smart glass foil. Energy Environ. Sci. 2012, 5, 8234–8237. [Google Scholar] [CrossRef]
- Choi, Y.; Sim, D.M.; Hur, Y.H.; Han, H.J.; Jung, Y.S. Synthesis of colloidal VO2 nanoparticles for thermochromic applications. Sol. Energy Mater. Sol. Cells 2018, 176, 266–272. [Google Scholar] [CrossRef]
- Manca, N.; Pellegrino, L.; Kanki, T.; Venstra, W.J.; Mattoni, G.; Higuchi, Y.; Tanaka, H.; Caviglia, A.D.; Marré, D. Selective high-frequency mechanical actuation driven by the VO2 electronic instability. Adv. Mater. 2017, 29, 1701618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warwick, M.E.; Ridley, I.; Binions, R. Thermochromic vanadium dioxide thin films prepared by electric field assisted atmospheric pressure chemical vapour deposition for intelligent glazing application and their energy demand reduction properties. Sol. Energy Mater. Sol. Cells 2016, 157, 686–694. [Google Scholar] [CrossRef]
- Jiazhen, Y.; Yue, Z.; Wanxia, H.; Mingjin, T. Effect of Mo-W Co-doping on semiconductor-metal phase transition temperature of vanadium dioxide film. Thin Solid Films 2008, 516, 8554–8558. [Google Scholar] [CrossRef]
- Warwick, M.E.; Binions, R. Thermochromic vanadium dioxide thin films from electric field assisted aerosol assisted chemical vapour deposition. Sol. Energy Mater. Sol. Cells 2015, 143, 592–600. [Google Scholar] [CrossRef]
- Gagaoudakis, E.; Michail, G.; Aperathitis, E.; Kortidis, I.; Binas, V.; Panagopoulou, M.; Raptis, Y.S.; Tsoukalas, D.; Kiriakidis, G. Low temperature rf-sputtered thermochromic VO2 films on flexible glass substrates. Adv. Mater. Lett. 2017, 8, 757–761. [Google Scholar] [CrossRef]
- Bukhari, S.A.; Kumar, S.; Kumar, P.; Gumfekar, S.P.; Chung, H.-J.; Thundat, T.; Goswami, A. The effect of oxygen flow rate on metal–insulator transition (MIT) characteristics of vanadium dioxide (VO2) thin films by pulsed laser deposition (PLD). Appl. Surf. Sci 2020, 529, 146995. [Google Scholar] [CrossRef]
- Garry, G.; Durand, O.; Lordereau, A. Structural, electrical and optical properties of pulsed laser deposited VO2 thin films on R-and C-sapphire planes. Thin Solid Films 2004, 453, 427–430. [Google Scholar] [CrossRef]
- Chae, J.-Y.; Lee, D.; Woo, H.-Y.; Kim, J.B.; Paik, T. Direct transfer of thermochromic tungsten-doped vanadium dioxide thin-films onto flexible polymeric substrates. Appl. Surf. Sci 2021, 545, 148937. [Google Scholar] [CrossRef]
- Lee, W.S.; Jeon, S.; Oh, S.J. Wearable sensors based on colloidal nanocrystals. Nano Converg. 2019, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, A.; Li, Y.; Ji, S.; Gao, Y.; Jin, P. Surface plasmon resonance induced excellent solar control for VO2@SiO2 nanorods-based thermochromic foils. Nanoscale 2013, 5, 9208–9213. [Google Scholar] [CrossRef]
- Guo, D.; Ling, C.; Wang, C.; Wang, D.; Li, J.; Zhao, Z.; Wang, Z.; Zhao, Y.; Zhang, J.; Jin, H. Hydrothermal one-step synthesis of highly dispersed M-phase VO2 nanocrystals and application to flexible thermochromic film. ACS Appl. Mater. Interfaces 2018, 10, 28627–28634. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Gao, Y.; Luo, H. Pure single-crystal rutile vanadium dioxide powders: Synthesis, mechanism and phase-transformation property. J. Phys. Chem. C 2008, 112, 18810–18814. [Google Scholar] [CrossRef]
- Zhang, J.; He, H.; Xie, Y.; Pan, B. Theoretical study on the tungsten-induced reduction of transition temperature and the degradation of optical properties for VO2. J. Chem. Phys. 2013, 138, 114705. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, Y.; Kang, L.; Cao, C.; Chen, S.; Luo, H. Fine crystalline VO2 nanoparticles: Synthesis, abnormal phase transition temperatures and excellent optical properties of a derived VO2 nanocomposite foil. J. Mater. Chem. A 2014, 2, 2718–2727. [Google Scholar] [CrossRef]
- Parkin, I.P.; Manning, T.D. Intelligent thermochromic windows. J. Chem. Educ. 2006, 83, 393. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Yang, C.; Jia, H.; Cui, X.; Zhao, S.; Xu, Y. Mesoporous SiO2/VO2 double-layer thermochromic coating with improved visible transmittance for smart window. Sol. Energy Mater. Sol. Cells 2017, 162, 134–141. [Google Scholar] [CrossRef]
- Howard, S.A.; Evlyukhin, E.; Páez Fajardo, G.; Paik, H.; Schlom, D.G.; Piper, L.F. Digital Tuning of the Transition Temperature of Epitaxial VO2 Thin Films on MgF2 Substrates by Strain Engineering. Adv. Mater. Interfaces 2021, 8, 2001790. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, D.; Song, S.; Kim, J.; Ju, T.S.; Kim, H.; Kim, J.; Yoon, S.; Kim, Y.; Phan, T.B. Correlation between Symmetry and Phase Transition Temperature of VO2 Films Deposited on Al2O3 Substrates with Various Orientations. Adv. Electron. Mater. 2021, 7, 2000874. [Google Scholar] [CrossRef]
- Yan, J.; Huang, W.; Zhang, Y.; Liu, X.; Tu, M. Characterization of preferred orientated vanadium dioxide film on muscovite (001) substrate. Phys. Status Solidi A 2008, 205, 2409–2412. [Google Scholar] [CrossRef]
- Li, C.-I.; Lin, J.-C.; Liu, H.-J.; Chu, M.-W.; Chen, H.-W.; Ma, C.-H.; Tsai, C.-Y.; Huang, H.-W.; Lin, H.-J.; Liu, H.-L. Van der Waal epitaxy of flexible and transparent VO2 film on muscovite. Chem. Mater. 2016, 28, 3914–3919. [Google Scholar] [CrossRef]
- Geim, A.K.; Grigorieva, I.V. Van der Waals heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef]
- Novoselov, K.; Mishchenko, O.A.; Carvalho, O.A.; Neto, A.C. 2D materials and van der Waals heterostructures. Science 2016, 353, aac9439. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Huang, Y.; Duan, X. Van der Waals integration before and beyond two-dimensional materials. Nature 2019, 567, 323–333. [Google Scholar] [CrossRef]
- Liang, W.; Jiang, Y.; Guo, J.; Li, N.; Qiu, W.; Yang, H.; Ji, Y.; Luo, S.N. Van der Waals heteroepitaxial VO2/mica films with extremely low optical trigger threshold and large THz field modulation depth. Adv. Opt. Mater. 2019, 7, 1900647. [Google Scholar] [CrossRef]
- Wang, J.N.; Xiong, B.; Peng, R.W.; Li, C.Y.; Hou, B.Q.; Chen, C.W.; Liu, Y.; Wang, M. Flexible Phase Change Materials for Electrically-Tuned Active Absorbers. Small 2021, 17, 2101282. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, L.; Fang, Q.; Xu, W.; Chen, S.; Zan, G.; Ren, H.; Song, L.; Zou, C. Free-standing SWNTs/VO2/Mica hierarchical films for high-performance thermochromic devices. Nano Energy 2017, 31, 144–151. [Google Scholar] [CrossRef]
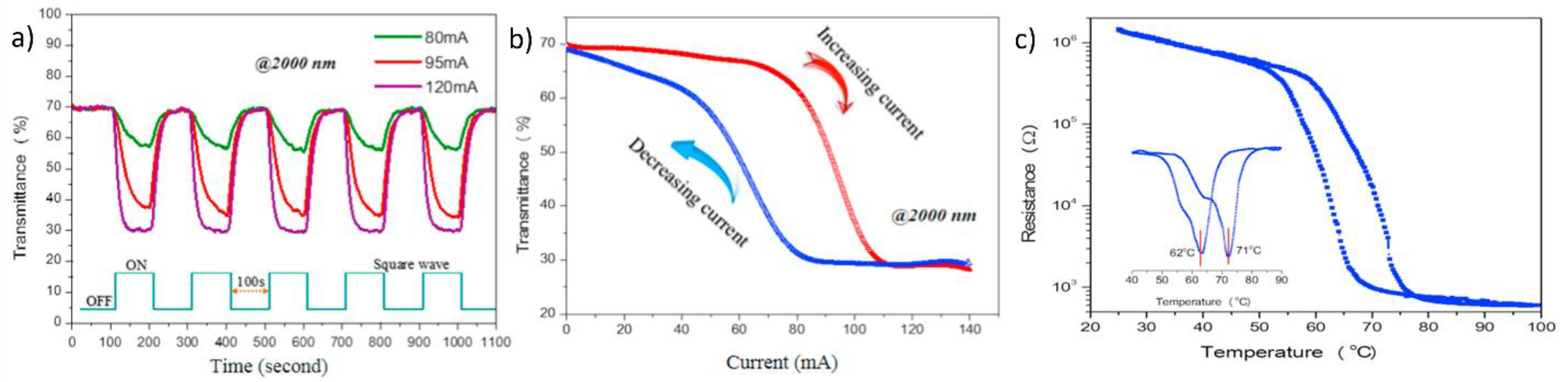
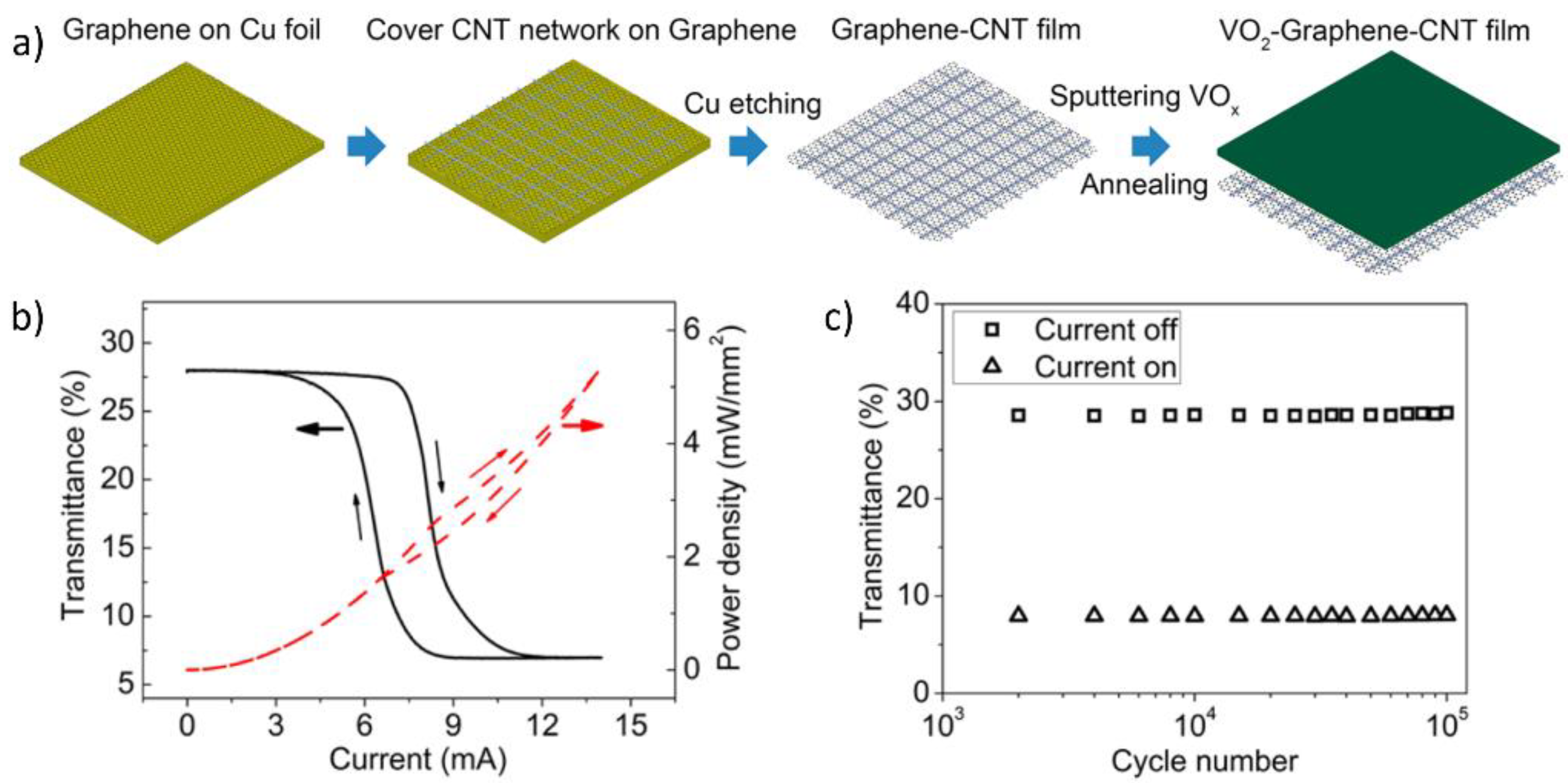
- Xiao, L.; Ma, H.; Liu, J.; Zhao, W.; Jia, Y.; Zhao, Q.; Liu, K.; Wu, Y.; Wei, Y.; Fan, S. Fast adaptive thermal camouflage based on flexible VO2/graphene/CNT thin films. Nano Lett. 2015, 15, 8365–8370. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Zhu, Y.; Huang, J.; Luo, H.; Jin, P.; Cao, X. Flexible VO2 thermochromic films with narrow hysteresis loops. Sol. Energy Mater. Sol. Cells 2021, 219, 110799. [Google Scholar] [CrossRef]
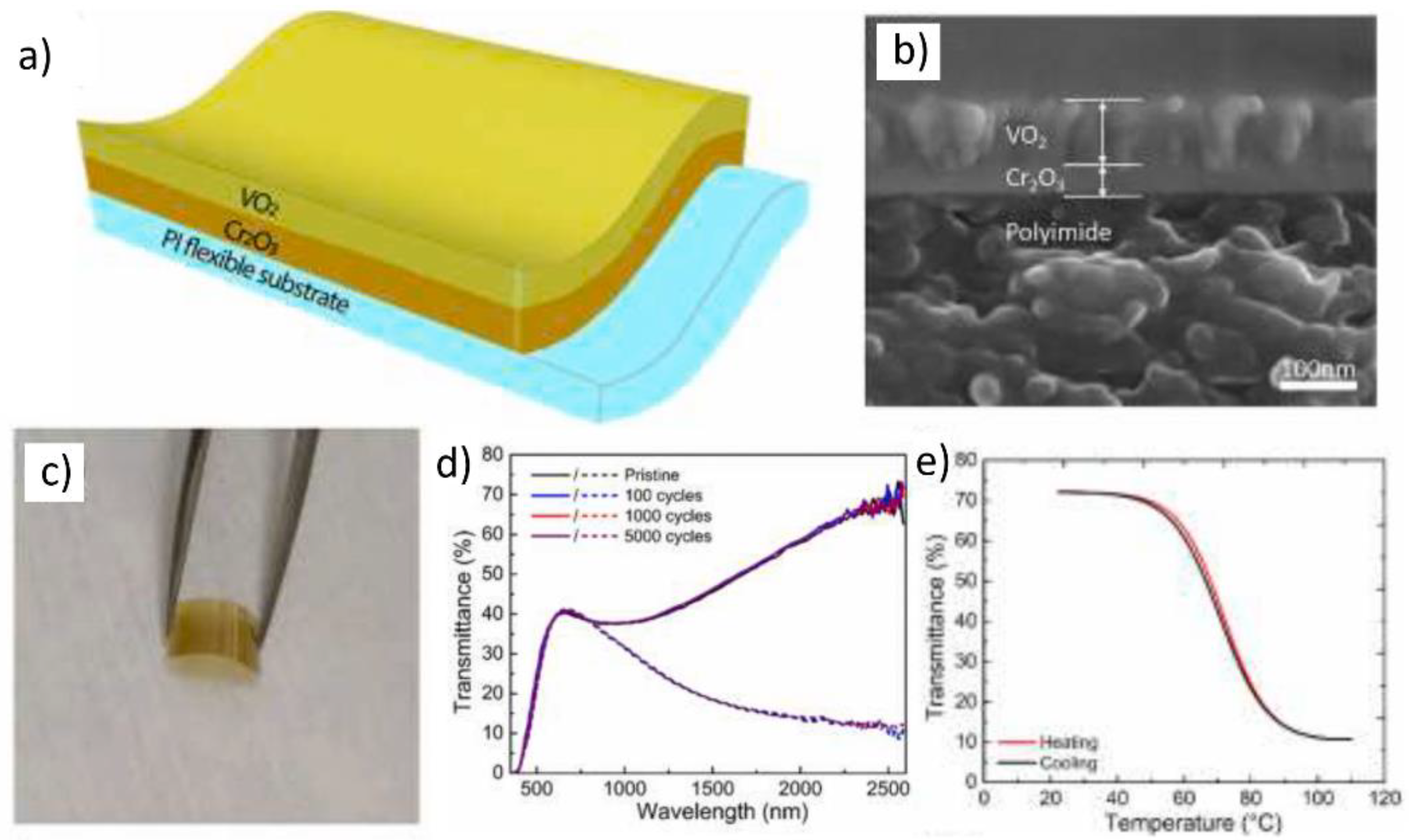
- Chang, T.; Cao, X.; Li, N.; Long, S.; Gao, X.; Dedon, L.R.; Sun, G.; Luo, H.; Jin, P. Facile and low-temperature fabrication of thermochromic Cr2O3/VO2 smart coatings: Enhanced solar modulation ability, high luminous transmittance and UV-shielding function. ACS Appl. Mater. Interfaces 2017, 9, 26029–26037. [Google Scholar] [CrossRef]
- Martins, L.G.; Song, Y.; Zeng, T.; Dresselhaus, M.S.; Kong, J.; Araujo, P.T. Direct transfer of graphene onto flexible substrates. Proc. Natl. Acad. Sci. USA 2013, 110, 17762–17767. [Google Scholar] [CrossRef] [Green Version]
- Malarde, D.; Powell, M.J.; Quesada-Cabrera, R.; Wilson, R.L.; Carmalt, C.J.; Sankar, G.; Parkin, I.P.; Palgrave, R.G. Optimized atmospheric-pressure chemical vapor deposition thermochromic VO2 thin films for intelligent window applications. ACS Omega 2017, 2, 1040–1046. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kim, Y.; Kim, K.S.; Jeong, H.Y.; Jang, A.-R.; Han, S.H.; Yoon, D.H.; Suh, K.S.; Shin, H.S.; Kim, T. Flexible thermochromic window based on hybridized VO2/graphene. ACS Nano 2013, 7, 5769–5776. [Google Scholar] [CrossRef]
- Paik, T.; Hong, S.-H.; Gaulding, E.A.; Caglayan, H.; Gordon, T.R.; Engheta, N.; Kagan, C.R.; Murray, C.B. Solution-processed phase-change VO2 metamaterials from colloidal vanadium oxide (VOx) nanocrystals. ACS Nano 2014, 8, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, C.; Gupta, D. Large area flexible pressure/strain sensors and arrays using nanomaterials and printing techniques. Nano Converg. 2019, 6, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Liu, M.; Kong, L.; Long, Y.; Jiang, X.; Yu, A. Recent progress in VO2 smart coatings: Strategies to improve the thermochromic properties. Prog. Mater. Sci. 2016, 81, 1–54. [Google Scholar] [CrossRef]
- Chae, B.-G.; Kim, H.-T.; Yun, S.-J.; Kim, B.-J.; Lee, Y.-W.; Youn, D.-H.; Kang, K.-Y. Highly oriented VO2 thin films prepared by sol-gel deposition. Electrochem. Solid-State Lett. 2005, 9, C12. [Google Scholar] [CrossRef]
- Speck, K.; Hu, H.-W.; Sherwin, M.; Potember, R. Vanadium dioxide films grown from vanadium tetra-isopropoxide by the sol-gel process. Thin Solid Films 1988, 165, 317–322. [Google Scholar] [CrossRef]
- Cao, X.; Wang, N.; Law, J.Y.; Loo, S.C.J.; Magdassi, S.; Long, Y. Nanoporous thermochromic VO2 (M) thin films: Controlled porosity, largely enhanced luminous transmittance and solar modulating ability. Langmuir 2014, 30, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.-R.; Lee, W.-J.; Yoon, M.-H.; Kim, B.-J. In Situ Tracking of Low-Temperature VO2 Crystallization via Photocombustion and Characterization of Phase-Transition Reliability on Large-Area Flexible Substrates. Chem. Mater. 2020, 32, 4013–4023. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, H.; Chen, Z.; Cao, M.; Chen, P.; Dou, Y.; Zhao, Y.; Li, J. Self-assembling VO2 nanonet with high switching performance at wafer-scale. Chem. Mater. 2015, 27, 7419–7424. [Google Scholar] [CrossRef]
- Zhong, L.; Luo, Y.; Li, M.; Han, Y.; Wang, H.; Xu, S.; Li, G. TiO2 seed-assisted growth of VO2 (M) films and thermochromic performance. CrystEngComm 2016, 18, 7140–7146. [Google Scholar] [CrossRef]
- Makarevich, A.; Makarevich, O.; Ivanov, A.; Sharovarov, D.; Eliseev, A.; Amelichev, V.; Boytsova, O.; Gorodetsky, A.; Navarro-Cia, M.; Kaul, A. Hydrothermal epitaxy growth of self-organized vanadium dioxide 3D structures with metal–insulator transition and THz transmission switch properties. CrystEngComm 2020, 22, 2612–2620. [Google Scholar] [CrossRef]
- Guo, D.; Zhao, Z.; Li, J.; Zhang, J.; Zhang, R.; Wang, Z.; Chen, P.; Zhao, Y.; Chen, Z.; Jin, H. Symmetric confined growth of superstructured vanadium dioxide nanonet with a regular geometrical pattern by a solution approach. Cryst. Growth Des. 2017, 17, 5838–5844. [Google Scholar] [CrossRef]
- Melnik, V.; Khatsevych, I.; Kladko, V.; Kuchuk, A.; Nikirin, V.; Romanyuk, B. Low-temperature method for thermochromic high ordered VO2 phase formation. Mater. Lett. 2012, 68, 215–217. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Makarevich, O.N.; Boytsova, O.V.; Tsymbarenko, D.M.; Eliseev, A.A.; Amelichev, V.A.; Makarevich, A.M. Citrate-assisted hydrothermal synthesis of vanadium dioxide textured films with metal-insulator transition and infrared thermochromic properties. Ceram. Int. 2020, 46, 19919–19927. [Google Scholar] [CrossRef]
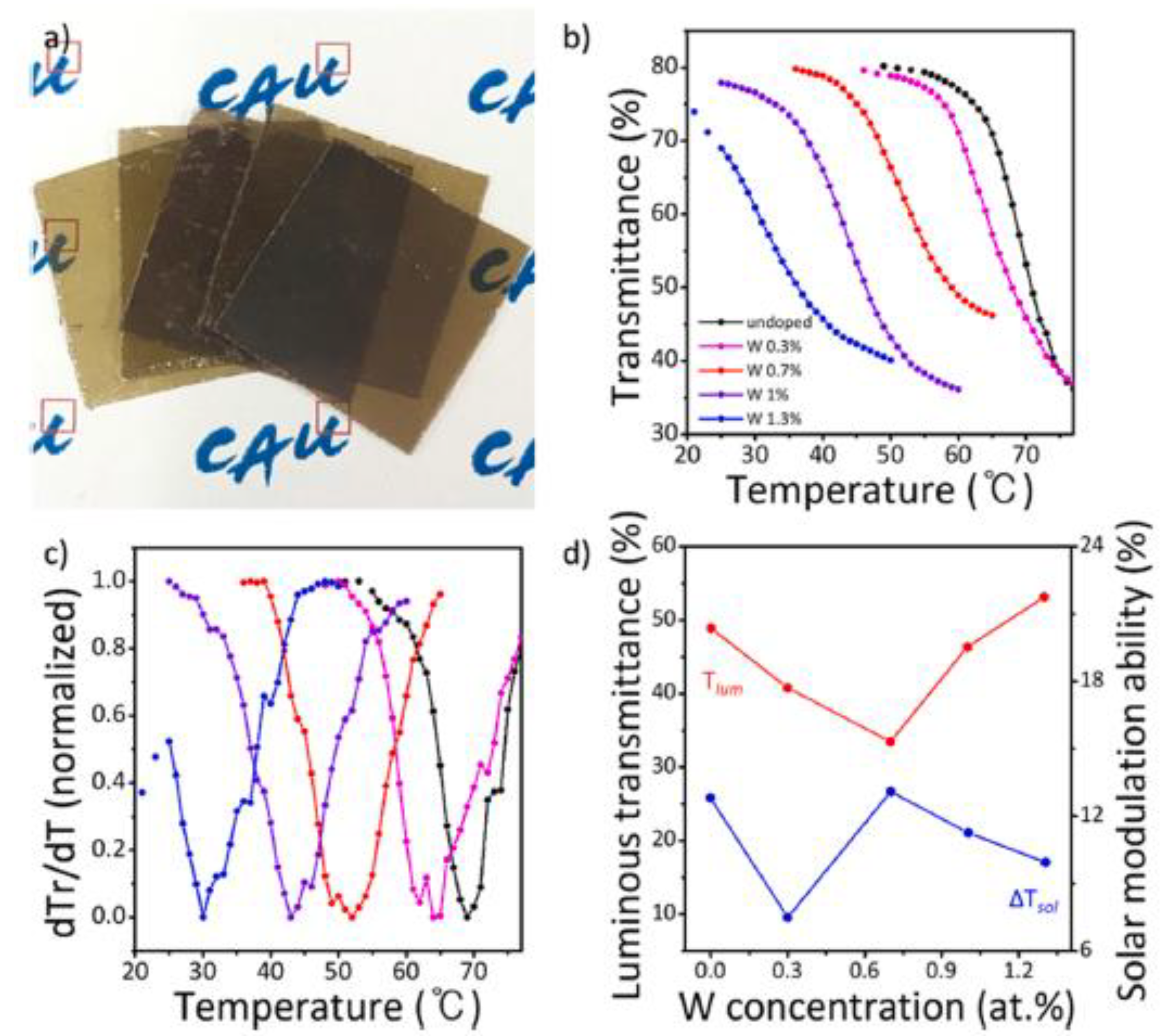
- Zhao, Z.; Liu, Y.; Yu, Z.; Ling, C.; Li, J.; Zhao, Y.; Jin, H. Sn–W Co-doping Improves Thermochromic Performance of VO2 Films for Smart Windows. ACS Appl. Energy Mater 2020, 3, 9972–9979. [Google Scholar] [CrossRef]
- Théobald, F. Étude hydrothermale du système VO2-VO2, 5-H2O. J. Less-Common Met. 1977, 53, 55–71. [Google Scholar] [CrossRef]
- Shi, J.; Zhou, S.; You, B.; Wu, L. Preparation and thermochromic property of tungsten-doped vanadium dioxide particles. Sol. Energy Mater. Sol. Cells 2007, 91, 1856–1862. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, K.; Gao, Y.; Wu, Q.; Liu, J.; Qiu, J.; Gu, Q.; Zheng, H. Ultra-long VO2 (A) nanorods using the high-temperature mixing method under hydrothermal conditions: Synthesis, evolution and thermochromic properties. CrystEngComm 2013, 15, 2753–2760. [Google Scholar] [CrossRef]
- Liu, L.; Cao, F.; Yao, T.; Xu, Y.; Zhou, M.; Qu, B.; Pan, B.; Wu, C.; Wei, S.; Xie, Y. New-phase VO2 micro/nanostructures: Investigation of phase transformation and magnetic property. New J. Chem. 2012, 36, 619–625. [Google Scholar] [CrossRef]
- Wu, C.; Hu, Z.; Wang, W.; Zhang, M.; Yang, J.; Xie, Y. Synthetic paramontroseite VO2 with good aqueous lithium–ion battery performance. Chem. Commun. 2008, 3891–3893. [Google Scholar] [CrossRef]
- Shen, N.; Chen, S.; Chen, Z.; Liu, X.; Cao, C.; Dong, B.; Luo, H.; Liu, J.; Gao, Y. The synthesis and performance of Zr-doped and W–Zr-codoped VO2 nanoparticles and derived flexible foils. J. Mater. Chem. A 2014, 2, 15087–15093. [Google Scholar] [CrossRef]
- Popuri, S.R.; Miclau, M.; Artemenko, A.; Labrugere, C.; Villesuzanne, A.; Pollet, M. Rapid hydrothermal synthesis of VO2 (B) and its conversion to thermochromic VO2 (M1). Inorg. Chem. 2013, 52, 4780–4785. [Google Scholar] [CrossRef]
- Corr, S.A.; Grossman, M.; Shi, Y.; Heier, K.R.; Stucky, G.D.; Seshadri, R. VO2 (B) nanorods: Solvothermal preparation, electrical properties, and conversion to rutile VO2 and V2O3. J. Mater. Chem. 2009, 19, 4362–4367. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, S.; Bi, W.; Long, R.; Tan, X.; Wu, C.; Wei, S.; Xie, Y. New aspects of size-dependent metal-insulator transition in synthetic single-domain monoclinic vanadium dioxide nanocrystals. Nanoscale 2011, 3, 4394–4401. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Li, M.; Wang, H.; Luo, Y.; Pan, J.; Li, G. Star-shaped VO2 (M) nanoparticle films with high thermochromic performance. CrystEngComm 2015, 17, 5614–5619. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, L.; Xia, F.; Webster, N.A.; Song, J.; Liu, B.; Luo, H.; Gao, Y. Controllable synthesis of VO2 (D) and their conversion to VO2 (M) nanostructures with thermochromic phase transition properties. Inorg. Chem. Front. 2016, 3, 1035–1042. [Google Scholar] [CrossRef]
- Li, M.; Ji, S.; Pan, J.; Wu, H.; Zhong, L.; Wang, Q.; Li, F.; Li, G. Infrared response of self-heating VO2 nanoparticles film based on Ag nanowires heater. J. Mater. Chem. A 2014, 2, 20470–20473. [Google Scholar] [CrossRef]
- Li, M.; Wu, X.; Li, L.; Wang, Y.; Li, D.; Pan, J.; Li, S.; Sun, L.; Li, G. Defect-mediated phase transition temperature of VO 2 (M) nanoparticles with excellent thermochromic performance and low threshold voltage. J. Mater. Chem. A 2014, 2, 4520–4523. [Google Scholar] [CrossRef]
- Chen, R.; Miao, L.; Liu, C.; Zhou, J.; Cheng, H.; Asaka, T.; Iwamoto, Y.; Tanemura, S. Shape-controlled synthesis and influence of W doping and oxygen nonstoichiometry on the phase transition of VO2. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Narayan, J.; Bhosle, V. Phase transition and critical issues in structure-property correlations of vanadium oxide. J. Appl. Phys 2006, 100, 103524. [Google Scholar] [CrossRef]
- Zeng, W.; Chen, N.; Xie, W. Research progress on the preparation methods for VO2 nanoparticles and their application in smart windows. CrystEngComm 2020, 22, 851–869. [Google Scholar] [CrossRef]
- Lopez, R.; Feldman, L.C.; Haglund Jr, R.F. Size-Dependent Optical Properties of VO2 Nanoparticle Arrays. Phys. Rev. Lett. 2004, 93, 177403. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Li, Y.; Wang, D.; Ren, L.; Zou, K. Effect of annealing temperature on the structure and properties of vanadium oxide films. Opt. Mater. Express 2016, 6, 1552–1560. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Z.; Wu, X.; Yang, W.; Jiang, Y. Transversal grain size effect on the phase-transition hysteresis width of vanadium dioxide films comprising spheroidal nanoparticles. Vacuum 2014, 104, 47–50. [Google Scholar] [CrossRef]
- Alie, D.; Gedvilas, L.; Wang, Z.; Tenent, R.; Engtrakul, C.; Yan, Y.; Shaheen, S.E.; Dillon, A.C.; Ban, C. Direct synthesis of thermochromic VO2 through hydrothermal reaction. J. Solid State Chem. 2014, 212, 237–241. [Google Scholar] [CrossRef]
- Li, W.; Ji, S.; Sun, G.; Ma, Y.; Guo, H.; Jin, P. Novel VO2 (M)–ZnO heterostructured dandelions with combined thermochromic and photocatalytic properties for application in smart coatings. New J. Chem. 2016, 40, 2592–2600. [Google Scholar] [CrossRef]
- Ji, S.; Zhang, F.; Jin, P. Preparation of high performance pure single phase VO2 nanopowder by hydrothermally reducing the V2O5 gel. Sol. Energy Mater. Sol. Cells 2011, 95, 3520–3526. [Google Scholar] [CrossRef]
- Chen, R.; Miao, L.; Cheng, H.; Nishibori, E.; Liu, C.; Asaka, T.; Iwamoto, Y.; Takata, M.; Tanemura, S. One-step hydrothermal synthesis of V1− xWxO2 (M/R) nanorods with superior doping efficiency and thermochromic properties. J. Mater. Chem. A 2015, 3, 3726–3738. [Google Scholar] [CrossRef]
- Kim, J.B.; Lee, D.; Yeo, I.H.; Woo, H.Y.; Kim, D.W.; Chae, J.-Y.; Han, S.H.; Paik, T. Hydrothermal synthesis of monoclinic vanadium dioxide nanocrystals using phase-pure vanadium precursors for high-performance smart windows. Sol. Energy Mater. Sol. Cells 2021, 226, 111055. [Google Scholar] [CrossRef]
- Dahiya, A.S.; Shakthivel, D.; Kumaresan, Y.; Zumeit, A.; Christou, A.; Dahiya, R. High-performance printed electronics based on inorganic semiconducting nano to chip scale structures. Nano Converg. 2020, 7, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Niklasson, G.A.; Granqvist, C.-G. Nanothermochromics with VO2-based core-shell structures: Calculated luminous and solar optical properties. J. Appl. Phys 2011, 109, 113515. [Google Scholar] [CrossRef] [Green Version]
- Shen, N.; Chen, S.; Wang, W.; Shi, R.; Chen, P.; Kong, D.; Liang, Y.; Amini, A.; Wang, J.; Cheng, C. Joule heating driven infrared switching in flexible VO2 nanoparticle films with reduced energy consumption for smart windows. J. Mater. Chem. A 2019, 7, 4516–4524. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, Y.; Liu, X.; Chen, Z.; Dai, L.; Cao, C.; Luo, H.; Kanahira, M.; Sun, C.; Yan, L. Mg-doped VO2 nanoparticles: Hydrothermal synthesis, enhanced visible transmittance and decreased metal–insulator transition temperature. Phys. Chem. Chem. Phys. 2013, 15, 7505–7511. [Google Scholar] [CrossRef]
- Nam, V.B.; Giang, T.T.; Koo, S.; Rho, J.; Lee, D. Laser digital patterning of conductive electrodes using metal oxide nanomaterials. Nano Converg. 2020, 7, 1–17. [Google Scholar] [CrossRef]
- Yang, S.; Vaseem, M.; Shamim, A. Fully inkjet-printed VO2-based radio-frequency switches for flexible reconfigurable components. Adv. Mater. Technol. 2019, 4, 1800276. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Liu, D.; Cheng, H.; Zhang, C. Inkjet printing of vanadium dioxide nanoparticles for smart windows. J. Mater. Chem. C 2018, 6, 2424–2429. [Google Scholar] [CrossRef]
- Ji, H.; Liu, D.; Cheng, H.; Tao, Y. Large area infrared thermochromic VO2 nanoparticle films prepared by inkjet printing technology. Sol. Energy Mater. Sol. Cells 2019, 194, 235–243. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, F.; Wang, J.; Khan, A.R.; Shi, Y.; Chen, Z.; Zhang, K.; Li, L.; Gao, Y.; Guo, X. VO2@SiO2/Poly(N-isopropylacrylamide) hybrid nanothermochromic microgels for smart window. Ind. Eng. Chem. Res. 2018, 57, 12801–12808. [Google Scholar] [CrossRef]
- Ji, H.; Liu, D.; Zhang, C.; Cheng, H. VO2/ZnS core-shell nanoparticle for the adaptive infrared camouflage application with modified color and enhanced oxidation resistance. Sol. Energy Mater. Sol. Cells 2018, 176, 1–8. [Google Scholar] [CrossRef]
- Wen, Z.; Ke, Y.; Feng, C.; Fang, S.; Sun, M.; Liu, X.; Long, Y. Mg-Doped VO2@ZrO2 Core—Shell Nanoflakes for Thermochromic Smart Windows with Enhanced Performance. Adv. Mater. Interfaces 2021, 8, 2001606. [Google Scholar] [CrossRef]
- Saini, M.; Dehiya, B.S.; Umar, A. VO2 (M)@CeO2 core-shell nanospheres for thermochromic smart windows and photocatalytic applications. Ceram. Int. 2020, 46, 986–995. [Google Scholar] [CrossRef]
- Moot, T.; Palin, C.; Mitran, S.; Cahoon, J.F.; Lopez, R. Designing Plasmon-Enhanced Thermochromic Films Using a Vanadium Dioxide Nanoparticle Elastomeric Composite. Adv. Opt. Mater. 2016, 4, 578–583. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Paik, T. Recent Advances in Fabrication of Flexible, Thermochromic Vanadium Dioxide Films for Smart Windows. Nanomaterials 2021, 11, 2674. https://doi.org/10.3390/nano11102674
Kim J, Paik T. Recent Advances in Fabrication of Flexible, Thermochromic Vanadium Dioxide Films for Smart Windows. Nanomaterials. 2021; 11(10):2674. https://doi.org/10.3390/nano11102674
Chicago/Turabian StyleKim, Jongbae, and Taejong Paik. 2021. "Recent Advances in Fabrication of Flexible, Thermochromic Vanadium Dioxide Films for Smart Windows" Nanomaterials 11, no. 10: 2674. https://doi.org/10.3390/nano11102674
APA StyleKim, J., & Paik, T. (2021). Recent Advances in Fabrication of Flexible, Thermochromic Vanadium Dioxide Films for Smart Windows. Nanomaterials, 11(10), 2674. https://doi.org/10.3390/nano11102674