High-Strength Regenerated Cellulose Fiber Reinforced with Cellulose Nanofibril and Nanosilica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dissolution and Regeneration Processing of Cellulose
2.3. Characterization of Regenerated Fibers
2.3.1. Tensile Properties of Fibers
2.3.2. Contact Angle Test
2.3.3. X-Ray Diffraction (XRD) Analysis
2.3.4. Fourier-Transform Infrared (FTIR) Spectroscopy
2.3.5. Scanning Electron microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDS)
2.3.6. Thermal Analysis
2.3.7. Rheological Measurements
3. Results and Discussion
3.1. Tensile Properties
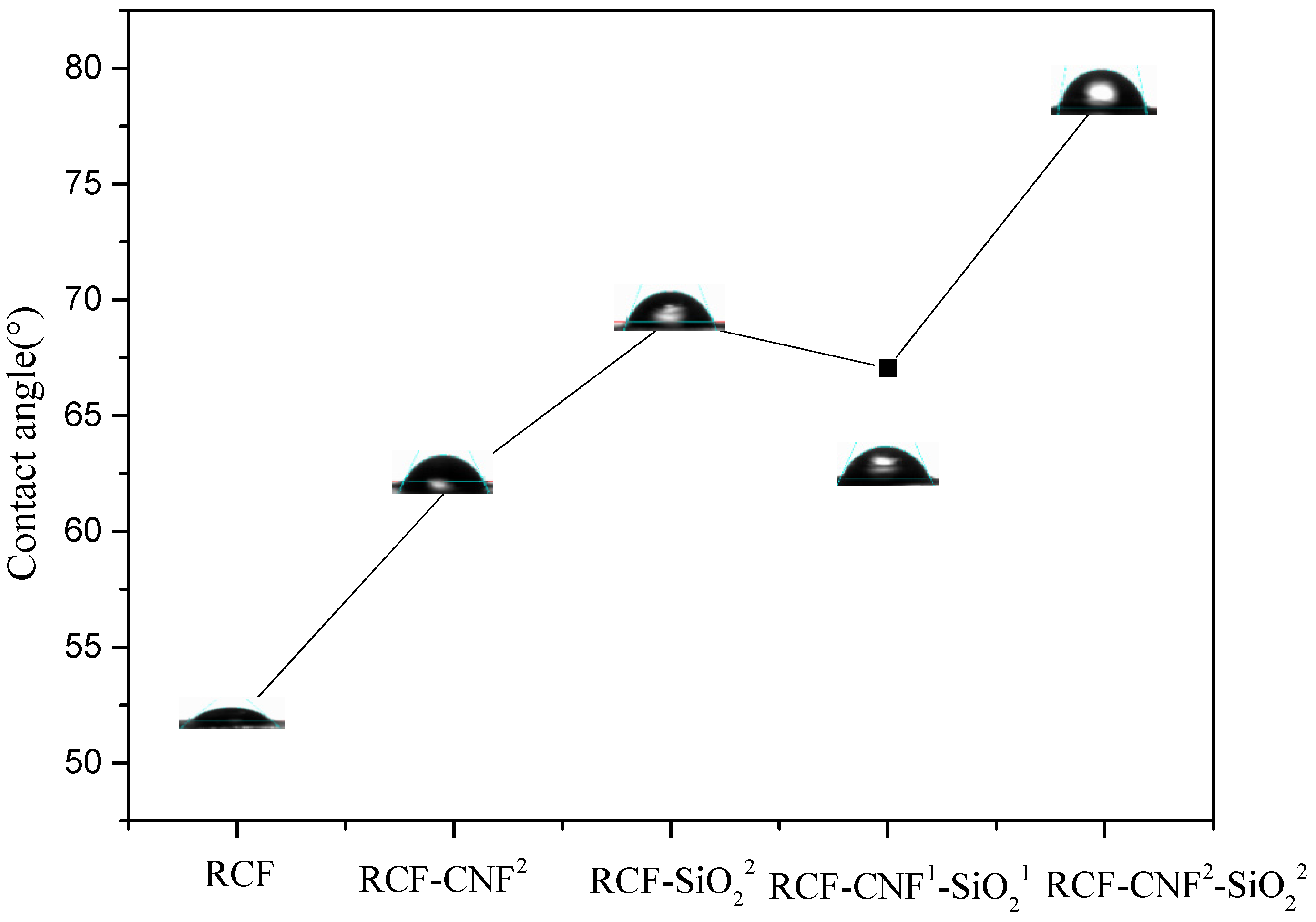
3.2. Water Contact Angle
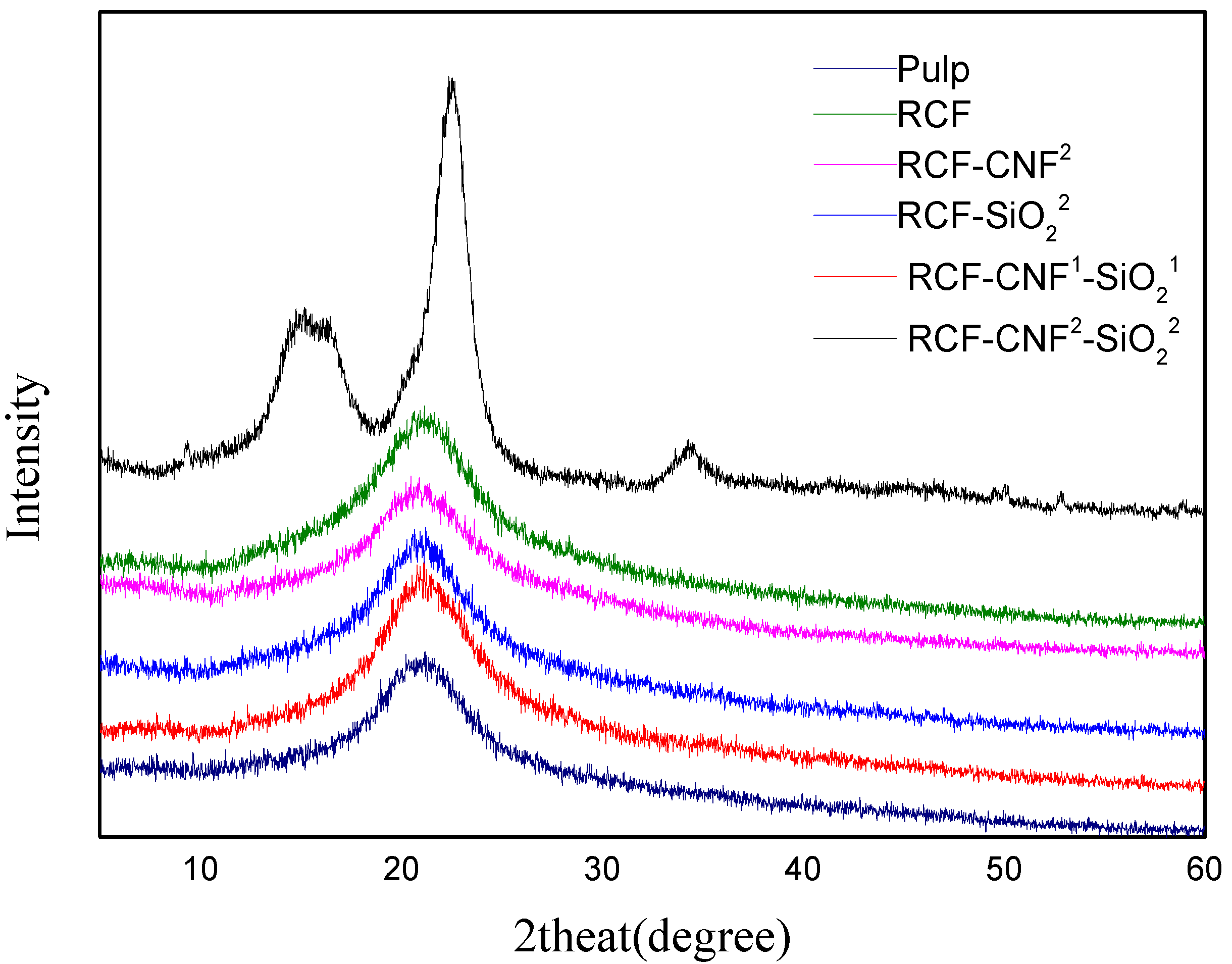
3.3. XRD Analysis
3.4. Fourier-Transform Infrared (FTIR) Spectroscopy Analysis
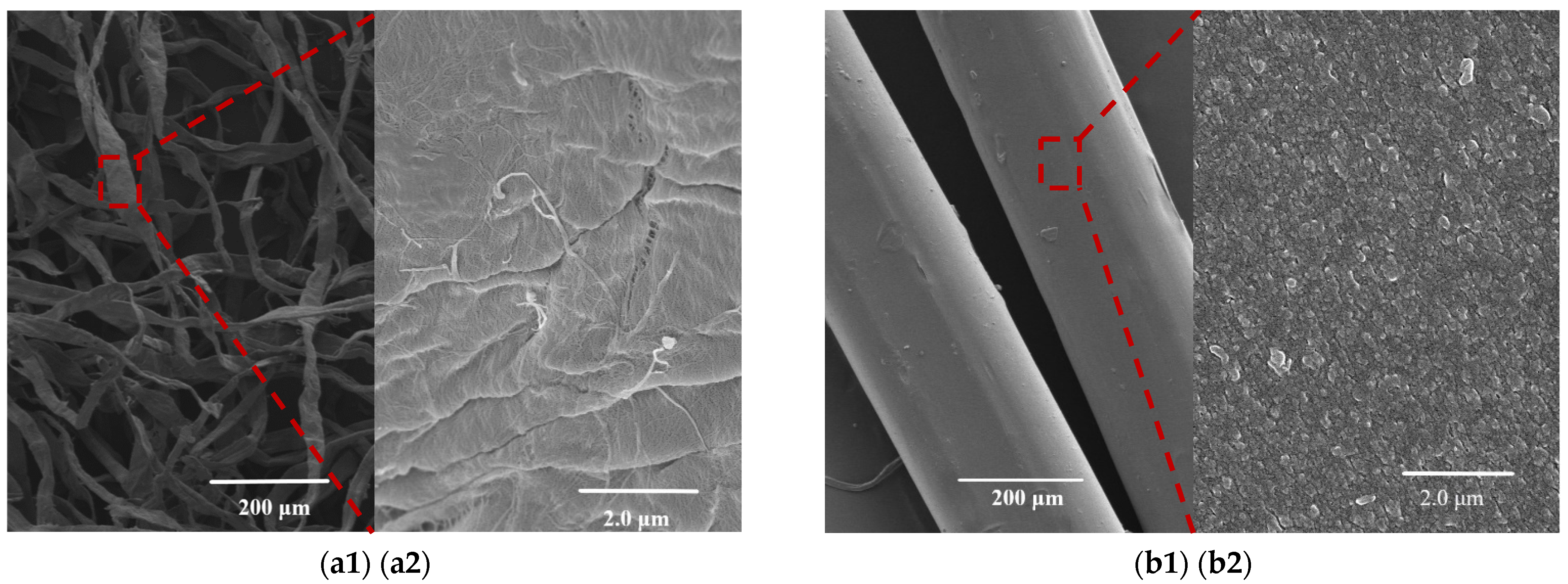
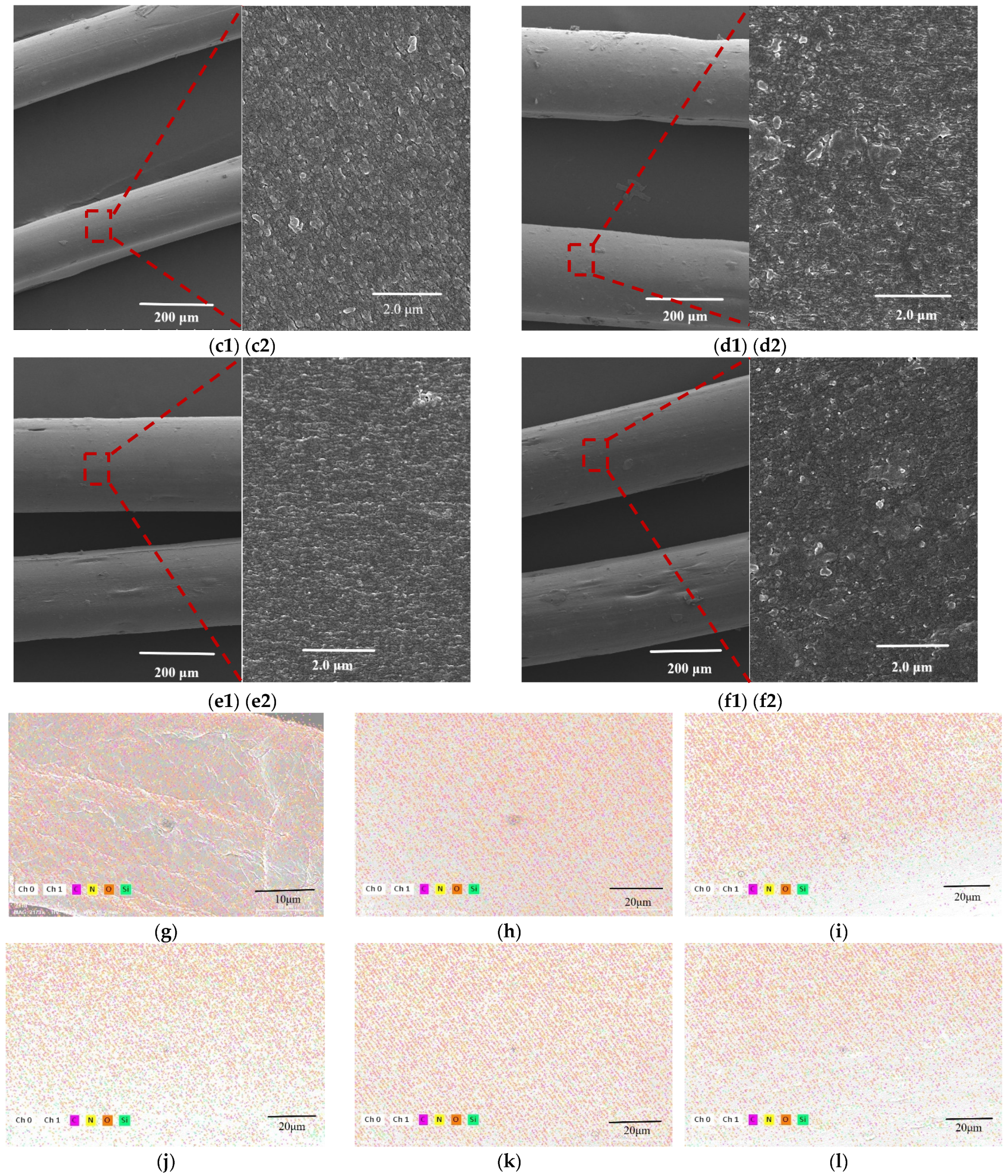
3.5. SEM and EDS Analysis
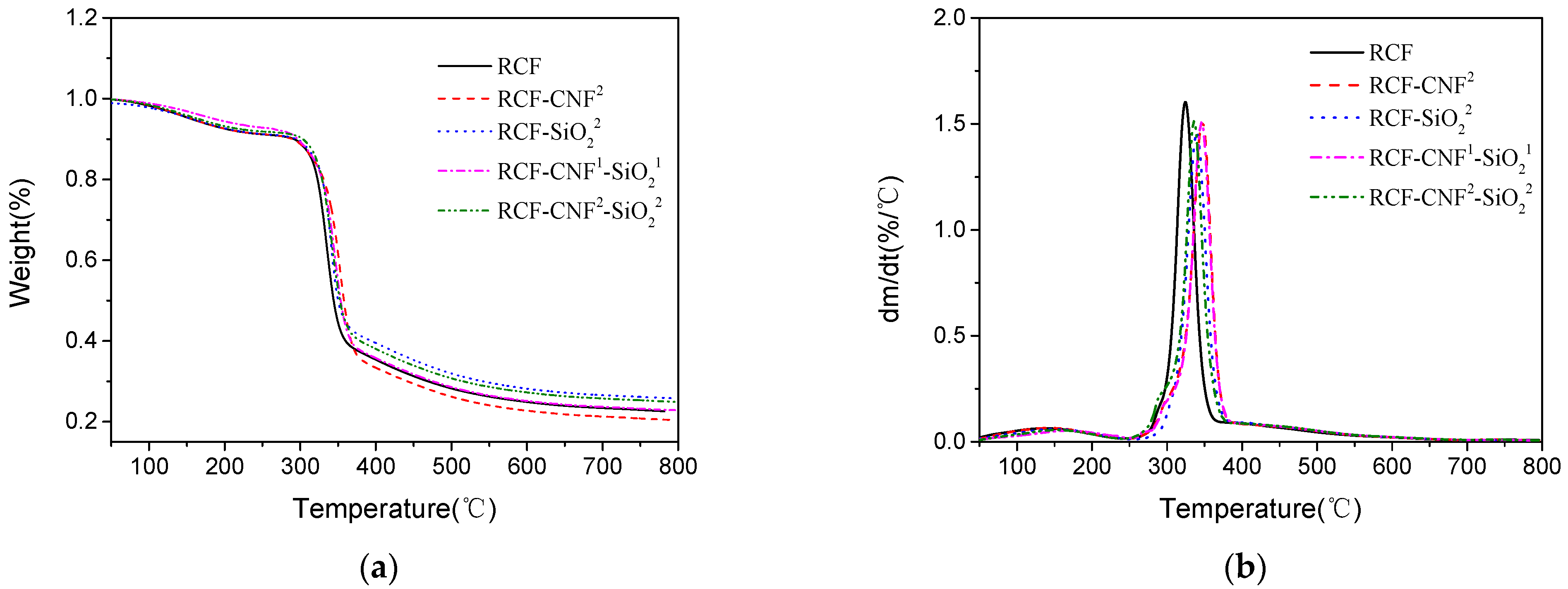
3.6. Thermal Analysis
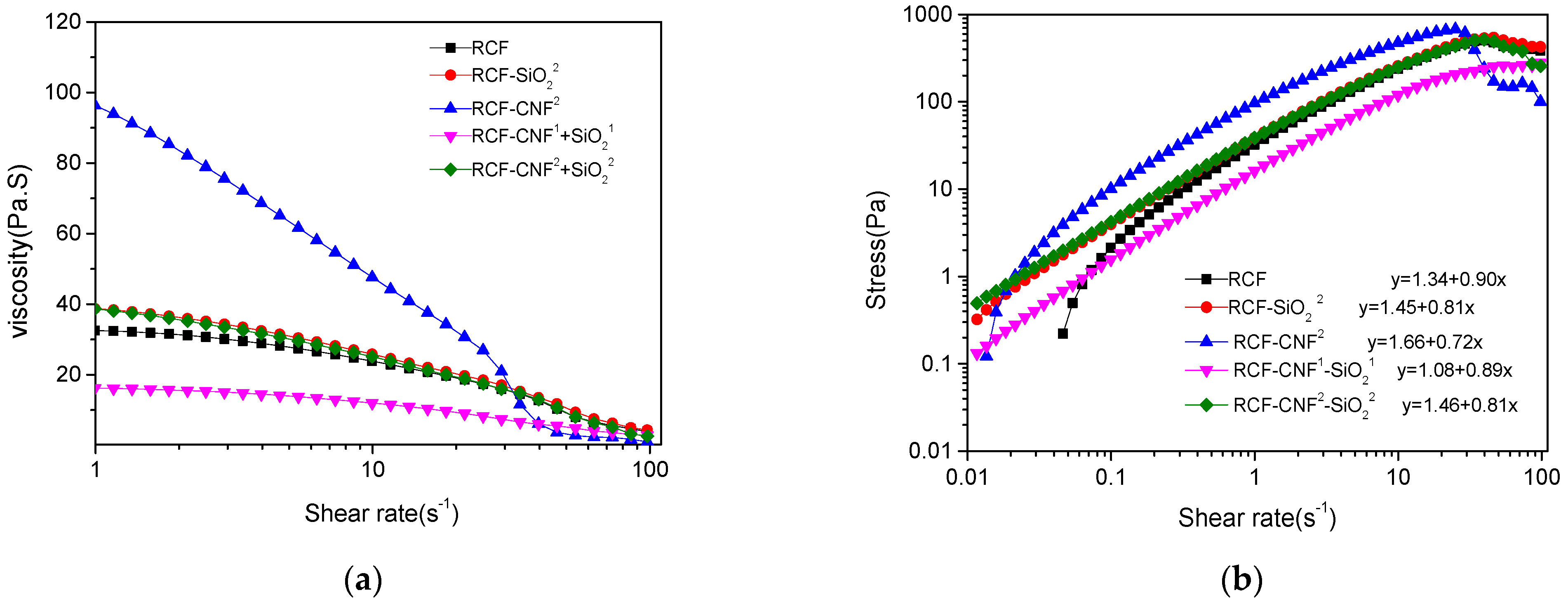
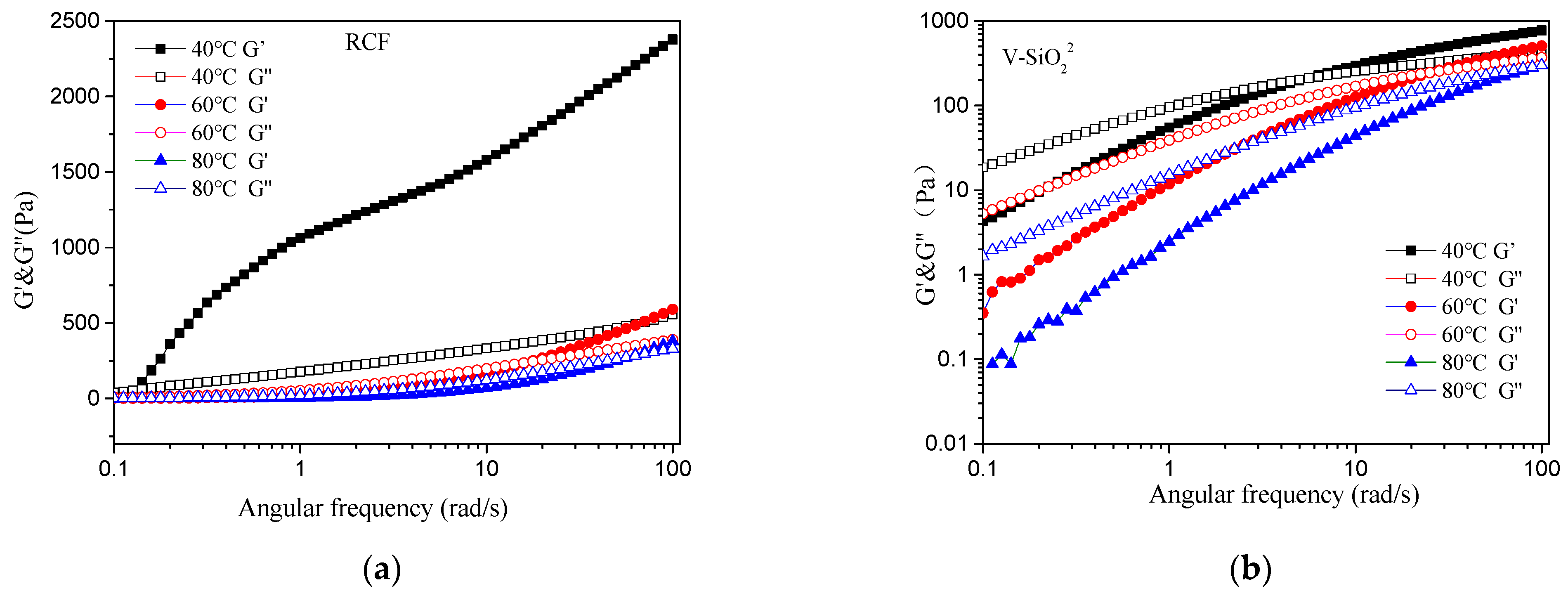
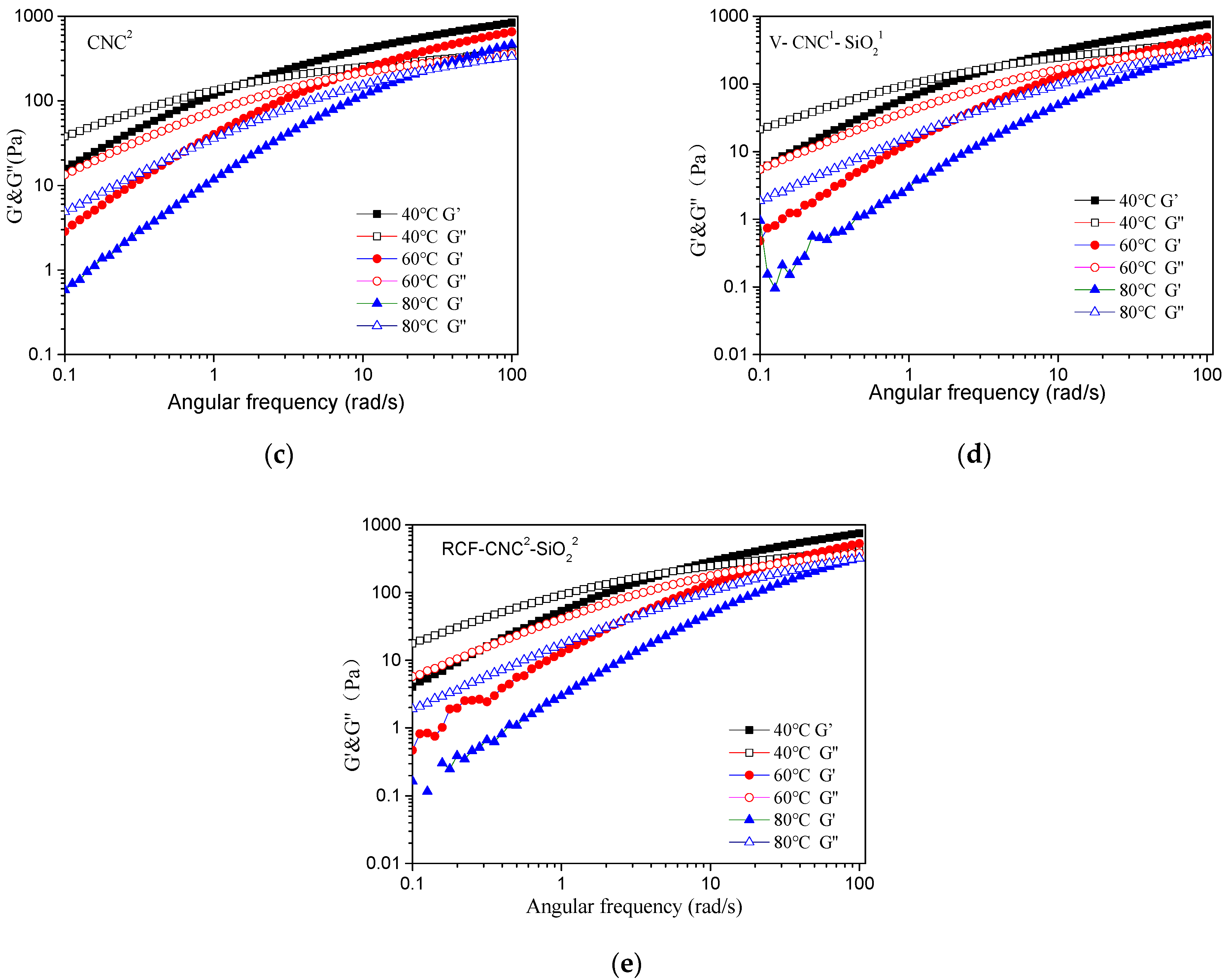
3.7. Rheological Properties of Cellulose/IL Solutions with Different Contents of Nanomaterial
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rojas, O.J. Cellulose chemistry and properties: Fibers, nanocelluloses and advanced materials preface. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Rojas, O.J., Ed.; Springer International Publishing: Cham, Switzerland, 2016; Volume 271, pp. V–VI, 3–9. [Google Scholar]
- Casado, U.; Mucci, V.; Aranguren, M.I. Cellulose nanocrystals suspensions: Liquid crystal anisotropy, rheology and films iridescence. Carbohydr. Polym. 2021, 261, 117848. [Google Scholar] [CrossRef]
- Shen, X.J.; Sun, R.C. Recent advances in lignocellulose prior-fractionation for biomaterials, biochemicals, and bioenergy. Carbohydr. Polym. 2021, 261, 117884. [Google Scholar] [CrossRef]
- Liu, X.; Huang, K.X.; Lin, X.X.; Li, H.X.; Tao, T.; Wu, Q.H.; Zheng, Q.H.; Huang, L.L.; Ni, Y.H.; Chen, L.H.; et al. Transparent and conductive cellulose film by controllably growing aluminum doped zinc oxide on regenerated cellulose film. Cellulose 2020, 27, 4847–4855. [Google Scholar] [CrossRef]
- Yousefi, H.; Faezipour, M.; Hedjazi, S.; Mousavi, M.M.; Azusa, Y.; Heidari, A.H. Comparative study of paper and nanopaper properties prepared from bacterial cellulose nanofibers and fibers/ground cellulose nanofibers of canola straw. Ind. Crop. Prod. 2013, 43, 732–737. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. Engl. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-allyl-3-methylimidazolium chloride room temperature ionic liquid a new and powerful nonderivatizing solvent for cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Protz, R.; Lehmann, A.; Ganster, J.; Fink, H.P. Solubility and spinnability of cellulose-lignin blends in aqueous nmmo. Carbohydr. Polym. 2021, 251, 117027. [Google Scholar] [CrossRef]
- Jadhav, S.; Lidhure, A.; Thakre, S.; Ganvir, V. Modified lyocell process to improve dissolution of cellulosic pulp and pulp blends in nmmo solvent. Cellulose 2021, 28, 973–990. [Google Scholar] [CrossRef]
- Yasin, S.; Hussain, M.; Zheng, Q.; Song, Y.H. Effects of ionic liquid on cellulosic nanofiller filled natural rubber bionanocomposites. J. Colloid Interface Sci. 2021, 591, 409–417. [Google Scholar] [CrossRef]
- Chen, F.; Bouvard, J.L.; Sawada, D.; Pradille, C.; Hummel, M.; Sixta, H.; Budtova, T. Exploring digital image correlation technique for the analysis of the tensile properties of all-cellulose composites. Cellulose 2021, 28, 4165–4178. [Google Scholar] [CrossRef]
- Menezes, D.B.; Diz, F.M.; Ferreira, L.F.R.; Corrales, Y.; Baudrit, J.R.V.; Costa, L.P.; Hernandez-Macedo, M.L. Starch-based biocomposite membrane reinforced by orange bagasse cellulose nanofibers extracted from ionic liquid treatment. Cellulose 2021, 28, 4137–4149. [Google Scholar] [CrossRef]
- Mamontov, E.; Osti, N.C.; Ryder, M.R. Order-disorder in room-temperature ionic liquids probed via methyl quantum tunneling. Struct. Dyn. 2021, 8, 024303. [Google Scholar] [CrossRef] [PubMed]
- Phadagi, R.; Singh, S.; Hashemi, H.; Kaya, S.; Venkatesu, P.; Ramjugernath, D.; Ebenso, E.E.; Bahadur, I. Understanding the role of dimethylformamide as co-solvents in the dissolution of cellulose in ionic liquids: Experimental and theoretical approach. J. Mol. Liq. 2021, 328, 115392. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, J.; Zhang, H.; He, J.; Ren, Q.; Guo, M. Homogeneous acetylation of cellulose in a new ionic liquid. Biomacromolecules 2004, 5, 266–268. [Google Scholar] [CrossRef]
- Song, H.Z.; Zhang, J.; Niu, Y.H.; Wang, Z.G. Phase transition and rheological behaviors of concentrated cellulose/ionic liquid solutions. J. Phys. Chem. B 2010, 114, 6006–6013. [Google Scholar] [CrossRef]
- Bendaoud, A.; Kehrbusch, R.; Baranov, A.; Duchemin, B.; Maigret, J.E.; Falourd, X.; Staiger, M.P.; Cathala, B.; Lourdin, D.; Leroy, E. Nanostructured cellulose-xyloglucan blends via ionic liquid/water processing. Carbohydr. Polym. 2017, 168, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Gabrys, T.; Fryczkowska, B.; Binias, D.; Slusarczyk, C.; Fabia, J. Preparation and properties of composite cellulose fibres with the addition of graphene oxide. Carbohydr. Polym. 2021, 254, 117436. [Google Scholar] [CrossRef]
- Cai, T.; Zhang, H.H.; Guo, Q.H.; Shao, H.L.; Hu, X.C. Structure and properties of cellulose fibers from ionic liquids. J. Appl. Polym. Sci. 2010, 115, 1047–1053. [Google Scholar] [CrossRef]
- Stark, N.M. Opportunities for cellulose nanomaterials in packaging films: A review and future trends. J. Renew. Mater. 2016, 4, 313–326. [Google Scholar] [CrossRef]
- Neves, R.M.; Ornaghi, H.L.; Zattera, A.J.; Amico, S.C. Recent studies on modified cellulose/nanocellulose epoxy composites: A systematic review. Carbohydr. Polym. 2021, 255, 117366. [Google Scholar] [CrossRef]
- Liu, J.X.; Chen, P.; Qin, D.J.; Jia, S.; Jia, C.; Li, L.; Bian, H.L.; Wei, J.; Shao, Z.Q. Nanocomposites membranes from cellulose nanofibers, sio2 and carboxymethyl cellulose with improved properties. Carbohydr. Polym. 2020, 233, 115818. [Google Scholar] [CrossRef]
- Song, H.Z.; Luo, Z.Q.; Wang, C.Z.; Hao, X.F.; Gao, J.G. Preparation and characterization of bionanocomposite fiber based on cellulose and nano-sio2 using ionic liquid. Carbohydr. Polym. 2013, 98, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Raabe, J.; Fonseca, A.D.; Bufalino, L.; Ribeiro, C.; Martins, M.A.; Marconcini, J.M.; Tonoli, G.H.D. Evaluation of reaction factors for deposition of silica (sio2) nanoparticles on cellulose fibers. Carbohydr. Polym. 2014, 114, 424–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.A.; Yoon, M.J.; Lee, E.S.; Lim, D.Y.; Kim, K.Y. Preparation and characterization of cellulose nanofibers (cnfs) from microcrystalline cellulose (mcc) and cnf/polyamide 6 composites. Macromol. Res. 2014, 22, 738–745. [Google Scholar] [CrossRef]
- He, M.; Duan, B.; Xu, D.F.; Zhang, L.N. Moisture and solvent responsive cellulose/sio2 nanocomposite materials. Cellulose 2015, 22, 553–563. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, S.; Wang, X.B.; Hao, J. Enhanced mechanical properties and thermal stability of cellulose insulation paper achieved by doping with melamine-grafted nano-sio2. Cellulose 2018, 25, 3619–3633. [Google Scholar] [CrossRef]
- Kim, U.J.; Kimura, S.; Wada, M. Facile preparation of cellulose-sio2 composite aerogels with high sio2 contents using a libr aqueous solution. Carbohydr. Polym. 2019, 222, 114975. [Google Scholar] [CrossRef]
- Segal, L.J.J.C.; Martin, A.E.J.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the x-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Olsson, C.; Westman, G. Wet spinning of cellulose from ionic liquid solutions-viscometry and mechanical performance. J. Appl. Polym. Sci. 2013, 127, 4542–4548. [Google Scholar] [CrossRef]
- Liu, L.; Ju, M.T.; Li, W.Z.; Hou, Q.D. Dissolution of cellulose from afex-pretreated zoysia japonica in amimcl with ultrasonic vibration. Carbohydr. Polym. 2013, 98, 412–420. [Google Scholar] [CrossRef]
- Xia, G.M.; Wan, J.Q.; Zhang, J.M.; Zhang, X.Y.; Xu, L.L.; Wu, J.; He, J.S.; Zhang, J. Cellulose-based films prepared directly from waste newspapers via an ionic liquid. Carbohydr. Polym. 2016, 151, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Reddy, J.P.; Rajulu, A.V.; Rhim, J.W.; Seo, J. Mechanical, thermal, and water vapor barrier properties of regenerated cellulose/nano-sio2 composite films. Cellulose 2018, 25, 7153–7165. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Thanikaivelan, P.; Srinivasn, K.; Mohan, D.; Rajendran, M. Synthesis, characterization and thermal studies on cellulose acetate membranes with additive. Eur. Polym. J. 2004, 40, 2153–2159. [Google Scholar] [CrossRef]
- Dlomo, K.; Mohomane, S.M.; Motaung, T.E. Influence of silica nanoparticles on the properties of cellulose composite membranes: A current review. Cell Chem. Technol. 2020, 54, 765–775. [Google Scholar] [CrossRef]
- Nelson, M.L.; O'Connor, R.T. Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part ii. A new infrared ratio for estimation of crystallinity in cellulose i and ii. J. Appl. Polym. Sci. 1964, 8, 1325–1341. [Google Scholar] [CrossRef]
- Langan, P.; Nishiyama, Y.; Chanzy, H. A revised structure and hydrogen-bonding system in cellulose ii from a neutron fiber diffraction analysis. J. Am. Chem. Soc. 1999, 121, 9940–9946. [Google Scholar] [CrossRef]
- Ashori, A.; Sheykhnazari, S.; Tabarsa, T.; Shakeri, A.; Golalipour, M. Bacterial cellulose/silica nanocomposites: Preparation and characterization. Carbohydr. Polym. 2012, 90, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.C.; Wu, C.J.; Yu, D.M.; Ding, Q.J.; Li, R.G. Tunable micro-structure of dissolving pulp-based cellulose nanofibrils with facile prehydrolysis process. Cellulose 2021, 28, 3759–3773. [Google Scholar] [CrossRef]
- Vallejo, M.; Cordeiro, R.; Dias, P.A.N.; Moura, C.; Henriques, M.; Seabra, I.J.; Malca, C.M.; Morouco, P. Recovery and evaluation of cellulose from agroindustrial residues of corn, grape, pomegranate, strawberry-tree fruit and fava. Bioresour. Bioprocess. 2021, 8, 25. [Google Scholar] [CrossRef]
- Liu, Y.; Jing, S.; Carvalho, D.; Fu, J.; Martins, M.; Cavaco-Paulo, A. Cellulose dissolved in ionic liquids for modification of the shape of keratin fibers. ACS Sustain. Chem. Eng. 2021, 9, 4102–4110. [Google Scholar] [CrossRef]
- Bai, L.M.; Liu, Y.T.; Ding, A.; Ren, N.Q.; Li, G.B.; Liang, H. Fabrication and characterization of thin-film composite (tfc) nanofiltration membranes incorporated with cellulose nanocrystals (cncs) for enhanced desalination performance and dye removal. Chem. Eng. J. 2019, 358, 1519–1528. [Google Scholar] [CrossRef]
- Xie, K.; Yu, Y.; Shi, Y. Synthesis and characterization of cellulose/silica hybrid materials with chemical crosslinking. Carbohydr. Polym. 2009, 78, 799–805. [Google Scholar] [CrossRef]
- Han, D.L.; Yan, L.F.; Chen, W.F.; Li, W.; Bangal, P.R. Cellulose/graphite oxide composite films with improved mechanical properties over a wide range of temperature. Carbohydr. Polym. 2011, 83, 966–972. [Google Scholar] [CrossRef]
- Yang, Y.P.; Zhang, Y.; Dawelbeit, A.; Deng, Y.; Lang, Y.X.; Yu, M.H. Structure and properties of regenerated cellulose fibers from aqueous naoh/thiourea/urea solution. Cellulose 2017, 24, 4123–4137. [Google Scholar] [CrossRef]
- Yang, Y.S.; Shan, L.; Shen, H.J.; Qiu, J. Green and facile fabrication of thermal superamphiphobic nanofibrillated-cellulose/chitosan/ots composites through mechano-chemical method. Fibers Polym. 2021, 22, 1407–1415. [Google Scholar] [CrossRef]
- Bunmechimma, L.; Leejarkpai, T.; Riyajan, S.A. Fabrication and physical properties of a novel macroporous poly(vinyl alcohol)/cellulose fibre product. Carbohydr. Polym. 2020, 240, 116215. [Google Scholar] [CrossRef]
- Okon, K.E.; Lin, F.C.; Chen, Y.D.; Huang, B. Effect of silicone oil heat treatment on the chemical composition, cellulose crystalline structure and contact angle of chinese parasol wood. Carbohydr. Polym. 2017, 164, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Collier, J.R.; Watson, J.L.; Collier, B.J.; Petrovan, S. Rheology of 1-butyl-3-methylimidazolium chloride cellulose solutions. Ii. Solution character and preparation. J. Appl. Polym. Sci. 2009, 111, 1019–1027. [Google Scholar] [CrossRef]
- Gericke, M.; Schlufter, K.; Liebert, T.; Heinze, T.; Budtova, T. Rheological properties of cellulose/ionic liquid solutions: From dilute to concentrated states. Biomacromolecules 2009, 10, 1188–1194. [Google Scholar] [CrossRef]
- Yao, Y.B.; Yan, Z.Y.; Li, Z.; Zhang, Y.M.; Wang, H.P. Viscoelastic behavior and sol-gel transition of cellulose/silk fibroin/1-butyl-3-methylimidazolium chloride extended from dilute to concentrated solutions. Polym. Eng. Sci. 2018, 58, 1931–1936. [Google Scholar] [CrossRef]
- Yao, Y.B.; Xia, X.L.; Mukuze, K.S.; Zhang, Y.M.; Wang, H.P. Study on the temperature-induced sol-gel transition of cellulose/silk fibroin blends in 1-butyl-3-methylimidazolium chloride via rheological behavior. Cellulose 2014, 21, 3737–3743. [Google Scholar] [CrossRef]
- Shakeel, A.; Mahmood, H.; Farooq, U.; Ullah, Z.; Yasin, S.; Iqbal, T.; Chassagne, C.; Moniruzzaman, M. Rheology of pure ionic liquids and their complex fluids: A review. ACS Sustain. Chem. Eng. 2019, 7, 13586–13626. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.B.; Mukuze, K.S.; Zhang, Y.M.; Wang, H.P. Rheological behavior of cellulose/silk fibroin blend solutions with ionic liquid as solvent. Cellulose 2014, 21, 675–684. [Google Scholar] [CrossRef]
- Xia, X.L.; Wang, J.N.; Wang, H.P.; Zhang, Y.M. Numerical investigation of spinneret geometric effect on spinning dynamics of dry-jet wet-spinning of cellulose/ bmim cl solution. J. Appl. Polym. Sci. 2016, 133, 49362. [Google Scholar] [CrossRef]
- Rajeev, A.; Deshpande, A.P.; Basavaraj, M.G. Rheology and microstructure of concentrated microcrystalline cellulose (mcc)/1-allyl-3-methylimidazolium chloride (amimcl)/water mixtures. Soft Matter 2018, 14, 7615–7624. [Google Scholar] [CrossRef]

| Sample | CNF (wt% to Dissolving Pulp) | Nano-SiO2 (wt% to Dissolving Pulp) |
|---|---|---|
| RCF | / | / |
| RCF-CNF2 | 2% | / |
| RCF-SiO22 | / | 2% |
| RCF-CNF1-SiO21 | 1% | 1% |
| RCF-CNF2-SiO22 | 2% | 2% |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Number of Broken in Spinning (/h) |
|---|---|---|---|
| RCF | 155.82 ± 5.23 | 8.73 ± 0.13 | 1 |
| RCF-CNF2 | 174.15 ± 2.36 | 7.43 ± 0.11 | 5 |
| RCF-SiO22 | 174.18 ± 1.26 | 7.65 ± 0.15 | 3 |
| RCF-CNF1-SiO21 | 229.78 ± 0.91 | 10.01 ± 0.15 | 2 |
| RCF-CNF2-SiO22 | 173.42 ± 3.35 | 6.33 ± 0.09 | 2 |
| Sample | Crystallinity (%) |
|---|---|
| Pulp | 50.88 ± 1.03% |
| RCF | 62.76 ± 1.21% |
| RCF-CNF2 | 50.11 ± 0.96% |
| RCF-SiO22 | 47.37 ± 1.54% |
| RCF-CNF1-SiO21 | 55.15 ± 0.53% |
| RCF-CNF2-SiO22 | 47.75 ± 0.75% |
| Sample | C (%) | O (%) | N (%) | Si (%) |
|---|---|---|---|---|
| Pulp | 28.2 | 46.32 | 25.35 | 0.14 |
| RCF | 28.05 | 46.63 | 25.16 | 0.15 |
| RCF-CNF2 | 27.48 | 47.51 | 24.85 | 0.15 |
| RCF-SiO22 | 27.42 | 47.35 | 24.67 | 0.56 |
| RCF-CNF1-SiO21 | 26.87 | 48.53 | 24.11 | 0.49 |
| RCF-CNF2-SiO22 | 27.51 | 47.23 | 24.56 | 0.70 |
| Sample | Ton (°C) | Mass Loss (%) | Tmax (°C) | Mass Loss (%) | Residue at 800 °C (%) |
|---|---|---|---|---|---|
| RCF | 307 | 12.28 | 329 | 27.16 | 22.48 |
| RCF-CNF2 | 300 | 10.96 | 344 | 30.44 | 20.39 |
| RCF-SiO22 | 308 | 11.76 | 343 | 30.42 | 25.80 |
| RCF-CNF1-SiO21 | 302 | 10.96 | 344 | 36.25 | 22.85 |
| RCF-CNF2-SiO22 | 313 | 11.76 | 335 | 26.42 | 24.93 |
| Sample | Temperature (°C) | Angular Frequency (Rad/s) | Modulus Values (Pa) |
|---|---|---|---|
| RCF | 40 | 0.13 | 56.45 |
| 60 | 13.39 | 220.34 | |
| 80 | 59.46 | 282.61 | |
| RCF-CNF2 | 40 | 1.59 | 161.68 |
| 60 | 7.117 | 195.78 | |
| 80 | 25.21 | 221.03 | |
| RCF-SiO22 | 40 | 5.27 | 207.32 |
| 60 | 25.41 | 237.24 | |
| 80 | 102.78 | 282.66 | |
| RCF-CNF1-SiO21 | 40 | 4.50 | 191.22 |
| 60 | 22.62 | 221.78 | |
| 80 | 90.28 | 283.62 | |
| RCF-CNF2-SiO22 | 40 | 5.67 | 207.325 |
| 60 | 25.25 | 251.22 | |
| 80 | 100.79 | 324.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Qi, L.; Lin, Z.; Yang, G.; He, M.; Chen, J. High-Strength Regenerated Cellulose Fiber Reinforced with Cellulose Nanofibril and Nanosilica. Nanomaterials 2021, 11, 2664. https://doi.org/10.3390/nano11102664
Xue Y, Qi L, Lin Z, Yang G, He M, Chen J. High-Strength Regenerated Cellulose Fiber Reinforced with Cellulose Nanofibril and Nanosilica. Nanomaterials. 2021; 11(10):2664. https://doi.org/10.3390/nano11102664
Chicago/Turabian StyleXue, Yu, Letian Qi, Zhaoyun Lin, Guihua Yang, Ming He, and Jiachuan Chen. 2021. "High-Strength Regenerated Cellulose Fiber Reinforced with Cellulose Nanofibril and Nanosilica" Nanomaterials 11, no. 10: 2664. https://doi.org/10.3390/nano11102664
APA StyleXue, Y., Qi, L., Lin, Z., Yang, G., He, M., & Chen, J. (2021). High-Strength Regenerated Cellulose Fiber Reinforced with Cellulose Nanofibril and Nanosilica. Nanomaterials, 11(10), 2664. https://doi.org/10.3390/nano11102664







