How to Make a Cocktail of Palladium Catalysts with Cola and Alcohol: Heteroatom Doping vs. Nanoscale Morphology of Carbon Supports
Abstract
:1. Introduction
2. Materials and Methods
2.1. Carbon Materials Preparation
2.2. Preparation of Pd/Sample Catalysts
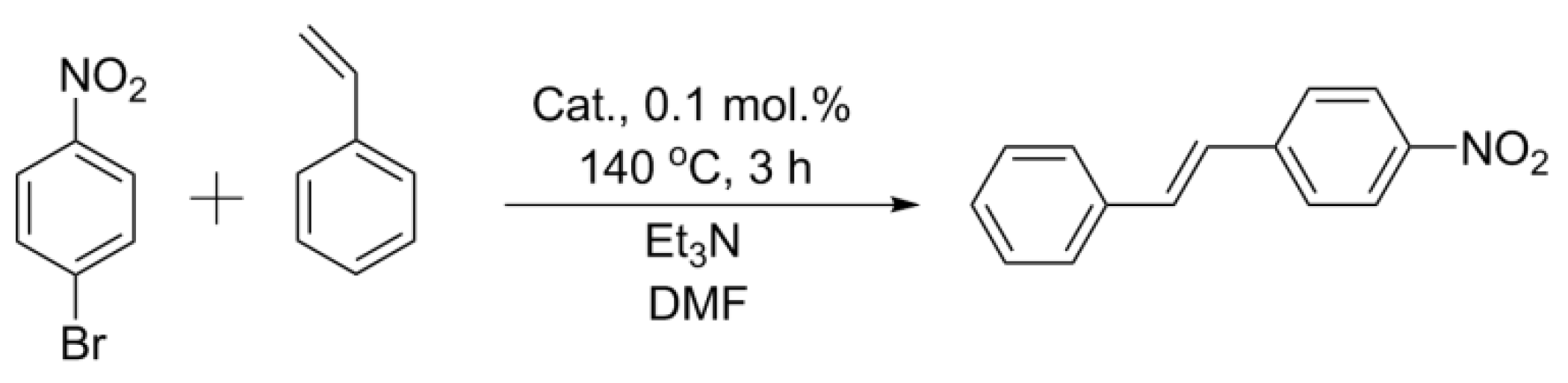
2.3. Mizoroki–Heck Reaction with the Obtained Catalysts
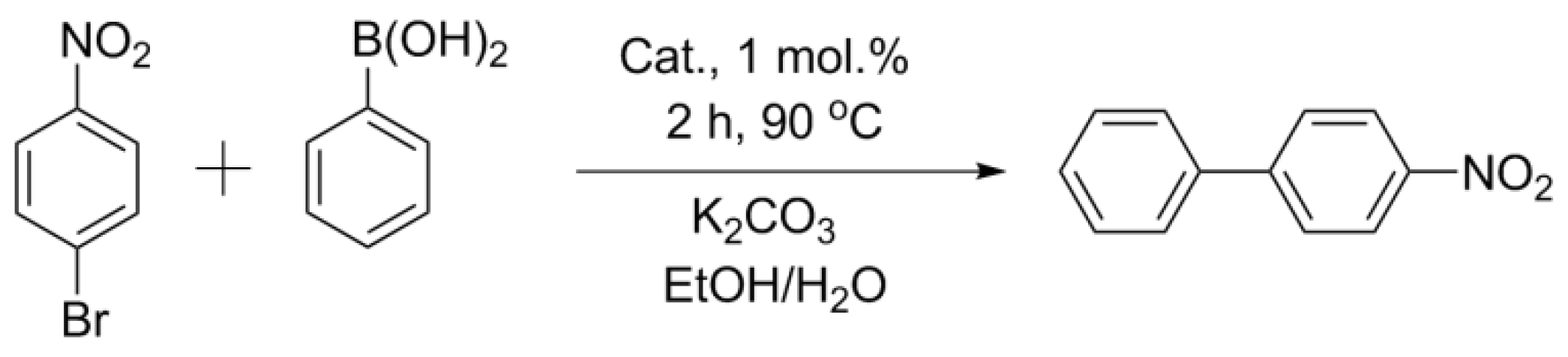
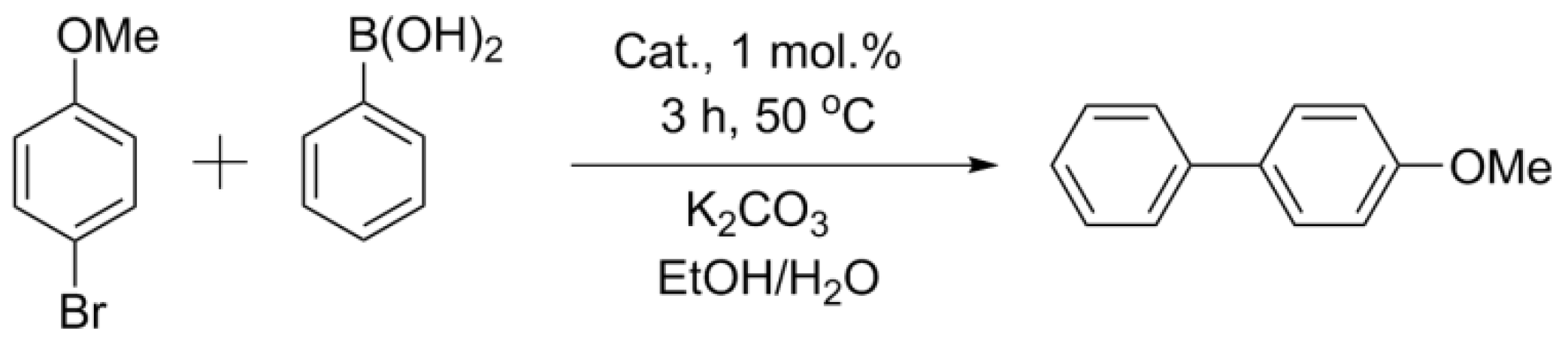
2.4. Suzuki–Miyaura Reaction with the Obtained Catalysts
2.5. Catalyst Recycling
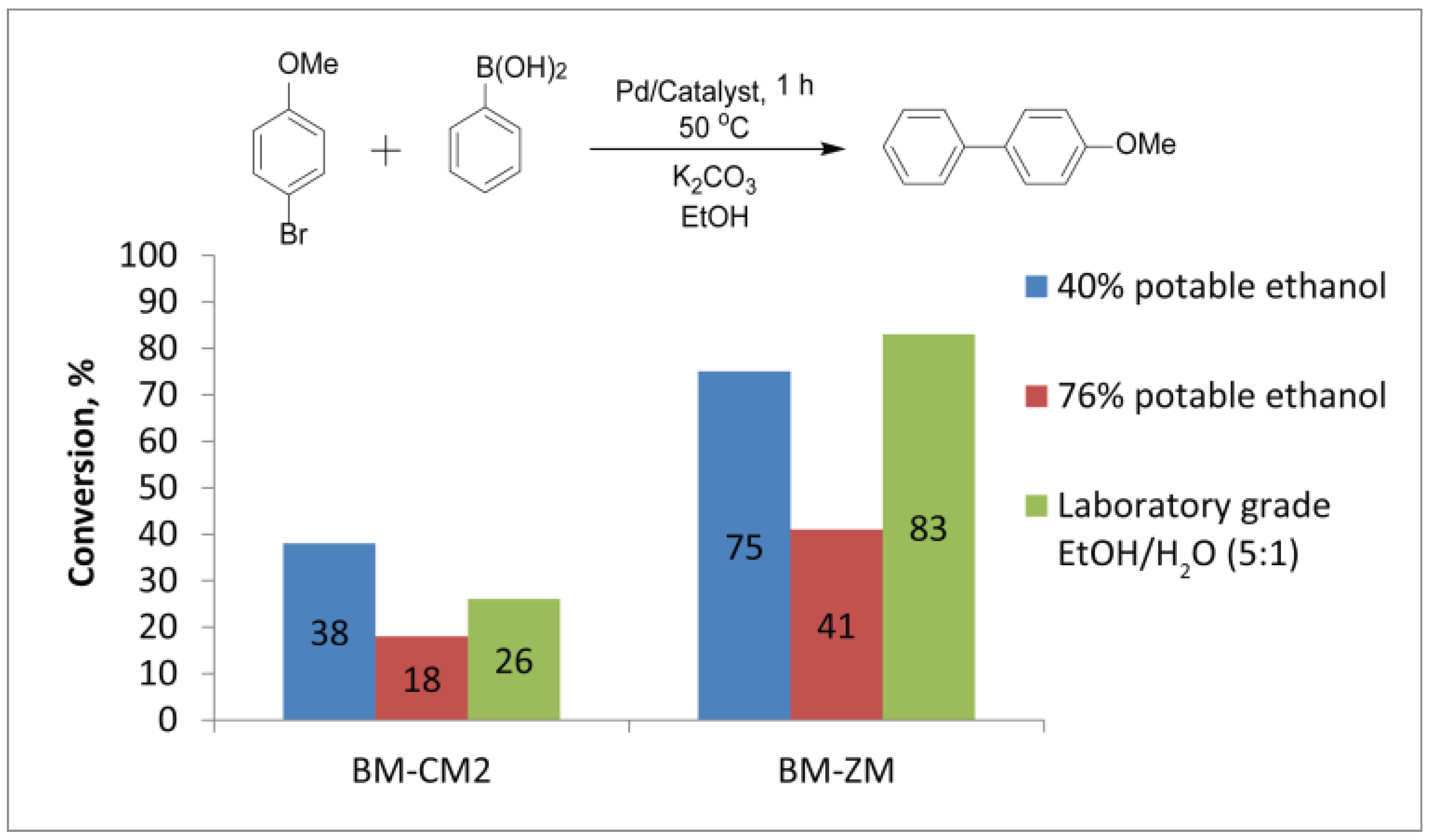
2.6. Suzuki–Miyaura Reactions in Different Media
2.7. Scanning Electron Microscope and Energy Dispersive X-ray Spectroscopy Studies
2.8. Transmission Electron Microscopy Measurements
2.9. X-ray Photoelectron Spectra Measurements and Analysis
2.10. Brunauer–Emmett–Teller Surface Area Analysis
2.11. Infra-Red Spectroscopy
2.12. Visualization of Catalyst Dynamics (Nanofishing Method)
2.13. Computational Details
2.14. Electrospray Ionization Mass Spectrometry
3. Results and Discussion
3.1. P-Doped Carbon Materials Preparation
3.2. Characterization of Prepared Carbon Materials
3.2.1. Surface Area Characterization
3.2.2. CM1 and CM2 Materials Characterization
3.2.3. ZM Material Characterization
3.2.4. Characterization of BM-CM1, BM-CM2 and BM-ZM Materials
3.3. Preparation of Palladium Catalysts Supported on Carbon Materials
3.3.1. Palladium Deposition on CM1 and BM-CM1
3.3.2. Palladium Deposition on CM2 and BM-CM2
3.3.3. Palladium Deposition on ZM and BM-ZM
3.4. Catalyst Activity in Suzuki–Miyaura and Mizoroki–Heck Reactions
3.4.1. Suzuki–Miyaura Reaction
3.4.2. Mizoroki–Heck Reactions
3.5. Which Properties of the Support Determine the Activity of the Catalyst?
3.5.1. Relationship between the Specific Surface Area and the Size of Deposited Nanoparticles
3.5.2. Relationship between the Average Diameter of Nanoparticles and the Catalytic Activity of the Supported Catalyst
3.5.3. Relationship between Doping with Heteroatoms and the Catalytic Activity of Supported Catalyst
3.6. Quantum Chemical Calculations of Pd Binding with Carbon Supports
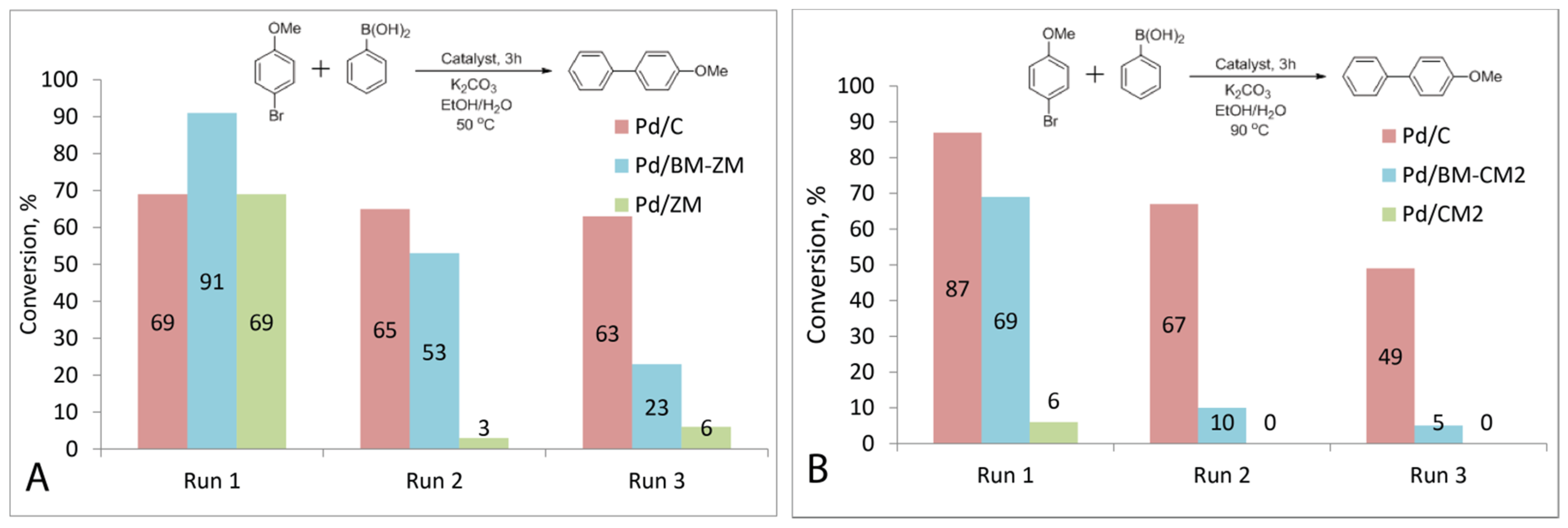
3.7. Recycling of Prepared Catalysts
3.8. SEM Study of Catalysts after Recycling
3.9. Suzuki–Miyaura Reactions in Ethanol/Water Mixtures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ananikov, V.; Beletskaya, I. Toward the Ideal Catalyst: From Atomic Centers to a “Cocktail” of Catalysts. Organometallics 2012, 31, 1595–1604. [Google Scholar] [CrossRef]
- Eremin, D.; Ananikov, V.P. Understanding active species in catalytic transformations: From molecular catalysis to nanoparticles, leaching, “Cocktails” of catalysts and dynamic systems. Coord. Chem. Rev. 2017, 346, 2–19. [Google Scholar] [CrossRef]
- Prima, D.O.; Kulikovskaya, N.S.; Galushko, A.S.; Mironenko, R.M.; Ananikov, V.P. Transition metal ‘cocktail’-type catalysis. Curr. Opin. Green Sustain. Chem. 2021, 31, 100502. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, Y.; Xu, J.; Sun, H.; Li, Z.; Liu, W.; Yuan, T.; Wang, X.; Cheong, W.-C.; Wang, Z.; et al. Recover the activity of sintered supported catalysts by nitrogen-doped carbon atomization. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Axet, M.R.; Dechy-Cabaret, O.; Durand, J.; Gouygou, M.; Serp, P. Coordination chemistry on carbon surfaces. Coord. Chem. Rev. 2016, 308, 236–345. [Google Scholar] [CrossRef]
- Ziccarelli, I.; Neumann, H.; Kreyenschulte, C.; Gabriele, B.; Beller, M. Pd-Supported on N-doped carbon: Improved heterogeneous catalyst for base-free alkoxycarbonylation of aryl iodides. Chem. Commun. 2016, 52, 12729–12732. [Google Scholar] [CrossRef]
- Sikora, E.; Kiss, A.; Göndör, Z.H.; Pekker, P.; Kristály, F.; Szőri, M.; Rágyanszki, A.; Viskolcz, B.; Fiser, B.; Vanyorek, L. Fine-tuning the catalytic activity by applying nitrogen-doped carbon nanotubes as catalyst supports for the hydrogenation of olefins. React. Kinet. Mech. Catal. 2019, 129, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Sadjadi, S.; Malmir, M.; Lazzara, G.; Cavallaro, G.; Heravi, M.M. Preparation of palladated porous nitrogen-doped carbon using halloysite as porogen: Disclosing its utility as a hydrogenation catalyst. Sci. Rep. 2020, 10, 2039. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Li, K.; Xiang, Y.; Zhou, Y.; Wang, Q.; Guo, L.; Ma, L.; Xu, X.; Lu, C.; Feng, F.; et al. Sulfur-doped porous carbon supported palladium catalyst for high selective o-chloro-nitrobenzene hydrogenation. Appl. Catal. A Gen. 2019, 581, 74–81. [Google Scholar] [CrossRef]
- Yang, J.; Xu, M.; Wang, J.; Jin, S.; Tan, B. A Facile Approach to Prepare Multiple Heteroatom-Doped Carbon Materials from Imine-Linked Porous Organic Polymers. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Shen, Z.; Yu, J. Phosphorus containing materials for photocatalytic hydrogen evolution. Green Chem. 2016, 19, 588–613. [Google Scholar] [CrossRef]
- Shao, Y.; Jiang, Z.; Zhang, Q.; Guan, J. Progress in Nonmetal-Doped Graphene Electrocatalysts for the Oxygen Reduction Reaction. ChemSusChem 2019, 12, 2133–2146. [Google Scholar] [CrossRef]
- Puziy, A.; Poddubnaya, O.; Gawdzik, B.; Tascón, J. Phosphorus-containing carbons: Preparation, properties and utilization. Carbon 2020, 157, 796–846. [Google Scholar] [CrossRef]
- Daifullah, A.; Girgis, B. Impact of surface characteristics of activated carbon on adsorption of BTEX. Colloids Surfaces A Physicochem. Eng. Asp. 2003, 214, 181–193. [Google Scholar] [CrossRef]
- Puziy, A.; Poddubnaya, O.; Martinez-Alonso, A.; Suárez-García, F.; Tascon, J.M.D. Synthetic carbons activated with phosphoric acid: I. Surface chemistry and ion binding properties. Carbon 2002, 40, 1493–1505. [Google Scholar] [CrossRef]
- Patel, M.A.; Luo, F.; Khoshi, M.R.; Rabie, E.; Zhang, Q.; Flach, C.R.; Mendelsohn, R.; Garfunkel, E.; Szostak, M.; He, H. P-Doped Porous Carbon as Metal Free Catalysts for Selective Aerobic Oxidation with an Unexpected Mechanism. ACS Nano 2016, 10, 2305–2315. [Google Scholar] [CrossRef]
- Some, S.; Kim, J.; Lee, K.; Kulkarni, A.; Yoon, Y.; Lee, S.; Kim, T.; Lee, H. Highly Air-Stable Phosphorus-Doped n-Type Graphene Field-Effect Transistors. Adv. Mater. 2012, 24, 5481–5486. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, B.; Li, X.; Ye, Y.; Ma, J.; Liu, C.; Zhou, X.; Xie, X. Enhancing thermal oxidation and fire resistance of reduced graphene oxide by phosphorus and nitrogen co-doping: Mechanism and kinetic analysis. Carbon 2019, 146, 650–659. [Google Scholar] [CrossRef]
- Song, P.; Wang, H.; Kang, L.; Ran, B.; Song, H.; Wang, R. Electrochemical nitrogen reduction to ammonia at ambient conditions on nitrogen and phosphorus co-doped porous carbon. Chem. Commun. 2019, 55, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Seredych, M.; Bandosz, T.J. Visible light photoactivity of sulfur and phosphorus doped nanoporous carbons in oxidation of dibenzothiophenes. Fuel 2013, 108, 846–849. [Google Scholar] [CrossRef]
- Abbas, Q.; Raza, R.; Shabbir, I.; Olabi, A. Heteroatom doped high porosity carbon nanomaterials as electrodes for energy storage in electrochemical capacitors: A review. J. Sci. Adv. Mater. Devices 2019, 4, 341–352. [Google Scholar] [CrossRef]
- Rusop, M.; Soga, T.; Jimbo, T. Photovoltaic characteristics of phosphorus-doped amorphous carbon films grown by r.f. plasma-enhanced CVD. Sol. Energy Mater. Sol. Cells 2006, 90, 3214–3222. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Li, J.; Sun, W.; Gao, J.; Guo, J.; Jiang, C. Nano-Structured Phosphorus Composite as High-Capacity Anode Materials for Lithium Batteries. Angew. Chem. Int. Ed. 2012, 51, 9034–9037. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Singh, P.K.; Bhattacharya, B.; Shulga, Y.M.; Shulga, N.Y.; Kumar, Y. Progress, status and prospects of non-porous, heteroatom-doped carbons for supercapacitors and other electrochemical applications. Appl. Phys. A 2019, 125, 122. [Google Scholar] [CrossRef]
- Chen, W.; Wan, M.; Liu, Q.; Xiong, X.; Yu, F.; Huang, Y. Heteroatom-Doped Carbon Materials: Synthesis, Mechanism, and Application for Sodium-Ion Batteries. Small Methods 2019, 3, 1800323. [Google Scholar] [CrossRef]
- Guillén, E.; Rico, R.; López-Romero, J.M.; Bedia, J.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Pd-activated carbon catalysts for hydrogenation and Suzuki reactions. Appl. Catal. A Gen. 2009, 368, 113–120. [Google Scholar] [CrossRef]
- Bedia, J.; Rosas, J.; Rodríguez-Mirasol, J.; Cordero, T. Pd supported on mesoporous activated carbons with high oxidation resistance as catalysts for toluene oxidation. Appl. Catal. B Environ. 2010, 94, 8–18. [Google Scholar] [CrossRef]
- Tiancun, X.; Lidun, A.; Weimin, Z.; Shishan, S.; Guoxin, X. Mechanism of sulfur poisoning on supported noble metal catalyst—The adsorption and transformation of sulfur on palladium catalysts with different supports. Catal. Lett. 1992, 12, 287–296. [Google Scholar] [CrossRef]
- Dunleavy, J.K. Sulfur as a Catalyst Poison. Platin. Met. Rev. 2006, 50, 110. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Y.; Xu, Y.; Wang, Q.; Huang, W.; Ying, J.; Zhou, J.; Ma, L.; Lu, C.; Feng, F.; et al. Regulation of sulfur doping on carbon-supported Pd particles and abnormal relationship between Pd particle size and catalytic performance in selective hydrogenation of o-chloronitrobenzene. Catal. Commun. 2020, 143, 106059. [Google Scholar] [CrossRef]
- Xu, S.-L.; Shen, S.-C.; Zhao, S.; Ding, Y.-W.; Chu, S.-Q.; Chen, P.; Lin, Y.; Liang, H.-W. Synthesis of carbon-supported sub-2 nanometer bimetallic catalysts by strong metal–sulfur interaction. Chem. Sci. 2020, 11, 7933–7939. [Google Scholar] [CrossRef]
- Zhou, Y.; Neyerlin, K.; Olson, T.S.; Pylypenko, S.; Bult, J.; Dinh, H.N.; Gennett, T.; Shao, Z.; O’Hayre, R. Enhancement of Pt and Pt-alloy fuel cell catalyst activity and durability via nitrogen-modified carbon supports. Energy Environ. Sci. 2010, 3, 1437–1446. [Google Scholar] [CrossRef]
- Chen, L.-F.; Huang, Z.-H.; Liang, H.-W.; Gao, H.-L.; Yu, S.-H. Three-Dimensional Heteroatom-Doped Carbon Nanofiber Networks Derived from Bacterial Cellulose for Supercapacitors. Adv. Funct. Mater. 2014, 24, 5104–5111. [Google Scholar] [CrossRef]
- Latorre-Sanchez, M.; Primo, A.; García, H. P-Doped Graphene Obtained by Pyrolysis of Modified Alginate as a Photocatalyst for Hydrogen Generation from Water–Methanol Mixtures. Angew. Chem. Int. Ed. Engl. 2013, 52, 11813–11816. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Zhang, J.; Mu, Y.; Gao, J.; Feng, Y.; Liu, H.; Guo, Z.; Zhang, C. Preparation and evaluation of activated carbon with different polycondensed phosphorus oxyacids (H3PO4, H4P2O7, H6P4O13 and C6H18O24P6) activation employing mushroom roots as precursor. J. Anal. Appl. Pyrolysis 2014, 108, 41–46. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Chen, S.; Wang, Z.; Hou, H.; Zhao, F. Cellulose-derived nitrogen and phosphorus dual-doped carbon as high performance oxygen reduction catalyst in microbial fuel cell. J. Power Sources 2015, 273, 1189–1193. [Google Scholar] [CrossRef]
- Nahata, M.; Seo, C.Y.; Krishnakumar, P.; Schwank, J. New approaches to water purification for resource-constrained settings: Production of activated biochar by chemical activation with diammonium hydrogenphosphate. Front. Chem. Sci. Eng. 2018, 12, 194–208. [Google Scholar] [CrossRef]
- Gun’Ko, V.; Seledets, O.; Skubiszewska-Zięba, J.; Zarko, V.; Leboda, R.; Janusz, W.; Chibowski, S. Phosphorus-containing carbon deposits on silica gel Si-100. Microporous Mesoporous Mater. 2005, 87, 133–145. [Google Scholar] [CrossRef]
- Ye, H.; Yin, Y.-X.; Guo, Y.-G. Insight into the loading temperature of sulfur on sulfur/carbon cathode in lithium-sulfur batteries. Electrochim. Acta 2015, 185, 62–68. [Google Scholar] [CrossRef]
- Wiśniewski, M.; Pacholczyk, A.; Terzyk, A.P.; Rychlicki, G. New phosphorus-containing spherical carbon adsorbents as promising materials in drug adsorption and release. J. Colloid Interface Sci. 2011, 354, 891–894. [Google Scholar] [CrossRef]
- Lysenko, N.D.; Yaremov, P.S.; Ovcharova, M.V.; Ilyin, V.G. Highly acidic phosphorus-containing porous carbons: Synthesis and physicochemical properties. J. Mater. Sci. 2012, 47, 3089–3095. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Reinish, C.A.; Tsyba, M.M.; Mikhalovska, L.I.; Mikhalovsky, S.V. One-pot preparation of functionalized nanostructured carbons. Carbon 2011, 49, 599–604. [Google Scholar] [CrossRef]
- Jin, H.; Feng, X.; Li, J.; Li, M.; Xia, Y.; Yuan, Y.; Yang, C.; Dai, B.; Lin, Z.; Wang, J.; et al. Heteroatom-Doped Porous Carbon Materials with Unprecedented High Volumetric Capacitive Performance. Angew. Chem. Int. Ed. 2019, 58, 2397–2401. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, L.; Shi, G.; Liu, J.; Chen, J.; Dai, L. N,P-Codoped Carbon Networks as Efficient Metal-free Bifunctional Catalysts for Oxygen Reduction and Hydrogen Evolution Reactions. Angew. Chem. Int. Ed. 2016, 55, 2230–2234. [Google Scholar] [CrossRef]
- Shena, X.; Huanga, Y.; Liua, Z.; Dinga, J.; Wanga, X.; Zhanga, Y.; Guoa, Y.; Tangb, X. Designed formation of nitrogen-doped caramel sheathed bilateral hybrid oxides nanoarrays as ultra-stable anode for high-areal-capacity lithium-ion batteries. J. Alloy. Compd. 2020, 834, 155069. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Cheng, Y.; Zhong, H.; Tian, H.; Pan, J.; Pareek, V.K.; Jiang, S.P.; Lamonier, J.-F.; Jaroniec, M.; Liu, J. From waste Coca Cola® to activated carbons with impressive capabilities for CO2 adsorption and supercapacitors. Carbon 2017, 116, 490–499. [Google Scholar] [CrossRef]
- Sun, S.; Gou, X.; Tao, S.; Cui, J.; Li, J.; Yang, Q.; Liang, S.; Yang, Z. Mesoporous graphitic carbon nitride (g-C3N4) nanosheets synthesized from carbonated beverage-reformed commercial melamine for enhanced photocatalytic hydrogen evolution. Mater. Chem. Front. 2019, 3, 597–605. [Google Scholar] [CrossRef]
- CHAPTER 1. Carbon (Nano)materials for Catalysis. In Nanostructured Carbon Materials for Catalysis; Royal Society of Chemistry (RSC): London, UK, 2015; pp. 1–45. [Google Scholar] [CrossRef]
- Holade, Y.; Morais, C.; Servat, K.; Napporn, T.W.; Kokoh, K.B. Enhancing the available specific surface area of carbon supports to boost the electroactivity of nanostructured Pt catalysts. Phys. Chem. Chem. Phys. 2014, 16, 25609–25620. [Google Scholar] [CrossRef] [PubMed]
- Takasu, Y.; Kawaguchi, T.; Sugimoto, W.; Murakami, Y. Effects of the surface area of carbon support on the characteristics of highly-dispersed Pt-Ru particles as catalysts for methanol oxidation. Electrochim. Acta 2003, 48, 3861–3868. [Google Scholar] [CrossRef]
- Borisov, V.A.; Iost, K.N.; Temerev, V.L.; Leont’eva, N.N.; Muromtsev, I.V.; Arbuzov, A.B.; Trenikhin, M.V.; Savel’eva, G.G.; Smirnova, N.S.; Shlyapin, D.A. The Influence of the Specific Surface Area of the Carbon Support on the Activity of Ruthenium Catalysts for the Ammonia-Decomposition Reaction. Kinet. Catal. 2018, 59, 136–142. [Google Scholar] [CrossRef]
- Yakukhnov, S.A.; Pentsak, E.O.; Galkin, K.I.; Mironenko, R.M.; Drozdov, V.A.; Likholobov, V.A.; Ananikov, V.P. Rapid “Mix-and-Stir” Preparation of Well-Defined Palladium on Carbon Catalysts for Efficient Practical Use. ChemCatChem 2018, 10, 1869–1873. [Google Scholar] [CrossRef]
- Kachala, V.V.; Khemchyan, L.L.; Kashin, A.S.; Orlov, N.V.; Grachev, A.A.; Zalesskiy, S.S.; Ananikov, V.P. Target-oriented analysis of gaseous, liquid and solid chemical systems by mass spectrometry, nuclear magnetic resonance spectroscopy and electron microscopy. Russ. Chem. Rev. 2013, 82, 648–685. [Google Scholar] [CrossRef]
- Kashin, A.S.; Ananikov, V.P. A SEM study of nanosized metal films and metal nanoparticles obtained by magnetron sputtering. Russ. Chem. Bull. 2011, 60, 2602–2607. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Eichkorn, K.; Treutler, O.; Öhm, H.; Häser, M.; Ahlrichs, R. Auxiliary basis sets to approximate Coulomb potentials (Chem. Phys. Letters 240 (1995) 283-290). Chem. Phys. Lett. 1995, 242, 652–660. [Google Scholar] [CrossRef]
- Eichkorn, K.; Weigend, F.; Treutler, O.; Ahlrichs, R. Auxiliary basis sets for main row atoms and transition metals and their use to approximate Coulomb potentials. Theor. Chem. Accounts 1997, 97, 119–124. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision, C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Peterson, S.C.; Jackson, M.A.; Kim, S.; Palmquist, D.E. Increasing biochar surface area: Optimization of ball milling parameters. Powder Technol. 2012, 228, 115–120. [Google Scholar] [CrossRef]
- Yin, S.-H.; Li, S.-W.; Xie, F.; Zhang, L.-B.; Peng, J.-H. Study on the aqueous solution behavior and extraction mechanism of Nd(III) in the presence of the complexing agent lactic acid with di-(2-ethylhexyl) phosphoric acid. RSC Adv. 2015, 5, 64550–64556. [Google Scholar] [CrossRef]
- Shen, X.; Li, X.; Zhao, F.; Wang, N.; Xie, C.; He, J.; Si, W.; Yi, Y.; Yang, Z.; Li, X.; et al. Preparation and structure study of phosphorus-doped porous graphdiyne and its efficient lithium storage application. 2D Mater. 2019, 6, 035020. [Google Scholar] [CrossRef]
- Yang, F.; He, X.; Wang, C.; Cao, Y.; Li, Y.; Yan, L.; Liu, M.; Lv, M.; Yang, Y.; Zhao, X.; et al. Controllable and eco-friendly synthesis of P-riched carbon quantum dots and its application for copper (II) ion sensing. Appl. Surf. Sci. 2018, 448, 589–598. [Google Scholar] [CrossRef]
- Li, X.; Pan, D.; Lin, S.; Zhuang, Z.; Lin, Z. Facile in vitro hydroxyapatite remineralization of human enamel with remarkable hardness. CrystEngComm 2013, 15, 4351–4356. [Google Scholar] [CrossRef]
- Feng, L.; Chen, X.; Cao, Y.; Chen, Y.; Wang, F.; Chen, Y.; Liu, Y. Pyridinic and pyrrolic nitrogen-rich ordered mesoporous carbon for efficient oxygen reduction in microbial fuel cells. RSC Adv. 2017, 7, 14669–14677. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Origin of the Electrocatalytic Oxygen Reduction Activity of Graphene-Based Catalysts: A Roadmap to Achieve the Best Performance. J. Am. Chem. Soc. 2014, 136, 4394–4403. [Google Scholar] [CrossRef] [PubMed]
- Boiko, D.A.; Pentsak, E.O.; Cherepanova, V.A.; Gordeev, E.G.; Ananikov, V.P. Deep neural network analysis of nanoparticle ordering to identify defects in layered carbon materials. Chem. Sci. 2021, 12, 7428–7441. [Google Scholar] [CrossRef] [PubMed]
- Valero-Romero, M.J.; Rodríguez-Cano, M.Á.; Palomo, J.; Rodríguez-Mirasol, J.; Cordero, T. Carbon-Based Materials as Catalyst Supports for Fischer–Tropsch Synthesis: A Review. Front. Mater. 2021, 7. [Google Scholar] [CrossRef]
- Goepel, M.; Kabir, H.; Küster, C.; Saraçi, E.; Zeigermann, P.; Valiullin, R.; Chmelik, C.; Enke, D.; Kärger, J.; Gläser, R. Improving mass-transfer in controlled pore glasses as supports for the platinum-catalyzed aromatics hydrogenation. Catal. Sci. Technol. 2015, 5, 3137–3146. [Google Scholar] [CrossRef] [Green Version]
- Rao, X.; Liu, C.; Qiu, J.; Jin, Z. A highly efficient and aerobic protocol for the synthesis of N-heteroaryl substituted 9-arylcarbazolyl derivatives via a palladium-catalyzed ligand-free Suzuki reaction. Org. Biomol. Chem. 2012, 10, 7875–7883. [Google Scholar] [CrossRef]
- Pentsak, E.O.; Ananikov, V.P. Pseudo-Solid-State Suzuki-Miyaura Reaction and the Role of Water Formed by Dehydration of Arylboronic Acids. Eur. J. Org. Chem. 2019, 2019, 4239–4247. [Google Scholar] [CrossRef]
- Carlone, A.; Marigo, M.; North, C.; Landa, A.; Jørgensen, K.A. A simple asymmetric organocatalytic approach to optically active cyclohexenones. Chem. Commun. 2006, 47, 4928–4930. [Google Scholar] [CrossRef] [PubMed]
- Galushko, A.S.; Gordeev, E.G.; Kashin, A.S.; Zubavichus, Y.V.; Ananikov, V.P. Visualization of catalyst dynamics and development of a practical procedure to study complex “cocktail”-type catalytic systems. Faraday Discuss. 2021, 229, 458–474. [Google Scholar] [CrossRef] [PubMed]


| Pd/CM1 | Pd/BM-CM1 | Pd/CM2 | Pd/BM-CM2 | Pd/ZM | Pd/BM-ZM | |
|---|---|---|---|---|---|---|
| Precursor of carbon material | Regular cola | Regular cola | Regular cola | Regular cola | Diet cola | Diet cola |
| Preparation of carbon material | Carbonization of solid | Carbonization of solid | Carbonization of viscous liquid | Carbonization of viscous liquid | Carbonization of viscous liquid | Carbonization of viscous liquid |
| Post-processing of carbon material | - | Ball-milling | - | Ball-milling | - | Ball-milling |
| Surface area of carbon material, m2/g | 289 | 319 | 332 | 459 | 3.4 | 232 |
| Average size of Pd NPs, nm | Crust | 4.7 ± 1.2 | 8.6 ± 3.0 | 5.3 ± 1.1 | 5.6 ± 1.0 49 ± 11 a | 6.4 ± 1.7 |
| Catalyst | Pd/BM-CM1 | Pd/CM2 | Pd/BM-CM2 | Pd/ZM | Pd/BM-ZM | Pd/Ccom |
|---|---|---|---|---|---|---|
| Conversion a, % (reaction 1) | 100 | 72 | 100 | 100 | 100 | 100 |
| Conversion a, % (reaction 2) | 14 b | 7 | 33 | 69 | 91 | 69 |
| Catalyst | Pd/CM2 | Pd/BM-CM2 | Pd/ZM | Pd/BM-ZM | Pd/Ccom |
|---|---|---|---|---|---|
| Conversion a, % | 2 | 17 | 10 | 65 | 67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pentsak, E.O.; Galushko, A.S.; Cherepanova, V.A.; Ananikov, V.P. How to Make a Cocktail of Palladium Catalysts with Cola and Alcohol: Heteroatom Doping vs. Nanoscale Morphology of Carbon Supports. Nanomaterials 2021, 11, 2599. https://doi.org/10.3390/nano11102599
Pentsak EO, Galushko AS, Cherepanova VA, Ananikov VP. How to Make a Cocktail of Palladium Catalysts with Cola and Alcohol: Heteroatom Doping vs. Nanoscale Morphology of Carbon Supports. Nanomaterials. 2021; 11(10):2599. https://doi.org/10.3390/nano11102599
Chicago/Turabian StylePentsak, Evgeniy O., Alexey S. Galushko, Vera A. Cherepanova, and Valentine P. Ananikov. 2021. "How to Make a Cocktail of Palladium Catalysts with Cola and Alcohol: Heteroatom Doping vs. Nanoscale Morphology of Carbon Supports" Nanomaterials 11, no. 10: 2599. https://doi.org/10.3390/nano11102599
APA StylePentsak, E. O., Galushko, A. S., Cherepanova, V. A., & Ananikov, V. P. (2021). How to Make a Cocktail of Palladium Catalysts with Cola and Alcohol: Heteroatom Doping vs. Nanoscale Morphology of Carbon Supports. Nanomaterials, 11(10), 2599. https://doi.org/10.3390/nano11102599








