Gold Nanostars with Reduced Fouling Facilitate Small Molecule Detection in the Presence of Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Nanoparticles
2.3. Characterization of Nanoparticles
2.3.1. Size and Morphology Analysis
2.3.2. Study of Optical Properties
2.4. Protein Corona Characterization
2.4.1. Cryogenic Transmission Electron Microscopy (Cryo-Tem)
2.4.2. Protein Secondary Structure Analysis
2.5. GNP Interaction with Small Molecules after Incubation with Protein
3. Results and Discussion
3.1. Synthesis and Nanoparticles Characterization
3.1.1. Nanoparticles Morphology
3.1.2. Optical Properties of Nanoparticles
3.1.3. Raman Signal Enhancement Ability of Nanoparticles
3.1.4. Changes of Nanoparticles Properties after Interaction with Protein
3.2. Protein Corona Characterization
3.2.1. Analysis of the Protein–Particle Interactions
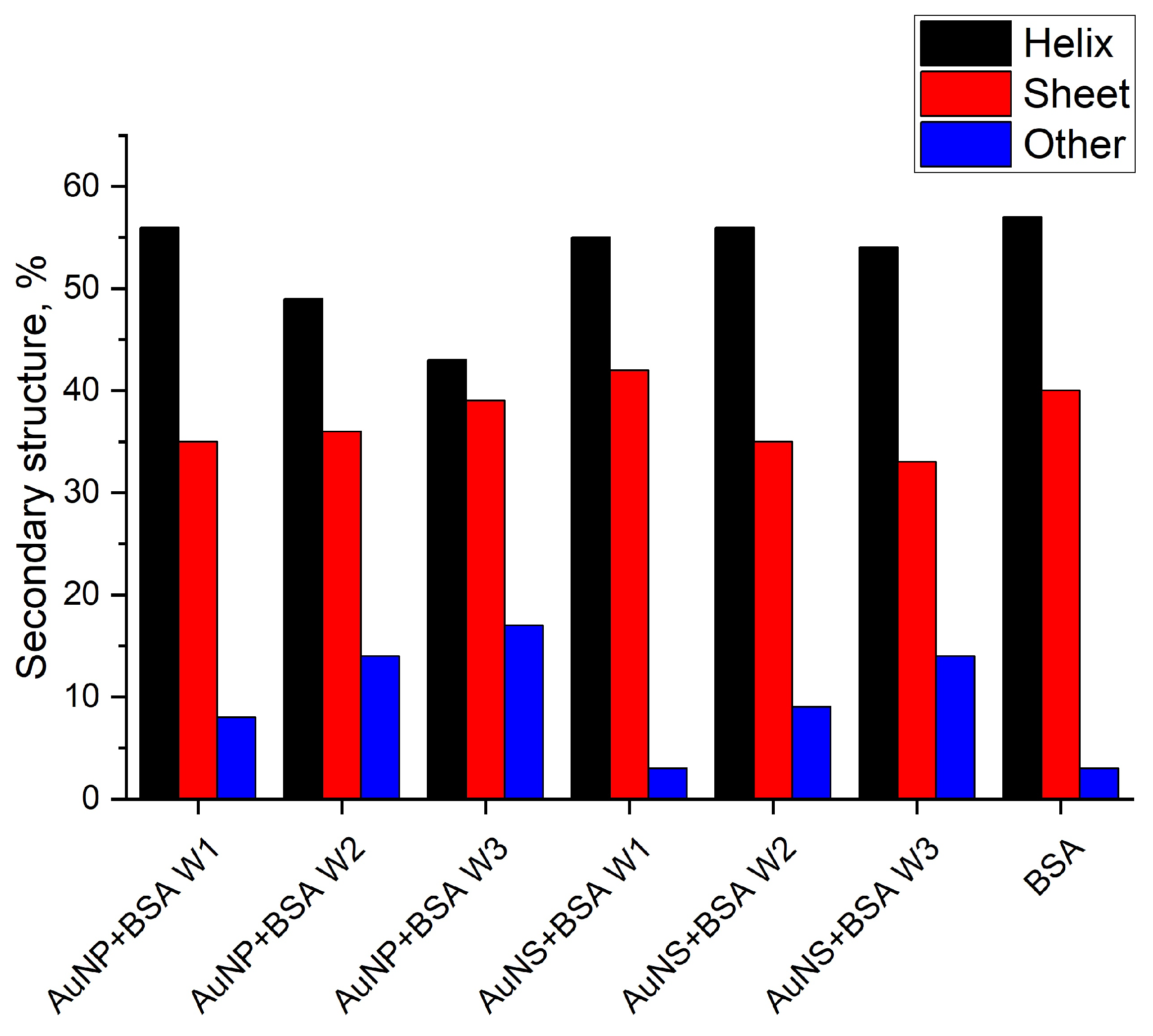
3.2.2. Conformational Changes of Protein
3.3. GNP Interaction with Small Molecules after Incubation with Protein
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AuNP | Spheroidal Gold Nanoparticles |
| AuNS | Star-shaped Gold Nanoparticle |
| BSA | Bovine Serum Albumin |
| CCK-8 | Cell Counting Kit-8 |
| DLS | Dynamic Light Scattering |
| DMSO | Dimethyl Sulfoxide |
| EF | Enhancement Factor |
| GNPs | Gold Nanoparticles |
| LSPR | Localized Surface Plasmon Resonance |
| MBA | 4-Mercaptobenzoic Acid |
| NIR | Near Infrared |
| NP | Nanoparticle |
| NTA | Nanoparticles Tracking Analysis |
| PBS | Phosphate-Buffered Saline |
| PC | Protein Corona |
| PDI | Polydispersity Index |
| SERS | Serface-Enhanced Raman Spectroscopy |
| SI | Support Information |
| SOM | Self-Organizing Map |
| TEM | Transmission Electron Microscopy |
| TFMBA | 2,3,5,6-Tetrafluoro-4-Mercaptobenzoic Acid |
| UV–Vis | Ultraviolet–Visible Absorption Spectroscopy |
| ZP | Zeta Potential |
References
- Heinzerling, P.; Oetken, M. Nanochemistry—A Split between 18th Century and Modern Times. World J. Chem. Educ. 2018, 6, 1–7. [Google Scholar] [CrossRef][Green Version]
- Faraday, M.X. The Bakerian Lecture. Experimental relations of gold (and other metals) to light. Philos. Trans. R. Soc. B 1857, 147, 145–181. [Google Scholar] [CrossRef]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Mugaka, B.P.; Hu, Y.; Ma, Y.; Ding, Y. Surface Modification of Gold Nanoparticles for Targeted Drug Delivery. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Pathak, Y.V., Ed.; Springer: Cham, Switzerland, 2019; pp. 391–403. [Google Scholar] [CrossRef]
- Zhang, Y.; Dasari, T.P.S.; Deng, H.; Yu, H. Antimicrobial Activity of Gold Nanoparticles and Ionic Gold. J. Environ. Sci. Health Part C 2015, 33, 286–327. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wang, Y.; Song, Z.; Feng, Y.; Chen, Y.; Zhang, D.; Feng, L. The Basic Properties of Gold Nanoparticles and their Applications in Tumor Diagnosis and Treatment. Int. J. Mol. Sci. 2020, 21, 2480. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, Y.; Mi, J.; Shen, Y.; Tu, Z.; Liu, L. Photothermal lysis of pathogenic bacteria by platinum nanodots decorated gold nanorods under near infrared irradiation. J. Hazard. Mater. 2018, 342, 121–130. [Google Scholar] [CrossRef]
- Govindaraju, S.; Yun, K. Synthesis of gold nanomaterials and their cancer-related biomedical applications: An update. 3 Biotech 2018, 8, 113. [Google Scholar] [CrossRef]
- Yang, T.; Wang, D.; Liu, X. Assembled gold nanorods for the photothermal killing of bacteria. Colloids Surfaces B Biointerfaces 2019, 173, 833–841. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Yang, D.; Hu, P.; Gao, L.; Chen, D.; Qiao, Y.; Wu, Y.; Jiang, X.; Li, G. Polydopamine-coated gold nanostars for near-infrared cancer photothermal therapy by multiple pathways. J. Mater. Sci. 2019, 54, 12036–12048. [Google Scholar] [CrossRef]
- Annavaram, V.; Posa, V.R.; Lakshmi, D.V.; Sumalatha, J.; Somala, A.R. Terminalia bellirica fruit extract-mediated synthesis of gold nanoparticles (AuNPs) and studies on antimicrobial and antioxidant activity. Inorg. Nano-Met. Chem. 2017, 47, 681–687. [Google Scholar] [CrossRef]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef]
- Yuan, H.; Khoury, C.G.; Hwang, H.; Wilson, C.M.; Grant, G.A.; Vo-Dinh, T. Gold nanostars: Surfactant-free synthesis, 3D modelling, and two-photon photoluminescence imaging. Nanotechnology 2012, 23, 75102. [Google Scholar] [CrossRef]
- Indrasekara, A.S.D.S.; Meyers, S.; Shubeita, S.; Feldman, L.C.; Gustafsson, T.; Fabris, L. Gold nanostar substrates for SERS-based chemical sensing in the femtomolar regime. Nanoscale 2014, 6, 8891–8899. [Google Scholar] [CrossRef]
- Khoury, C.G.; Vo-Dinh, T. Gold Nanostars for Surface-Enhanced Raman Scattering: Synthesis, Characterization and Optimization. J. Phys. Chem. C 2008, 112, 18849–18859. [Google Scholar] [CrossRef]
- Hrelescu, C.; Sau, T.K.; Rogach, A.L.; Jäckel, F.; Feldmann, J. Single gold nanostars enhance Raman scattering. Appl. Phys. Lett. 2009, 94, 153113. [Google Scholar] [CrossRef]
- Barbosa, S.; Agrawal, A.; Rodríguez-Lorenzo, L.; Pastoriza-Santos, I.; Alvarez-Puebla, R.A.; Kornowski, A.; Weller, H.; Liz-Marzán, L.M. Tuning Size and Sensing Properties in Colloidal Gold Nanostars. Langmuir 2010, 26, 14943–14950. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.L.; Zhang, M.; Diagaradjane, P.; Peddibhotla, S.; Contreras, A.; Hilsenbeck, S.G.; Woodward, W.A.; Krishnan, S.; Chang, J.C.; Rosen, J.M. Thermal Enhancement with Optically Activated Gold Nanoshells Sensitizes Breast Cancer Stem Cells to Radiation Therapy. Sci. Transl. Med. 2010, 2, 55ra79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z. Biomedical Applications of Shape-Controlled Plasmonic Nanostructures: A Case Study of Hollow Gold Nanospheres for Photothermal Ablation Therapy of Cancer. J. Phys. Chem. Lett. 2010, 1, 686–695. [Google Scholar] [CrossRef]
- Xia, Y.; Li, W.; Cobley, C.M.; Chen, J.; Xia, X.; Zhang, Q.; Yang, M.; Cho, E.C.; Brown, P.K. Gold Nanocages: From Synthesis to Theranostic Applications. Acc. Chem. Res. 2011, 44, 914–924. [Google Scholar] [CrossRef]
- Huang, X.; Neretina, S.; El-Sayed, M.A. Gold Nanorods: From Synthesis and Properties to Biological and Biomedical Applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef]
- Hahn, M.A.; Singh, A.K.; Sharma, P.; Brown, S.C.; Moudgil, B.M. Nanoparticles as contrast agents for in vivo bioimaging: Current status and future perspectives. Anal. Bioanal. Chem. 2011, 399, 3–27. [Google Scholar] [CrossRef]
- Rodríguez-Lorenzo, L.; Álvarez-Puebla, R.A.; Pastoriza-Santos, I.; Mazzucco, S.; Stéphan, O.; Kociak, M.; Liz-Marzán, L.M.; García de Abajo, F.J. Zeptomol Detection Through Controlled Ultrasensitive Surface-Enhanced Raman Scattering. J. Am. Chem. Soc. 2009, 131, 4616–4618. [Google Scholar] [CrossRef]
- Alvarez-Puebla, R.; Liz-Marzán, L.M.; García de Abajo, F.J. Light Concentration at the Nanometer Scale. J. Phys. Chem. Lett. 2010, 1, 2428–2434. [Google Scholar] [CrossRef]
- Reguera, J.; Langer, J.; Jiménez de Aberasturi, D.; Liz-Marzán, L.M. Anisotropic metal nanoparticles for surface enhanced Raman scattering. Chem. Soc. Rev. 2017, 46, 3866–3885. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Åberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef]
- Sloan-Dennison, S.; Schultz, Z.D. Label-free plasmonic nanostar probes to illuminate in vitro membrane receptor recognition. Chem. Sci. 2019, 10, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Chen, C. The Crown and the Scepter: Roles of the Protein Corona in Nanomedicine. Adv. Mater. 2019, 31, 1805740. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Langer, J.; de Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed]
- Bailly, A.L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.A. In vivo evaluation of safety, biodistribution and pharmacokinetics of laser-synthesized gold nanoparticles. Sci. Rep. 2019, 9, 12890. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kuang, H.; Zhang, W.; Aguilar, Z.P.; Wei, H.; Xu, H. Comparisons of the biodistribution and toxicological examinations after repeated intravenous administration of silver and gold nanoparticles in mice. Sci. Rep. 2017, 7, 3303. [Google Scholar] [CrossRef]
- Charbgoo, F.; Nejabat, M.; Abnous, K.; Soltani, F.; Taghdisi, S.M.; Alibolandi, M.; Thomas Shier, W.; Steele, T.W.J.; Ramezani, M. Gold nanoparticle should understand protein corona for being a clinical nanomaterial. J. Control. Release 2018, 272, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, J.; García, I.; Liz-Marzán, L.M. Cellular Uptake of Nanoparticles versus Small Molecules: A Matter of Size. Acc. Chem. Res. 2018, 51, 2305–2313. [Google Scholar] [CrossRef]
- Feliu, N.; Docter, D.; Heine, M.; del Pino, P.; Ashraf, S.; Kolosnjaj-Tabi, J.; Macchiarini, P.; Nielsen, P.; Alloyeau, D.; Gazeau, F.; et al. In Vivo degeneration and the fate of inorganic nanoparticles. Chem. Soc. Rev. 2016, 45, 2440–2457. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, S.B.; Bernfur, K.; Mikkelsen, A.; Cedervall, T. Analysis of nanoparticle biomolecule complexes. Nanoscale 2018, 10, 4246–4257. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, G.P.; Kneipp, J. Different binding sites of serum albumins in the protein corona of gold nanoparticles. Analyst 2018, 143, 6061–6068. [Google Scholar] [CrossRef] [PubMed]
- Docter, D.; Westmeier, D.; Markiewicz, M.; Stolte, S.; Knauer, S.K.; Stauber, R.H. The nanoparticle biomolecule corona: Lessons learned— Challenge accepted? Chem. Soc. Rev. 2015, 44, 6094–6121. [Google Scholar] [CrossRef]
- Das, A.; Chakrabarti, A.; Das, P. Probing Protein Adsorption on Nanoparticle Surface by Second Harmonic Light Scattering. Phys. Chem. Chem. Phys. 2016, 18, 24325–24331. [Google Scholar] [CrossRef] [PubMed]
- Cabaleiro-Lago, C.; Szczepankiewicz, O.; Linse, S. The Effect of Nanoparticles on Amyloid Aggregation Depends on the Protein Stability and Intrinsic Aggregation Rate. Langmuir 2012, 28, 1852–1857. [Google Scholar] [CrossRef]
- García-Álvarez, R.; Hadjidemetriou, M.; Sánchez-Iglesias, A.; Liz-Marzán, L.M.; Kostarelos, K. In Vivo formation of protein corona on gold nanoparticles. the effect of their size and shape. Nanoscale 2018, 10, 1256–1264. [Google Scholar] [CrossRef]
- Wang, G.; Wang, W.; Shangguan, E.; Gao, S.; Liu, Y. Effects of gold nanoparticle morphologies on interactions with proteins. Mater. Sci. Eng. C 2020, 111, 110830. [Google Scholar] [CrossRef] [PubMed]
- Wuithschick, M.; Birnbaum, A.; Witte, S.; Sztucki, M.; Vainio, U.; Pinna, N.; Rademann, K.; Emmerling, F.; Kraehnert, R.; Polte, J. Turkevich in New Robes: Key Questions Answered for the Most Common Gold Nanoparticle Synthesis. ACS Nano 2015, 9, 7052–7071. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Liu, Y.; Lyu, N.; Rajendran, V.K.; Piper, J.; Rodger, A.; Wang, Y. Sensitive and Direct DNA Mutation Detection by Surface-Enhanced Raman Spectroscopy Using Rational Designed and Tunable Plasmonic Nanostructures. Anal. Chem. 2020, 92, 5708–5716. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. Available online: https://imagej.net/Welcome (accessed on 28 September 2020). [CrossRef]
- Hall, V.; Sklepari, M.; Rodger, A. Protein Secondary Structure Prediction from Circular Dichroism Spectra Using a Self-Organizing Map with Concentration Correction. Chirality 2014, 26, 471–482. [Google Scholar] [CrossRef]
- Harmsen, S.; Wall, M.A.; Huang, R.; Kircher, M.F. Cancer imaging using surface-enhanced resonance Raman scattering nanoparticles. Nat. Protoc. 2017, 12, 1400–1414. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dixit, C.K. 3-Methods for characterization of nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Woodhead Publishing: Duxford, UK, 2017. [Google Scholar] [CrossRef]
- Shnoudeh, A.J.; Hamad, I.; Abdo, R.W.; Qadumii, L.; Jaber, A.Y.; Surchi, H.S.; Alkelany, S.Z. Chapter 15—Synthesis, Characterization, and Applications of Metal Nanoparticles. In Advances in Pharmaceutical Product Development and Research; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 527–612. [Google Scholar] [CrossRef]
- Kuschnerus, I.; Lau, M.; Giri, K.; Bedford, N.; Biazik, J.; Ruan, J.; Garcia-Bennett, A. Effect of a protein corona on the fibrinogen induced cellular oxidative stress of gold nanoparticles. Nanoscale 2020, 12, 5898–5905. [Google Scholar] [CrossRef]
- Liu, X.; Atwater, M.; Wang, J.; Huo, Q. Extinction coefficient of gold nanoparticles with different sizes and different capping ligands. Colloids Surfaces B Biointerfaces 2007, 58, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Casals, E.; Pfaller, T.; Duschl, A.; Oostingh, G.J.; Puntes, V. Time Evolution of the Nanoparticle Protein Corona. ACS Nano 2010, 4, 3623–3632. [Google Scholar] [CrossRef] [PubMed]
- Azzazy, H.M.E.; Mansour, M.M.H.; Samir, T.M.; Franco, R. Gold nanoparticles in the clinical laboratory: Principles of preparation and applications. Clin. Chem. Lab. Med. 2011, 50, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle—Protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef]
- Miyake, N.; Sato, T.; Maki, Y. Effect of Zeta Potentials on Bovine Serum Albumin Adsorption to Hydroxyapatite Surfaces. Bull. Tokyo Dent. Coll. 2013, 54, 97–101. [Google Scholar] [CrossRef][Green Version]
- Szekeres, G.P.; Kneipp, J. SERS Probing of Proteins in Gold Nanoparticle Agglomerates. Front. Chem. 2019, 7, 30. [Google Scholar] [CrossRef]
- Hernández, B.; Tinacci, L.; Coïc, Y.M.; Chenal, A.; Cohen, R.; Sanchez-Cortes, S.; Ghomi, M. Tryptophan Tight Binding to Gold Nanoparticles Induces Drastic Changes in Indole Ring Raman Markers. J. Phys. Chem. C 2018, 122, 13034–13046. [Google Scholar] [CrossRef]
- Sweeting, L. Organic Structural Spectroscopy. J. Chem. Educ. 1998, 75, 1218–1219. [Google Scholar] [CrossRef]
- Greenfield, N.J. Applications of circular dichroism in protein and peptide analysis. TrAC Trends Anal. Chem. 1999, 18, 236–244. [Google Scholar] [CrossRef]
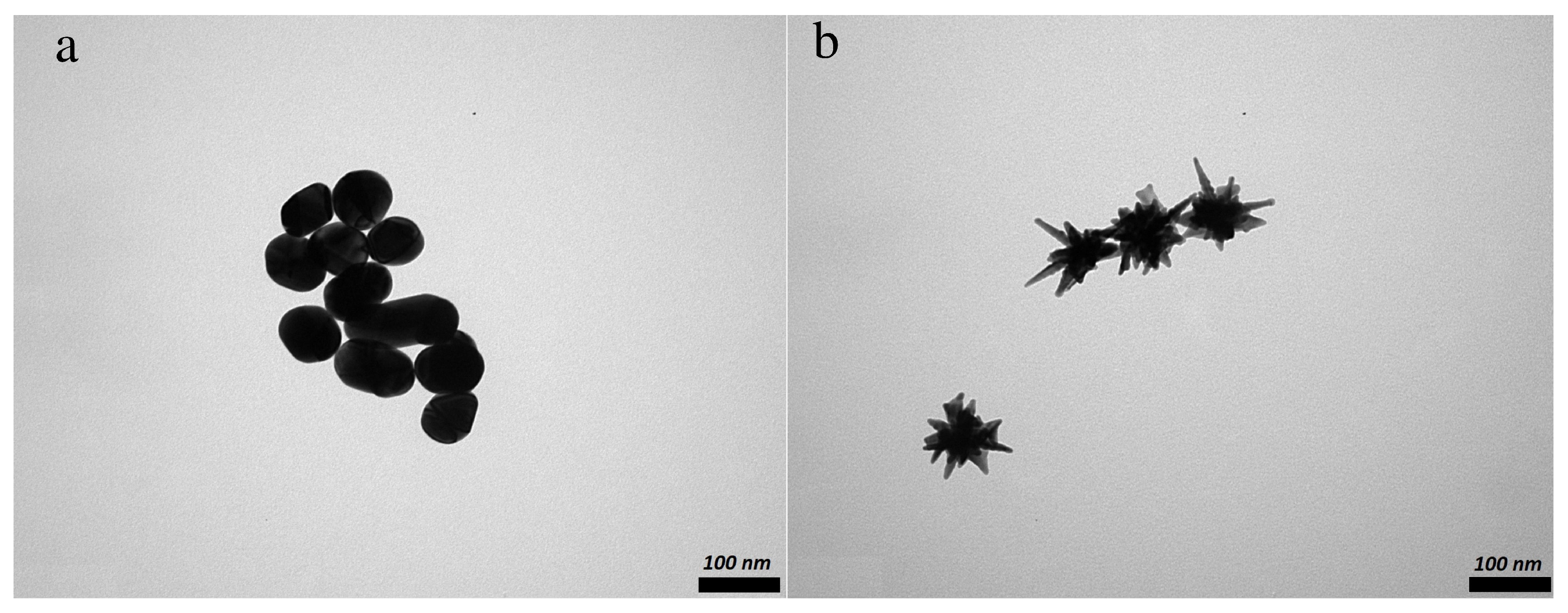
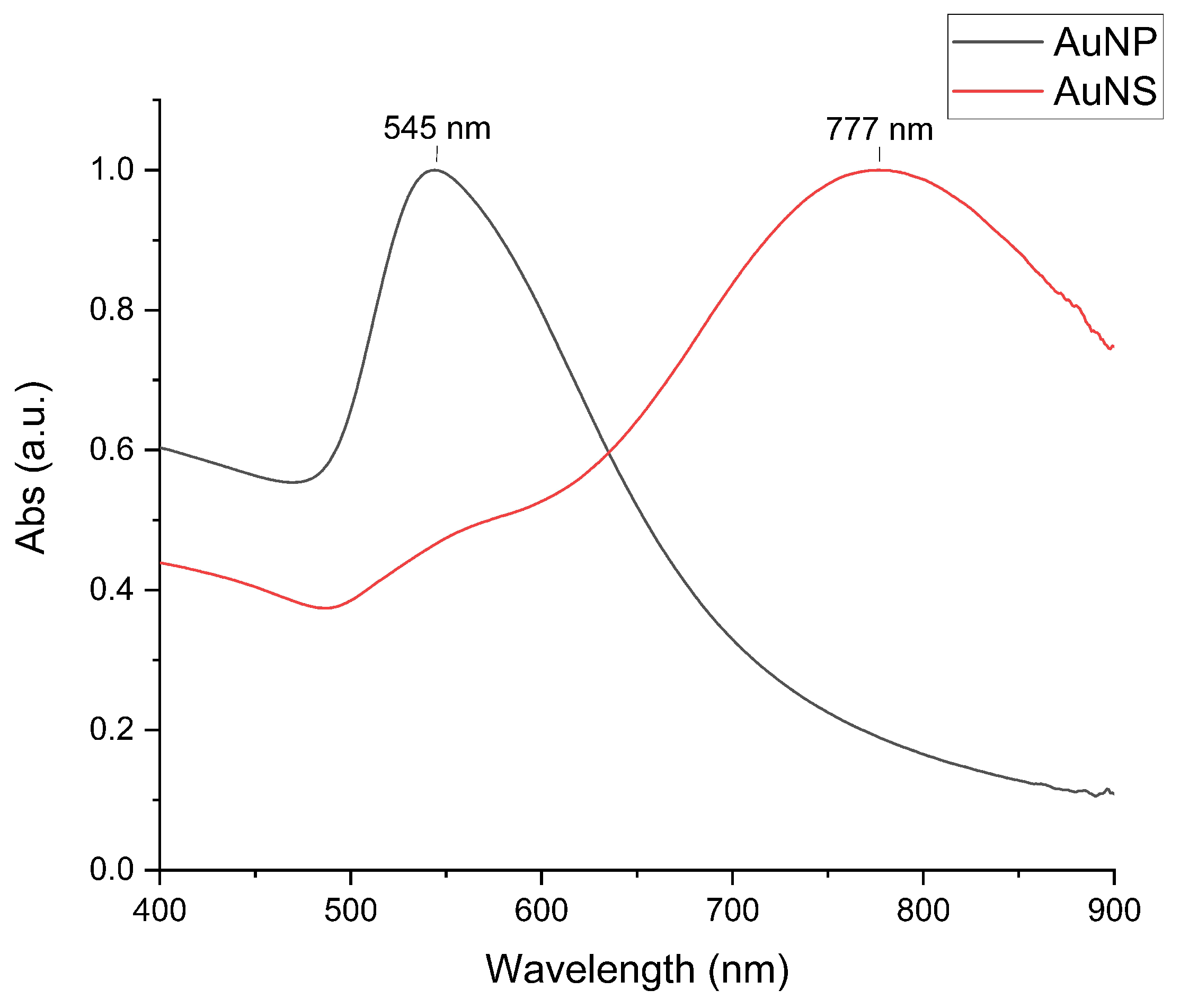
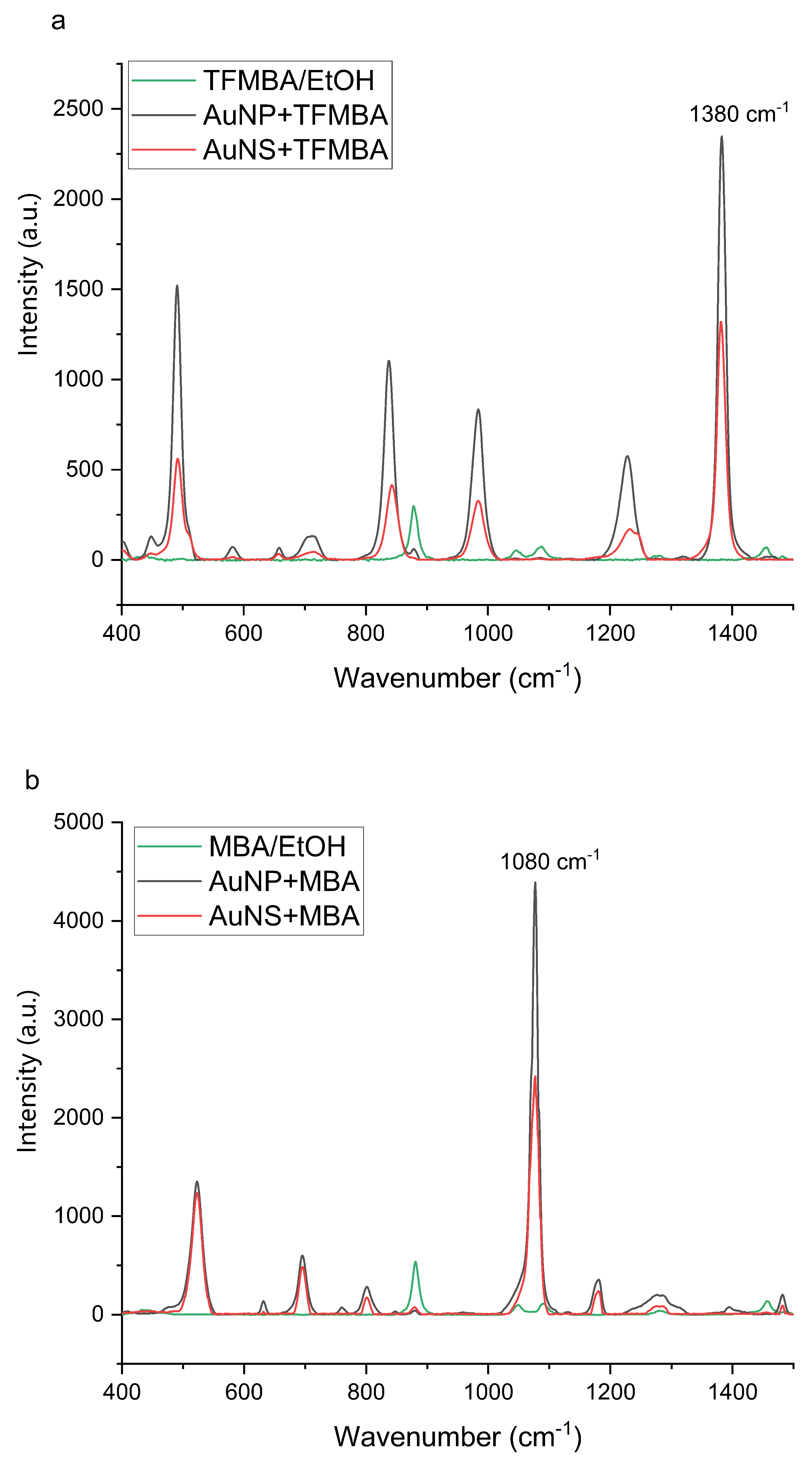
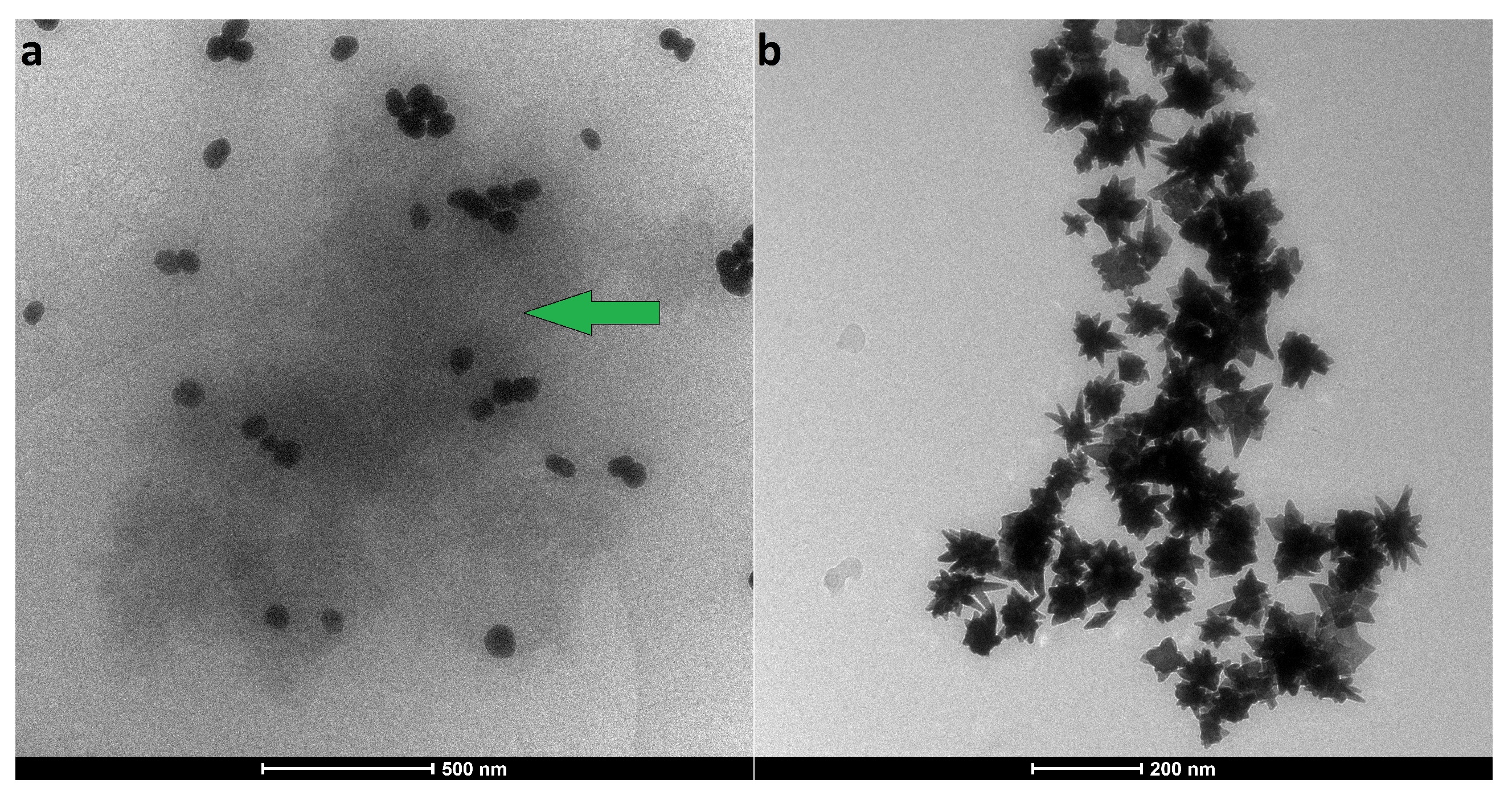
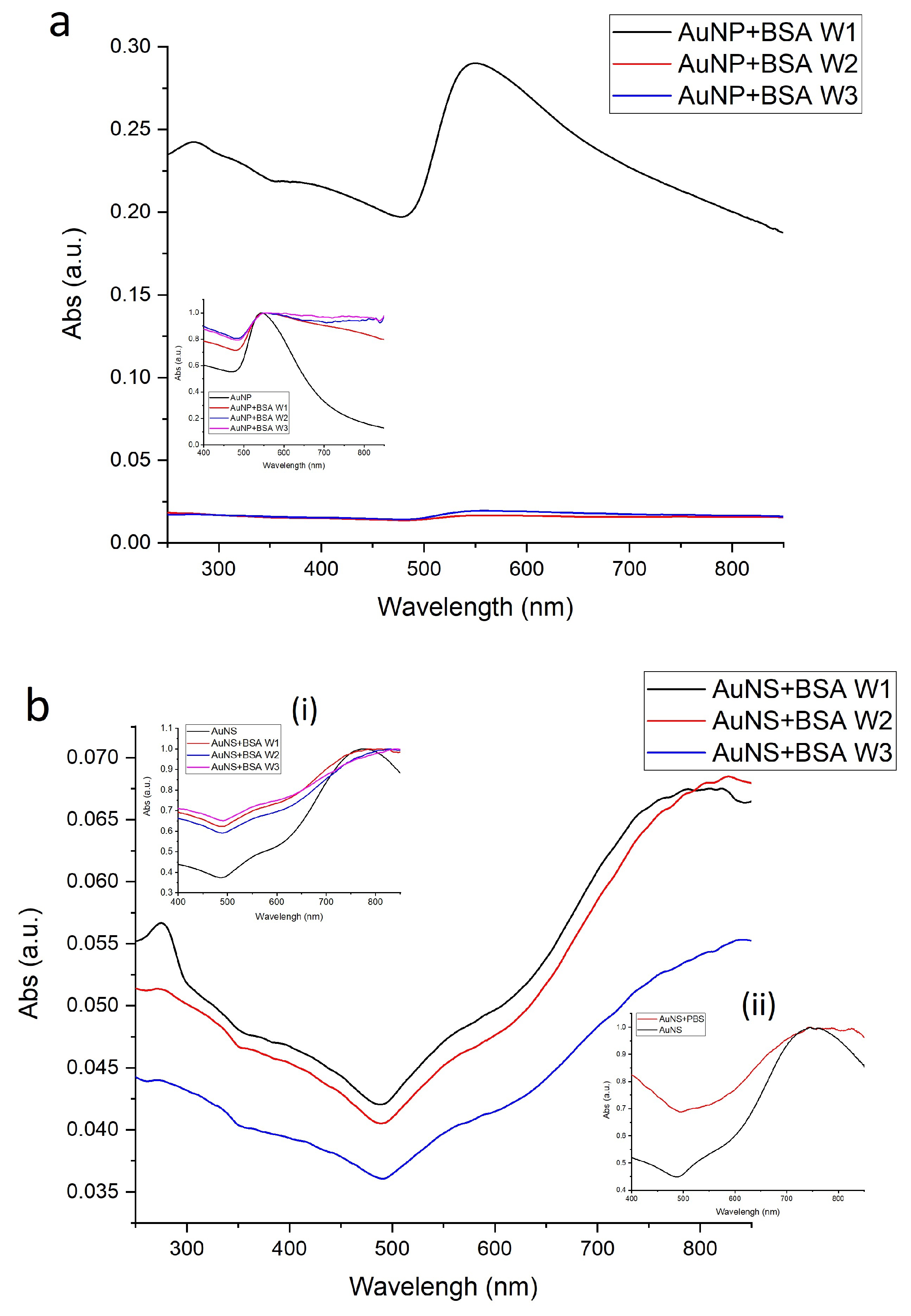
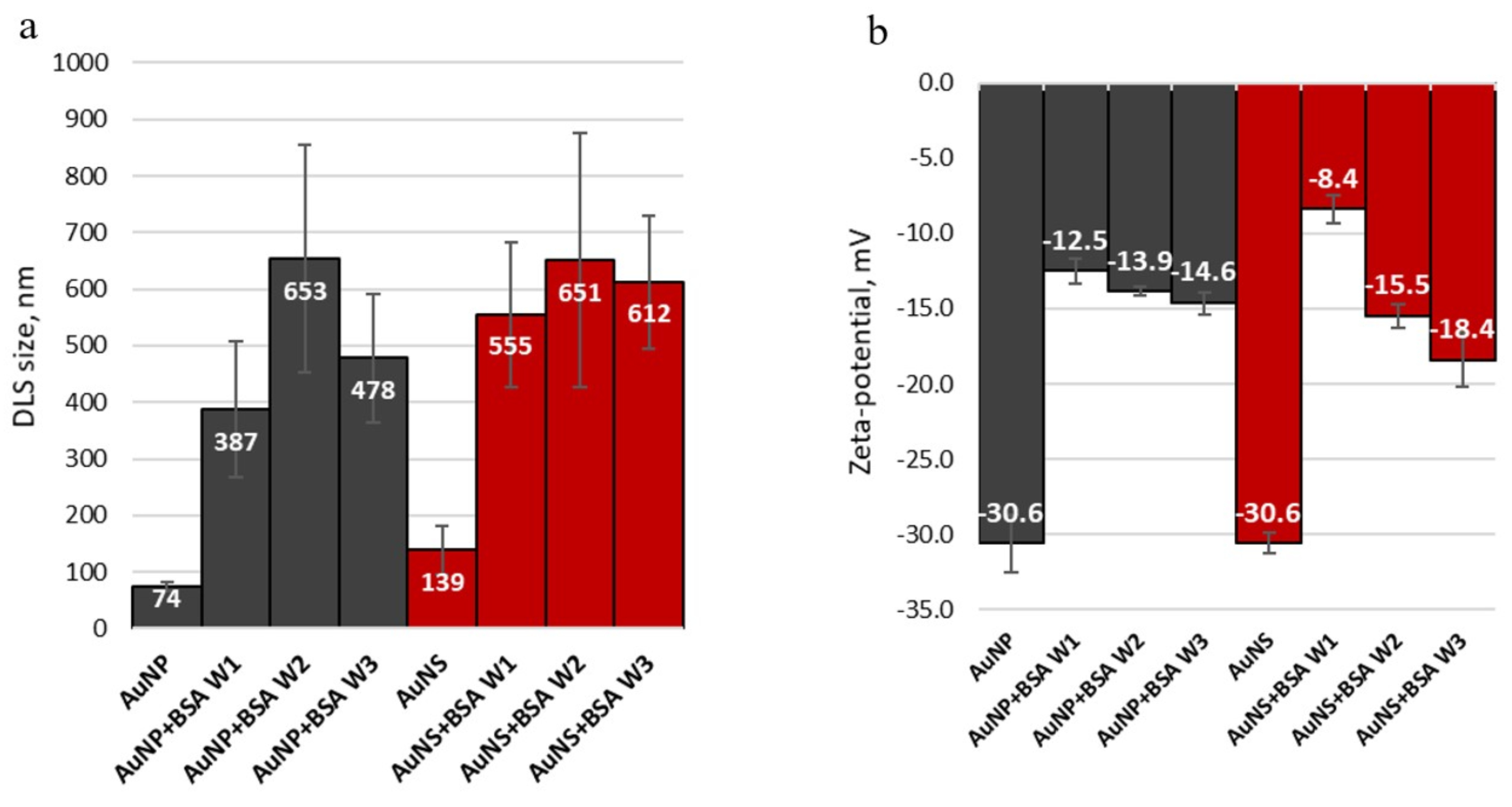
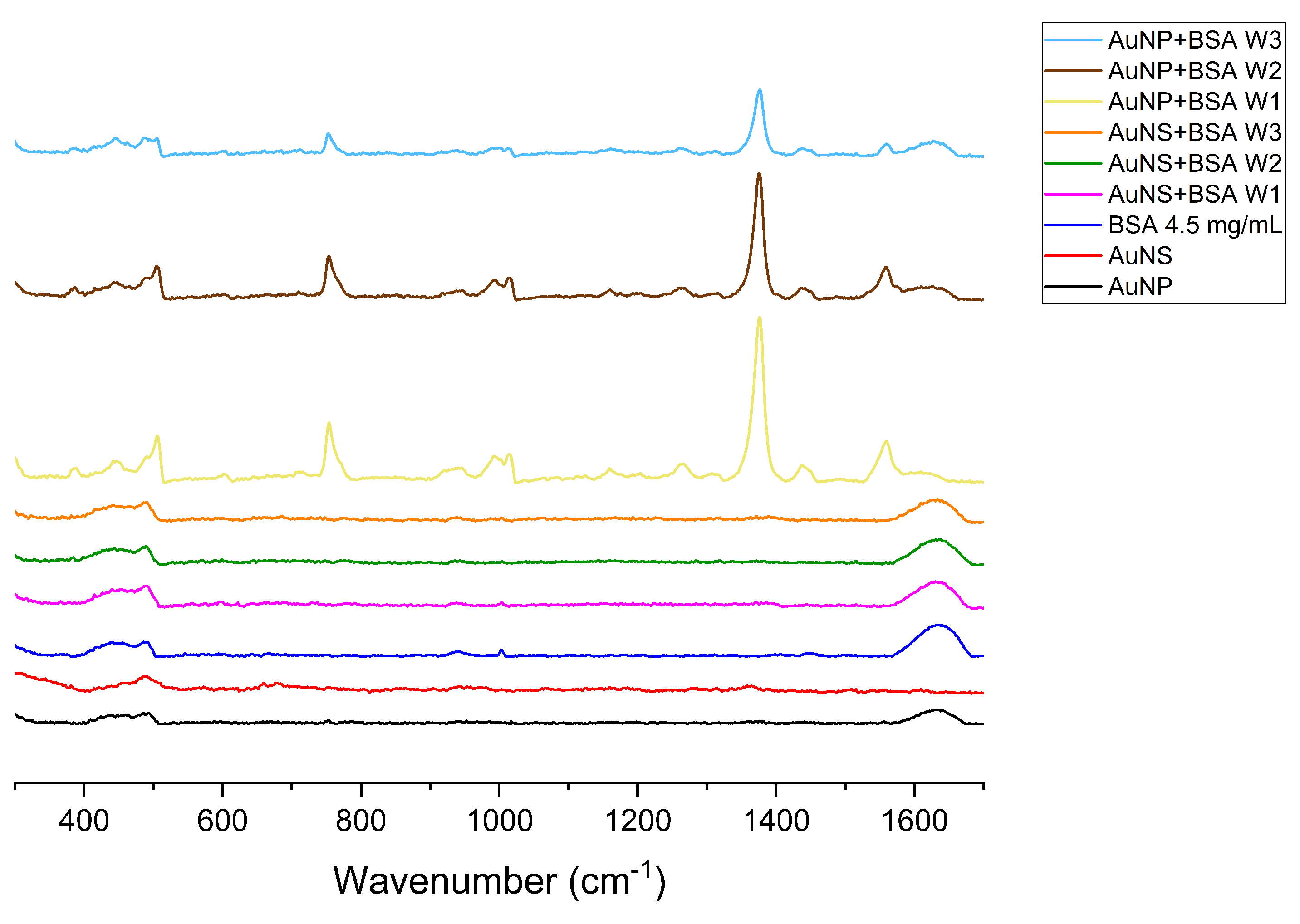
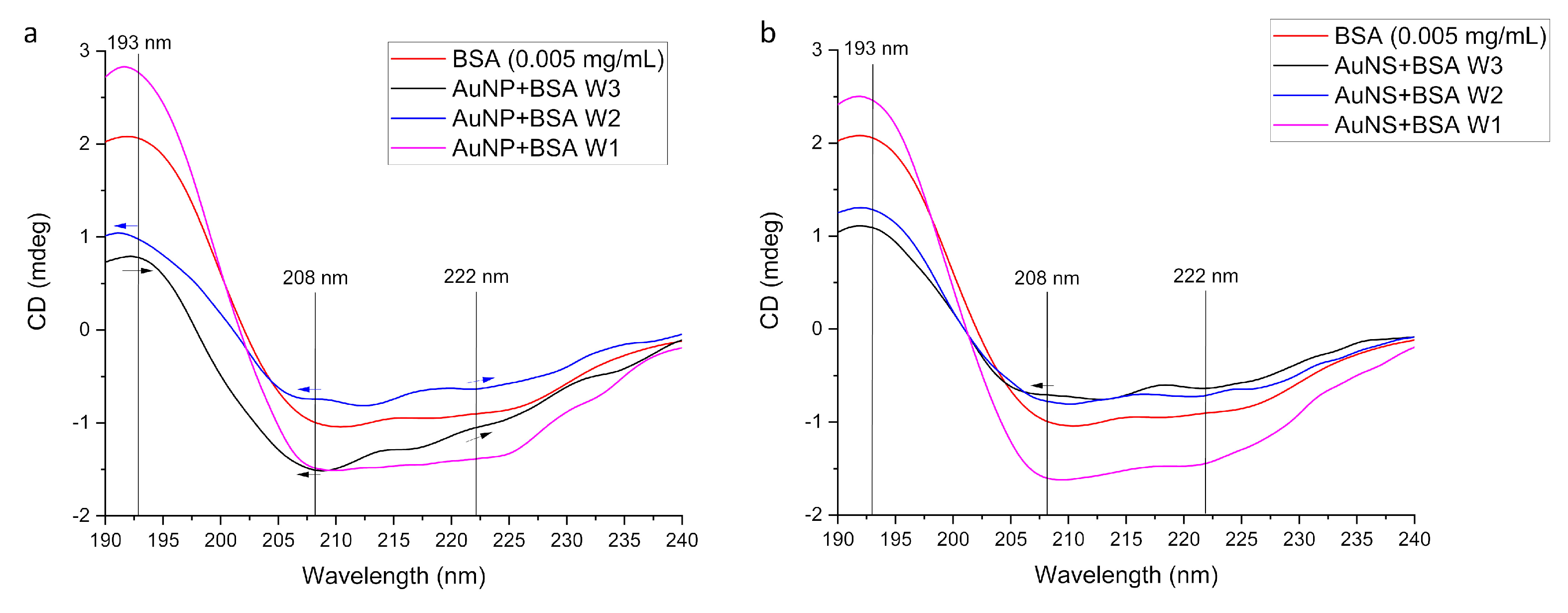

| Sample | Size, nm | Surface Charge (ZP), mV | |
|---|---|---|---|
| DLS | TEM | ||
| AuNP | 94 ± 5 (PDI: 0.254) | 93 ± 17 | −30.6 ± 1.9 |
| AuNS | 139 ± 27 (PDI: 0.396) | 92 ± 21, core size ∼ 50 nm | −30.6 ± 0.7 |
| Sample | EF |
|---|---|
| AuNP + TFMBA | |
| AuNS+TFMBA | |
| AuNP + MBA | |
| AuNS+MBA |
| Wavelength, | Assignment [57,58,59] |
|---|---|
| 490 | (S–S) |
| 506 | (S–S) |
| 602 | (COO–) |
| 753 | Trp, (C–S) |
| 941 | (C–C–N) symm, -helical skeletal |
| 996 | R breathing |
| 1003 | Phe: indole asymm ring |
| 1183 | Tyr, (–C–N) |
| 1300–1200 | Trp, Phe: (R), Amide III—region |
| 1309 | Wag (CH2) |
| 1360–1340 | Trp, doublet |
| 1383 | (CH3), (COO–) |
| 1437 | asymm (CH3), bend (CH2) |
| 1560 | Trp: (R), (r), amide II |
| Sample | EF | EF Ratios | |
|---|---|---|---|
| Comparison between Protein Incubated and bare GNP | Comparison between AuNS and AuNP | ||
| AuNP+TFMBA | 4.4 × 104 | 44-fold decrease | 10-fold larger |
| AuNP+BSA+TFMBA | 1.0 × 103 | ||
| AuNS+TFMBA | 6.5 × 104 | 7-fold decrease | |
| AuNS+BSA+TFMBA | 9.5 × 103 | ||
| AuNP+MBA | 8.2 × 104 | 39-fold decrease | 12-fold larger |
| AuNP+BSA+MBA | 2.1 × 103 | ||
| AuNS+MBA | 1.2 × 105 | 6-fold decrease | |
| AuNS+BSA+MBA | 2.6 × 104 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tukova, A.; Kuschnerus, I.C.; Garcia-Bennett, A.; Wang, Y.; Rodger, A. Gold Nanostars with Reduced Fouling Facilitate Small Molecule Detection in the Presence of Protein. Nanomaterials 2021, 11, 2565. https://doi.org/10.3390/nano11102565
Tukova A, Kuschnerus IC, Garcia-Bennett A, Wang Y, Rodger A. Gold Nanostars with Reduced Fouling Facilitate Small Molecule Detection in the Presence of Protein. Nanomaterials. 2021; 11(10):2565. https://doi.org/10.3390/nano11102565
Chicago/Turabian StyleTukova, Anastasiia, Inga Christine Kuschnerus, Alfonso Garcia-Bennett, Yuling Wang, and Alison Rodger. 2021. "Gold Nanostars with Reduced Fouling Facilitate Small Molecule Detection in the Presence of Protein" Nanomaterials 11, no. 10: 2565. https://doi.org/10.3390/nano11102565
APA StyleTukova, A., Kuschnerus, I. C., Garcia-Bennett, A., Wang, Y., & Rodger, A. (2021). Gold Nanostars with Reduced Fouling Facilitate Small Molecule Detection in the Presence of Protein. Nanomaterials, 11(10), 2565. https://doi.org/10.3390/nano11102565










