Effect of Copper Doping on Electronic Structure and Optical Absorption of Cd33Se33 Quantum Dots
Abstract
1. Introduction
2. Methodologies
3. Results and Discussion
3.1. Geometry Distortions of Cu-Doped Cd33Se33 Quantum Dots
3.2. Stability of Cu-Doped Cd33Se33 QDs
3.3. Oxidation State for Cu Dopants in Cd33Se33 Quantum Dots
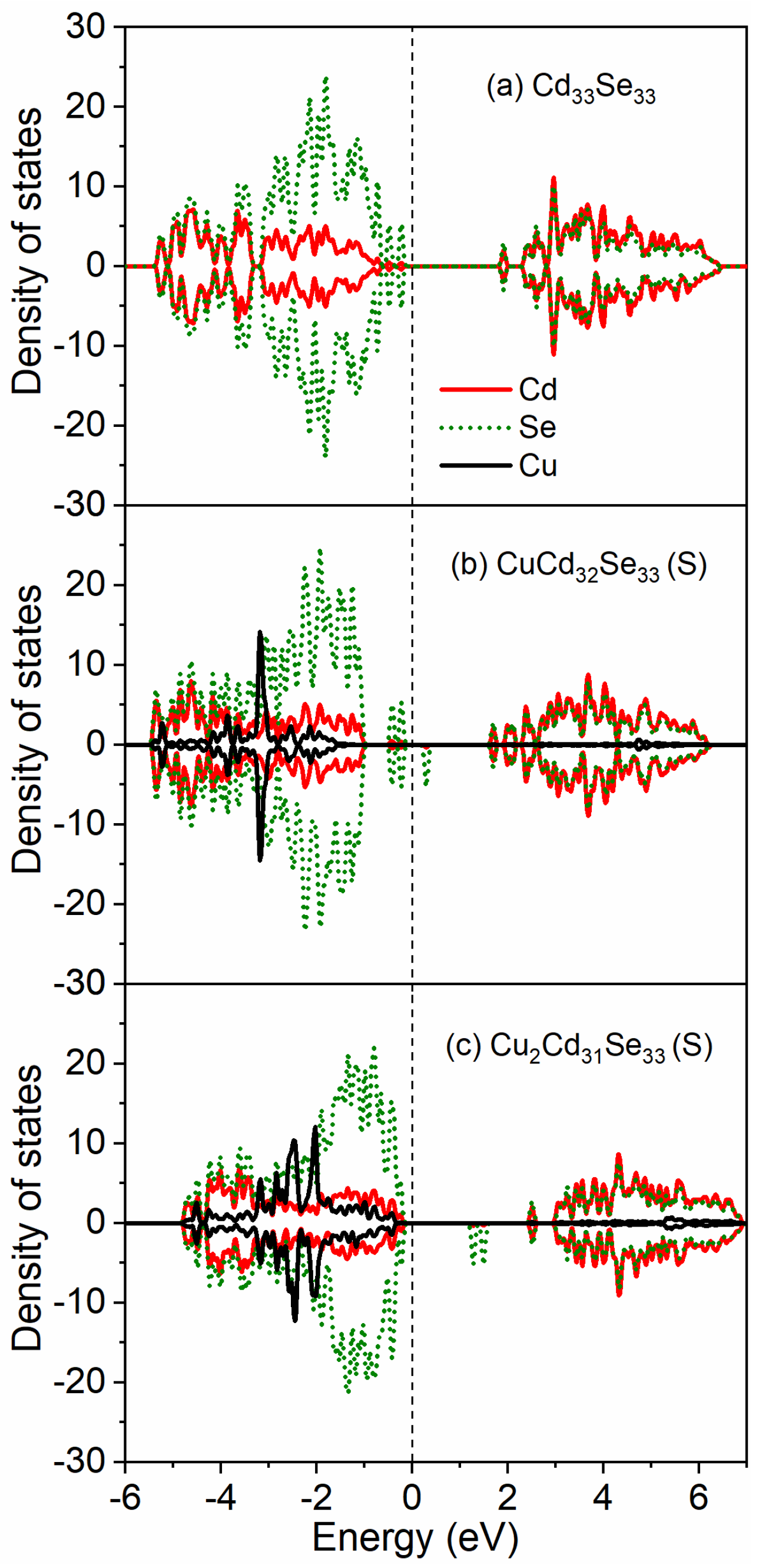
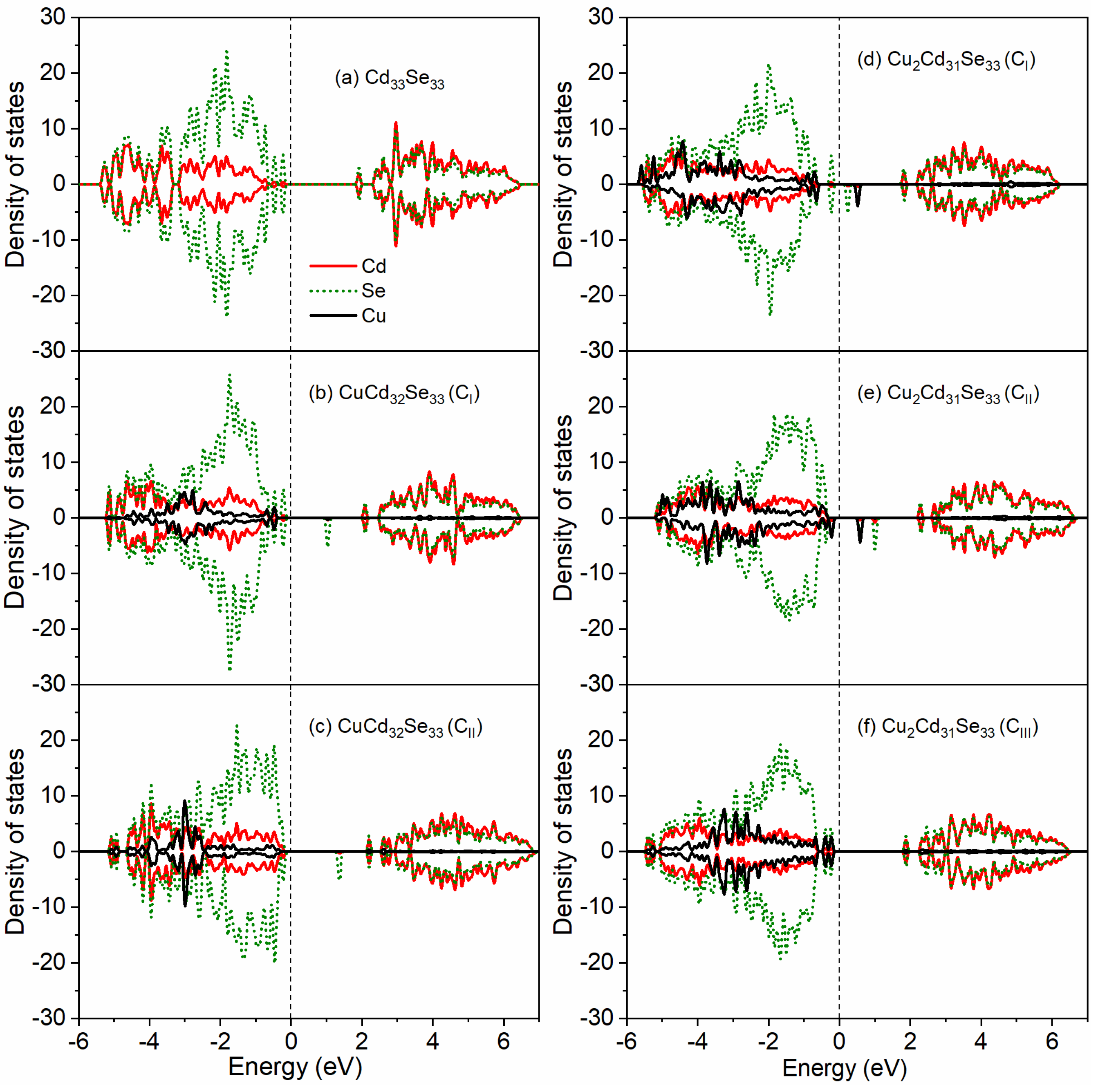
3.4. Electronic Properties for Cu-Doped Cd33Se33 Quantum Dots
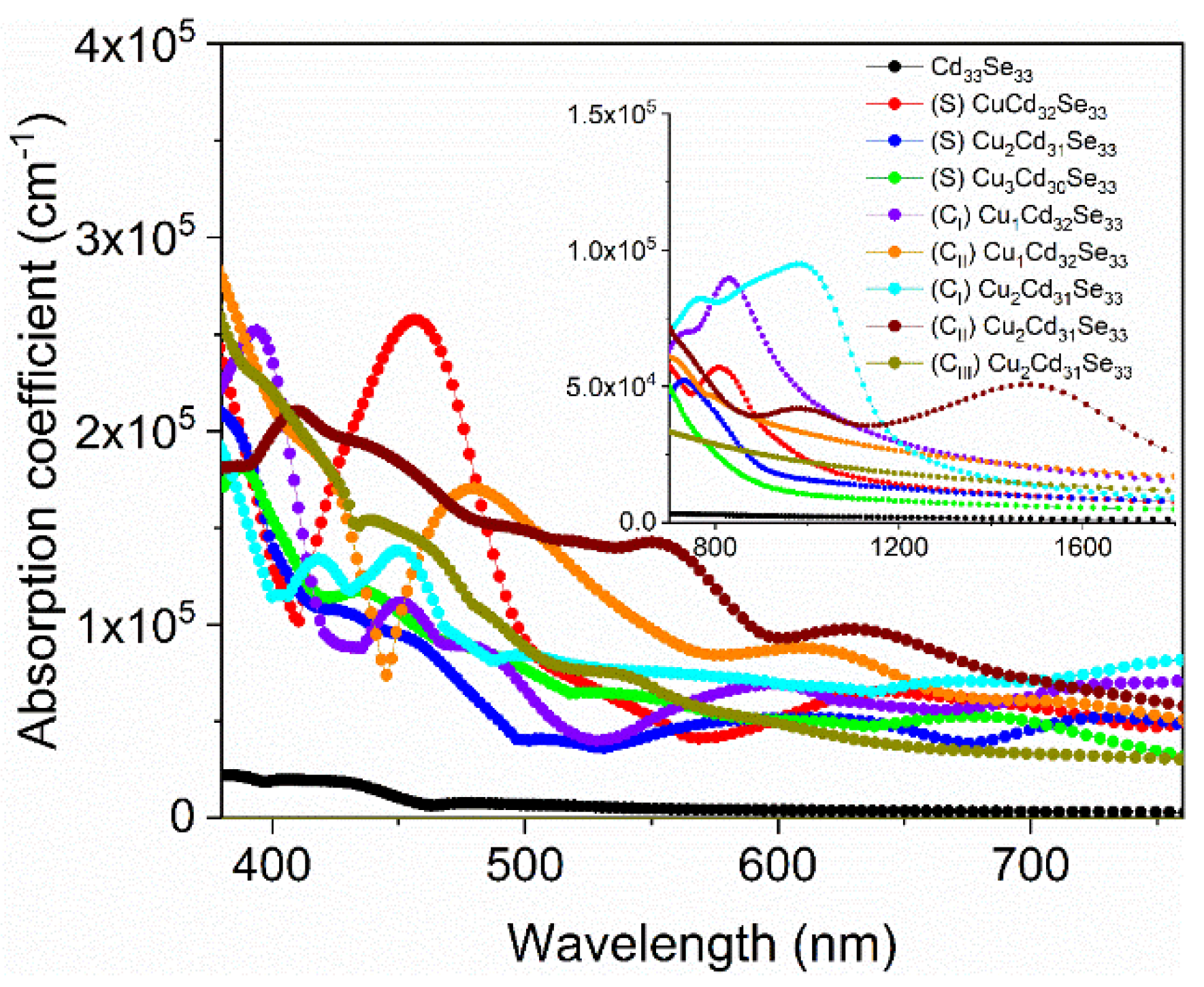
3.5. Optical Absorption Spectra for Cu-Doped Cd33Se33 Quantum Dots
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klimov, V.I.; Ivanov, S.A.; Nanda, J.; Achermann, M.; Bezel, I.; McGuire, J.A.; Piryatinski, A. Single-Exciton Optical Gain in Semiconductor Nanocrystals. Nature 2007, 447, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Mocatta, D.; Cohen, G.; Schattner, J.; Millo, O.; Rabani, E.; Banin, U. Heavily Doped Semiconductor Nanocrystal Quantum Dots. Science 2011, 332, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Mattoussi, H.; Mauro, J.M.; Goldman, E.R.; Anderson, G.P.; Sundar, V.C.; Mikulec, F.V.; Bawendi, M.G. Self-Assembly of CdSe−ZnS Quantum Dot Bioconjugates Using an Engineered Recombinant Protein. J. Am. Chem. Soc. 2000, 122, 12142–12150. [Google Scholar] [CrossRef]
- Mattoussi, H.; Mauro, J.; Goldman, E.; Green, T.; Anderson, G.; Sundar, V.; Bawendi, M. Bioconjugation of Highly Luminescent Colloidal CdSe-ZnS Quantum Dots with an Engineered Two-Domain Recombinant Protein. Phys. Status Solidi B 2001, 224, 277–283. [Google Scholar] [CrossRef]
- Santra, S.; Yang, H.; Holloway, P.H.; Stanley, J.T.; Mericle, R.A. Synthesis of Water-Dispersible Fluorescent, Radio-Opaque, and Paramagnetic CdS:Mn/ZnS Quantum Dots: A Multifunctional Probe for Bioimaging. J. Am. Chem. Soc. 2005, 127, 1656–1657. [Google Scholar] [CrossRef]
- Pradhan, N.; Battaglia, D.M.; Liu, Y.; Peng, X. Efficient, Stable, Small, and Water-Soluble Doped ZnSe Nanocrystal Emitters as Non-Cadmium Biomedical Labels. Nano Lett. 2007, 7, 312–317. [Google Scholar] [CrossRef]
- Wood, V.; Halpert, J.E.; Panzer, M.J.; Bawendi, M.G.; Bulović, V. Alternating Current Driven Electroluminescence from ZnSe/ZnS:Mn/ZnS Nanocrystals. Nano Lett. 2009, 9, 2367–2371. [Google Scholar] [CrossRef] [PubMed]
- Janβen, N.; Whitaker, K.M.; Gamelin, D.R.; Bratschitsch, R. Ultrafast Spin Dynamics in Colloidal ZnO Quantum Dots. Nano Lett. 2008, 8, 1991–1994. [Google Scholar] [CrossRef]
- Yang, H.; Holloway, P.H.; Ratna, B.B. Photoluminescent and Electroluminescent Properties of Mn-Doped ZnS Nanocrystals. J. Appl. Phys. 2003, 93, 586–592. [Google Scholar] [CrossRef]
- Warkentin, M.; Bridges, F.; Carter, S.A.; Anderson, M. Electroluminescence Materials ZnS:Cu,Cl and ZnS:Cu,Mn,Cl Studied by EXAFS Spectroscopy. Phys. Rev. B 2007, 75, 075301. [Google Scholar] [CrossRef]
- Smith, B.A.; Zhang, J.Z.; Joly, A.; Liu, J. Luminescence Decay Kinetics of Mn2+-Doped ZnS Nanoclusters Grown in Reverse Micelles. Phys. Rev. B 2000, 62, 2021–2028. [Google Scholar] [CrossRef]
- Lu, W.; Gao, P.; Jian, W.B.; Wang, Z.L.; Fang, J. Perfect Orientation Ordered In-Situ One-Dimensional Self-Assembly of Mn-Doped PbSe Nanocrystals. J. Am. Chem. Soc. 2004, 126, 14816–14821. [Google Scholar] [CrossRef]
- Norberg, N.S.; Parks, G.L.; Salley, G.M.; Gamelin, D.R. Giant Excitonic Zeeman Splittings in Colloidal Co2+-Doped ZnSe Quantum Dots. J. Am. Chem. Soc. 2006, 128, 13195–13203. [Google Scholar] [CrossRef] [PubMed]
- Fainblat, R.; Barrows, C.J.; Gamelin, D.R. Single Magnetic Impurities in Colloidal Quantum Dots and Magic-Size Clusters. Chem. Mater. 2017, 29, 8023–8036. [Google Scholar] [CrossRef]
- Sahu, A.; Kang, M.S.; Kompch, A.; Notthoff, C.; Wills, A.W.; Deng, D.; Winterer, M.; Frisbie, C.D.; Norris, D.J. Electronic Impurity Doping in CdSe Nanocrystals. Nano Lett. 2012, 12, 2587–2594. [Google Scholar] [CrossRef]
- Chen, D.; Viswanatha, R.; Ong, G.L.; Xie, R.; Balasubramaninan, M.; Peng, X. Temperature Dependence of “Elementary Processes” in Doping Semiconductor Nanocrystals. J. Am. Chem. Soc. 2009, 131, 9333–9339. [Google Scholar] [CrossRef]
- Viswanatha, R.; Brovelli, S.; Pandey, A.; Crooker, S.A.; Klimov, V.I. Copper-Doped Inverted Core/Shell Nanocrystals with “Permanent” Optically Active Holes. Nano Lett. 2011, 11, 4753–4758. [Google Scholar] [CrossRef] [PubMed]
- Brovelli, S.; Galland, C.; Viswanatha, R.; Klimov, V.I. Tuning Radiative Recombination in Cu-Doped Nanocrystals via Electrochemical Control of Surface Trapping. Nano Lett. 2012, 12, 4372–4379. [Google Scholar] [CrossRef]
- Dutta, A.; Bera, R.; Ghosh, A.; Patra, A. Ultrafast Carrier Dynamics of Photo-Induced Cu-Doped CdSe Nanocrystals. J. Phys. Chem. C 2018, 122, 16992–17000. [Google Scholar] [CrossRef]
- Ning, J.; Liu, J.; Levi-Kalisman, Y.; Frenkel, A.I.; Banin, U. Controlling Anisotropic Growth of Colloidal ZnSe Nanostructures. J. Am. Chem. Soc. 2018, 140, 14627–14637. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Knowles, K.E.; Gopalan, A.; Hughes, K.E.; James, M.C.; Gamelin, D.R. One-Pot Synthesis of Monodisperse Colloidal Copper-Doped CdSe Nanocrystals Mediated by Ligand–Copper Interactions. Chem. Mater. 2016, 28, 7375–7384. [Google Scholar] [CrossRef]
- Alperson, B.; Rubinstein, I.; Hodes, G. Identification of Surface States on Individual CdSe Quantum Dots by Room-Temperature Conductance Spectroscopy. Phys. Rev. B 2001, 63, 081303. [Google Scholar] [CrossRef]
- Jana, S.; Srivastava, B.B.; Acharya, S.; Santra, P.K.; Jana, N.R.; Sarma, D.D.; Pradhan, N. Prevention of Photooxidation in Blue–Green Emitting Cu Doped ZnSe Nanocrystals. Chem. Commun. 2010, 46, 2853. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mandal, P.; Talwar, S.S.; Major, S.S.; Srinivasa, R.S. Orange-Red Luminescence from Cu Doped CdS Nanophosphor Prepared Using Mixed Langmuir–Blodgett Multilayers. J. Chem. Phys. 2008, 128, 114703. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, N.; Goorskey, D.; Thessing, J.; Peng, X. An Alternative of CdSe Nanocrystal Emitters: Pure and Tunable Impurity Emissions in ZnSe Nanocrystals. J. Am. Chem. Soc. 2005, 127, 17586–17587. [Google Scholar] [CrossRef]
- Xie, F.; Xie, R.; Wang, Q. Synthesis and Characterization of CuIn(SxSe1–x)2 Nanocrystals. Adv. Sci. Lett. 2011, 4, 3624–3628. [Google Scholar] [CrossRef]
- Buonsanti, R.; Milliron, D.J. Chemistry of Doped Colloidal Nanocrystals. Chem. Mater. 2013, 25, 1305–1317. [Google Scholar] [CrossRef]
- Luo, H.; Tuinenga, C.; Guidez, E.B.; Lewis, C.; Shipman, J.; Roy, S.; Aikens, C.M.; Chikan, V. Synthesis and Characterization of Gallium-Doped CdSe Quantum Dots. J. Phys. Chem. C 2015, 119, 10749–10757. [Google Scholar] [CrossRef]
- Meulenberg, R.W.; van Buuren, T.; Hanif, K.M.; Willey, T.M.; Strouse, G.F.; Terminello, L.J. Structure and Composition of Cu-Doped CdSe Nanocrystals Using Soft X-Ray Absorption Spectroscopy. Nano Lett. 2004, 4, 2277–2285. [Google Scholar] [CrossRef][Green Version]
- Kasuya, A.; Sivamohan, R.; Barnakov, Y.A.; Dmitruk, I.M.; Nirasawa, T.; Romanyuk, V.R.; Kumar, V.; Mamykin, S.V.; Tohji, K.; Jeyadevan, B.; et al. Ultra-Stable Nanoparticles of CdSe Revealed from Mass Spectrometry. Nat. Mater. 2004, 3, 99–102. [Google Scholar] [CrossRef]
- Whitham, P.J.; Knowles, K.E.; Reid, P.J.; Gamelin, D.R. Photoluminescence Blinking and Reversible Electron Trapping in Copper-Doped CdSe Nanocrystals. Nano Lett. 2015, 15, 4045–4051. [Google Scholar] [CrossRef]
- Corrado, C.; Jiang, Y.; Oba, F.; Kozina, M.; Bridges, F.; Zhang, J.Z. Synthesis, Structural, and Optical Properties of Stable ZnS:Cu,Cl Nanocrystals. J. Phys. Chem. A 2009, 113, 3830–3839. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, B.B.; Jana, S.; Pradhan, N. Doping Cu in Semiconductor Nanocrystals: Some Old and Some New Physical Insights. J. Am. Chem. Soc. 2011, 133, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Isarov, A.V.; Chrysochoos, J. Optical and Photochemical Properties of Nonstoichiometric Cadmium Sulfide Nanoparticles: Surface Modification with Copper(II) Ions. Langmuir 1997, 13, 3142–3149. [Google Scholar] [CrossRef]
- Lilhare, D.; Sinha, T.; Khare, A. Influence of Cu Doping on Optical Properties of (Cd–Zn)S Nanocrystalline Thin Films: A Review. J. Mater. Sci. Mater. Electron. 2018, 29, 688–713. [Google Scholar] [CrossRef]
- Harb, M.; Masih, D.; Takanabe, K. Screened Coulomb Hybrid DFT Investigation of Band Gap and Optical Absorption Predictions of CuVO3, CuNbO3 and Cu5Ta11O30 Materials. Phys. Chem. Chem. Phys. 2014, 16, 18198–18204. [Google Scholar] [CrossRef]
- Deák, P.; Aradi, B.; Frauenheim, T.; Janzén, E.; Gali, A. Accurate Defect Levels Obtained from the HSE06 Range-Separated Hybrid Functional. Phys. Rev. B 2010, 81, 153203. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid Functionals Based on a Screened Coulomb Potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Kilina, S.; Ivanov, S.; Tretiak, S. Effect of Surface Ligands on Optical and Electronic Spectra of Semiconductor Nanoclusters. J. Am. Chem. Soc. 2009, 131, 7717–7726. [Google Scholar] [CrossRef]
- Kuznetsov, A.E.; Beratan, D.N. Structural and Electronic Properties of Bare and Capped Cd33Se33 and Cd33Te33 Quantum Dots. J. Phys. Chem. C 2014, 118, 7094–7109. [Google Scholar] [CrossRef]
- Puzder, A.; Williamson, A.J.; Zaitseva, N.; Galli, G.; Manna, L.; Alivisatos, A.P. The Effect of Organic Ligand Binding on the Growth of CdSe Nanoparticles Probed by Ab Initio Calculations. Nano Lett. 2004, 4, 2361–2365. [Google Scholar] [CrossRef]
- Puzder, A.; Williamson, A.J.; Gygi, F.; Galli, G. Self-Healing of CdSe Nanocrystals: First-Principles Calculations. Phys. Rev. Lett. 2004, 92, 217401. [Google Scholar] [CrossRef] [PubMed]
- Hafner, J. ChemInform Abstract: Ab-Initio Simulations of Materials Using VASP: Density-Functional Theory and Beyond. J. Comput. Chem. 2008, 29, 2044–2078. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Wang, Y. Generalized Gradient Approximation for the Exchange-Correlation Hole of a Many-Electron System. Phys. Rev. B 1996, 54, 16533–16539. [Google Scholar] [CrossRef]
- Winkler, U.; Eich, D.; Chen, Z.H.; Fink, R.; Kulkarni, S.K.; Umbach, E. Detailed Investigation of CdS Nanoparticle Surfaces by High-Resolution Photoelectron Spectroscopy. Chem. Phys. Lett. 1999, 306, 95–102. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Zhao, F.A.; Xiao, H.Y.; Bai, X.M.; Zu, X.T. Effects of Ag Doping on the Electronic and Optical Properties of CdSe Quantum Dots. Phys. Chem. Chem. Phys. 2019, 21, 16108–16119. [Google Scholar] [CrossRef] [PubMed]
- Suyver, J.F.; van der Beek, T.; Wuister, S.F.; Kelly, J.J.; Meijerink, A. Luminescence of Nanocrystalline ZnSe:Cu. Appl. Phys. Lett. 2001, 79, 4222–4224. [Google Scholar] [CrossRef]
- Ganesan, P.; Lakshmipathi, S. Influence of Dopants Cu, Ga, In, Hg on the Electronic Structure of CdnSn (n = 6, 15) Clusters–a DFT Study. RSC Adv. 2016, 6, 93056–93067. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Liu, J.; Wang, S.; Cheng, Y.; Ji, M.; Zhou, Y.; Xu, M.; Hao, W.; Zhang, J. From Cu2S Nanocrystals to Cu Doped CdS Nanocrystals through Cation Exchange: Controlled Synthesis, Optical Properties and Their p-Type Conductivity Research. Sci. China Mater. 2015, 58, 693–703. [Google Scholar] [CrossRef]
- Nelson, H.D.; Hinterding, S.O.M.; Fainblat, R.; Creutz, S.E.; Li, X.; Gamelin, D.R. Mid-Gap States and Normal vs Inverted Bonding in Luminescent Cu+- and Ag+-Doped CdSe Nanocrystals. J. Am. Chem. Soc. 2017, 139, 6411–6421. [Google Scholar] [CrossRef] [PubMed]
- Gajdoš, M.; Hummer, K.; Kresse, G.; Furthmüller, J.; Bechstedt, F.J.P.R.B. Linear optical properties in the projector-augmented wave methodology. Phys. Rev. B 2006, 73, 045112. [Google Scholar] [CrossRef]
- Schmidt, W.G. Calculation of reflectance anisotropy for semiconductor surface exploration. Phys. Status Sol. B 2005, 242, 2751–2764. [Google Scholar] [CrossRef]
- Kudera, S.; Zanella, M.; Giannini, C.; Rizzo, A.; Li, Y.; Gigli, G.; Cingolani, R.; Ciccarella, G.; Spahl, W.; Parak, W.J.; et al. Sequential Growth of Magic-Size CdSe Nanocrystals. Adv. Mater. 2007, 19, 548–552. [Google Scholar] [CrossRef]
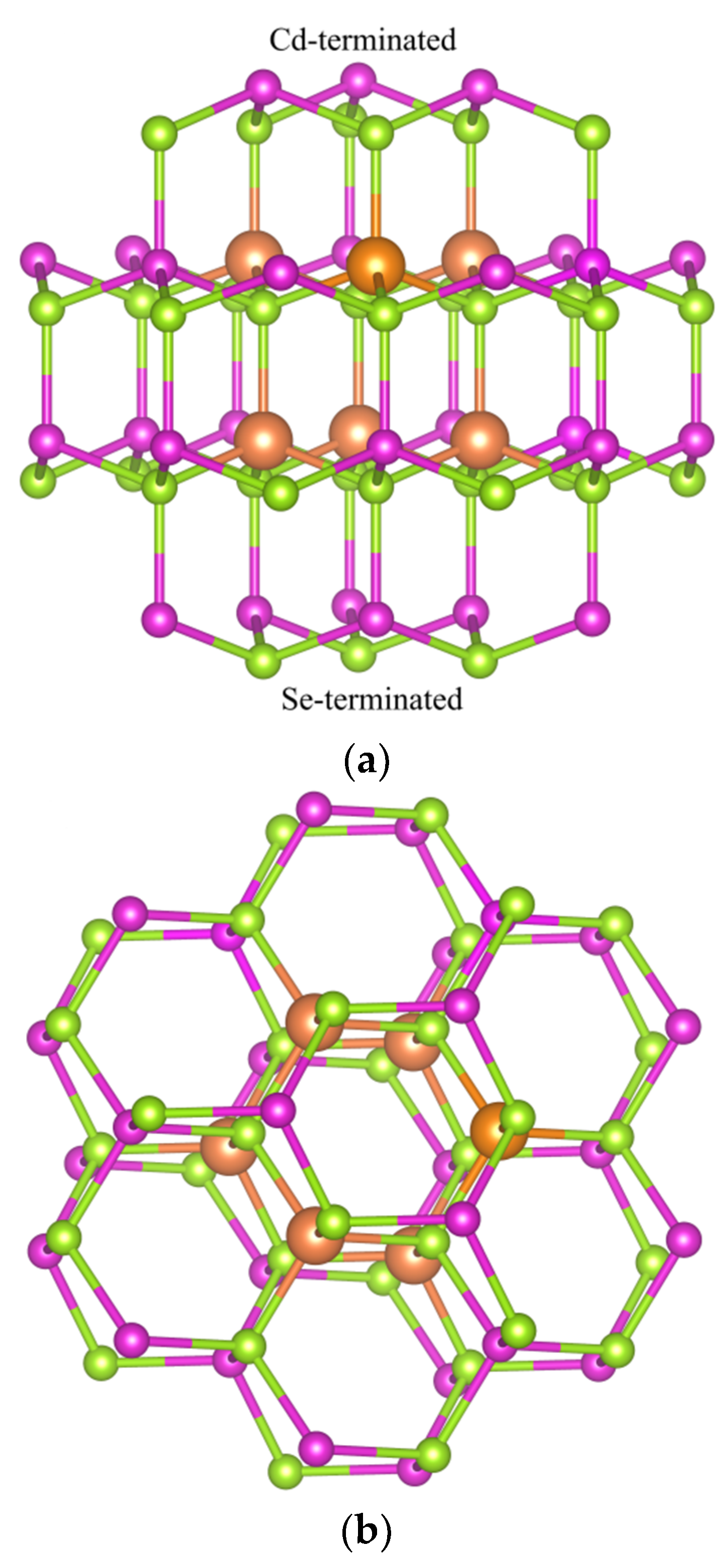
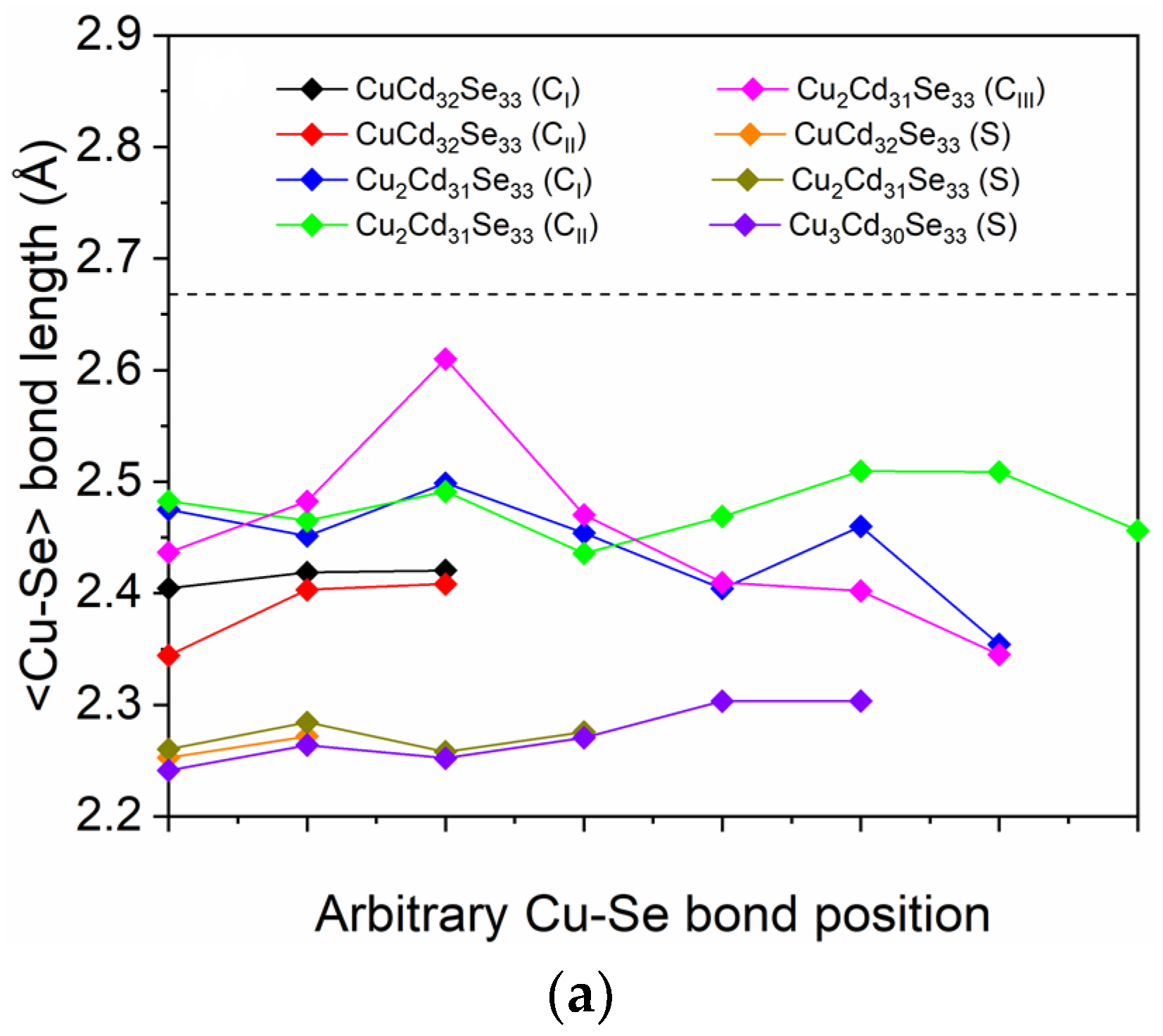
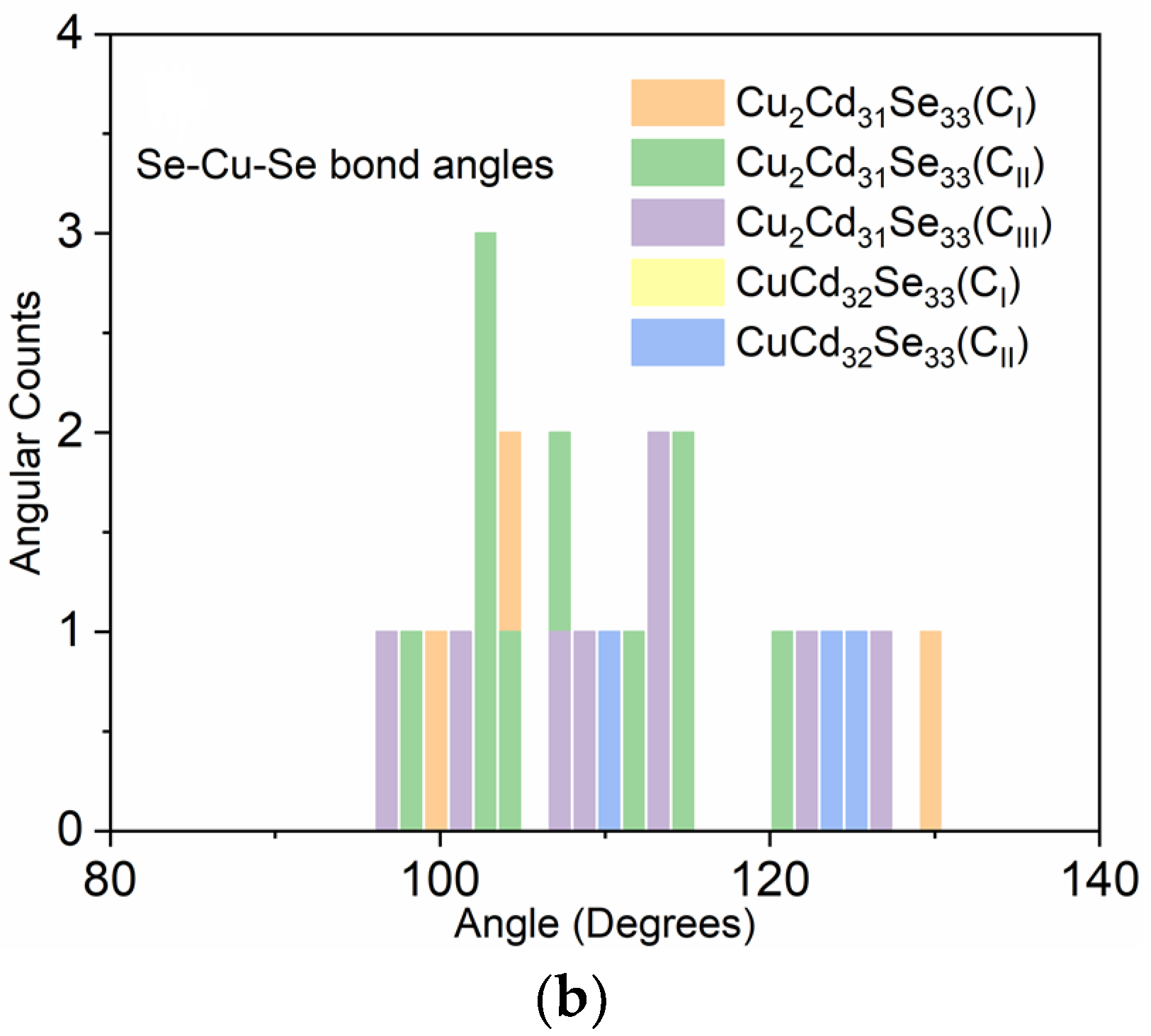
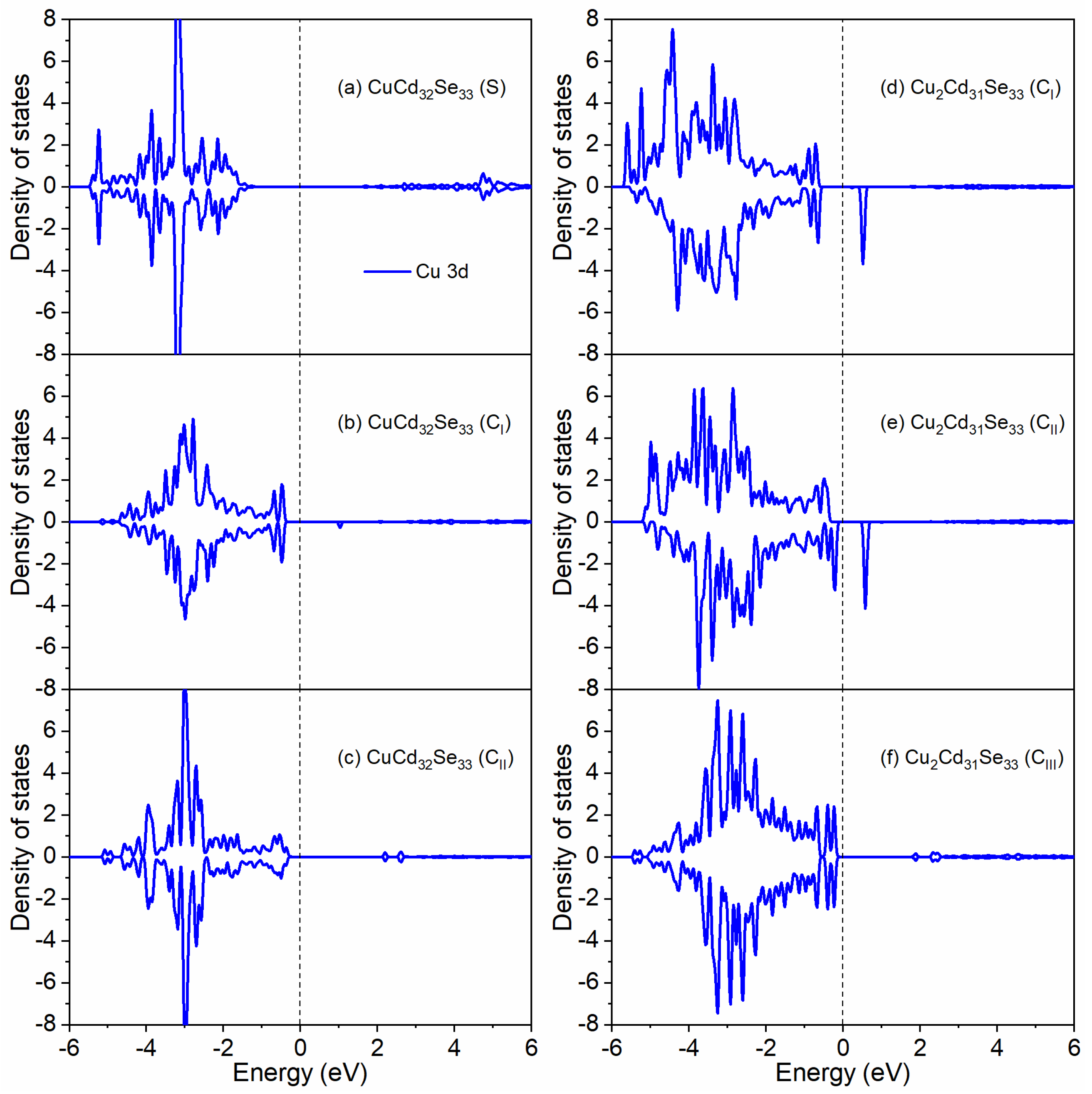
| d<Cd-Se> (Å) | d<Cu-Se> (Å) | ∠Se-Cd-Se (°) | ∠Se-Cu-Se (°) | |
|---|---|---|---|---|
| Cd33Se33 (C) | 2.678 | 109.04 | ||
| Cd33Se33 (S) | 2.657 | 119.78 | ||
| CuCd32Se33 (S) | 2.262 | 177.83 | ||
| Cu2Cd31Se33 (S) | 2.269 | 171.93 | ||
| Cu3Cd30Se33 (S) | 2.272 | 168.50 | ||
| CuCd32Se33 (CI) | 2.414 | 120.0 | ||
| CuCd32Se33 (CII) | 2.385 | 119.8 | ||
| Cu2Cd31Se33 (CI) | 2.442 | 113.28 | ||
| Cu2Cd31Se33S (CI) | 2.477 | 109.39 | ||
| Cu2Cd31Se33 (CII) | 2.522 | 109.85 | ||
| Cu2Cd31Se33S(CII) | 2.486 | 104.85 | ||
| Cu2Cd31Se33 (CIII) | 2.451 | 112.51 |
| Total Binding Energy (eV) | Binding Energy/Cu Atom (eV) | |
|---|---|---|
| CuCd32Se33 (S) | 1.824 | 1.824 |
| CuCd32Se33 (CI) | 1.726 | 1.726 |
| CuCd32Se33 (CII) | 1.466 | 1.466 |
| Cu2Cd31Se33 (S) | 0.538 | 0.269 |
| Cu2Cd31Se33 (CI) | 0.267 | 0.134 |
| Cu2Cd31Se33 (CII) | 0.497 | 0.248 |
| Cu2Cd31Se33 (CIII) | 0.797 | 0.398 |
| Cu3Cd30Se33 (S) | −0.397 | −0.466 |
| Cu | Cd | Se | |
|---|---|---|---|
| CuCd32Se33 (S) | 0.204 | 0.647 | −0.634 |
| CuCd32Se33 (CI) | 0.288 | 0.671 | −0.660 |
| CuCd32Se33 (CII) | 0.261 | 0.671 | −0.659 |
| Cu2Cd31Se33 (S) | 0.266 | 0.673 | −0.648 |
| Cu2Cd31Se33 (CI) | 0.345 | 0.674 | −0.654 |
| Cu2Cd31Se33 (CII) | 0.390 | 0.670 | −0.653 |
| Cu2Cd31Se33 (CIII) | 0.287 | 0.670 | −0.647 |
| Cu3Cd30Se33 (S) | 0.260 | 0.667 | −0.630 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Hu, S.; Xu, C.; Xiao, H.; Zhou, X.; Zu, X.; Peng, S. Effect of Copper Doping on Electronic Structure and Optical Absorption of Cd33Se33 Quantum Dots. Nanomaterials 2021, 11, 2531. https://doi.org/10.3390/nano11102531
Zhao F, Hu S, Xu C, Xiao H, Zhou X, Zu X, Peng S. Effect of Copper Doping on Electronic Structure and Optical Absorption of Cd33Se33 Quantum Dots. Nanomaterials. 2021; 11(10):2531. https://doi.org/10.3390/nano11102531
Chicago/Turabian StyleZhao, Fengai, Shuanglin Hu, Canhui Xu, Haiyan Xiao, Xiaosong Zhou, Xiaotao Zu, and Shuming Peng. 2021. "Effect of Copper Doping on Electronic Structure and Optical Absorption of Cd33Se33 Quantum Dots" Nanomaterials 11, no. 10: 2531. https://doi.org/10.3390/nano11102531
APA StyleZhao, F., Hu, S., Xu, C., Xiao, H., Zhou, X., Zu, X., & Peng, S. (2021). Effect of Copper Doping on Electronic Structure and Optical Absorption of Cd33Se33 Quantum Dots. Nanomaterials, 11(10), 2531. https://doi.org/10.3390/nano11102531





