Titanium Nitride Nanodonuts Synthesized from Natural Ilmenite Ore as a Novel and Efficient Thermoplasmonic Material
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Chemicals
2.2. Synthesis of TiO2 Nanoparticles from Ilmenite Ore
2.3. Synthesis of TiN by Nitridation of TiO2 in NH3
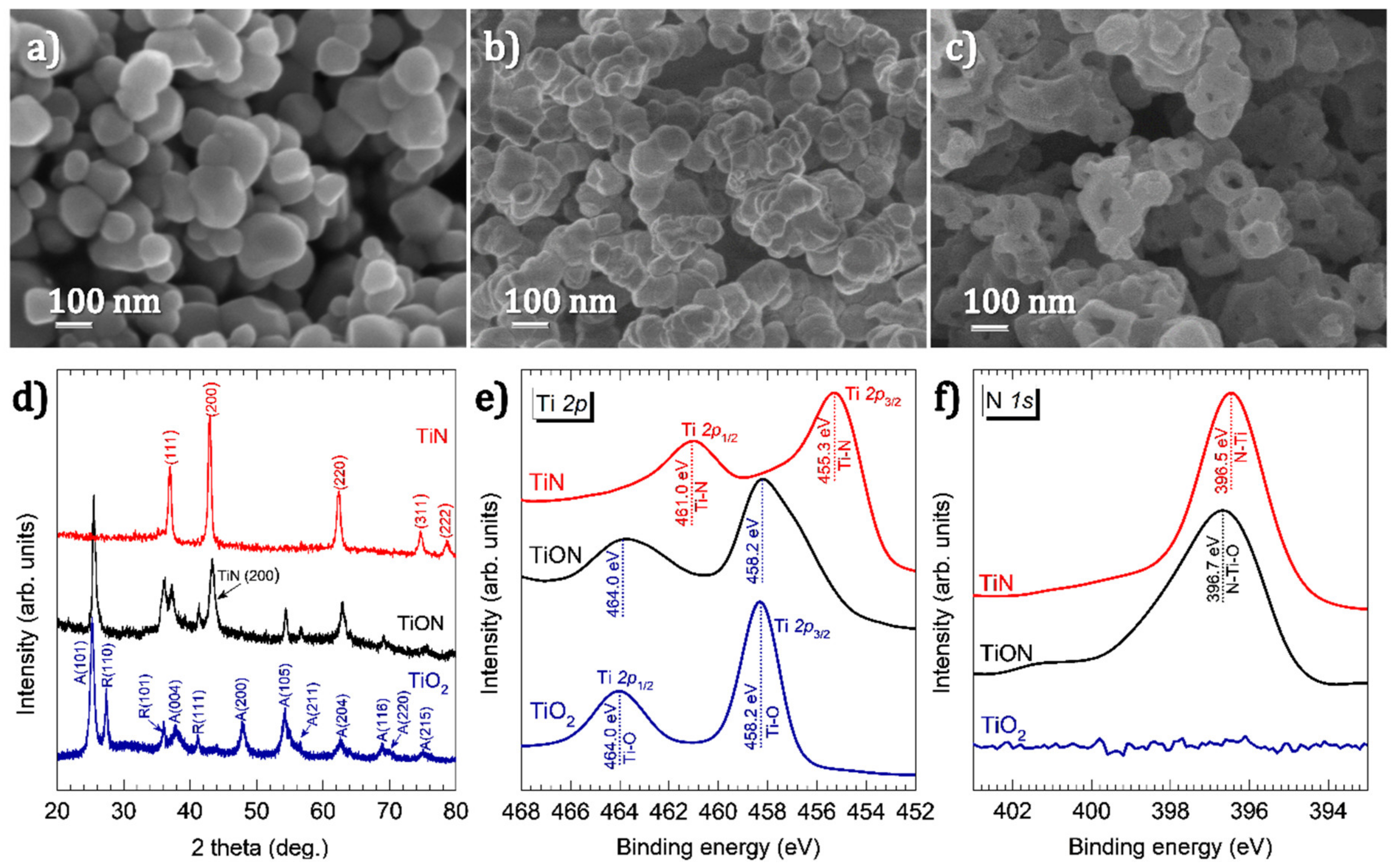
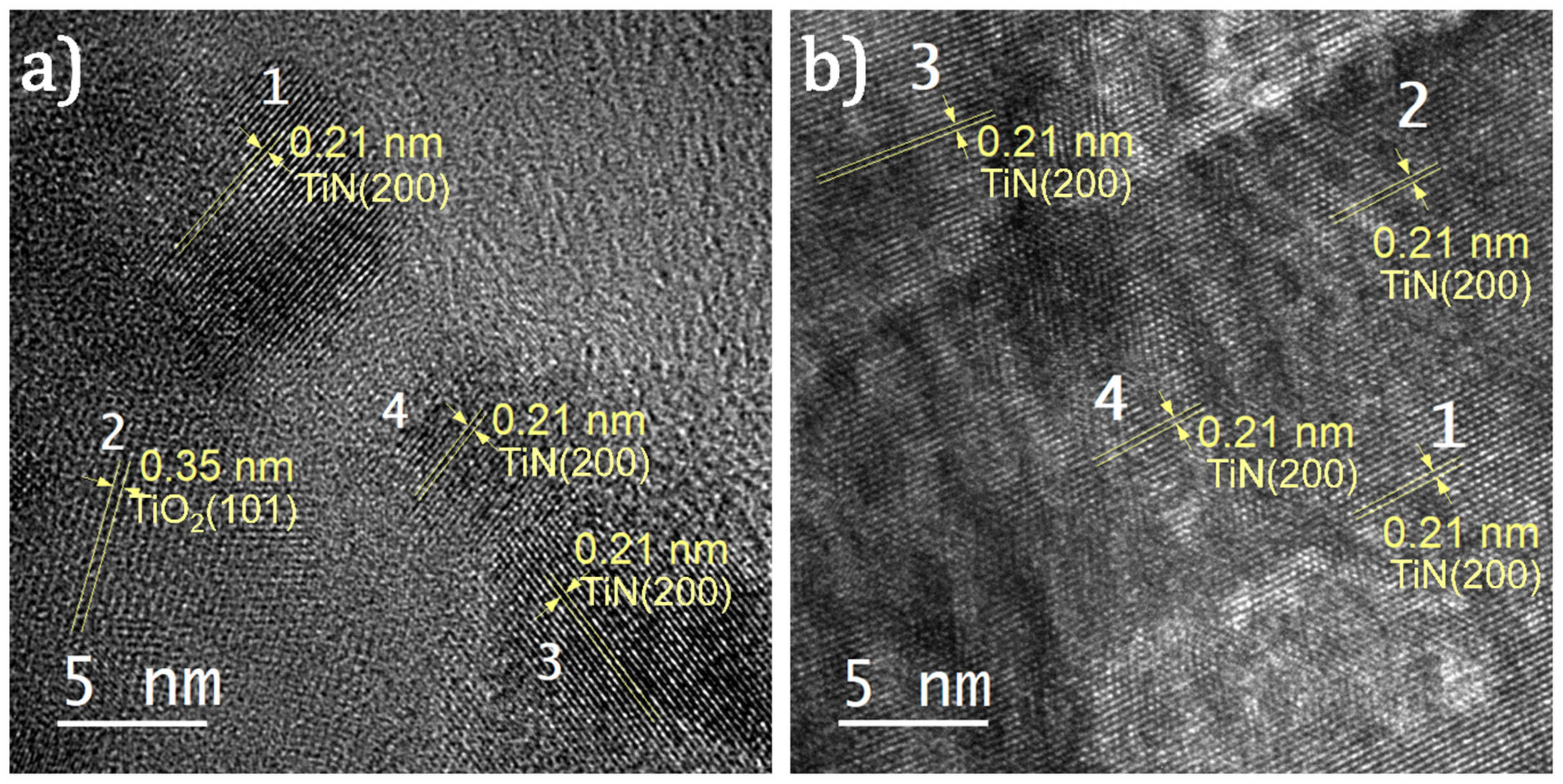
2.4. Material Characterizations
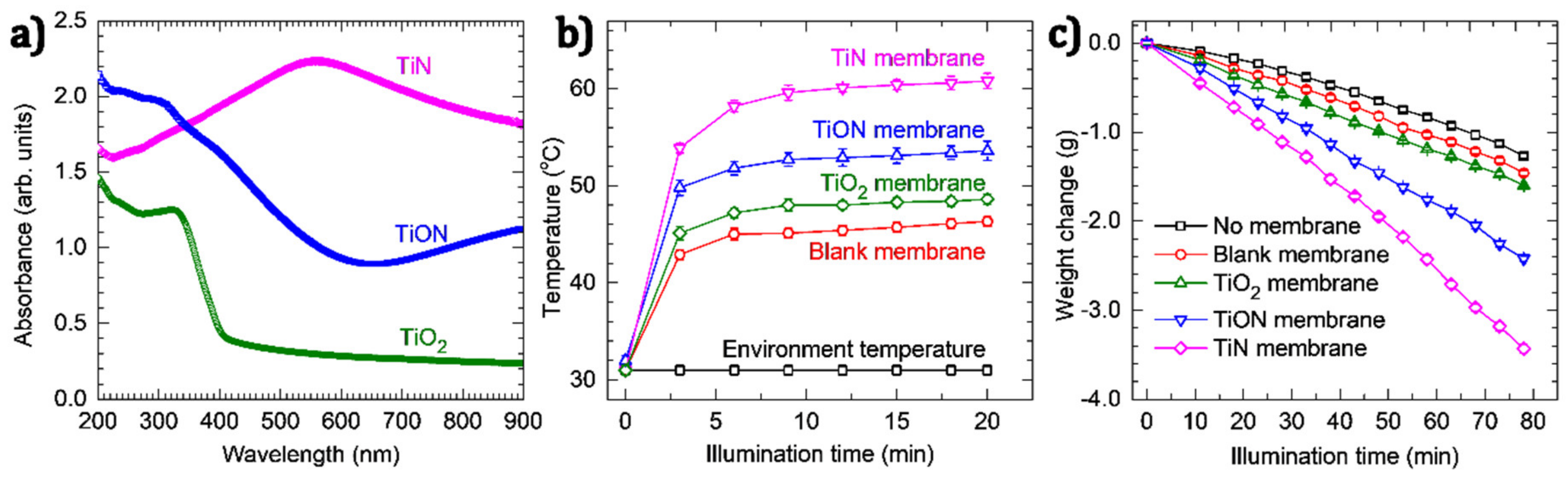
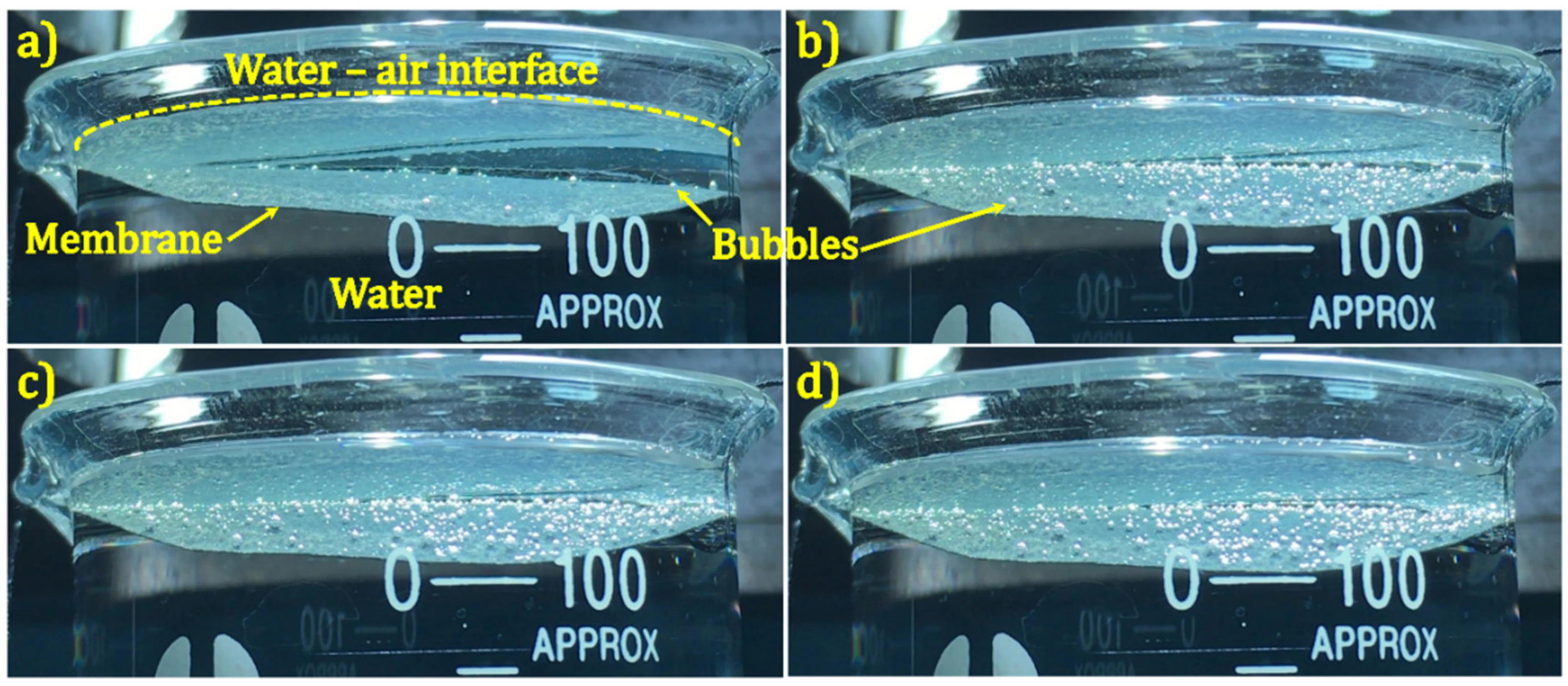
2.5. Solar Water Evaporation Experiments
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baffou, G.; Quidant, R. Thermo-plasmonics: Using metallic nanostructures as nano-sources of heat. Laser Photonics Rev. 2013, 7, 171–187. [Google Scholar] [CrossRef]
- Baffou, G.; Polleux, J.; Rigneault, H.; Monneret, S. Super-Heating and Micro-Bubble Generation around Plasmonic Nanoparticles under cw Illumination. J. Phys. Chem. C 2014, 118, 4890–4898. [Google Scholar] [CrossRef]
- Baffou, G.; Cichos, F.; Quidant, R. Applications and challenges of thermoplasmonics. Nat. Mater. 2020, 19, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kuang, Y.; Hu, L. Challenges and Opportunities for Solar Evaporation. Joule 2019, 3, 683–718. [Google Scholar] [CrossRef]
- Zhao, F.; Guo, Y.; Zhou, X.; Shi, W.; Yu, G. Materials for solar-powered water evaporation. Nat. Rev. Mater. 2020, 5, 388–401. [Google Scholar] [CrossRef]
- Tao, P.; Ni, G.; Song, C.; Shang, W.; Wu, J.; Zhu, J.; Chen, G.; Deng, T. Solar-driven interfacial evaporation. Nat. Energy 2018, 3, 1031–1041. [Google Scholar] [CrossRef]
- Pang, Y.; Zhang, J.; Ma, R.; Qu, Z.; Lee, E.; Luo, T. Solar–Thermal Water Evaporation: A Review. ACS Energy Lett. 2020, 5, 437–456. [Google Scholar] [CrossRef]
- Yang, P.; Liu, K.; Chen, Q.; Li, J.; Duan, J.; Xue, G.; Xu, Z.; Xie, W.; Zhou, J. Solar-driven simultaneous steam production and electricity generation from salinity. Energy Environ. Sci. 2017, 10, 1923–1927. [Google Scholar] [CrossRef]
- Ni, G.; Li, G.; Boriskina, S.V.; Li, H.; Yang, W.; Zhang, T.J.; Chen, G. Steam generation under one sun enabled by a floating structure with thermal concentration. Nat. Energy 2016, 1, 16126. [Google Scholar] [CrossRef]
- Zhu, L.; Gao, M.; Peh, C.K.N.; Ho, G.W. Recent progress in solar-driven interfacial water evaporation: Advanced designs and applications. Nano Energy 2019, 57, 507–518. [Google Scholar] [CrossRef]
- Ren, H.; Tang, M.; Guan, B.; Wang, K.; Yang, J.; Wang, F.; Wang, M.; Shan, J.; Chen, Z.; Wei, D.; et al. Hierarchical Graphene Foam for Efficient Omnidirectional Solar–Thermal Energy Conversion. Adv. Mater. 2017, 29, 1702590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liao, Q.; Yao, H.; Cheng, H.; Huang, Y.; Yang, C.; Jiang, L.; Qu, L. Three-dimensional water evaporation on a macroporous vertically aligned graphene pillar array under one sun. J. Mater. Chem. A 2018, 6, 15303–15309. [Google Scholar] [CrossRef]
- Hu, X.; Xu, W.; Zhou, L.; Tan, Y.; Wang, Y.; Zhu, S.; Zhu, J. Tailoring Graphene Oxide-Based Aerogels for Efficient Solar Steam Generation under One Sun. Adv. Mater. 2017, 29, 1604031. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, H.; Ni, G.; Marconnet, A.M.; Loomis, J.; Yerci, S.; Miljkovic, N.; Chen, G. Solar steam generation by heat localization. Nat. Commun. 2014, 5, 4449. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Zhen, Y.R.; Neumann, O.; Polman, A.; De Abajo, F.J.G.; Nordlander, P.; Halas, N.J. Evolution of light-induced vapor generation at a liquid-immersed metallic nanoparticle. Nano Lett. 2013, 13, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Zeiny, A.; Jin, H.; Lin, G.; Song, P.; Wen, D. Solar evaporation via nanofluids: A comparative study. Renew Energy 2018, 122, 443–454. [Google Scholar] [CrossRef]
- Neumann, O.; Urban, A.S.; Day, J.; Lal, S.; Nordlander, P.; Halas, N.J. Solar vapor generation enabled by nanoparticles. ACS Nano 2013, 7, 42–49. [Google Scholar] [CrossRef]
- Li, W.; Guler, U.; Kinsey, N.; Naik, G.V.; Boltasseva, A.; Guan, J.; Shalaev, V.M.; Kildishev, A.V. Refractory plasmonics with titanium nitride: Broadband. Adv. Mater. 2014, 26, 7959–7965. [Google Scholar] [CrossRef]
- Guler, U.; Ndukaife, J.C.; Naik, G.V.; Nnanna, A.G.A.; Kildishev, A.V.; Shalaev, V.M.; Boltasseva, A. Local heating with lithographically fabricated plasmonic titanium nitride nanoparticles. Nano Lett. 2013, 13, 6078–6083. [Google Scholar] [CrossRef]
- Guler, U.; Suslov, S.; Kildishev, A.V.; Boltasseva, A.; Shalaev, V.M. Colloidal Plasmonic Titanium Nitride Nanoparticles: Properties and Applications. Nanophotonics 2015, 4, 269–276. [Google Scholar] [CrossRef]
- Kaur, M.; Ishii, S.; Shinde, S.L.; Nagao, T. All-Ceramic Microfibrous Solar Steam Generator: TiN Plasmonic Nanoparticle-Loaded Transparent Microfibers. ACS Sustain. Chem. Eng. 2017, 5, 8523–8528. [Google Scholar] [CrossRef]
- Lalisse, A.; Tessier, G.; Plain, J.; Baffou, G. Plasmonic efficiencies of nanoparticles made of metal nitrides (TiN, ZrN) compared with gold. Sci. Rep. 2016, 6, 38647. [Google Scholar] [CrossRef] [PubMed]
- Traver, E.; Karaballi, R.A.; Monfared, Y.E.; Daurie, H.; Gagnon, G.A.; Dasog, M. TiN, ZrN, and HfN Nanoparticles on Nanoporous Aluminum Oxide Membranes for Solar-Driven Water Evaporation and Desalination. ACS Appl. Nano Mater. 2020, 3, 2787–2794. [Google Scholar] [CrossRef]
- Guler, U.; Shalaev, V.M.; Boltasseva, A. Nanoparticle plasmonics: Going practical with transition metal nitrides. Mater. Today 2015, 18, 227–237. [Google Scholar] [CrossRef]
- Naik, G.V.; Schroeder, J.L.; Ni, X.; Kildishev, A.V.; Sands, T.D.; Boltasseva, A. Titanium nitride as a plasmonic material for visible and near-infrared wavelengths. Opt. Mater. Express 2012, 2, 478–489. [Google Scholar] [CrossRef]
- Naldoni, A.; Kudyshev, Z.A.; Mascaretti, L.; Sarmah, S.P.; Rej, S.; Froning, J.P.; Tomanec, O.; Yoo, J.E.; Wang, D.; Kment, Š.; et al. Solar thermoplasmonic nanofurnace for high-temperature heterogeneous catalysis. Nano Lett. 2020, 20, 3663–3672. [Google Scholar] [CrossRef]
- Ishii, S.; Sugavaneshwar, R.P.; Nagao, T. Titanium Nitride Nanoparticles as Plasmonic Solar Heat Transducers. J. Phys. Chem. C 2016, 120, 2343–2348. [Google Scholar] [CrossRef]
- Kaur, M.; Ishii, S.; Shinde, S.L.; Nagao, T. All-ceramic solar-driven water purifier based on anodized aluminum oxide and plasmonic titanium nitride. Adv. Sustain. Syst. 2019, 3, 1800112. [Google Scholar] [CrossRef]
- Bian, Y.; Tang, K.; Xu, Z.; Ma, J.; Shen, Y.; Hao, L.; Chen, X.; Nie, K.; Li, J.; Ma, T.; et al. Highly efficient solar steam generation by hybrid plasmonic structured TiN/mesoporous anodized alumina membrane. J. Mater. Res. 2018, 33, 3857–3869. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, K.; Liu, L.; Wang, K.; Xiang, J.; Hou, D.; Wang, J. Titanium nitride nanoparticle embedded membrane for photothermal membrane distillation. Chemosphere 2020, 256, 127053. [Google Scholar] [CrossRef]
- Chen, M.; Wu, Y.; Song, W.; Mo, Y.; Lin, X.; He, Q.; Guo, B. Plasmonic nanoparticle-embedded poly(p-phenylene benzobisoxazole) nanofibrous composite films for solar steam generation. Nanoscale 2018, 10, 6186–6193. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, Y.; Chen, F.; Zhu, X.; Dai, J.; Li, Y.; Yang, Z.; Yan, X.; Song, J.; Wang, Y.; et al. Plasmonic Wood for High-Efficiency Solar Steam Generation. Adv. Energy Mater. 2018, 8, 1701028. [Google Scholar] [CrossRef]
- Guler, U.; Zemlyanov, D.; Kim, J.; Wang, Z.; Chandrasekar, R.; Meng, X.; Stach, E.; Kildishev, A.V.; Shalaev, V.M.; Boltasseva, A. Plasmonic Titanium Nitride Nanostructures via Nitridation of Nanopatterned Titanium Dioxide. Adv. Opt. Mater. 2017, 5, 1600717. [Google Scholar] [CrossRef]
- Moon, G.D.; Joo, J.B.; Dahl, M.; Jung, H.; Yin, Y. Nitridation and layered assembly of hollow TiO2 shells for electrochemical energy storage. Adv. Funct. Mater. 2014, 24, 848–856. [Google Scholar] [CrossRef]
- Samiee, M.; Luo, J. A facile nitridation method to improve the rate capability of TiO2 for lithium-ion batteries. J. Power Sources 2014, 245, 594–598. [Google Scholar] [CrossRef]
- Howell, I.R.; Giroire, B.; Garcia, A.; Li, S.; Aymonier, C.; Watkins, J.J. Fabrication of plasmonic TiN nanostructures by nitridation of nanoimprinted TiO2 nanoparticles. J. Mater. Chem. C 2018, 6, 1399–1406. [Google Scholar] [CrossRef]
- Romero-Gómez, P.; Rico, V.; Espinós, J.P.; González-Elipe, A.R.; Palgrave, R.G.; Egdell, R.G. Nitridation of nanocrystalline TiO2 thin films by treatment with ammonia. Thin Solid Film. 2011, 519, 3587–3595. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, M.; Wang, L.; Wang, F.; Yang, L.; Li, X.; Wang, C. Plasmonic Ag-TiO2-x nanocomposites for the photocatalytic removal of NO under visible light with high selectivity: The role of oxygen vacancies. Appl. Catal. B Environ. 2017, 204, 67–77. [Google Scholar] [CrossRef]
- Oktay, S.; Kahraman, Z.; Urgen, M.; Kazmanli, K. XPS investigations of tribolayers formed on TiN and (Ti, Re)N coatings. Appl. Surf. Sci. 2015, 328, 255–261. [Google Scholar] [CrossRef]
- Hao, Q.; Li, W.; Xu, H.; Wang, J.; Yin, Y.; Wang, H.; Ma, L.; Ma, F.; Jiang, X.; Schmidt, O.G.; et al. VO2/TiN Plasmonic Thermochromic Smart Coatings for Room-Temperature Applications. Adv. Mater. 2018, 30, 1705421. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, X.; Zhan, P.; Wang, W.; Zhang, Z. Tuning the optical bandgap of TiO2-TiN composite films as photocatalyst in the visible light. AIP Adv. 2013, 3, 062129. [Google Scholar] [CrossRef]
- Gou, H.P.; Zhang, G.H.; Chou, K.C. Phase evolution and reaction mechanism during reduction–nitridation process of titanium dioxide with ammonia. J. Mater. Sci. 2017, 52, 1255–1264. [Google Scholar] [CrossRef]
- Al-Thabaiti, S.A.; Hahn, R.; Liu, N.; Kirchgeorg, R.; So, S.; Schmuki, P.; Basahel, S.N.; Bawaked, S.M. NH3 treatment of TiO2 nanotubes: From N-doping to semimetallic conductivity. Chem. Commun. 2014, 50, 7960–7963. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Kim, H., II; Chung, D.Y.; Yoo, J.M.; Weon, S.; Choi, W.; Sung, Y.E. Scaffold-like titanium nitride nanotubes with a highly conductive porous architecture as a nanoparticle catalyst support for oxygen reduction. ACS Catal. 2016, 6, 3914–3920. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Deng, L.; Wei, N.; Weng, Y.; Dong, S.; Qi, D.; Qiu, J.; Chen, X.; Wu, T. High-Performance Photothermal Conversion of Narrow-Bandgap Ti2O3 Nanoparticles. Adv. Mater. 2017, 29, 1603730. [Google Scholar] [CrossRef]
- Li, X.; Xu, W.; Tang, M.; Zhou, L.; Zhu, B.; Zhu, S.; Zhu, J. Graphene oxide-based efficient and scalable solar desalination under one sun with a confined 2D water path. Proc. Natl. Acad. Sci. USA 2016, 113, 13953–13958. [Google Scholar] [CrossRef]
- Xu, W.; Hu, X.; Zhuang, S.; Wang, Y.; Li, X.; Zhou, L.; Zhu, S.; Zhu, J. Flexible and Salt Resistant Janus Absorbers by Electrospinning for Stable and Efficient Solar Desalination. Adv. Energy Mater. 2018, 8, 1702884. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Y.; Zhang, L.; Wang, P. Rational design of a bi-layered reduced graphene oxide film on polystyrene foam for solar-driven interfacial water evaporation. J. Mater. Chem. A 2017, 5, 16212–16219. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Wang, P. Self-Floating Carbon Nanotube Membrane on Macroporous Silica Substrate for Highly Efficient Solar-Driven Interfacial Water Evaporation. ACS Sustain. Chem. Eng. 2016, 4, 1223–1230. [Google Scholar] [CrossRef]
- Xue, G.; Liu, K.; Chen, Q.; Yang, P.; Li, J.; Ding, T.; Duan, J.; Qi, B.; Zhou, J. Robust and Low-Cost Flame-Treated Wood for High-Performance Solar Steam Generation. ACS Appl. Mater. Interfaces 2017, 9, 15052–15057. [Google Scholar] [CrossRef]
- Xu, N.; Hu, X.; Xu, W.; Li, X.; Zhou, L.; Zhu, S.; Zhu, J. Mushrooms as Efficient Solar Steam-Generation Devices. Adv. Mater. 2017, 29, 1606762. [Google Scholar] [CrossRef] [PubMed]
- Baffou, G. Thermoplasmonics: Heating Metal Nanoparticles Using Light; Cambridge University Press: Cambridge, UK, 2018; ISBN 978-1-108-41832-4. [Google Scholar]
- Carlson, M.T.; Green, A.J.; Richardson, H.H. Superheating water by CW excitation of gold nanodots. Nano Lett. 2012, 12, 1534–1537. [Google Scholar] [CrossRef] [PubMed]
| Photothermal Material | Floating Substrate | Light Intensity (kW m−2) | Evaporation Rate (kg h−1 m−2) | Reference |
|---|---|---|---|---|
| TiN nanodonuts | Polymer membrane | 0.55 | 1.38 | This work |
| TiN NPs | Ceramic fiber wool | 1.0 | 1.847 | [21] |
| TiN NPs | Mesoporous anodized alumina membrane | 1.21 | 1.606 | [29] |
| Ti2O3 NPs | Cellulose membrane | 1.0 | 1.32 | [45] |
| RGO-Sodium alginate-CNT aerogel | Self-floating | 1.0 | 1.622 | [13] |
| 2D GO film | Cellulose-wrapped Polystyrene foam | 1.0 | 1.45 | [46] |
| Carbon black coated PMMA nanofiber on PAN nanofiber | Self-floating | 1.0 | 1.3 | [47] |
| Bi-layered rGO film | Polystyrene foam | 1.0 | 1.31 | [48] |
| Carbon nanotubes | Porous Silica | 1.0 | 1.32 | [49] |
| Flame-treated wood | Self-floating | 1.0 | 1.05 | [50] |
| Carbonized mushrooms | Polystyrene foam | 1.0 | 1.475 | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thi Le, T.-L.; Nguyen, L.T.; Nguyen, H.-H.; Nghia, N.V.; Vuong, N.M.; Hieu, H.N.; Thang, N.V.; Le, V.T.; Nguyen, V.H.; Lin, P.-C.; et al. Titanium Nitride Nanodonuts Synthesized from Natural Ilmenite Ore as a Novel and Efficient Thermoplasmonic Material. Nanomaterials 2021, 11, 76. https://doi.org/10.3390/nano11010076
Thi Le T-L, Nguyen LT, Nguyen H-H, Nghia NV, Vuong NM, Hieu HN, Thang NV, Le VT, Nguyen VH, Lin P-C, et al. Titanium Nitride Nanodonuts Synthesized from Natural Ilmenite Ore as a Novel and Efficient Thermoplasmonic Material. Nanomaterials. 2021; 11(1):76. https://doi.org/10.3390/nano11010076
Chicago/Turabian StyleThi Le, Thanh-Lieu, Lam Tan Nguyen, Hoai-Hue Nguyen, Nguyen Van Nghia, Nguyen Minh Vuong, Hoang Nhat Hieu, Nguyen Van Thang, Viet Thong Le, Viet Huong Nguyen, Pin-Cheng Lin, and et al. 2021. "Titanium Nitride Nanodonuts Synthesized from Natural Ilmenite Ore as a Novel and Efficient Thermoplasmonic Material" Nanomaterials 11, no. 1: 76. https://doi.org/10.3390/nano11010076
APA StyleThi Le, T.-L., Nguyen, L. T., Nguyen, H.-H., Nghia, N. V., Vuong, N. M., Hieu, H. N., Thang, N. V., Le, V. T., Nguyen, V. H., Lin, P.-C., Yadav, A., Madarevic, I., Janssens, E., Bui, H. V., & Ngoc, L. L. T. (2021). Titanium Nitride Nanodonuts Synthesized from Natural Ilmenite Ore as a Novel and Efficient Thermoplasmonic Material. Nanomaterials, 11(1), 76. https://doi.org/10.3390/nano11010076








