Coloration and Chromatic Sensing Behavior of Electrospun Cellulose Fibers with Curcumin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
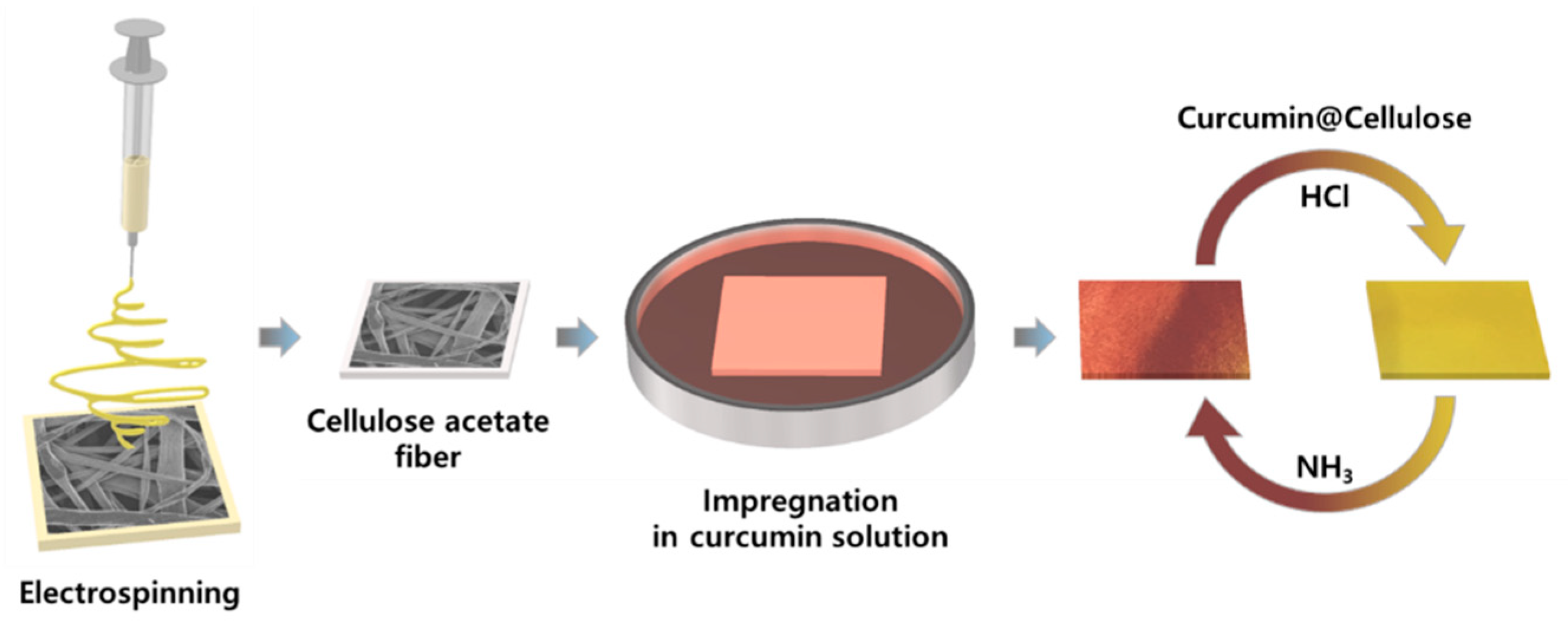
2.3. Coloration Process for Cellulose Fabrics with Curcumin
2.4. Chromatic Sensing Experiment
3. Results and Discussion
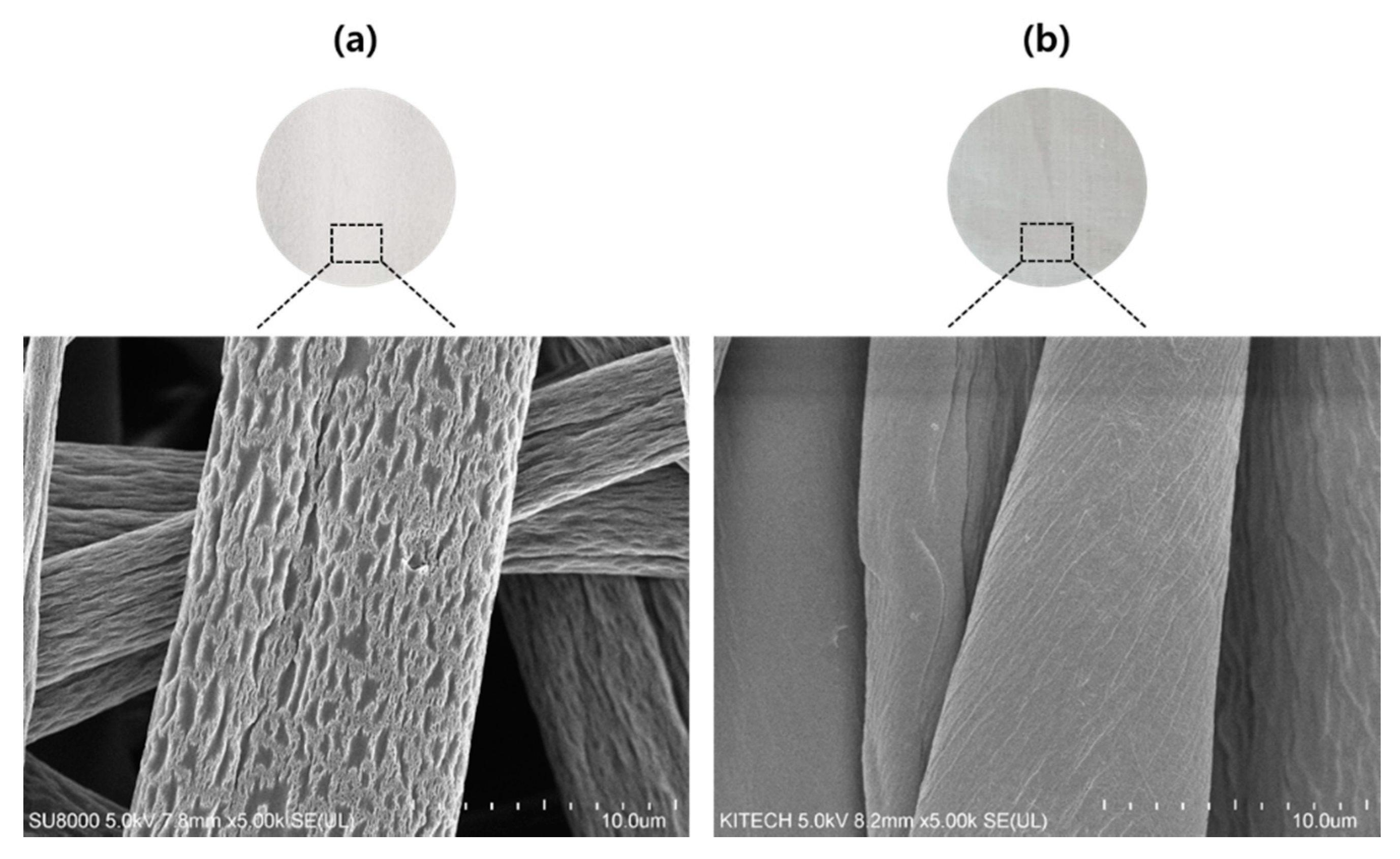
3.1. Morphologies of Cotton Fibers and Electrospun CA Fibers
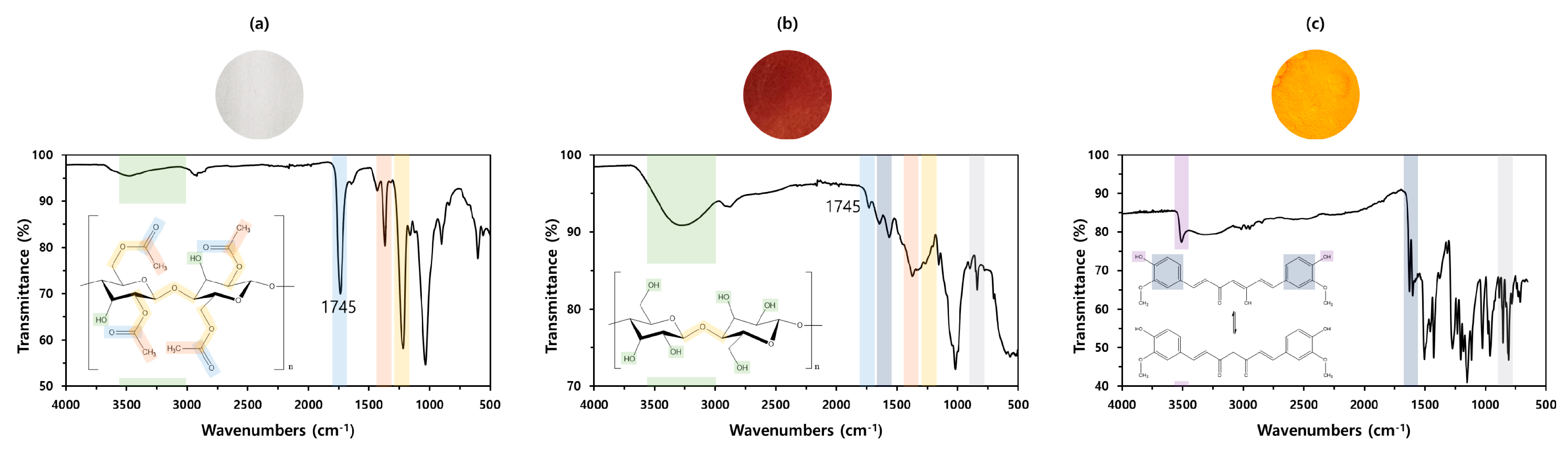
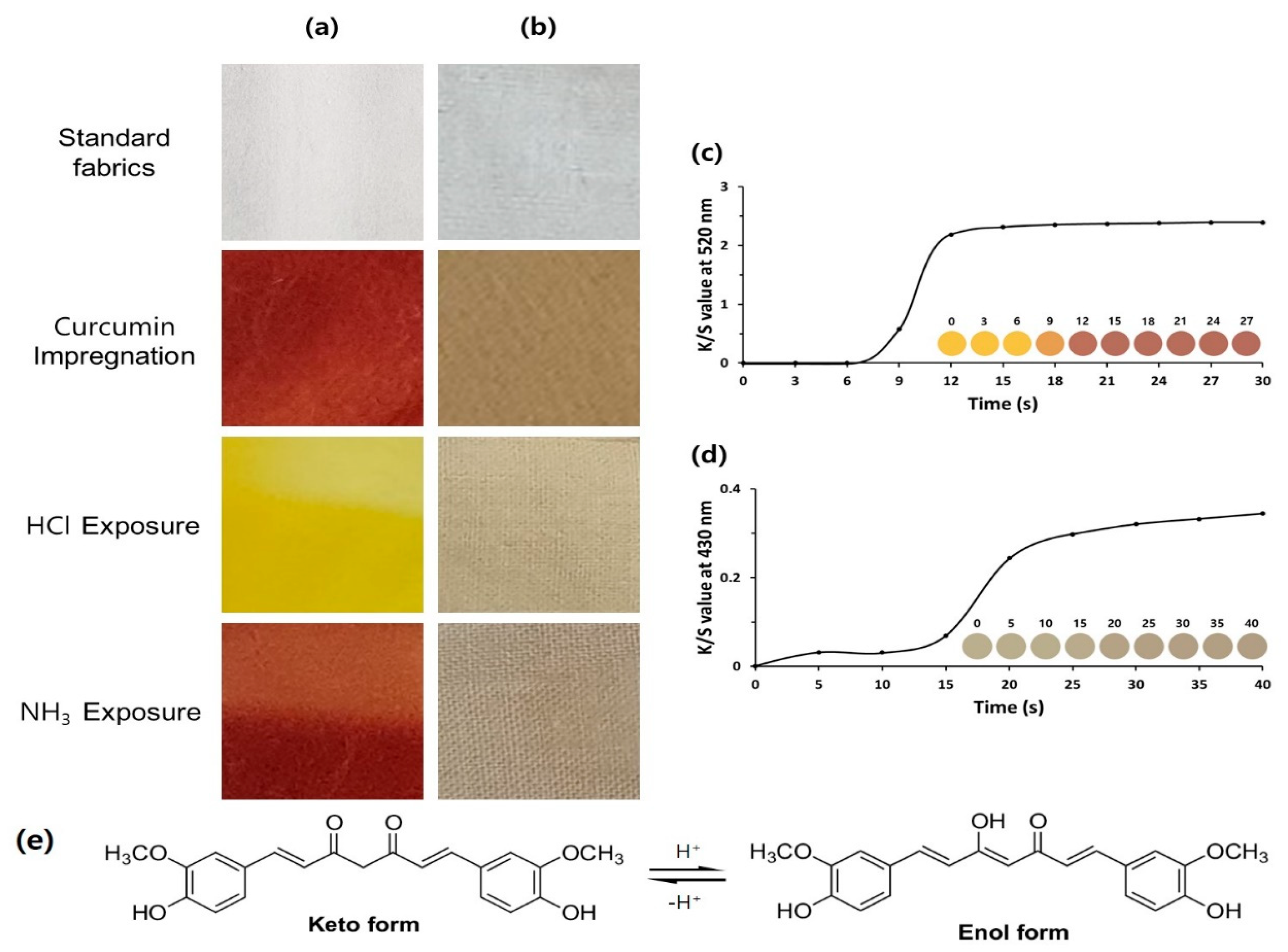
3.2. Coloration Behavior of Cellulose Fabric with Curcumin
3.3. Chromatic Gas Sensing Behaviors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Fu, Y. Hydrolysis of cellulose to glucose by solid acid catalysts. Green Chem. 2013, 15, 1095–1111. [Google Scholar] [CrossRef]
- Cai, T.; Zhang, H.; Guo, Q.; Shao, H.; Hu, X. Structure and properties of cellulose fibers from ionic liquids. J. Appl. Polym. Sci. 2010, 115, 1047–1053. [Google Scholar] [CrossRef]
- Khatri, M.; Ahmed, F.; Shaikh, I.; Phan, D.-N.; Khan, Q.; Khatri, Z.; Lee, H.; Kim, I.S. Dyeing and characterization of regenerated cellulose nanofibers with vat dyes. Carbohydr. Polym. 2017, 174, 443–449. [Google Scholar] [CrossRef]
- Bouloton, J.; Morton, T.H. The Dyeing of Cellulosic Materials: A Review of the Physics and Chemistry of the Dyeing Process. J. Soc. Dye. Colour. 1940, 56, 145–159. [Google Scholar] [CrossRef]
- Shahid, M.; Shahid-ul, I.; Mohammad, F. Recent advancements in natural dye applications: A review. J. Clean. Prod. 2013, 53, 310–331. [Google Scholar] [CrossRef]
- Roman, B.; Retajczyk, M.; Sałaciński, Ł.; Pełech, R. Curcumin—Properties, Applications and Modification of Structure. Mini Rev. Org. Chem. 2020, 17, 486–495. [Google Scholar] [CrossRef]
- Han, S.; Yang, Y. Antimicrobial activity of wool fabric treated with curcumin. Dye. Pigment. 2005, 64, 157–161. [Google Scholar] [CrossRef]
- Varaprasad, K.; Mohan, Y.M.; Vimala, K.; Mohana Raju, K. Synthesis and characterization of hydrogel-silver nanoparticle-curcumin composites for wound dressing and antibacterial application. J. Appl. Polym. Sci. 2011, 121, 784–796. [Google Scholar] [CrossRef]
- Boonkanon, C.; Phatthanawiwat, K.; Wongniramaikul, W.; Choodum, A. Curcumin nanoparticle doped starch thin film as a green colorimetric sensor for detection of boron. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117351. [Google Scholar] [CrossRef]
- Larik, S.A.; Khatri, A.; Ali, S.; Kim, S.H. Batchwise dyeing of bamboo cellulose fabric with reactive dye using ultrasonic energy. Ultrason. Sonochem. 2015, 24, 178–183. [Google Scholar] [CrossRef]
- Lee, H.; Nishino, M.; Sohn, D.; Lee, J.S.; Kim, I.S. Control of the morphology of cellulose acetate nanofibers via electrospinning. Cellulose 2018, 25, 2829–2837. [Google Scholar] [CrossRef]
- Song, J.; Kim, M.; Lee, H. Recent Advances on Nanofiber Fabrications: Unconventional State-of-the-Art Spinning Techniques. Polymers 2020, 12, 1386. [Google Scholar] [CrossRef]
- An, S.; Jeon, B.; Bae, J.H.; Kim, I.S.; Paeng, K.; Kim, M.; Lee, H. Thiol-based chemistry as versatile routes for the effective functionalization of cellulose nanofibers. Carbohydr. Polym. 2019, 226, 115259. [Google Scholar] [CrossRef]
- Liu, H.; Tang, C. Electrospinning of Cellulose Acetate in Solvent Mixture N,N-Dimethylacetamide (DMAc)/Acetone. Polym. J. 2007, 39, 65–72. [Google Scholar] [CrossRef]
- Phan, D.-N.; Lee, H.; Choi, D.; Kang, C.-Y.; Im, S.S.; Kim, I.S. Fabrication of Two Polyester Nanofiber Types Containing the Biobased Monomer Isosorbide: Poly (Ethylene Glycol 1,4-Cyclohexane Dimethylene Isosorbide Terephthalate) and Poly (1,4-Cyclohexane Dimethylene Isosorbide Terephthalate). Nanomaterials 2018, 8, 56. [Google Scholar] [CrossRef]
- Tungprapa, S.; Puangparn, T.; Weerasombut, M.; Jangchud, I.; Fakum, P.; Semongkhol, S.; Meechaisue, C.; Supaphol, P. Electrospun cellulose acetate fibers: Effect of solvent system on morphology and fiber diameter. Cellulose 2007, 14, 563–575. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Tan, N.C.B. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Choi, H.Y.; Bae, J.H.; Hasegawa, Y.; An, S.; Kim, I.S.; Lee, H.; Kim, M. Thiol-functionalized cellulose nanofiber membranes for the effective adsorption of heavy metal ions in water. Carbohydr. Polym. 2020, 234, 115881. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Lee, T.S.; Park, W.H. Electrospinning of ultrafine cellulose acetate fibers: Studies of a new solvent system and deacetylation of ultrafine cellulose acetate fibers. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 5–11. [Google Scholar] [CrossRef]
- Liu, H.; Hsieh, Y.-L. Ultrafine fibrous cellulose membranes from electrospinning of cellulose acetate. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2119–2129. [Google Scholar] [CrossRef]
- Liu, H.; Hsieh, Y.-L. Surface methacrylation and graft copolymerization of ultrafine cellulose fibers. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 953–964. [Google Scholar] [CrossRef]
- Ma, Z.; Kotaki, M.; Ramakrishna, S. Electrospun cellulose nanofiber as affinity membrane. J. Membr. Sci. 2005, 265, 115–123. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, M.; Liu, R.; Li, Y.; Wang, D.; Tan, J.; Wu, R.; Huang, Y. Electrospun membrane of cellulose acetate for heavy metal ion adsorption in water treatment. Carbohydr. Polym. 2011, 83, 743–748. [Google Scholar] [CrossRef]
- Mangolim, C.S.; Moriwaki, C.; Nogueira, A.C.; Sato, F.; Baesso, M.L.; Neto, A.M.; Matioli, G. Curcumin–β-cyclodextrin inclusion complex: Stability, solubility, characterisation by FT-IR, FT-Raman, X-ray diffraction and photoacoustic spectroscopy, and food application. Food Chem. 2014, 153, 361–370. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, R.R.M.; Senna, A.M.; Botaro, V.R. Influence of degree of substitution on thermal dynamic mechanical and physicochemical properties of cellulose acetate. Ind. Crops Prod. 2017, 109, 452–458. [Google Scholar] [CrossRef]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of pH, Temperature, and Molecular Environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef]
- Angelini, G.; Pasc, A.; Gasbarri, C. Curcumin in silver nanoparticles aqueous solution: Kinetics of keto-enol tautomerism and effects on AgNPs. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125235. [Google Scholar] [CrossRef]
- Bhatia, N.K.; Kishor, S.; Katyal, N.; Gogoi, P.; Narang, P.; Deep, S. Effect of pH and temperature on conformational equilibria and aggregation behaviour of curcumin in aqueous binary mixtures of ethanol. RSC Adv. 2016, 6, 103275–103288. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Lee, H.; Kim, M.; Park, Y.C. Coloration and Chromatic Sensing Behavior of Electrospun Cellulose Fibers with Curcumin. Nanomaterials 2021, 11, 222. https://doi.org/10.3390/nano11010222
Kim M, Lee H, Kim M, Park YC. Coloration and Chromatic Sensing Behavior of Electrospun Cellulose Fibers with Curcumin. Nanomaterials. 2021; 11(1):222. https://doi.org/10.3390/nano11010222
Chicago/Turabian StyleKim, Minhee, Hoik Lee, Myungwoong Kim, and Yoon Cheol Park. 2021. "Coloration and Chromatic Sensing Behavior of Electrospun Cellulose Fibers with Curcumin" Nanomaterials 11, no. 1: 222. https://doi.org/10.3390/nano11010222
APA StyleKim, M., Lee, H., Kim, M., & Park, Y. C. (2021). Coloration and Chromatic Sensing Behavior of Electrospun Cellulose Fibers with Curcumin. Nanomaterials, 11(1), 222. https://doi.org/10.3390/nano11010222






